Abstract
Introduction
The near-infrared wavelengths (700nm–900nm) are the most suitable optical window for light penetration and deep tissue imaging in small animals. Herein we report a near-infrared fluorescent contrast agent, crimson carrier, which acts as a blood pool contrast agent to detect and quantify injury and disease in live animals.
Methods
After determining the excitation-emission spectra and pharmacokinetics, crimson carrier was injected into myoinjured mice to monitor their recovery. Crimson carrier was also used to image transgenic mice with spontaneous tumors.
Results
Crimson carrier has maximal excitation and emission wavelengths of 745 nm and 820 nm, respectively. Elimination occurs predominantly via urinary excretion.
Discussion
We demonstrate the utility of this contrast agent for serial imaging of traumatized muscle as well as muscle tumors. The unique long-acting pharmacokinetics and urinary excretion route characteristics make crimson carrier a contrast agent of choice for the visualization of tumors and injured muscle or other tissues in live animal studies.
Keywords: crimson carrier, optical imaging, muscle injury, near-infrared contrast agent, tumor imaging
INTRODUCTION
Preclinical and clinical fluorescence optical imaging instruments are increasingly utilized to obtain biological information on the path from gene to clinic.1 Skin and red blood cell autofluorescence occurs to some extent at all excitation wavelengths in the visible wavelength range of light (400–700 nm). Due to this background signal, the quantification of fluorescence signals for in vivo imaging can be difficult when working in this range. To achieve better signal:background and increasingly accurate quantitative imaging in vivo, working within the near-infrared (NIR) wavelength range (700–900 nm) is advantageous. In this spectral region, light absorption and scattering by physiologically abundant molecules such as hemoglobin, oxyhemoglobin, and deoxyhemoglobin is minimized.2 NIR fluorescent contrast agents developed for in vivo optical imaging provide novel opportunities for pre-clinical diagnostic imaging in deep tissues.2 In this report we describe the use of crimson carrier, a novel, near-infrared blood pool contrast agent in detecting muscle injury and muscle tumors in well characterized animal models.
MATERIALS AND METHODS
Contrast agent
Crimson carrier (C93H132N4O38S) is composed of a β-cyclodextrin ring (a common carrier for hydrophobic compounds) conjugated to Indocyanine Green (ICG) via a short linker (CH2)6. It was developed and commercialized by Numira Biosciences, Inc. Crimson carrier has a molecular weight of 1946.11 atomic mass unit (amu). For each experiment, 0.3 mg of powdered crimson carrier was dissolved in 30 µl Dimethyl Sulfoxide (DMSO, Sigma D8418, St. Louis, MO, USA) by pipeting up and down, followed by addition of 970 µl of PBS pH 7.4 to achieve a final concentration of 0.15 mM.
Live animal imaging
Optical imaging for all experiments was performed using the Xenogen IVIS® Spectrum system (Caliper – Xenogen, Alameda, CA, USA). All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Health Science Center at San Antonio. All images were acquired using epiillumination at an excitation wavelength of 745 nm and an emission wavelength of 820 nm unless otherwise stated. The camera settings were kept constant at 1 sec exposure time, 1×1 binning, 12.6 cm or 6.5 cm field of view, and f/stop of 1/2. The data was acquired and analyzed using the manufacturer’s Living Image 3.2© software. All animals were imaged using the same anesthesia protocol of 2% isoflurane in 100% oxygen at 2.5 liters per minute. Body temperature was maintained at 37°C by a heated stage.
Determination of excitation and emission spectra
To determine the excitation and emission spectra for crimson carrier, the solution was loaded into a clean cuvette and imaged after 15 minutes using the Xenogen IVIS® Spectrum system. Images were captured using a combined set of excitations ranging from 435 nm to 745 nm (windows of 35 nm) and emission filters ranging from 500 nm to 840 nm (windows of 20 nm).
In vivo pharmacokinetics of crimson carrier
Hairless SKH1/SKH1 mice were obtained from Charles River Laboratories (Wilmington, MA, USA). To determine the pharmacokinetics of crimson carrier, Hairless SKH1/SKH1 mice (n=3) were injected with 100 µl of 0.15 mM crimson carrier in PBS by intraperitoneal injection and serially imaged in the supine position for 10 days using the same parameters.
