Summary
In humans, aspects of working memory capacity (i.e., resistance to interference or selective attention) correlate strongly with measures indicative of general intelligence [1–7], and likewise, the efficacy of working memory capacity and its related process, selective attention, are each strongly predictive of the aggregate performance of individual mice in cognitive test batteries [8,9]. Since by its nature, working memory is taxed during most cognitive tasks, it has been suggested that the efficacy of working memory may have a causal influence on individuals’ performance on common tests of “intelligence” [10,11]. Despite the attention that this possibility has received, supporting evidence based on studies of humans has been largely correlational in nature (but see [12]). Here, genetically heterogeneous mice were assessed on a battery of five learning tasks. Animals’ aggregate performance across the five tasks was used to estimate their general cognitive abilities, a trait that is in some respects analogous to intelligence [13,14]. In two experiments, we demonstrate that repetitive working memory training (concurrent performance in two eight-choice mazes) promoted an increase in selective attention and had a commensurately positive effect on the animals’ aggregate performance on a battery of five learning tasks. The enhancement of general cognitive performance by working memory exercise was attenuated if the nominal selective attention demands of that working memory training were reduced. In total, these results provide initial evidence that the efficacy of working memory capacity and selective attention are causally related to an animal’s general cognitive performance, and suggest behavioral strategies to promote those abilities. Furthermore, these results suggest a conservation of the processes that regulate general cognitive performance in humans and infrahuman animals.
Results
Working Memory Exercise Promotes Selective Attention and General Cognitive Performance
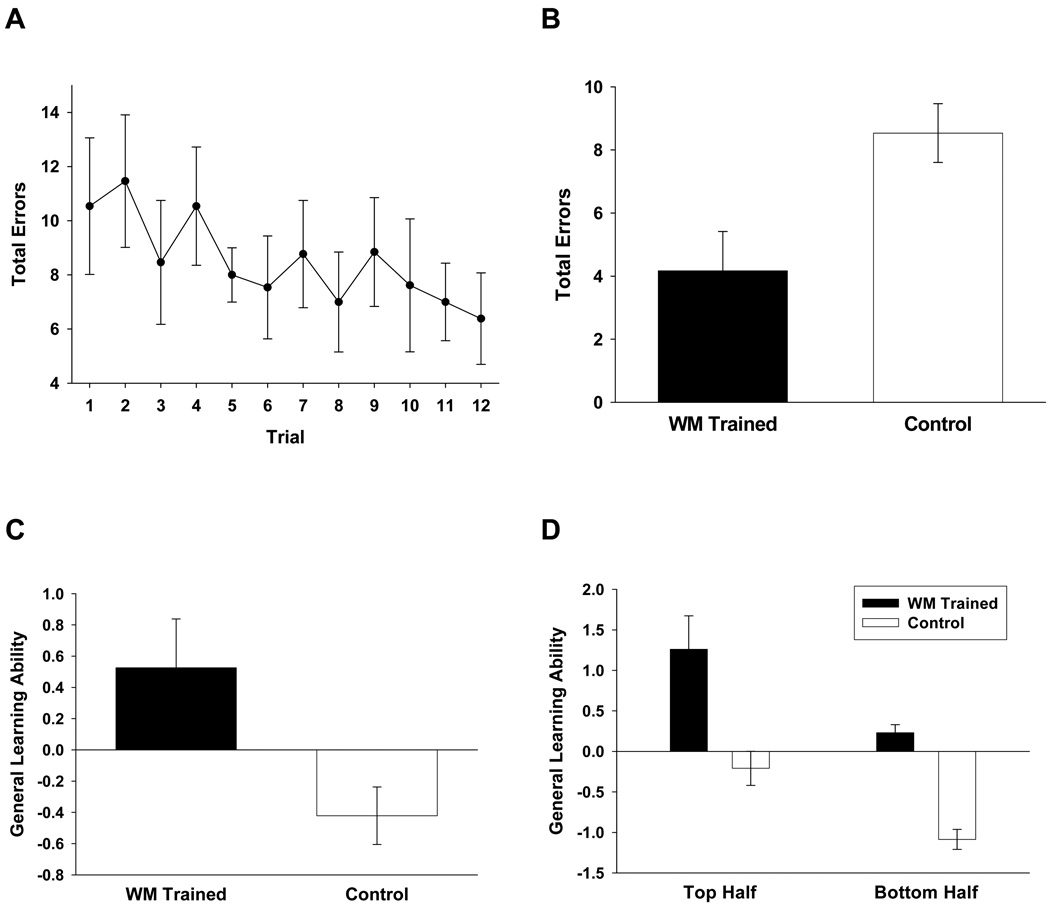
Procedures and results from individual tests are presented in supplementary material. One group of animals received 12 days of training wherein they performed concurrently in two eight-choice radial arm mazes. This task was designed to promote a heavy reliance on all aspects of working memory (including aspects of short-term maintenance, capacity, and selective attention) and has been previously described [8,9]. A second group received comparable handling absent any working memory training. When both groups of mice were subsequently tested on a mouse analog [9] of the human Stroop test, mice that received this working memory training performed significantly better than animals that had not undergone training. That is, task-relevant interference provoked fewer errors in animals that had previously undergone working memory training (Figure 1B), F(1, 25) = 8.20, p < .01, suggesting that this training promoted an increase in the efficacy of selective attention.
