Abstract
Background
Osborne-Mendel (OM) rats are prone to obesity when fed a high fat diet, while S5B/Pl (S5B) rats are resistant to diet-induced obesity when fed the same diet. OM rats have a decreased satiation response to fatty acids infused in the gastrointestinal tract, compared to S5B rats. One possible explanation is that OM rats are less sensitive to the satiating hormone, glucagon-like peptide 1 (GLP-1). GLP-1 is produced in the small intestine and is released in response to a meal. The current experiments examined the role of GLP-1 in OM and S5B rats.
Methods
Experiment 1 examined preproglucagon mRNA expression in the ileum of OM and S5B rats fed a high fat (55% kcal) or low fat (10% kcal) diet. Experiment 2 investigated the effects of a 2h high fat meal following a 24h fast in OM and S5B rats on circulating GLP-1 (active) levels. Experiment 3 examined the effects of Exendin-4 (GLP-1 receptor agonist) administration on the intake of a high fat or a low fat diet in OM and S5B rats.
Results
Preproglucagon mRNA levels were increased in the ileum of OM rats compared to S5B rats and were increased by high fat diet in OM and S5B rats. OM and S5B rats exhibited a similar meal-initiated increase in circulating GLP-1 (active) levels. Exendin-4 dose-dependently decreased food intake to a greater extent in S5B rats, compared to OM rats. The intake of low fat diet, compared to the intake of high fat diet, was more sensitive to the effects of Exendin-4 in these strains.
Conclusions
These results suggest that though OM and S5B rats have similar preproglucagon mRNA expression in the ileum and circulating GLP-1 levels, OM rats are less sensitive to the satiating effects of GLP-1. Therefore, dysregulation of the GLP-1 system may be a mechanism through which OM rats overeat and gain weight.
Keywords: obesity-prone, obesity-resistant, preproglucagon, GLP-1, High fat diet, Exendin 4
Osborne-Mendel (OM) and S5B/Pl (S5B) rats are established animals models used to examine individual differences in the response to dietary fat and mechanisms which lead to the over-consumption of dietary fat and subsequent obesity in animals that are prone to obesity (1–8). OM rats are more susceptible to diet-induced obesity and gain more weight and body fat when fed a high fat diet, than S5B rats, which are resistant to diet-induced obesity. Individual differences in the response to dietary fat between OM and S5B rats have been examined in a variety of paradigms, however, the response to dietary fat in the gastrointestinal (GI) tract is of particular interest since the GI tract produces hormonal signals that influence hunger and satiety. The response to nutrients in the GI tract has been examined in OM and S5B rats. Duodenal infusions of Intralipid and sodium linoleate on sham-fed animals (rats with a gastric cannula to drain gastric contents) suppressed food intake more completely and for a longer period of time in S5B rats compared to OM rats (2). These data suggest that OM rats have a decreased satiation response to a high fat diet and that differences in GI satiating mechanisms may contribute to the increased susceptibility to obesity in OM rats.
A number of GI hormones modulate food intake. Most of these GI hormones inhibit feeding (e.g. cholecystokinin, glucagon-like peptide 1, glucose-insulin stimulating peptide, peptide YY and oxyntomodulin); only one, ghrelin, increases feeding (9–14). The effects of ghrelin have been investigated in OM and S5B rats (4). Injection of growth hormone releasing peptide-2 (GHRP-2), which binds to the same growth hormone secretogogue receptor as ghrelin had differential effects in OM and S5B rats. In satiated S5B rats, GHRP-2 stimulated intake of low fat diet, but not high fat diet. In contrast, the satiated OM rats showed an increase in high fat diet intake after GHRP-2, with no effect on intake of low fat diet. Thus ghrelin may be an important component of the afferent signaling that differentiates these strains and their choice of a high fat diet.
The inhibitory effects of cholecystokinin (CCK-8) on feeding behavior have also been examined in OM and S5B rats (8). Though it was predicted that there would be a strain difference in the response to CCK-8, whose inhibitory effects on feeding are relayed to the brain by the vagus nerve, the inhibition of food intake by CCK-8 was similar in OM and S5B rats (8).
Glucagon-like peptide 1 (GLP-1) is one of several peptide hormones produced peripherally in the L-cells of the small intestine, primarily in the distal small intestine (ileum) that induces satiation (15;16). GLP-1 levels are increased within minutes of consuming a meal, remain elevated for 3 hours and provide a negative feedback signal to the brain, which leads to meal termination. GLP-1 is released in proportion to the caloric content of the meal. Studies suggest that circulating GLP-1 levels are lower after a meal in obese people than in lean controls (12;17–19). GLP-1 receptor agonists reduce blood glucose and food intake in rodents and chronic treatment with GLP-1 agonists results in loss of body weight (20). GLP-1 receptors are expressed in a variety of peripheral tissues, including vagal afferent fibers (9;15;21–23) and in various regions of the brain, including the brainstem and hypothalamus. The anorectic effects of GLP-1 are preserved in obesity (24).
