Abstract
The E46K is a point mutation in α-synuclein (α-syn) that causes familial Parkinsonism with Lewy body dementia. We have now generated a cell model of Parkinsonism/Parkinson’s disease (PD) and demonstrated cell toxicity after expression of E46K in the differentiated PC12 cells. E46K α-syn inhibited proteasome activity and induced mitochondrial depolarization in the cell model. Baicalein has been reported to inhibit fibrillation of wild type α-syn in vitro, and to protect neurons against several chemical-induced models of PD. We now report that baicalein significantly attenuated E46K-induced mitochondrial depolarization and proteasome inhibition, and protected cells against E46K-induced toxicity in a cell model of PD. Baicalein also reduced E46K fibrilization in vitro, with a concentration-dependent decrease in beta sheet conformation, though it increased some oligomeric species, and decreased formation of E46K α-syn-induced aggregates and rescued toxicity in N2A cells. Taken together, these data indicate that mitochondrial dysfunction, proteasome inhibition and specific aspects of abnormal E46K aggregation accompany E46K α-syn-induced cell toxicity, and baicalein can protect as well as altering aggregation properties. Baicalein has potential as a tool to understand the relation between different aggregation species and toxicity, and might be a candidate compound for further validation by using in vivo α-syn genetic PD models.
Keywords: Parkinson’s disease, proteasome inhibition, baicalein, α-synuclein, mitochondria depolarization, aggregation
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease, causing tremor, rigidity and bradykinesia, postural and autonomic instability, and cognitive and emotional disorders among other cardinal signs (Betarbet et al. 2006; Fahn 2003; Jankovic 2008; Zarranz et al. 2004). It is characterized by the loss of dopaminergic neurons in the substantia nigra of the brain and cytosolic inclusions known as Lewy bodies (Chinta and Andersen 2005; Eriksen et al. 2003; Wakabayashi and Takahashi 2007). Although there are effective symptomatic agents to treat the motor features of PD, there is no neuroprotective treatment to prevent the progressive loss of dopaminergic neurons.
α-Synuclein (α-syn) is a protein of unclear function that localizes within presynaptic terminals in the CNS (Clayton and George 1998; Clayton and George 1999) and, together with ubiquitin and synphilin-1, is among the major components of Lewy bodies in PD (Spillantini et al. 1997). Three mutations of α-syn were identified to cause familial Parkinsonism (Kruger et al. 1998; Polymeropoulos et al. 1997; Zarranz et al. 2004). Although mutation of α-syn only occurs rarely, the similarity between the genetic and sporadic forms of PD indicates the genetic PD models help us to dissect the key biochemical and pathogenic pathways of PD. Thus, the genetic forms of Parkinsonism have opened new avenues for research in PD and disorders characterized by accumulation of α-syn (Brice 2006; Corti and Brice 2003; Papapetropoulos et al. 2003). Furthermore, altered expression of normal α-syn (due to duplication or triplication at the α-syn locus, and possibly due to promoter variants) appears to be a risk factor for sporadic PD (Singleton 2005; Singleton et al. 2003). Therefore, investigation of α-syn-induced PD pathogenesis may not only shed light on genetic forms of PD, but may also elucidate the pathophysiology of sporadic PD, and led to the development of potential therapeutics.
The E46K mutation was identified after the A30P and A53T mutations, and helped to confirm the association of α-syn mutations with familial Parkinsonism. E46K mutation patients have dementia with Lewy bodies, Parkinsonism or PD. In addition, E46K α-syn forms insoluble fibrils in vitro more rapidly than the wild-type protein (Fredenburg et al. 2007). E46K α-syn shows both an increase in lipid binding and in filament assembly compared to wild-type α-syn (Choi et al. 2004). Electron microscopy revealed that E46K α-syn fibrils possess a typical amyloid ultra structure (Zarranz et al. 2004), and E46K α-syn increases amyloid fibril formation (Greenbaum et al. 2005). In mammalian cell culture, E46K α-syn aggregates more efficiently than the wild-type protein and both A30P and A53T mutations (Pandey et al. 2006). Thus, the E46K mutation may offer advantages for examining PD models. In previous studies (Smith et al. 2005; Tanaka et al. 2001), we developed cell models with inducible expression of A30P and A53T α-syn. In our current study, we generated an inducible cell model of PD by expressing E46K α-syn in PC12 cells using the Tet-off system. We found that E46K α-syn induction resulted in significant toxicity in these cells. We also created a second cell model of PD by transient expression of E46K in N2A cells. In this system, we were able to detect E46K-induced α-syn aggregates and toxicity.
Baicalein, a major flavonoid from a traditional Chinese herb Scutellaria baicalensis Georgi (Huangqin), possesses potent anti-inflammatory and antioxidant properties. It has been reported that baicalein prevents β-amyloid-induced aggregation and cell death (Wang et al. 2004; Zhu et al. 2004), inhibits fibrillation, disaggregates existing fibrils of wild type α-syn in vitro (Zhu et al. 2004), and leads to a reduced number of cells with microtubular retraction induced by α-syn aggregation in oligodendrocytes (Kragh et al. 2009). A more recent study (Hong et al. 2008) indicates that baicalein-stabilized α-syn oligomers are able to inhibit fibrillation of non-baicalein-treated α-syn. These highly stable α-syn oligomers that inhibit fibrillation do not disrupt the integrity of the biological membrane, suggesting that some forms of soluble oligomer formation can be beneficial (Hong et al. 2008), though others may be toxic. However, the effect of baicalein on mutant α-syn-induced aggregation has not been studied.