Figure 1.
A. Animals were first trained to asymptote in two radial arm mazes, then during single trials, alternated choices in the two mazes (working memory training). A second group received no training during this phase. Plotted is the total number of errors committed on each trial (across the two mazes) during 12 such trials. Brackets indicate standard errors. B. Animals performed an odor discrimination and a visual discrimination in each of two distinct contexts. At the time of six test trials, the odor and visual cues were simultaneously presented in the context that signaled the odor discrimination or the visual discrimination. This constituted a mouse analog of the human Stroop test, where task-relevant distracters must be ignored in order for the animal to perform efficiently. The average number of errors across the six test trials are plotted as a function of group (i.e., either working memory trained or not). These results indicate that working memory training facilitated selective attention performance. Brackets indicate standard errors. C. From principal components analysis of all learning tasks, each animal was assigned a factor score to represent its general learning ability. Average factor scores for each group are plotted; brackets indicate standard errors. Prior working memory training promoted an improvement in general learning performance. D. From principal components analysis of all learning tasks, general learning ability (primary factor score) is plotted as a function of group for both the top and bottom half of the distribution of general learning abilities. Working memory training supported improved general learning abilities in animals drawn from both halves of the distribution. Brackets indicate standard errors.
All animals were then tested on five independent learning tasks (passive avoidance, odor discrimination, associative fear conditioning, egocentric navigation in a Lashley Maze, and spatial navigation in a water maze). Results from individual tasks in the learning battery are presented in supplementary material. To obtain general learning scores for all mice, acquisition data from the five learning tasks was subjected to a principal components analysis. A primary factor was extracted with an eiganvalue of 1.51, which accounted for 30% of the variance across animals. From this primary factor, factor scores were extracted to represent each animal’s general learning ability. Factor scores, which are analogous to an average z score of an animal’s performance across all tasks, provide a sensitive index of an individual’s aggregate performance in the learning battery. However, the number of subjects used here is somewhat small by factor analytic standards. Therefore, to verify the accuracy of these factor scores as descriptors of general learning performance, the factor scores were compared to each animal’s average rank performance (relative to others in the sample) across all five learning tasks (a method that has previously been reported [13]). Factor scores and average ranks were significantly correlated, r(25) = .61, p < .001. Since factor scores are presumed to be a more precise measure of an animal’s aggregate performance across all tasks, these scores were used in all subsequent analyses to compare the performance of groups of animals.
Mice trained on the concurrent radial arm maze task had significantly higher general learning scores than did animals that were not subjected to this working memory training (Figure 1C), F(1, 25) = 4.48, p < .05 (Cohen’s d effect size = .94). It was possible that working memory training differentially improved general learning scores in a subset of mice (e.g., mice from different regions of the distribution of general learning abilities). To assess this, factor scores for the top and bottom performers on the learning battery were compared across animals that had or not had working memory training (Figure 1D). The top half of mice that had undergone working memory training had significantly higher general learning abilities than the top half of animals that had not received working memory training, F(1, 11) = 6.88, p < .05. A similar difference was observed in those animals from the bottom halves of the distribution of learning abilities, F(1, 11) = 13.92, p < .005, suggesting that working memory training promoted an improvement in general learning performance regardless of an animal’s position in the distribution of learning abilities.
In this experiment, animals that underwent working memory training exhibited a commensurate increase in their aggregate performance in a battery of diverse learning tasks. Two alternative explanations of these results are possible. First, it is conceivable that any manipulation outside of the home cage would have resulted in an increase in general learning abilities because it simply rescues the animals from the environmental deprivation of the home cage. However, this result stands in contrast to previous attempts to manipulate general learning abilities of mice through extensive and repeated exposure to novel environments, a treatment that had no effect on general learning performance [15]. Relatedly, we have observed that working memory training promotes an increase in exploration (data not shown), raising the possibility that learning is facilitated as a consequence of the increase in exploration (and thus contact with environmental contingencies that support learning). However, we have tested this possibility by directly increasing exploratory tendencies by adapting mice to a series of novel environments. The widespread increase in exploratory behaviors promoted by this adaptation to novel environments supported no increase in general learning abilities (measured identically to that reported here; [15]). Therefore, we can conclude that simply increasing exploration is insufficient to increase general learning abilities. (It is worth noting that in work presently under review, we have reported that the relationship between exploration and general learning abilities is actually a consequence of variations in learning abilities, wherein learning impacts the rate of habituation to a novel environment.) In total, these results indicate that working memory training promotes an improvement in general cognitive abilities, and it does so independently of any effect of that training on exploratory tendencies or other nonspecific consequences of exposure to environments outside of the home cage.