Muscle injury model using cardiotoxin
For myoinjury experiments, twelve-week-old male Hairless SKH1/SKH1 mice were injected intraperitoneally with 100 µl of crimson carrier. Sixty minutes later, each mouse received intramuscular injections to the anterior compartment muscles below the knee of the right hind limb with 100 µl of cardiotoxin (2.5µM, Calbiochem, San Diego, CA, USA), while the left hind limbs served as non-injected controls. Imaging was performed in the prone position within intervals of 1–240 hours after crimson carrier injection.
In selected experiments, mice were injected with cardiotoxin, sacrificed at various timepoints, and the muscles of the lower anterior compartment were removed, fixed in 10% neutral buffered formalin, and processed for routine light microscopic examination after paraffin embedding, sectioning, and hematoxylin and eosin staining. Images were captured using a Nikon microscope (Eclipse 80i) (Melville, NY, USA) equipped with a digital camera (DS-Fi1) (Melville, NY, USA) using NIS-Elements F software (Melville, NY, USA).
Image Analysis
Image analysis was performed using Living Image 3.2® software. To calculate fluorescence signal intensity (in photons/sec), regions of interest were made on the serial images acquired using the Xenogen IVIS® Spectrum system. The calculated signal intensities were serially plotted using Graph Pad Prism® software (Graphpad Software, La Jolla, CA).
Statistical Analysis
Mean contrasts with baseline were carried out with a repeated measures linear model with an autoregressive order one autocorrelation assumption. All statistical testing was two-sided with a significance level of 5%. SAS Version 9.1.3 for Windows (SAS Institute, Cary North Carolina, USA) was used throughout.
In vivo tumor imaging
Mice carrying conditional Pax3:Fkhr oncogene or the Patched1 tumor suppressor gene have been described previously.3, 4 In this study, three transgenic mice with spontaneous tumors were evaluated. These animals were a Myf6 ICNm/WT Pax3 P3Fm/ P3Fm Trp53 F2-10/ F2-10 Rb1 Flox/Flox mouse bearing a neck tumor, a Pax7 CreERp/WT Trp53 F2-10/ F2-10 mouse bearing a back tumor and a Pax7 CreERp/WT PTC1 F1-2m/WT Trp53 F2-10/ F2-10 mouse with a facial tumor. All animals were injected with 100 µl of 0.15 mM crimson carrier intravenously and imaged 24 hours after injection. The animals were euthanized, and tumor tissue was collected for pathology studies.
RESULTS