Baicalein also attenuated 6-hydroxydopamine- and MPTP-induced neurotoxicity in cells and mice (Cheng et al. 2008; Im et al. 2005; Lee et al. 2005; Mu et al. 2009; Wu et al. 2006) and inhibited methamphetamine-induced loss of dopamine transporter in mouse striatum (Wu et al. 2006). Our previous study provided some initial data that baicalein can inhibit E46K α-syn-induced LDH release in PC12 cells (Kostka et al. 2008), suggesting an effect on toxicity. However, definitive evidence for an effect on toxicity was not provided, and mechanisms by which baicalein protects cells against mutant α-syn are unknown. The effect of baicalein on E46K aggregation in cells has not been investigated. In our current study, we found that E46K α-syn induced mitochondria depolarization and proteasome inhibition in differentiated PC12 cells, formed aggregates both in vitro and in cell cultures, and resulted in cell death. Baicalein decreased E46K α-syn aggregation in vitro and in cultured cells, attenuated mitochondria depolarization and proteasome inhibition, and decreased E46K-induced cell death
Materials and methods
Materials
Media and N2 supplements for cell culture were purchased from Invitrogen Inc(Carlsbad, CA, USA). NGF was purchased from Roche (Indianapolis, IN, USA). Hoechst 33342, calcein-AM, and ethidium homodimer-2 were purchased from Molecular Probes (Eugene, OR, USA) and cyclosporine A (CsA) was purchased from BioMol (Plymouth Meeting, PA, USA). Anti α-synucleinmonoclonal antibody was purchased from BD Pharmingen (Palo Alto, CA, USA). The caspase inhibitor z-VAD-fmk was purchased from Enzyme Systems products (Livermore, CA, USA). Baicalein, and Thioflavin T (ThT) were obtained from Sigma. Uranyl Acetate was obtained from Electron Microscopy Sciences (Hatfield, PA, USA).
Generation of inducible PC12 cell lines by expression of E46K mutant α-synuclein
Tet-off PC12 cells (Clontech, Palo Alto, CA), stably expressing tTA, were co-transfected with 2 μg of E46K α-syn construct and 0.2 μg pTK-Hyg (Clontech, Palo Alto, CA) plasmid by using Lipofectamine Plus (Life Technologies Inc., Gaithersburg, MD). The cells were selected in the DMEM (GIBCO) with 5% fetal bovine serum (FBS), 10% horse serum, 100 μg/ml G418, 200 μg/ml hygromycin, 200 ng/ml doxycycline (Dox), 100 units/ml penicillin and 100 units/ml streptomycin. After a 4- to 6-week selection at 37°C in a humidified 7% CO2 incubator, G418/hygromycin-resistant colonies were isolated and screened for transgene expression by Western blot analysis with an anti-α syn antibody (1:500, BD Pharmingen). We characterized three cell lines with higher levels of E46K expression, and chose a line that highly expressed E46K α-syn with most significant toxicity for this study (cell line 34, Fig. 1). Cells were maintained in the presence of Dox (200 ng/ml) and media was changed every 48 h. Expression of the transgene and induction of differentiation were obtained by withdrawing Dox and adding NGF to medium at the same time.
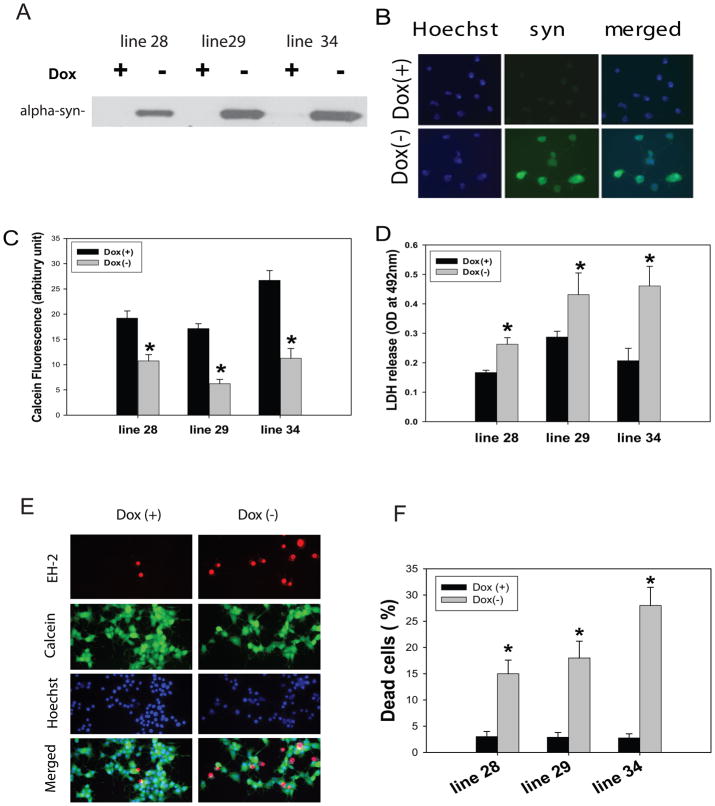
Fig 1. E46K α-synuclein expression leads to cell death in PC12 cells.
Tet-off PC12 cells were maintained in the presence of doxycycline (Dox, 200 ng/ml) to inhibit E46K α-synuclein expression. Expression of E46K α-synuclein was induced by withdrawal of Dox, and PC12 cells were differentiated by adding NGF to the medium. Three G418/hygromycin-resistant lines (line 28, 29, 34) showed higher expression of E46K α-synuclein by Western blots (A) and immunostaining (B); Cell toxicity was measured by calcein fluorescent cell viability assay (C), LDH release assay (D), and LIVE/DEAD assay (E and F) at day 7 following transgene induction. *p<0.01 compared to the values of corresponding Dox(+) group by standard student’s t-test.
N2A cell culture and transient transfection of E46K α-synuclein
Mouse N2A cells (American-type culture collection, ATCC) were maintained in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum(Invitrogen Inc) and 1% penicillin/streptomycin. Cells were grown on coverslips in 24-well plates (Corning Incorporated Life Sciences) to 50% confluence at the time of transfection. Cells were then transfected with Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s protocol. Briefly, 0.5 μg of DNA and 0.5 μl of Lipofectamine™ 2000 were diluted in 25 μl of Opti-MEM Medium, respectively. After incubation for 5 min at room temperature, DNA and Lipofectamine™ 2000 were mixed and incubated for 20 min at room temperature. 50 μl of the mixture of DNA and Lipofectamine™ 2000 were added to cultures. The medium containing DMSO or baicalein was replaced at 4 h after transfection. Cells were then fixed and processed for immunofluorescent staining at the indicated times.