The current experiments were conducted to assess the role of GLP-1 on the intake of a high fat diet in OM and S5B rats. In Experiment 1, the effect of either a high fat or a low fat diet on preproglucagon mRNA levels in the ileum of OM and S5B rats was investigated. It was hypothesized that preproglucagon mRNA levels would be increased following high fat food intake in S5B/Pl rats, but not OM rats. In Experiment 2, plasma levels of active GLP-1 from fed and fasted OM and S5B rats fed a high fat diet were analyzed. It was hypothesized that OM rats would release less GLP-1 (active) after a high fat meal, than S5B rats. In Experiment 3, Exendin 4 (Ex-4), a GLP-1 receptor agonist was administered to 24h fasted OM and S5B rats fed either a high fat or a low fat diet. It was hypothesized that OM rats would be less sensitive to the satiating effects of Ex-4.
Methods
Animals
The 8–9 week old male Osborne-Mendel (OM) and S5B/Pl (S5B) rats used in these studies were bred in Pennington Biomedical Research Center breeding colonies. Rats were individually housed in an AAALAC approved animal facility on a 12/12h light/dark cycle (lights on at 0700) with food and water available. Animals were given access to a pelleted high fat (55% kcal from fat)/low carbohydrate (21% kcal from carbohydrate) diet or a pelleted low fat (10% kcal from fat)/high carbohydrate (66% kcal from carbohydrate) (25–27). All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Experiment 1: Real Time PCR analysis of preproglucagon mRNA levels in the ileum
OM and S5B rats (8–9 weeks old) were fed either high fat or low fat diet for 2 weeks prior to sacrifice (sacrificed at 10–11 weeks of age). Food intake was measured daily, body weight was measured weekly and an index of body fat was determined at the time of sacrifice by measurement of the retroperitoneal and epididymal fat pads ((fat pad weight (g)/body weight (g))*100). For Real-time PCR, animals were euthanized by decapitation. A one inch section of the ileum was removed, thoroughly cleaned and the enterocytes were removed by gentle scraping with a clean metal spatula. Excised enterocytes were immediately frozen on dry ice and stored at −80°C until further processing.
Real-time Polymerase Chain Reaction (PCR)
RNA was isolated from enterocytes using Tri-Reagent (Molecular Research Ctr, Cincinnati, OH USA) and RNeasy Minikit procedures (Qiagen, Valencia, CA USA) and based on previous experiments (27). Briefly, enterocytes were homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform was added to the lysate, and the mixture was centrifuged (12,000×g) in phase lock tubes to separate RNA. Ethanol (70%) was added to the upper aqueous phase, which was filtered by centrifugation (8000×g). Following multiple washes, the samples were subjected to an elution step using RNAase-free water. Reverse transcription (RT) was conducted using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). For RT, 2.0μg of RNA from each sample was added to random primers (10×), dNTP (25×), MultiScribe Reverse Transcriptase (50U/μl) and RT buffer (10×) and incubated in a thermal cycler (PTC-100, MJ Research, Inc, Watertown, MA, USA) for 10 min at 27°C, then for 120 min at 37°C. Taqman Gene Expression Assays (Applied Biosystems) were used to assess levels of preproglucagon and the housekeeping gene, 18s. For Real-time PCR, Taqman Universal PCR Master Mix (Applied Biosystems), gene expression assay, and RT product (10ng) were added to a 384 well plate. The cycling parameters consisted of an initial 2 min incubation at 50 °C, followed by 10 min at 95 °C, then 15 sec at 95 °C, and a 1 min annealing/extension step at 60 °C (40 cycles). The quantity of prepro-glucagon mRNA levels were based on a standard curve and normalized to 18S levels (ABI Prism 7900 Sequence Detection System, Applied Biosystems).
Experiment 2: Circulating GLP-1(active) levels
OM and S5B rats (8–9 weeks old) were fed a high fat diet for 2 weeks prior to sacrifice. All rats were subjected to a 24h fast. At the end of the 24 fast, half of the rats were given access to high fat diet for 2h. At the end of this 2h period, rats (10–11 weeks old) were sacrificed. Animals were extensively handled during this experiment to habituate them to the experimental procedures.. At the time of sacrifice, trunk blood was collected to ensure adequate amounts of plasma for future assays. DPP-IV inhibitor was added to each sample (10μl DPP-IV inhibitor per 1ml blood), and samples were placed on ice. Blood was centrifuged at 3000rpm for 10minutes at 4°C and plasma was removed from tubes and stored at −80°C until processing. A GLP-1 (active, 7–36) amide) ELISA kit was used to assess plasma GLP-1 in this experiment (Linco Research, St. Charles, MO). The GLP-1 assay was conducted as described in the protocol provided with the ELISA kit. Briefly, on Day 1, plasma and buffer were added to the ELISA plate and the plate was incubated overnight. On Day 2, following a series of washes, detection conjugate and substrate were added to each well as described in the protocol and the plate was read on a fluorescence plate reader with an excitation/emission wavelength of 355nm/460nm. Individual values were determined from a standard curve based on standards provided by the manufacturer.