Crimson carrier is a near-infrared contrast agent
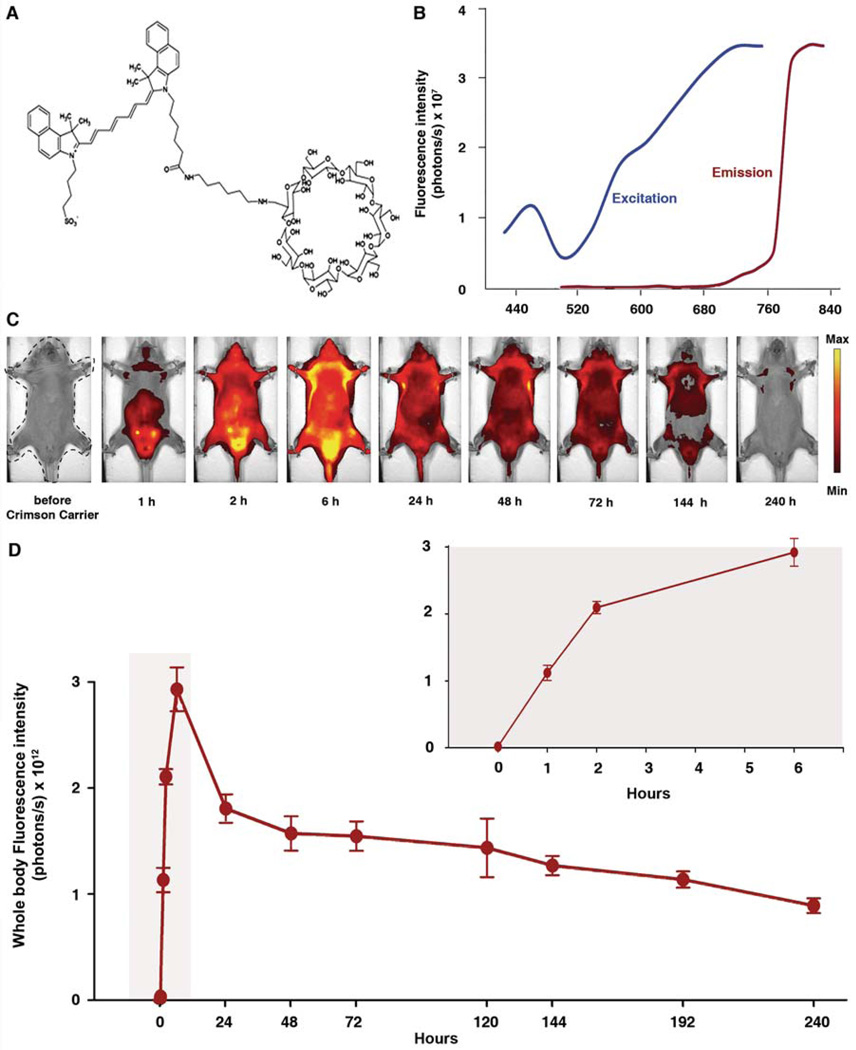
Crimson carrier is a chimerical molecule consisting of a β-cyclodextrin ring commonly used for lipophilic drug delivery, attached to a near infrared moiety modified for improved urinary excretion (Figure 1A). The peaks for excitation and emission wavelengths for crimson carrier were measured to be 745 nm and 820 nm, respectively (Figure 1B).
Figure 1.
(A) Chemical structure of crimson carrier. (B) Spectra for free crimson carrier reveal excitation and emission maxima at 745 nm and 820 nm respectively. (C) Pharmacokinetics study for crimson carrier in HairlessSKH1/SKH1 mice. Mice were injected with 100 µl of crimson carrier intraperitoneally and serially imaged for 10 days in supine position. The first image was captured prior to injection of crimson carrier; time thereafter in reference to crimson carrier injection. The images are displayed on a minimum – maximum scale of 3 × 109 to 1 × 1010 photons/s/cm2/steradian. (D) Whole body fluorescence signal intensity (in photons/sec), was calculated by placing a regions of interest covering entire body, which is the dashed region shown in the 1st picture of panel C. Inset graph demonstrates the expanded view of the early time points (highlighted area) immediately following crimson carrier injection. Clearance half-life of crimson carrier was approximately 144 hours. Error bars, s.d.
Pharmacokinetics of crimson carrier clearance
Pharmacokinetic experiments were performed to determine distribution and clearance under baseline conditions. Following intraperitoneal injection, the fluorescence signal intensity of crimson carrier steadily increased and peaked 6 hours after administration (Figure 1C). The pharmacokinetic studies show the clearance half-life of this agent to be 144 hours with intraperitoneal administration (Figure 1D). Clearance was primarily urinary (data not shown). No overt toxicity was observed in crimson carrier-injected animals monitored as long as 10 weeks. Even though the pharmacokinetic plots for intraperitoneal injection (Figure 1D) and intravenous administration (Supplementary Figure S1) had very distinct absolute whole body fluorescence values and showed different trends, contrast performance characteristics by both routes were comparable with respect to the timing of maximal signal detection (6 h) and the half-life of crimson carrier (144 h).