In vitro aggregation assay
Lyophilyzed E46K α-syn protein was dissolved in double distilled water, diluted to a concentration of 0.8 mg/ml (50 μM) in PBS (pH 7.4), and centrifuged at 15,000 × g to remove aggregated material. A 10 mM stock solution of baicalein was prepared in 100% dimethylsulfoxide (DMSO). To carry out aggregation experiments, a 50 μM α-syn aliquot was mixed with baicalein at a final concentration of 0.1, 1, or 10 μM (final DMSO concentration of 0.1%) and incubated at 37 °C without agitation. A control reaction containing only E46K α-syn and 0.1% DMSO was also prepared.
Light scattering measurements
A 10 μL aliquot of each aggregation reaction was added to 0.99 ml of PBS (pH 7.4). A 90° light scattering measurement of each solution was taken in a 0.5 mm QS Helma cuvette using a FluoroMax-3 spectrofluorometer (Jobin Yvon Horiba) with both excitation and emission wavelengths set at 450 nm and a 1 nm slit width. Data are reported as an average of three scans and were determined following subtraction of the buffer signal.
Thioflavin T fluorescence assay
Thioflavin T (ThT) fluorescence was carried out on the samples described above using a FluoroMax-3 spectrofluorometer. Following addition of ThT to a final concentration of 50 μM and an excitation at 450 nm (slit width of 2.5 nm), fluorescence was measured at an emission wavelength of 482 nm (and slit width of 5 nm). Data are reported as an average of three scans and were determined following subtraction of the buffer signal.
Circular Dichroism measurements
Far-UV CD spectra were collected on a Chirascan spectrometer (Applied Biophysics) using a QS Helma cuvette with a 0.5 mm path length. CD spectra were recorded with a step size of 1 nm, a bandwidth of 1 nm, and an averaging time of 1 s. Prior to CD analysis, each E46K α-syn sample was diluted in PBS (pH 7.4) to a final concentration of 0.25 mg/ml. CD data was converted to mean residue ellipticity using Pro-data software.
Electron microscopy
Aliquots of 5 μL aggregation reaction diluted 2 times with PBS (pH 7.4) were applied to formvar- and carbon-coated copper grids and incubated for 3 min. Salts were washed out with distilled water, and samples were dried, negatively stained with 2% (w/v) uranyl acetate, and visualized on a HITACHI 7600 TEM operated at 80 kV.
Statistics
Quantitative data are expressed as mean ± SE based on at least three independent experiments performed in triplicate. The difference between two groups was statistically analyzed by Student’s t-test or an analysis of variance (one-way ANOVA). A p-value <0.05 was considered significant.
Results
Expression of E46K α-syn results in cell death in differentiated PC12 cells
To investigate the role of E46K α-syn in PD pathogenesis, we generated an inducible PC12 cell model expressing E46K α-syn. Using the Tet-off gene expression system, E46K α-syn expression was induced by withdrawal of doxycycline. Expression of E46K α-syn led to progressive cell death from day 4 to day 7, and most significant cell toxicity was detected at day 7 following transgene induction(supplemental Fig 1). To quantify cytoxicity in cells expressing E46K α-syn, we employed three different assays, including the LDH release assay, a calcein fluorescent cell viability assay, and the LIVE/DEAD assay in three cell lines with high levels of E46K α-syn expression (Fig 1). There were significant cell death at day 7 in these three lines, namely line28, 29 and 34 using all three assays, with line 34 showing the greatest cell toxicity (Fig 1); therefore, we used line 34 line in the remainder of this study. In comparing assay sensitivity, the LIVE/DEAD assay showed the highest signal/noise ratio; therefore, we used this assay to evaluate the effects of baicalein on cell toxicity.
Baicalein attenuates E46K α-syn induced cell death in differentiated PC12 cells in a concentration-dependent manner
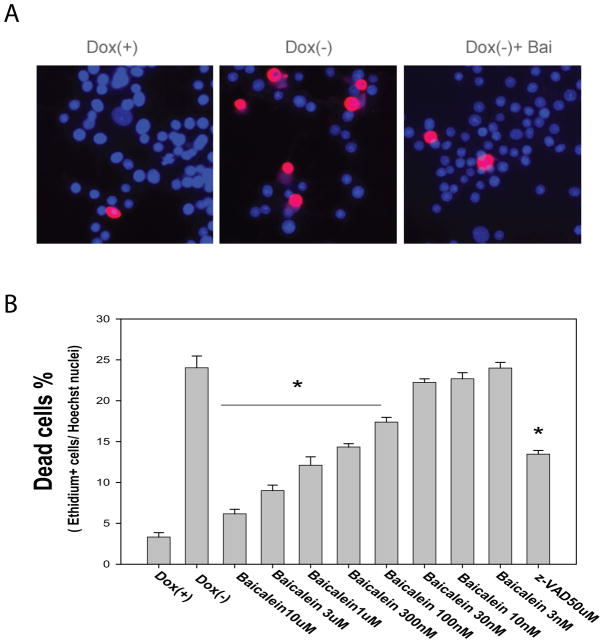
Induction of E46Kα-syn expression in differentiated PC12 cells resulted in approximately 25% cell death at day 7 following transgene expression. We found that baicalein treatment significantly attenuated cell death in a concentration-dependent manner within a concentration range of 100 nM to 10 μM (Fig. 2). The inhibition of cell death by baicalein was more potent than that of caspase inhibitor z-VAD. As we have previously observed in the Tet-Off system, compounds found to inhibit transgene expression might also attenuate toxicity (Wang et al. 2005). To determine whether baicalein affects E46K transgene expression, we conducted a Western blot assay and found that baicalein treatment had no effect on E46K α-syn expression (data not shown).
Fig. 2. Baicalein protects cells against E46 K α-synuclein induced toxicity in PC12 cells.
Cells maintained in medium containing doxycycline (Dox +) served as control, E46K α-synuclein was induced by withdrawal of Dox (Dox −) and cell differentiation was induced by adding NGF to the medium. Cells were treated with baicalein at a range from 3 nM to 10 μM or caspase inhibitor z-VAD (50 μM) at day 2 and the medium was refreshed every 48hrs with baicalein. Cell toxicity was measured by LIVE/DEAD assay at day 7 following transgene induction. (A) Representative pictures of dead cells stained with ethidium homodimer (red) and nucleus labeled by Hoechst 33342 (blue); (B) Baicalein concentration-dependently inhibited cell death induced by E46K α-synuclein, *p< 0.01 compared with the value of Dox (−) group (with Standard Student’s t-tests).