Experiment 3: Exendin 4 administration on food intake
OM and S5B rats (8–9 weeks old) were fed either the high fat or the low fat diet for 4 weeks prior to testing. During this period, all animals were habituated to periods of fasting, in which food was removed for 24 hours. Animals were also habituated to intraperitoneal injection procedures and during habituation, received an intraperitoneal injection of saline ten minutes prior to the return of food. Animals were tested using a Latin square design in which each rat received each dose of Ex- 4. Injections were given every 6 days. Body weight measurements indicated that the rats were gaining weight between injections. This design was used to control for any carryover effects from the drug. Prior to testing, rats were subjected to a 24h fast immediately prior to administration of varying doses of Ex-4 (1μg/kg, 5μg/kg, 10μg/kg; Sigma-Aldrich) or saline. All injections were administered by intraperitoneal injection. Ten minutes following Ex-4 administration, fresh food (high fat or low fat) was returned to the rat. Food intake was measured at 1h, 2h, 4h, and 24h following refeeding. Rats were 15–16 weeks old at the end of the experiment.
Statistical Analysis
In Experiment 1, a mixed ANOVA was conducted to assess differences in daily food intake (kcal) and weekly body weight changes (strain × diet × time). A between subjects ANOVA (strain × diet) was used to compare the index of body adiposity ((retroperitoneal + epididymal fat pad weight (g)/body weight) * 100) and preproglucagon mRNA levels in the enterocytes of the ileum in high fat and low fat fed OM and S5B rats. Bonferroni post-hoc tests were used to assess differences between individual groups. In Experiment 2, GLP-1 values were based on a standard curve. Individual sample values were analyzed by a between subjects ANOVA (strain × nutritional status). In Experiment 3, high fat food intake (kcal) and low fat food intake (kcal) following Ex-4 administration were analyzed by a mixed ANOVA (time × Ex-4 concentration × strain). An additional mixed ANOVA was conducted for each strain (time × Ex-4 concentration). Bonferroni post-hoc tests were used to compare saline to each dose of Ex-4, when a significant main effect or interaction was detected. A significance level of p<.05 was used for all tests.
Results
Experiment 1: Real Time PCR analysis of preproglucagon mRNA levels in the ileum
Food intake was measured daily for 2 weeks prior to sacrifice. A strain × day × diet interaction (F(13,442)=2.04, p<.02, Figure 1) and strain × diet interaction (F(1,34)=24.79,p<.0001) were detected. Post-hoc analyses revealed that OM rats consumed significantly more high fat diet (kcal) than low fat diet (kcal) throughout the experiment (p<.05). S5B rats consumed significantly more high fat diet than low fat diet on most days (days 1, 2, 3, 4, 7, 9, 10, 11, 12, and 14; p<.05). OM rats consumed more high fat diet than S5B rats throughout the experiment, except for Days 1 and 14, while OM rats consumed more low fat diet than S5B rats on Days 1, 10 and 12 (p<.05). Body weight was measured weekly prior to sacrifice.
Figure 1.
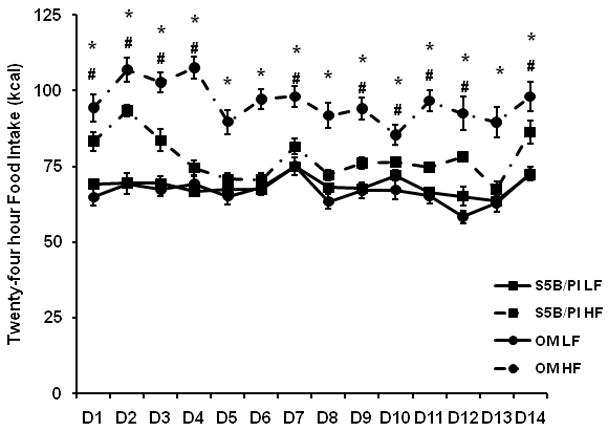
Daily low fat and high fat food intake was measured for 14 days. OM rats ate more high fat food (HF) than low fat food (LF) throughout the 14 days. S5B rats ate more high fat food (HF) than low fat food (LF) on most days. OM rats consumed more high fat diet than S5B rats from Days 2–13. Data is expressed in kilocalories (kcal) and shown as mean ± SEM. * OM high fat diet vs. OM low fat diet; # S5B high fat diet vs. S5B low fat diet; (p<.05).