Crimson carrier can be used to monitor the time course of muscle injury and regeneration
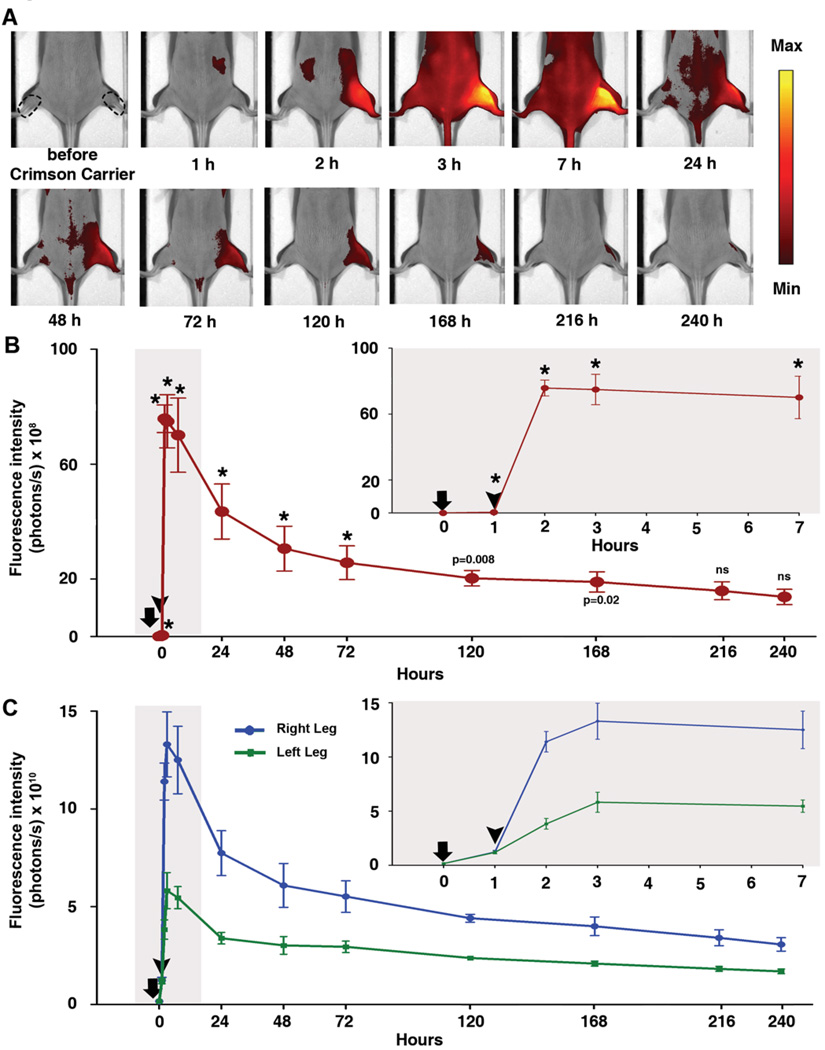
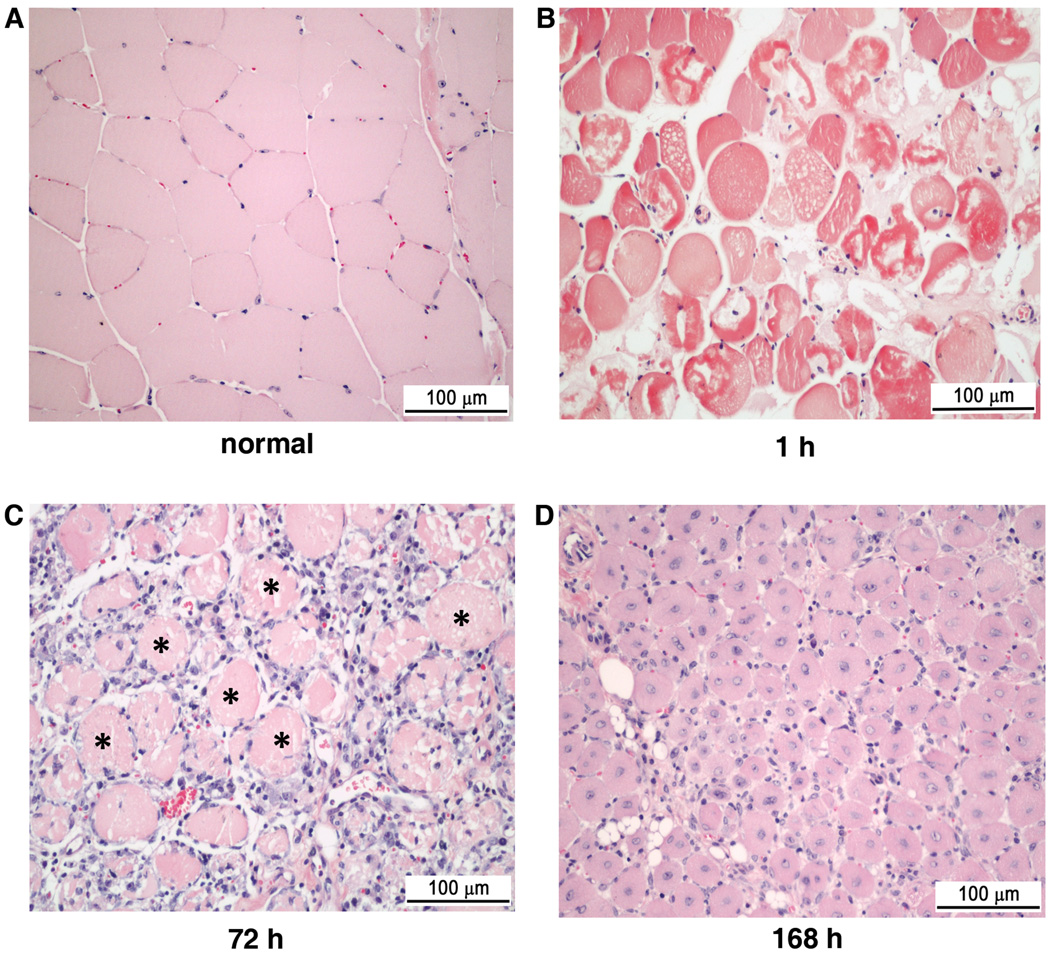
A well-established cardiotoxin muscle injury model5 was used to determine the efficacy of crimson carrier in monitoring tissue injury and regeneration. Cardiotoxin contains lytic factors with a primary mechanism of action of forming pores that depolarize and degrade the muscle plasma membrane.6 Cardiotoxin was injected intramuscularly into the anterior compartment of the right hind limb muscles of HairlessSKH1/SKH1 mice while the left, non-injected hind limb was used as a control in each mouse. To determine the extent of injury-induced crimson carrier extravasation into the extravascular space, intravascular crimson carrier was measured by the intensity of the non-injected, control leg and the crimson carrier that was extravasated into the injured tissues was represented by the pair difference of the injected (right) hind limb to the non-injected (left) hind limb signal (Figure 2B). At and beyond the initial peak at 2 hours (1 hour after cardiotoxin) up to 168 hours after baseline, the paired difference remained significantly elevated from baseline. The injected (right) leg had signal intensity approximately double that for non-injected (left) leg from cardiotoxin injection to the end of 240 h time point. The ratio of injected:non-injected reached 2.98 at one hour after cardiotoxin injection, indicating maximal damage to the tissue and maximal contrast agent extravasation at that time point (Figure 2C). Light microscopy confirmed the sequential, cardiotoxin-induced skeletal muscle alterations, i.e., immediate and widespread myonecrosis, inflammatory cell accumulation, and myofiber regeneration (Figure 3 A–D).