Baicalein attenuates decreased proteasome activity induced by expression of E46K α-syn in differentiated PC12 cells
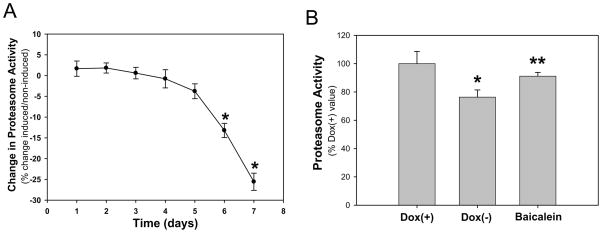
Other mutations of α-syn, such as A30P and A53T, have been shown to resemble, in some respects, the phenotype of cells exposed to proteasomal inhibitors. To investigate the possibility that cells expressing E46K had diminished proteasome function, we measured proteasomal activity. We found that expression of E46K α-syn resulted in decreased proteasome activity in a time-dependent manner: a significantly reduction in proteasome activity was detected at day 6 following E46K expression, and continued to decline with time (Fig 3A). Baicalein treatment significantly attenuated the E46K-induced proteasome inhibition (Fig 3B).
Fig. 3. Baicalein attenuates proteasome inhibition induced by E46K α-synuclein in PC12 cells.
(A) Chymotryptic proteasomal activity was measured at the indicated time points in E46K-induced and noninduced cultures. Time-dependent decline of proteasomal activity occurred in E46K-induced cells, *p<0.05 compared to the values of day 1 with Student’s t-tests. (B) Baicalein (10 μM) attenuated proteasomal inhibition induced by expression of E46K α-synuclein. Proteasomal activity was measured on day 6 following E46K induction. *p<0.05 compared to values of Dox(+) group and **p< 0.05 compared to the values of Dox(−) group with Student’s t-tests.
Baicalein preserves mitochondrial membrane potential in differentiated PC12 cells
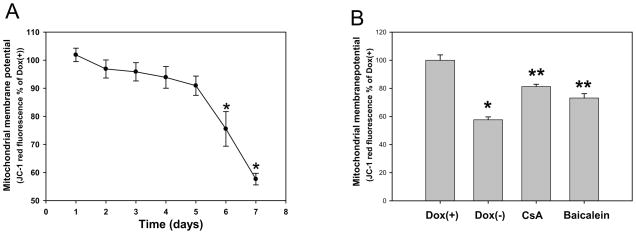
Mitochondrial dysfunction has been implicated in the pathogenesis of PD. Therefore, it was deemed important to examine the relationship between mitochondrial function and E46K α-syn expression to elucidate the pathological mechanisms of PD. Previous studies have shown that other mutations of α-syn induced mitochondrial depolarization (Elkon et al. 2002; Hsu et al. 2000; Smith et al. 2005; Stefanis et al. 2001; Tanaka et al. 2001). To identify whether E46K α-syn led to mitochondria depolarization, we used the dye JC-1 to measure the mitochondrial membrane potential. Expression of E46K caused mitochondrial depolarization in a time-dependent manner (Fig 4A). Significant mitochondrial depolarization was detected at day 6 following E46K induction, one day before the detection of significant cell death. Baicalein significantly attenuated mitochondrial depolarization induced by E46K α-syn in differentiated PC12 cells, as did cyclosporin A treatment, a mitochondrial permeability transition pore inhibitor (Fig. 4B).
Fig. 4. Baicalein attenuates mitochondrial depolarization induced by E46K α-synuclein in PC12 cells.
(A) Mitochondrial membrane potential was measured at the indicated time points in E46K-induced and noninduced cultures; E46K α-synuclein expression results in mitochondria depolarization. *p<0.05 compared to the values of day 1 with Student’s t-tests. (B) Baicalein (10 μM) preserved mitochondrial membrane potential, which was measured on day 6 following E46K α-synuclein induction. Cyclosporin A (CsA) served as a positive control. * p< 0.05 compared to the value of Dox (+) group, **p<0.05 compared to the values of Dox(−) group with Student’s t-tests.
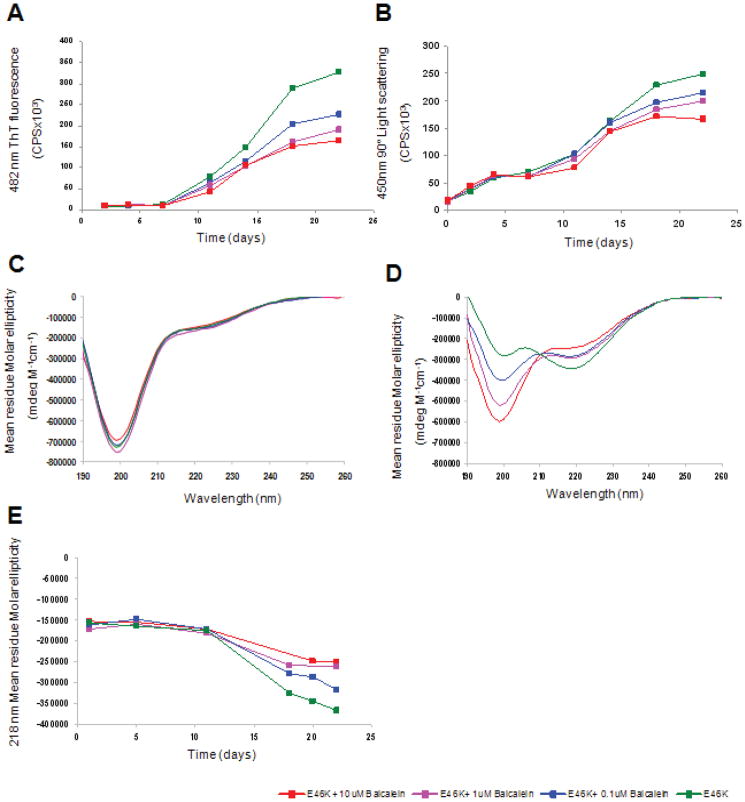
Baicalein inhibits E46K α-syn aggregation in vitro
To analyze the effect of Baicalein on E46K α-syn aggregation, we first carried out a Thioflavin T (ThT) binding assay. Purified soluble E46K α-syn was treated with increasing concentrations of Baicalein and fibrillogenesis was monitored by ThT fluorescence emission at 482 nm. ThT is a reagent known to become strongly fluorescent upon binding to amyloid fibrils. In the absence of baicalein, an increase in ThT fluorescence, consistent with the formation of E46K α-syn fibrils, was observed after a lag phase of 10 days (Fig 5A). This observation is consistent with a nucleated polymerization mechanism (Fredenburg et al. 2007; Zhu et al. 2004) Addition of baicalein to the sample lead to a reduction in ThT fluorescence, as compared to the control, consistent with a decrease in E46K fibrillization (Fig 5A). The effect of baicalein was concentration-dependent, with a baicalein IC50 of approximately 10 μM, and a measurable ThT fluorescence signal reduction with addition of 1 μM baicalein.