Change in body weight from pre-diet weight was calculated each week. Main effects for strain and diet for Week 1 were detected (F(1,34)=82.31, p<.0001; F(1,34)=43.90, p<.0001, respectively; See Figure 2A) and a strain × diet interaction was detected for Week 2 (F(1,34)=20.28, p<.0001). OM rats gained more body weight than S5B rats. OM rats consuming high fat diet gained more weight than OM rats consuming low fat diet and S5B rats consuming high fat diet gained more weight than S5B rats consuming low fat diet (p<.05). An index of body fat was determined at the time of sacrifice by weighing the retroperitoneal and epididymal fat pads. A strain × diet interaction was detected for body fat index in these animals (F(1,34)=21.95, p<.0001; See Figure 2B). Post-hoc tests revealed that OM rats had a higher index of body fat than S5B rats and OM rats consuming a high fat diet had a higher index of body fat than OM rats consuming a low fat diet (p<.05).
Figure 2.
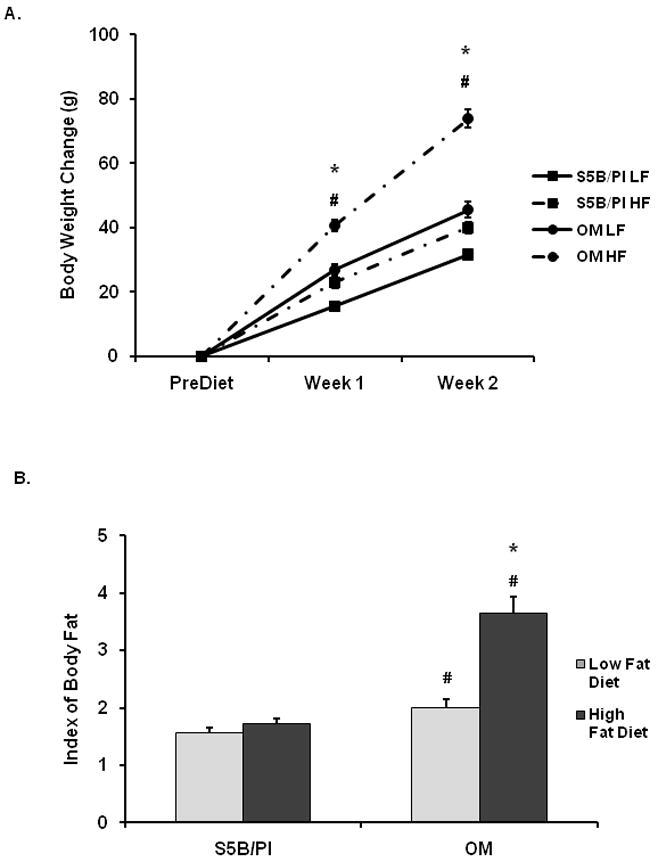
A. Weekly body weight change indicated that OM rats fed a high fat diet (HF) gained more weight than OM rats fed a low fat diet (LF). S5B rats fed a high fat diet (HF) gained more weight than S5B rats fed a low fat diet (LF). Data are expressed as the cumulative weekly change from pre-diet body weight and shown as mean ± SEM. * OM high fat diet vs. OM low fat diet; # S5B high fat diet vs. S5B low fat diet; (p<.05). B. OM rats had a higher index of body fat than S5B rats. High fat diet increased the index of body fat in OM rats, but not S5B rats. The index of body fat is based on retroperitoneal and epididymal fat pad weight and shown as mean ± SEM. * OM low fat diet vs. OM high fat diet; # OM vs. S5B: (p<.05).
Preproglucagon mRNA levels were measured in ileal enterocytes of OM and S5B rats following 2 weeks access to either a high fat or a low fat diet. A between-subjects ANOVA revealed a main effect for strain (F(1,35) = 16.07, p< .0005; See Figure 3). Post-hoc analyses indicated significantly higher levels of preproglucagon mRNA in the ileal enterocytes of OM rats fed a low fat diet compared to S5B rats fed a low fat diet (p<.05). Additionally, the consumption of a high fat diet increased preproglucagon mRNA levels in both OM and S5B rats (p<.05). There was a 20.5% ± 0.15 vs. 93.3% ± 0.25% (mean ± SEM) increase in preproglucagon mRNA levels in high fat fed OM and S5B rats compared to low fat fed OM and S5B rats, respectively (t(17)=2.39, p<.05).
Figure 3.