Figure 2.
Monitoring myoinjury using crimson carrier. (A) HairlessSKH1/SKH1 mouse serial images in the prone position after injury of right (R) hind limb with intramuscular cardiotoxin injection; the left (L) hind limb was not injected. The images are displayed with a minimum – maximum scale of 5 × 109 to 2 × 1010 photons/s/cm2/steradian. On the first image right and left regions of interest are shown as dotted lines. The first image is prior to intraperitoneal injection of crimson carrier; times thereafter are labeled in reference to crimson carrier injection, and cardiotoxin was injected 1 hour after crimson carrier injection. (B) Paired difference of injected hind limb and non-injected hind limb, * indicates p<0.001 (compared to baseline signal), ns stands for not statistically significant. Error bars, s.d.; n= 3 mice. Time on x-axis measured after crimson carrier injection (arrow); cardiotoxin injection into the right hind limb denoted by the arrowhead. Inset graph demonstrates the expanded view of the early time points (highlighted area) immediately following crimson carrier and cardiotoxin injections. (C) Absolute fluorescence intensity values of injected hind limb (right leg, blue curve) and non-injected hind limb (left leg, green curve).
Figure 3.
Cardiotoxin-induced histopathological alterations in HairlessSKH1/SKH1 mouse tibialis anterior muscle. In comparison to normal (A), uninjured muscle with peripherally displaced myonuclei, widespread myofiber disruption was present within 1 hour (B) after intramuscular cardiotoxin injection. While a mononuclear cell infiltrate around necrotic myofibers (asterisks) prevailed at 72 hours (C) post-cardiotoxin, regenerated myofibers with centrally-located nuclei predominated within 168 hours (D). Scale bar, 100 µm, hematoxylin and eosin.