Fig. 5. Baicalein inhibits fibril formation and beta sheet conversion of E46K α-synuclein.
(A) Thioflavin T assay reveals a concentration-dependent inhibition of 482 nm fluorescence in the presence of baicalein. (B) 90° static light scattering follows the increase in particle size over time (C, D) Far UV CD spectra of E46K α-synuclein in the presence or absence of baicalein collected on day 1 (C) and day 22 (D), follows conformational changes associated with E46K fibril formation, and the effect of baicalein on these changes. (E) Comparison of beta-sheet structure of E46K, as evident by a negative 218 nm peak, in the presence of increasing concentrations of baicalein. Green: E46K, Blue: E46K + 0.1 μM baicalein, Light red: E46K + 1 μM baicalein, Red: E46K+10μM baicalein.
To confirm these observations, we used static light scattering (SLS) to monitor the effect of baicalein on E46K α-syn aggregation. SLS yields the average size and mass of particles and, via the angular-dependent scattering curves, can provide information on particle shape. As shown in Fig 5B, a dose-dependent decrease in light scattering of E46K α-syn, attributed to a decrease in large aggregate formation, was observed in the presence of baicalein as compared to the control. These data are consistent with those from the ThT binding assay, and demonstrate that baicalein inhibited E46K α-syn aggregation in a dose-dependent manner.
Baicalein prevents E46K α-synuclein conversion to beta –sheet conformation
Previous studies have demonstrated that monomeric wild type α-syn is unstructured in solution, but adopts beta-sheet structure upon conversion to amyloid fibrils (Zhu et al. 2004). To determine the effect of baicalein on secondary structure of E46K, we used Circular Dichroism (CD) Spectroscopy. As shown in Fig 5C, soluble E46K displayed a CD spectrum characteristic of a random coil, or unstructured, protein with a major negative peak observed at 198 nm. The presence of baicalein at 1 day had no significant effect on α-syn E46K secondary structure (Fig 5C). After 22 days, E46K displayed a CD spectrum characteristic of a beta-sheet protein, including disappearance of the 198 nm negative peak and coincident appearance of a negative peak at 218 nm (Fig 5D). These changes are consistent with the formation of E46K amyloid fibrils. A concentration-dependent inhibition of E46K conversion to beta-sheet, as demonstrated by the negative 218 nm peak, was observed in the presence of baicalein after 22 days (Fig 5D, E). Similar observations were recently reported for wild-type α-syn aggregation studies carried out in the presence of baicalein (38) These data demonstrate that baicalein prevents the conversion of E46K from random coil to beta-sheet-rich amyloid fibrils.
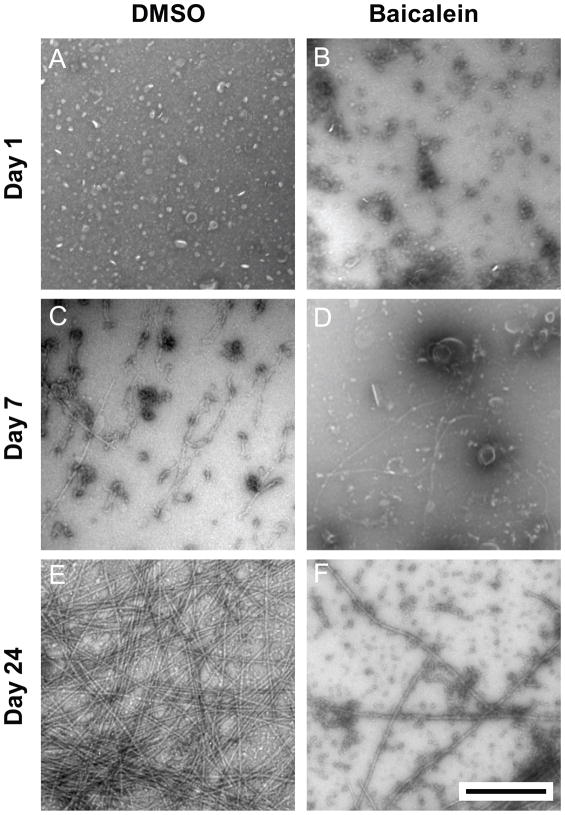
Baicalein inhibits E46K α-syn fibril formation and alters the structure of oligomeric structures visible by Transmission Electron Microscopy
To visualize the effect of baicalein on E46K α-syn aggregation at the morphological level, we used Transmission Electron Microscopy (TEM). As shown in Fig. 6, elongated fibrillar structures 5–25 nm in diameter were visible beginning at day 7, with a peak after 24 days (Fig 6C, E). In contrast, treatment of 50 μM E46K α-syn with 10 μM baicalein lead to a dramatic decrease in fibril formation, relative to the control sample, in favor of amorphous protein aggregates and spheroid structures with a diameter of 20–40 nm (Fig 6D, F). These data are consistent with ThT binding, SLS, and CD Spectroscopy results, and, taken together, demonstrate that baicalein inhibits fibrillization of E46K α-syn.
Fig. 6. Morphology of E46K α-synuclein fibrillar and spheroid species by Transmission Electron Microscopy.