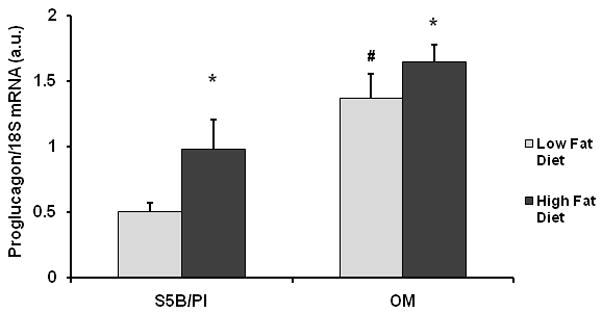
Preproglucagon mRNA levels were higher in the ileum of OM than S5B rat following 14d access to a high fat or low fat diet. Access to a high fat diet increased preproglucagon mRNA levels in both OM and S5B rats. Preproglucagon mRNA levels were normalized to 18S and are shown as mean ± SEM. * high fat diet vs. low fat diet; # OM vs. S5B; (p<.05).
Experiment 2: Circulating GLP-1(active) levels
Circulating active GLP-1 levels in OM and S5B rats were measured following either a 24h fast or a 24h fast followed by 2h access to a high fat diet. As expected, a significant main effect was detected for nutritional status (F(1,13)=18.5, p<.001; See Figure 4). Post-hoc analyses revealed that refeeding for 2h with a high fat diet increased circulating active GLP-1 levels in OM and S5B rats (p<.05). No differences were detected between the response to the high fat meal in OM and S5B rats.
Figure 4.
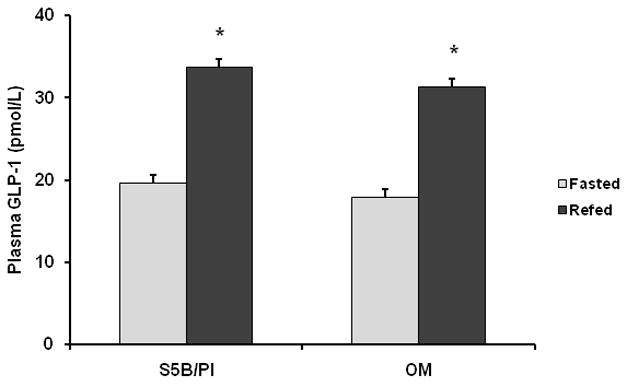
Plasma GLP-1 (active) levels were measured in OM and S5B rats fed a high fat diet and either fasted for 24h or fasted for 24h and then refed for 2h. In OM and S5B rats, circulating GLP-1 (active) levels were increased following refeeding. Data are shown as mean ± SEM. * Fasted vs. Refed, (p<.05).
Experiment 3: Exendin 4 administration on food intake
Food intake was measured following Ex-4 administration in OM and S5B rats fed either a high fat or a low fat diet. Several significant interaction effects were detected between time following injection, dose of Ex-4 and rat strain (time × Ex-4 × strain, F(9,147) = 4.38, p<.0001; time × Ex-4, F(9,147) = 3.94, p< .0001; time × strain F(3,147) = 3.27, p<.02; Ex-4 × strain F(3,49) = 11.18, p<.00001). Post-hoc tests revealed that 1h, 2h and 4h following injection, obesity-resistant S5B rats receiving 1μg/kg, 5μg/kg and 10μg/kg Ex-4 ate significantly less high fat and low fat diet than saline-treated control S5B rats (p<.05; See Figure 5A). Additionally, post-hoc analyses revealed decreases in low fat food intake in obesity-prone OM rats at 5μg/kg and 10μg/kg of Ex-4 (p<.05; See Figure 5B) compared to saline-treated control OM rats at 1h, 2h and 4h. High fat food intake was decreased in OM rats receiving 5μg/kg and 10μg/kg Ex-4 at 1h following administration and in OM rats receiving 10μg/kg Ex-4 at 2h following administration (p<.05; See Figure 5B).
Figure 5.
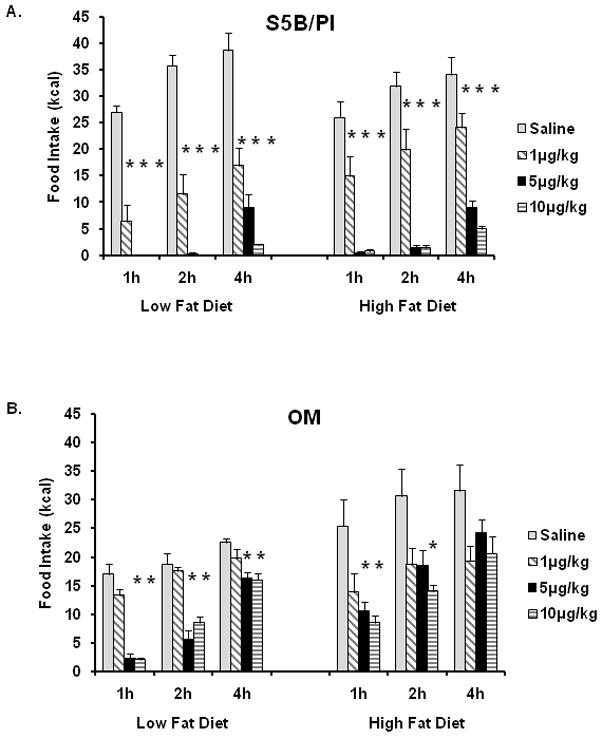
A. S5B rats were administered varying doses of Ex-4 following a 24h fast and given access to either low fat or high fat diet. Ex-4 dose dependently decreased high fat and low fat diet intake at 1h, 2h, and 4h. B. Following a 24h fast, OM were administered Ex-4 and low fat and high fat intake were measured. The two highest doses of Ex-4 (5μg/kg and 10μg/kg) decreased low fat diet intake at 1h, 2h, and 4h and high fat diet intake at 1h. The highest dose of Ex-4 decreased high fat food intake at 2h, but not at 4h. Data are expressed as cumulative food intake in kilocalories (kcal) and shown as mean ± SEM. * saline vs. Ex-4, (p<.05).