Crimson carrier as a contrast agent for diseased muscle
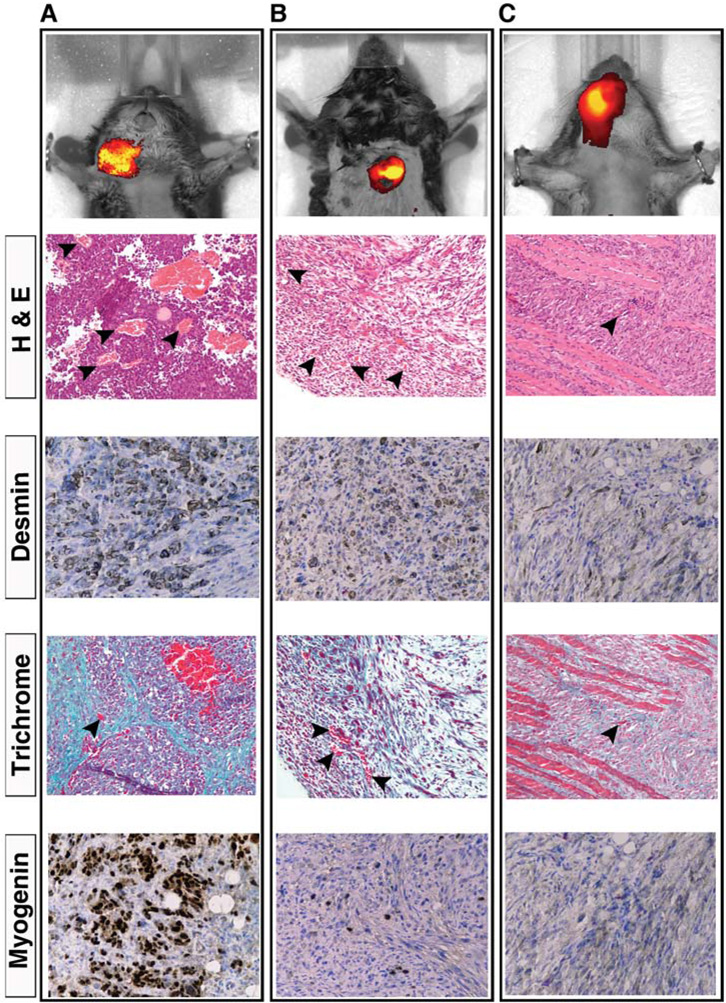
To detect perfusion abnormalities, such as capillary leak, which are often associated with cancers, three transgenic mice with solid tumors (sarcomas) were injected intravenously with 100 µl of crimson carrier and imaged 24 hours later. Results in Figure 4A–C show the rate of clearance of crimson carrier in case of altered vascular conditions.
Figure 4.
In vivo imaging of tumor tissues using crimson carrier. Black arrowheads, capillaries. (A) Myf6 ICNm/WT Pax3 P3Fm/ P3Fm Trp53 F2-10/ F2-10 Rb1 Flox/Flox mouse bearing an alvelolar rhabdomyosarcomas within the neck (tumor volume 0.818 cc). Scale bar: 5.5 × 109 – 9 × 109 photons/s/cm2/steradian. Desmin and Myogenin confirm diagnosis, whereas Trichrome demonstrates collagen rich stroma. (B) Pax7 CreER/WT Trp53 F2-10/ F2-10 mouse with an embryonal rhabdomyosarcoma in the back (tumor volume 0.272 cc). The diagnosis was confirmed by desmin and myogenin immunoreactivity. Scale bar: 6.42 × 109 – 1.3 × 1010 photons/s/cm2/steradian. (C) Pax7 CreERp/WT PTC1 F1-2m/WT Trp53 F2-10/ F2-10 mouse with a poorly differentiated spindle cell sarcoma involving the face (tumor volume 0.467 cc). Scale bar: 3 × 109 – 6 × 109 photons/s/cm2/steradian. Myogenin was negative for this tumor.