Aggregation samples at days 1, 7, and 24 were negatively stained with Uranyl Acetate. TEM micrographs depict alpha-synuclein E46K in the absence (A, C, E) and presence (B, D, F) of 10 μM baicalein. Scale bar represents 500 nm for all micrographs.
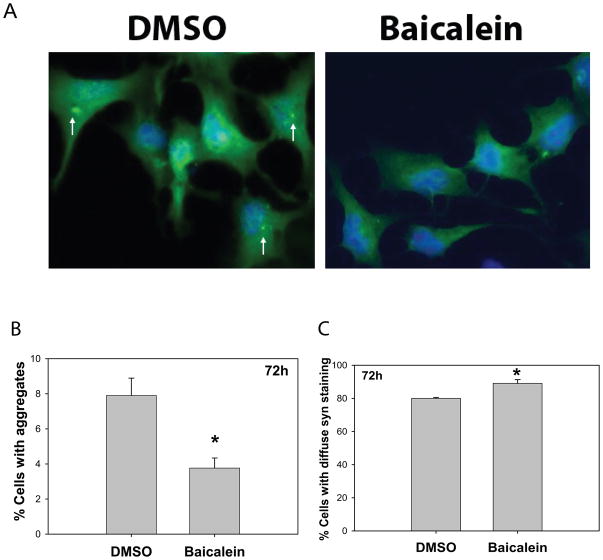
Baicalein inhibits E46K expression-induced α-synuclein aggregation in N2A cells
It has been reported that the E46K mutation formed insoluble fibrils in vitro more rapidly than the wild type protein, and resulted in widespread pathology (Zarranz et al. 2004). To further investigate the effects of baicalein on E46K α-syn aggregation in mammalian cell culture, we transfected E46K α-syn into neuronal N2A cells. In E46K-transfected N2A cells, α-syn aggregates were detected at 72 h after transfection (Fig 7), whereas we could not detect similar aggregates in PC12 cells. Baicalein treatment significantly decreased the number of cells with α-synuclein aggregates and increased cells with diffuse (soluble) α-syn staining in live cells (Fig. 7).
Fig. 7. Balicalein inhibits α-synuclein aggregation in neuronal N2A cells transiently transfected with E46K α-synuclein.
N2A cells were transiently transfected with E46K α-synuclein and cells were fixed at 72 hrs after transfection and immunostained with anti-α-synuclein antibody. (A) Representative cell images of cells treated with vehicle (DMSO) or baicalein (10 μM). Green fluorescent color represents E46K α-synuclein staining, and blue color represents nucleus. Note the arrows point to aggregates. (B quantification of live cells with aggregates or (C) diffuse immuostaining of E46K α-synuclein at 72 h after transfection. *p<0.05 compared to the values of vehicle (DMSO) group with Student’s t-tests.
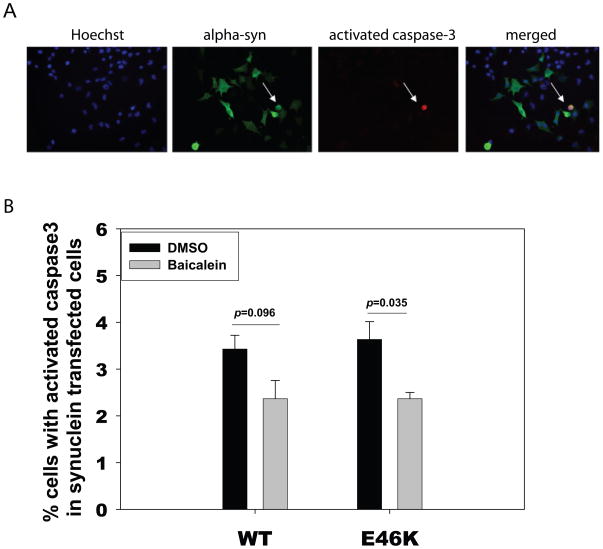
Baicalein inhibits E46K α-syn-induced toxicity in N2A cells
To determine whether a baicalein-induced decrease in α-syn aggregation in N2A cells is beneficial or detrimental, we used activated caspase-3 staining as an early marker for cell toxicity. N2A cells transfected with E46K α-syn or wild type α-syn were treated with DMSO (vehicle) had a higher percentage of activated caspase-3 staining compared to cells treated with baicalein (Fig. 8).
Fig. 8. Baicalein inhibits E46K α-synuclein-induced toxicity in neuronal cells.
N2A cells were transiently transfected with E46 K α-synuclein or wild type α-synuclein. Cells were fixed at 72hrs and immunostained with anti-activated caspase 3 antibody (Red), anti-alpha-synuclein antibody (green) and nucleus was labeled by Hoechst 33342 (blue). (A) Representative pictures of staining. (B) Quantification of cells with activated caspase 3 in α-synuclein transfected N2A cells. P values were determined by Student t-tests.
Discussion
We have investigated the effects of the flavonoid baicalein on E46K α-syn aggregation using recombinant expressed protein, and on aggregation and cell toxicity in two cell culture models of PD. Baicalein reduced fiber formation and beta sheet composition, while increasing the abundance of certain oligomeric assemblies of E46K α-syn. Baicalein strikingly ameliorated E46K-induced toxicity in mammalian cell cultures.
The effect of baicalein on wild-type α-syn in vitro has been previously studied, though its effect on mutant α-syn has not been investigated. Previous studies have demonstrated that baicalein is a potent inhibitor of α-syn fibrillization (Bomhoff et al. 2006; Hong et al. 2008; Kostka et al. 2008; Kragh et al. 2009; Lee et al. 2009; Luk et al. 2007; Meng et al. 2009; Zhu et al. 2004). We found comparable effects on E46K mutant α-syn protein. using ThT fluorescence, static light scattering, and TEM, we show that baicalein treatment lead to a dramatic reduction in E46K α-syn fibril formation. We found effects at substantially lower concentrations than most previous studies (around 1–10 μM, while previous aggregation studies employed 100 μM baicalein or higher concentrations) (Hong et al. 2008; Kragh et al. 2009; Lee et al. 2009; Luk et al. 2007; Zhu et al. 2004). We used CD spectroscopy to demonstrate that baicalein stabilized an unfolded conformation of E46K, consistent with two recent studies by Uversky and coworkers using wild-type synuclein protein (Hong et al., 2008; Meng et al., 2009). Previous studies have suggested that baicalein can form a covalent linkage with wild-type α-syn. Interestingly, while baicalein- α-syn oligomers have a well-packed globular structure, these species cannot form fibrils, but can interact with monomeric, unmodified α-syn to prevent fibril formation and stabilize its unfolded conformation (Hong et al, 2008).