High fat and low fat food intake were also measured at 24h following administration of Ex-4. S5B rats ate less low fat diet at 24h when administered 1μg/kg, 5μg/kg and 10μg/kg Ex-4 compared to saline. High fat diet intake in S5B rats was reduced at 24h following administration of 5μg/kg and 10μg/kg Ex-4 (See Figure 6A, p<.05). Twenty-four hour intake of low fat diet was decreased in OM rats following administration of 5μg/kg and 10μg/kg Ex-4. However, in high fat fed rats, only 10μg/kg Ex-4 decreased high fat food intake in OM rats compared to saline-injected controls (See Figure 6B, p<.05).
Figure 6.
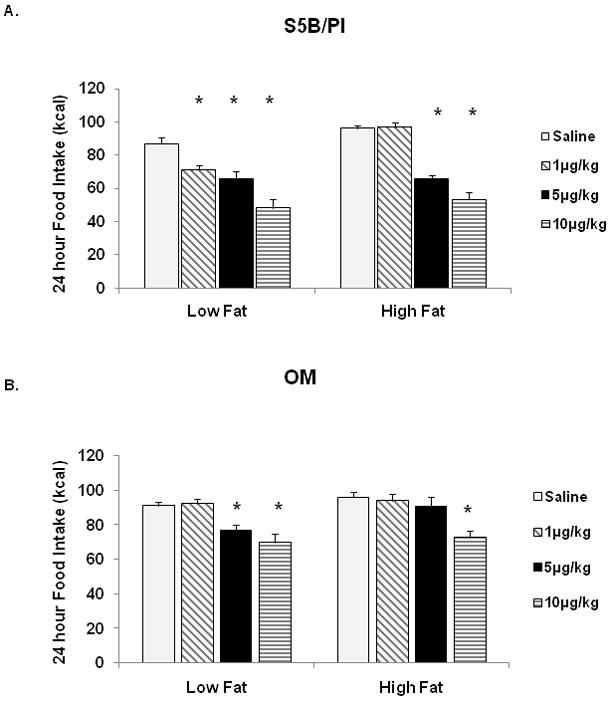
High fat and low fat food intake was measured 24h following Ex-4 administration. A. In S5B rats, low fat and high fat diet intake was suppressed by Ex- 4 (1μg/kg, 5μg/kg, 10μg/kg and 5μg/kg, 10μg/kg). B. In OM rats, Ex-4 administration suppressed low fat intake and high fat food intake (5μg/kg, 10μg/kg and 10μg/kg). Data are expressed as cumulative food intake in kilocalories (kcal) and shown as mean ± SEM. * saline vs. Ex-4, (p<.05).
Discussion
OM rats are prone to diet-induced obesity, whereas S5B rats are resistant to diet-induced obesity. Therefore, these rat strains have been used to examine individual differences in the response to a high fat diet. When given ad libitum access to a high fat diet, OM rats consume more high fat diet, gain more weight, and gain more body adiposity than S5B rats (1–8). In the current experiments, we were particularly interested in the individual differences in the response to dietary fat by the GI tract in OM and S5B rats. Previous studies indicate that OM rats are less responsive to the satiating effects of intraduodenal infusions of sodium linoleate and Intralipid than S5B rats (2). This suggests that the satiation response to fatty acids is blunted in obesity-prone OM rats. One possible mechanism mediating the decreased satiation response to fatty acids in OM rats is the hormone, GLP-1. The current experiments were conducted to assess the role of GLP-1 in the intake of a high fat diet in an established animal model of obesity.