DISCUSSION
In this study we have characterized a novel, long-acting near-infrared contrast agent, crimson carrier (λexcitation 745 nm, λemission 820 nm) that has an experimentally favorable mode of excretion (urinary). To demonstrate the use of crimson carrier to monitor tissue injury and recovery over time, a well-characterized cardiotoxin injury model was utilized.6 With cardiotoxin injection, an immediate 1500-fold increase in the paired difference, compared to baseline, occurred at the 2-hour time point suggesting an increased extravascular crimson carrier signal in the injected hind limb, which far exceeded the intravascular crimson carrier signal of the contralateral, non-injected hind limb. These data suggest that capillary leak/extravasation begins within 1 hour after injury and that ongoing leak and/or decreased clearance continues in the regenerating muscle. This time course is consistent with the present histopathological findings as well as the inflammatory and regenerative response previously reported for the cardiotoxin injury model.5
Our choice of Hairless mice for these studies was not accidental. In unpublished studies we have found the hyperpigmented macules (i.e. freckles) of C57BL/6 mice to significantly impair the sensitivity and accuracy of infrared contrast agent detection (Supplementary Figure S2). Fortunately, however, we have characterized the response to muscle injury in the Hairless model and found this response to be comparable to C5BL/6 animals7.
For tumor imaging, as confirmed at necropsy, the bright fluorescence in live animal imaging emanates from the tumor and not from the skin surface overlying the tumor (data not shown). Histological analysis confirmed the presence of neoplasms corresponding to the crimson carrier signal in all three mice. Histological analysis also confirmed the presence of numerous small blood vessels and capillaries within the tumors. Thus, crimson carrier has utility not only for determining the time course of muscle injury and regeneration but also for detection of altered tumor vascular beds such as can be found in cancers.
When we compare/contrast this work on crimson carrier with our previous work on a similar near-infrared contrast agent IR-8207, we observe that crimson carrier has a longer half-life (144 hours) and is eliminated by urinary excretion, while IR-820 has a shorter half-life (35 hours) and is eliminated by hepatobiliary excretion. IR-820 is known to bind to albumin; therefore, IR-820 distribution follows albumin kinetics. Whether serum protein binding occurs for crimson carrier is still unknown. Both contrast agents are useful in tumor imaging as well as tissue injury and regeneration, and the nature of each experiment (e.g., tissue of interest overlying the liver v/s the bladder) can help define which agent is optimal for a specific application.
Supplementary Material
Acknowledgments
Supported in part by HL090196 (to C.O.M.), HL074236 and the Veterans Administration (to P.K.S.) and a sponsored research agreement with Numira Biosciences Inc. (C.K.).
Footnotes
COMPETING INTERESTS STATEMENT
Crimson carrier is the property of Numira Biosciences Inc. and its use as a blood pool agent is the property of UTHSCSA. CK is a co-founder of Numira Biosciences Inc. which is developing and commercializing crimson carrier. Numira has licensed the use of crimson carrier as a blood pool agent from the UTHSCSA. The work described herein was supported in part by a sponsored research agreement with Numira Biosciences Inc.
REFERENCES
- 1.Grimm J, Kirsch DG, Windsor SD, Kim CF, Santiago PM, Ntziachristos V, et al. Use of gene expression profiling to direct in vivo molecular imaging of lung cancer. Proc Natl Acad Sci U S A. 2005;102:14404–14409. doi: 10.1073/pnas.0503920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luker GD, Luker KE. Optical imaging: current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi E, Cho MJ, Arenkiel BR, Hansen MS, Rivera OJ, McCleish AT, et al. Bortezomib reverses a post-translational mechanism of tumorigenesis for patched1 haploinsufficiency in medulloblastoma. Pediatr Blood Cancer. 2009 Aug;53(2):131–132. doi: 10.1002/pbc.21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijo K, Hosoyama T, Bjornson CR, Schaffer BS, Prajapati SI, Bahadur AN, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009 Aug;23(8):2681–2690. doi: 10.1096/fj.08-128116. Epub 2009 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, et al. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2007;293:R651–R661. doi: 10.1152/ajpregu.00069.2007. [DOI] [PubMed] [Google Scholar]
- 6.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–945. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Prajapati SI, Martinez CO, Bahadur AN, Wu IQ, Zheng W, Lechleiter JD, et al. Near-infrared Imaging of Injured Tissue in Living Subjects Using IR-820. Mol Imaging. 2009;8:45–54. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.