Based on the more recent paper, α-synuclein Y125/Y133/Y136 within wild-type are important for baicalein inhibition (Meng et al, 2009), suggesting that oxidized (quinone) baicalein can form a covalent linkage with E46K α-syn, and that this baicalein-modified protein can prevent fibrillization of the non-modified protein. These studies were done with a higher concentration of baicalein than the current experiments (100 μM vs 10μM). Our aggregation studies were carried out without agitation while Meng et al stirred the WT α-syn aggregation samples. This may account in part for differences in rates of aggregate formation between the two studies (hours to days for Meng et al vs several days for our studies).
In previous cell culture studies (Smith et al. 2005; Tanaka et al. 2001), we developed cell models with inducible expression of A30P and A53T α-syn. The A30P mutant was not toxic on-its-own, but caused enhanced toxicity in concert with cell stress, such as proteasome inhibition. The A53T mutation was capable of causing cell toxicity on its own, via several different pathways, including mitochondrial inhibition, free radical toxicity, and ER stress (Smith et al., 2005). Our new data show that E46K can also cause toxicity on its own. Since patients with the E46K mutation have widespread brain pathology, and since the E46K mutation shows robust fibrilization in cell free systems, this may be an excellent model for study.
The mechanism of baicalein inhibition of toxicity is unclear. Baicalein is a free radical scavenger and xanthine oxidase inhibitor (Lapchak et al. 2007; Shieh et al. 2000). In addition, it has been shown to protect neurons from 6-hydroxydopamine-induced toxicity (Mu et al. 2009), MPTP neurotoxicity (Cheng et al. 2008), and inhibited methamphetamine-induced loss of dopamine transporter in mouse striatum (Wu et al. 2006). In the current studies, baicalein was able to attenuate mitochondria depolarization and proteasome inhibition induced by E46K, and protect against E46K-induced cell toxicity in a concentration-dependent manner in stably-transfected PC12 cells. Consistent with these observations, baicalein treatment resulted in a two-fold reduction in toxicity in transiently-transfected N2A cells, as demonstrated by activated caspase 3 staining.
Our results demonstrate that baicalein decreased fibril formation and reduced toxicity. However, this observation does not imply a simple causative relationship. The fibrilization pathway is likely to be complex, and several intermediates, including different kinds of oligomeric species, may have very different effects on toxicity (Fink 2006; Irvine et al. 2008; Kazantsev and Kolchinsky 2008; Ross and Poirier 2004; Uversky 2003; Uversky 2008; Waxman and Giasson 2009). As in previous cell free system studies, we found an increase in some oligomeric species, though this increase was not investigated in detail in the current study. We found that baicalein caused an increase in annular oligomers. This finding, in concert with the minimal effects of oligomers on membranes in the Hong et al study, suggests that pore formation by annular intermediates (Lashuel et al. 2002a; Lashuel et al. 2002b) may not be a major mechanism of toxicity in our systems.
Multiple lines of evidence (El-Agnaf et al. 1998; Moussa et al. 2008; Uversky 2008) indicate that abnormal conformation and aggregation of both wild-type and mutant α-syn, and accumulation in Lewy bodies are relevant to both familial and sporadic PD brains (Baba et al. 1998; Ono et al. 2008). Therefore, identification of specific steps in the process of α-syn aggregation may be useful for understanding pathogenesis of both genetic and sporadic forms of PD.
Previous studies have demonstrated that proteasomal activity was inhibited in PC12 cells expressing other α-syn mutations (Betarbet et al. 2006; Emmanouilidou et al. 2008; Smith et al. 2005; Tanaka et al. 2001) The present data demonstrate that E46K α-syn-expressing cells have diminished proteasome activity. Taken together, these data support a link between mutant α-syn expression and proteasomal inhibition. Here, we show that baicalein treatment can partially restore proteasome function. If a baicalein-E46K adduct is formed in cells, as is observed in in vitro aggregation studies, this modified protein might be less capable of inhibiting the proteasome. Alternatively non-covalent interactions might alter the conformation of α-syn, making a less effective proteasome inhibitor. Mitochondrial dysfunction is believed to play an important role in PD-induced cell death (Yao and Wood 2009). Here, we show that cells expressing E46Kmutant α-syn displayed a selective increase in mitochondrial depolarization and cell death. Baicalein treatment resulted in improved mitochondrial membrane potential and reduced cell death.
In conclusion, our current results provide evidence for a striking neuroprotective effect of baicalein on E46K mutant α-syn-induced toxicity and aggregation in cell cultures. In combination with other studies involving chemically-induced PD models, these findings suggest the value of further investigation of the mechanism of cell protection by baicalein, and analogs. Baicalen may be a useful tool for studying the role of different conformational forms and oligomerization states of α-syn in cell toxicity. These studies also provide support for baicalein as a potential therapeutic agent in preclinical trials carried out in α-syn transgenic mouse models.
Supplementary Material
Acknowledgments
This research was supported by NINDS NS 055971 (to WD), NS38377 and NS16375 (to CAR), and NS 053679 (to MAP).