In Experiment 1, OM and S5B rats were fed either a high fat diet (55% kcal from fat) or a low fat diet (10% kcal from fat) for 14 days. Daily food intake, weekly body weight and an index of body adiposity were assessed. As expected OM rats consumed more high fat diet, than low fat diet, and OM rats consumed more high fat diet than S5B rats (See Figure 1). OM rats given access to a high fat diet were hyperphagic throughout the experiment. As shown in Figure 2A, OM rats fed the high fat diet gained more weight than OM rats fed the low fat diet (73.9 ± 2.8g vs. 45.5 ± 2.4g, mean ± SEM, respectively). S5B rats fed the high fat diet gained more weight than S5B rats fed the low fat diet (40.0 ± 1.9g vs. 31.6 ± 1.7g, mean ± SEM, respectively). Based on our index of body fat, OM rats had level of body fat than S5B rats (See Figure 2B), which was exaggerated by consumption of the high fat diet.
Using Real-Time PCR, preproglucagon mRNA levels in the ileum were measured in OM and S5B rats fed the low fat or high fat diets. Based on the previous report (2) that OM rats exhibited a decreased satiety response to intraduodenal infusions of fatty acids, we expected preproglucagon mRNA levels to be similar or even lower in OM than S5B rats and that these two strains would exhibit a differential response to the high fat diet. Our data suggest that preproglucagon mRNA levels in the ileal enterocytes were higher in OM rats compared to S5B rats and that high fat diet increased preproglucagon mRNA expression in both OM and S5B rats (See Figure 3). The relative increase in preproglucagon expression in response to the high fat diet differed between strains. S5B rats exhibited a greater increase in preproglucagon expression in response to high fat diet (93.3 ± 0.25%, mean ± SEM), than OM rats (20.5 ± 0.15%, mean ± SEM). One possible explanation for these results is that the precursor protein, preproglucagon, can be processed into several different biological peptides including, GLP-1, GLP-2, glucagon, glucose-dependent insulinotropic peptide (GIP), glicentin-related pancreatic peptide (GRPP) and glicentin, which is later cleaved into oxyntomodulin and GRPP (28–30). The enzymes, prohormone convertase 1 and 2, cleave proglucagon into different products depending on the tissue (31). In the pancreas, glucagon is the major product and in the brain and intestine, GLP-1, GLP-2 and oxyntomodulin are the major products (32). Therefore, our data suggest that the precursor for GLP-1, GLP-2 and oxyntomodulin is elevated in OM rats and is increased to differing degrees by a high fat diet in OM and S5B rats.
Experiment 2 was conducted to determine if circulating levels of GLP-1 differed between OM and S5B rats fed a high fat diet and to determine if the meal-initiated release of GLP-1 differed between the two strains. GLP-1 levels are increased within minutes of consuming a meal, remain elevated for 3 hours and provide a negative feedback signal to the brain, which leads to meal termination. Our data suggest that OM and S5B rats exhibit a similar meal-initiated increase in circulating GLP-1 following 2h access to a high fat diet (See Figure 4). Circulating GLP-1 binds to GLP-1 receptors in the central nervous system and the peripheral nervous system (9;15;21–23). The data from Experiments 1 and 2, suggest that obesity-prone OM rats do not exhibit deficits in preproglucagon mRNA expression or in meal-initiated GLP-1 release, compared to obesity-resistant S5B rats. Therefore, it is possible that a dysregulation of central and peripheral GLP-1 receptors mediate the decreased satiation response to fatty acids that has been found in OM rats, compared to S5B rats. To begin to examine this possibility, we conducted Experiment 3.
In Experiment 3, Ex-4 (Ex-4), a GLP-1 receptor agonist, was administered to 24h fasted OM and S5B rats fed either a high fat or a low fat diet. It was hypothesized that OM rats would be less sensitive to the satiating effects of Ex-4. The data generated in Experiment 3 suggest that Ex-4 administration dose-dependently decreased high fat and low fat food intake in fasted S5B rats for up to 24h following administration (See Figure 5A). As hypothesized, OM rats were less sensitive to the satiating effects of Ex-4 than S5B rats. Ex-4 administration transiently decreased high fat food intake in fasted OM rats (See Figure 5B). Unlike S5B rats, only the two highest doses of Ex-4 had any effect on either low fat or high fat food intake in OM rats. At the two highest doses of Ex-4, S5B rats had an almost complete suppression of food intake.
The current findings that obesity-prone OM rats exhibit higher levels of preproglucagon mRNA in the ileal enterocytes than S5B rats, exhibit a similar release of GLP-1 following a meal as S5B rats, but are less sensitive to the satiating effects of the GLP-1 receptor agonist, Ex-4, than S5B rats, suggest that dysregulation of GLP-1 receptors may mediate the decreased satiation response to fatty acids in OM rats. This decreased satiation response would then lead to the increased consumption of fatty acids/high fat diets, which subsequently leads to obesity in obesity-prone OM rats. Therefore, deficits in GLP-1 signaling are likely a mechanism by which OM rats become obese and a mechanism that requires further investigation.