References
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22(2):404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bomhoff G, Sloan K, McLain C, Gogol EP, Fisher MT. The effects of the flavonoid baicalein and osmolytes on the Mg 2+ accelerated aggregation/fibrillation of carboxymethylated bovine 1SS-alpha-lactalbumin. Arch Biochem Biophys. 2006;453(1):75–86. doi: 10.1016/j.abb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Brice A. What can we learn from genes responsible for familial forms of Parkinson’s disease? Bull Acad Natl Med. 2006;190(2):485–496. discussion 497–488. [PubMed] [Google Scholar]
- Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441(1):16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37(5):942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576(3):363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21(6):249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58(1):120–129. [PubMed] [Google Scholar]
- Corti O, Brice A. Parkinson’s disease: what have we learned from the genes responsible for familial forms? Med Sci (Paris) 2003;19(5):613–619. doi: 10.1051/medsci/2003195613. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Jakes R, Curran MD, Wallace A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson’s disease. FEBS Lett. 1998;440(1–2):67–70. doi: 10.1016/s0014-5793(98)01419-7. [DOI] [PubMed] [Google Scholar]
- Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J Mol Neurosci. 2002;18(3):229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the act: alpha-synuclein is the culprit in Parkinson’s disease. Neuron. 2003;40(3):453–456. doi: 10.1016/s0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39(9):628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT., Jr The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46(24):7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280(9):7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- Hong DP, Fink AL, Uversky VN. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008;383(1):214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157(2):401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Joo WS, Nam E, Lee ES, Hwang YJ, Kim YS. Baicalein prevents 6-hydroxydopamine-induced dopaminergic dysfunction and lipid peroxidation in mice. J Pharmacol Sci. 2005;98(2):185–189. doi: 10.1254/jphs.sc0050014. [DOI] [PubMed] [Google Scholar]
- Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008;14(7–8):451–464. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Kazantsev AG, Kolchinsky AM. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch Neurol. 2008;65(12):1577–1581. doi: 10.1001/archneur.65.12.1577. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Dimayuga ER, Maragos WF. Dopamine induces proteasome inhibition in neural PC12 cell line. Free Radic Biol Med. 2000;29(10):1037–1042. doi: 10.1016/s0891-5849(00)00412-3. [DOI] [PubMed] [Google Scholar]
- Kostka M, Hogen T, Danzer KM, Levin J, Habeck M, Wirth A, Wagner R, Glabe CG, Finger S, Heinzelmann U, Garidel P, Duan W, Ross CA, Kretzschmar H, Giese A. Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J Biol Chem. 2008;283(16):10992–11003. doi: 10.1074/jbc.M709634200. [DOI] [PubMed] [Google Scholar]
- Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El-Agnaf O, Richter-Landsberg C, Jensen PH. {alpha}-Synuclein Aggregation and Ser-129 Phosphorylation-dependent Cell Death in Oligodendroglial Cells. J Biol Chem. 2009;284(15):10211–10222. doi: 10.1074/jbc.M809671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150(3):585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002a;418(6895):291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002b;322(5):1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Noh YH, Lee DY, Kim YS, Kim KY, Chung YH, Lee WB, Kim SS. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur J Cell Biol. 2005;84(11):897–905. doi: 10.1016/j.ejcb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee IH, Choe YJ, Kang S, Kim HY, Gai WP, Hahn JS, Paik SR. Real-time analysis of amyloid fibril formation of alpha-synuclein using a fibrillation-state-specific fluorescent probe of JC-1. Biochem J. 2009;418(2):311–323. doi: 10.1042/BJ20081572. [DOI] [PubMed] [Google Scholar]
- Luk KC, Hyde EG, Trojanowski JQ, Lee VM. Sensitive fluorescence polarization technique for rapid screening of alpha-synuclein oligomerization/fibrillization inhibitors. Biochemistry. 2007;46(44):12522–12529. doi: 10.1021/bi701128c. [DOI] [PubMed] [Google Scholar]
- Meng X, Munishkina LA, Fink AL, Uversky VN. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry. 2009;48(34):8206–8224. doi: 10.1021/bi900506b. [DOI] [PubMed] [Google Scholar]
- Moussa CE, Mahmoodian F, Tomita Y, Sidhu A. Dopamine differentially induces aggregation of A53T mutant and wild type alpha-synuclein: insights into the protein chemistry of Parkinson’s disease. Biochem Biophys Res Commun. 2008;365(4):833–839. doi: 10.1016/j.bbrc.2007.11.075. [DOI] [PubMed] [Google Scholar]
- Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 2009;92(4):642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Ono K, Hirohata M, Yamada M. Alpha-synuclein assembly as a therapeutic target of Parkinson’s disease and related disorders. Curr Pharm Des. 2008;14(30):3247–3266. doi: 10.2174/138161208786404191. [DOI] [PubMed] [Google Scholar]
- Pandey N, Schmidt RE, Galvin JE. The alpha-synuclein mutation E46K promotes aggregation in cultured cells. Exp Neurol. 2006;197(2):515–520. doi: 10.1016/j.expneurol.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, Ellul J, Paschalis C, Athanassiadou A, Papadimitriou A, Papapetropoulos T. Clinical characteristics of the alpha-synuclein mutation (G209A)-associated Parkinson’s disease in comparison with other forms of familial Parkinson’s disease in Greece. Eur J Neurol. 2003;10(3):281–286. doi: 10.1046/j.1468-1331.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20(5A):2861–2865. [PubMed] [Google Scholar]
- Singleton AB. Altered alpha-synuclein homeostasis causing Parkinson’s disease: the potential roles of dardarin. Trends Neurosci. 2005;28(8):416–421. doi: 10.1016/j.tins.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005;14(24):3801–3811. doi: 10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21(24):9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, VLD, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10(9):919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21(2):211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9(5):507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Pathology of familial Parkinson’s disease. Brain Nerve. 2007;59(8):851–864. [PubMed] [Google Scholar]
- Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on beta-amyloid peptide-(25–35)-induced amnesia in mice. Eur J Pharmacol. 2004;506(1):55–61. doi: 10.1016/j.ejphar.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Wang W, Duan W, Igarashi S, Morita H, Nakamura M, Ross CA. Compounds blocking mutant huntingtin toxicity identified using a Huntington’s disease neuronal cell model. Neurobiol Dis. 2005;20(2):500–508. doi: 10.1016/j.nbd.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta. 2009;1792(7):616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PH, Shen YC, Wang YH, Chi CW, Yen JC. Baicalein attenuates methamphetamine-induced loss of dopamine transporter in mouse striatum. Toxicology. 2006;226(2–3):238–245. doi: 10.1016/j.tox.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Yao Z, Wood NW. Cell death pathways in Parkinson’s disease: Role of mitochondria. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2624. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279(26):26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.