Acknowledgments
This research was supported by NIH NIDDK 32089 to G. A. Bray. This work used the Animal Models and Phenotyping Core and the Genomics Core facilities that are supported in part by COBRE (NIH P20-RR021945) and NORC (1P30-DK072476) center grants from the National Institute of Health. The authors would like to thank Christine Blackmon and Katherine Pyburn for their technical assistance on this project.
This research was supported by NIDDK 32089 to G.A. Bray and NIDDK1P0DK072476.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Reference List
- 1.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides. 2006;27:3292–3298. doi: 10.1016/j.peptides.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg D, McCaffery J, Potack JZ, Bray GA, York DA. Differential satiating effects of fats in the small intestine of obesity-resistant and obesity-prone rats. Physiol Behav. 1999;66:621–626. doi: 10.1016/s0031-9384(98)00336-9. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara Y, White CL, Kageyama H, Kageyama A, York DA, Bray GA. Effects of diet and time of the day on serum and CSF leptin levels in Osborne-Mendel and S5B/Pl rats. Obes Res. 2004;12:1067–1076. doi: 10.1038/oby.2004.134. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, York DA, Bray GA. Regulation of ghrelin gene expression in stomach and feeding response to a ghrelin analogue in two strains of rats. Peptides. 2004;25:2171–2177. doi: 10.1016/j.peptides.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. Differences in adiposyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes. 2008;32:853–862. doi: 10.1038/sj.ijo.0803792. [DOI] [PubMed] [Google Scholar]
- 6.Pittman D, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, Gilbertson TA. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Sen. 2008;33:449–460. doi: 10.1093/chemse/bjn012. [DOI] [PubMed] [Google Scholar]
- 7.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone, but not obesity-resistant rats. Behav Brain Res. 2007;180:190–196. doi: 10.1016/j.bbr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White CL, Ishihara Y, York DA, Bray GA. Effect of meta-chlorophenylpiperazine and cholecystokinin on food intake of Osborne-Mendel and S5B/Pl rats. Obesity. 2007;15:624–631. doi: 10.1038/oby.2007.579. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhri O, Bloom SR. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heijboer AC, Piji H, van den Hoek AM, Havekes LM, Romijn JA, Corssmit EP. Gut-brain axis: regulation of glucose metabolism. J Neuroendocrinol. 2006;18:883–894. doi: 10.1111/j.1365-2826.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 11.Moran TH. Gut peptide signaling in the control of food intake. Obesity. 2006;14(Suppl 5):250S–253S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 12.Moran TH. Gut peptides in the control of food intake. Int J Obes. 2009;33:S7–S10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 13.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterol. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterol. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 15.Beglinger C, Degen C. Gastrointestinal satiety signals in humans - physiologic roles for GLP-1 and PYY ? Physiol Behav. 2006;89:460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Druce MR, Small CJ, Bloom SR. Minireview: Gut peptides regulating satiety. Endocrinol. 2004;145:2660–2665. doi: 10.1210/en.2004-0089. [DOI] [PubMed] [Google Scholar]
- 17.Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterol. 1994;107:1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 18.Moran TH, Dailey MJ. Minireview: Gut peptides: Targets for antiobesity drug development? Endocrinol. 2009;150:2526–2530. doi: 10.1210/en.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naslund E, Hellstrom PM. Glucagon-like peptide-1 in the pathogenesis of obesity. Drug News Perspect. 1998;11:92–97. doi: 10.1358/dnp.1998.11.2.659947. [DOI] [PubMed] [Google Scholar]
- 20.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinol. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- 23.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 24.Davis HR, Mullins DE, Pines JM, Hoos LM, France CF, Compton DS, Graziano MP, Sybertz EJ, Strader CD, Van Heek M. Effect of chronic central administration of glucagon-like peptide 1 (7–36) amide on food consumption and body weight in normal and obese rats. Obes Res. 1998;6:147–156. doi: 10.1002/j.1550-8528.1998.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Chen J, York DA. Chronic ICV enterostatin preferentially reduced fat intake and lowered body weight. Peptides. 1997;18:657–661. doi: 10.1016/s0196-9781(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 26.Primeaux SD, York DA, Bray GA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27:1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Primeaux SD, Blackmon C, Barnes MJ, Braymer HD, Bray GA. Central administration of the RFamide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides. 2008;29:1994–2000. doi: 10.1016/j.peptides.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinol. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 29.Lopez LC, Frazier ML, Su CJ, Kumar A, Saunders GF. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983;80:5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25 (Suppl 5):S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ. Glucagon-like peptide 1 (GLP-1): An intestinal hormone, signalling nutritional abundance, with an unusual therapeutic potential. Trends Endocrinol Metab. 1999;10:229–235. doi: 10.1016/s1043-2760(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 32.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]