The Effectiveness of Working Memory Exercise is Dependent on its Taxation of Selective Attention
A second experiment was designed to determine the component of working memory that is most relevant to the impact of working memory training on general cognitive performance. Previous research in our laboratory indicates that performance indicative of selective attention is more highly correlated with general learning abilities than are measures of simple memory span or duration [9]. Consequently, reducing the selective attention component of working memory training should reduce the effect of that training on general learning abilities. In the working memory training described above, animals were required to maintain a memory of two sets of choices that were guided by a common, overlapping set of visual cues, thus taxing both the animals’ ability to maintain information while simultaneously segregating that information according to the task (maze) that it was specific to. In humans, cue overlap (and the selective attention that it demands) has been shown by Conway and Engle [16] to be highly relevant to the relationship between working memory and performance on tests indicative of general fluid intelligence. In combination with the results of Kolata et al. [9], these data suggest that in the previous experiment, the presumed demands on selective attention imposed by the overlapping cues is a particularly strong candidate for the component of working memory training responsible for the observed increase in general learning abilities.
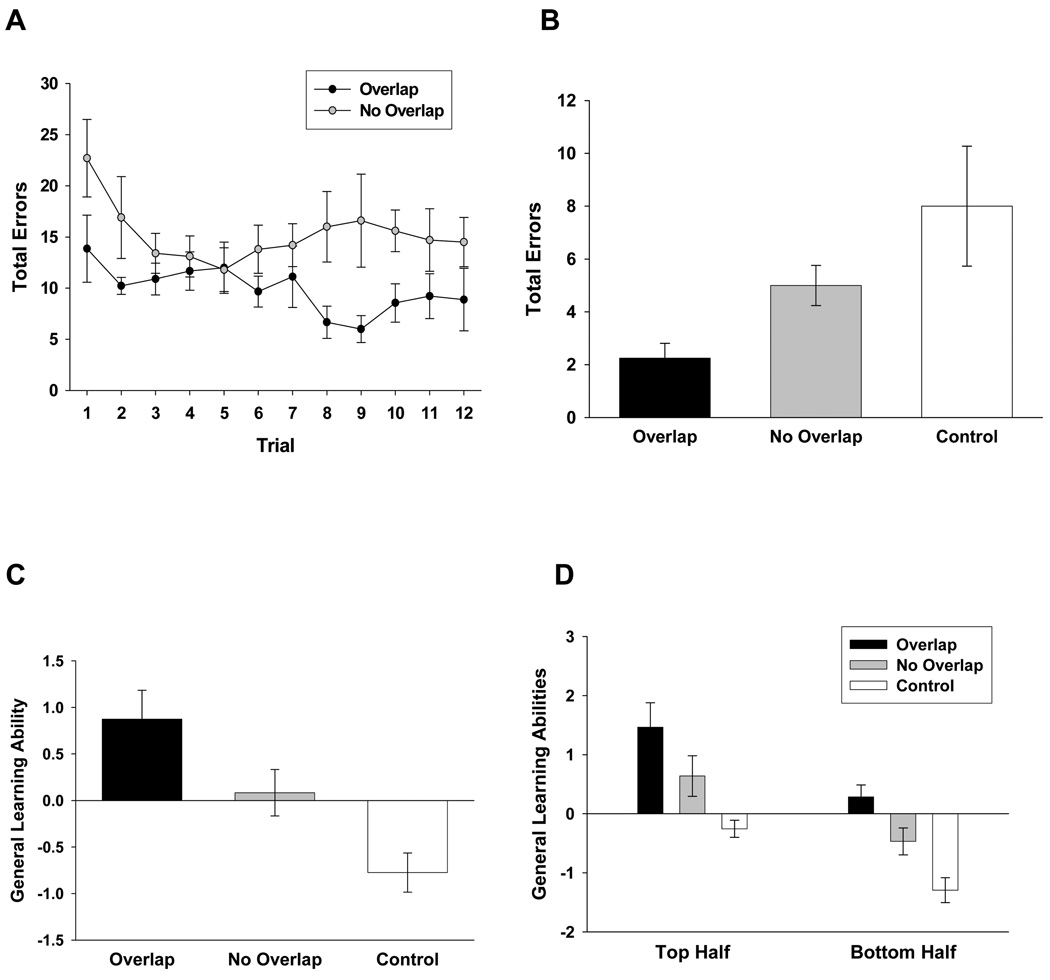
In a second experiment, two groups received 12 days of working memory training (Figure 2A), and a third group received comparable handling and deprivation conditions. Of the two groups that received working memory training, one was treated identically to the comparable group in the previous experiment, i.e., trained on two mazes in a single room such that the mazes shared overlapping cues. The second was trained on the same two mazes, but the mazes were physically separated by an opaque (floor-to-ceiling) curtain with distinct cues on each side. When so arranged, the two mazes shared no cues in common. When the three groups of mice were subsequently tested in the mouse analog of the human Stroop test, there was a strong trend towards a main effect of training history (Figure 2B), F(2, 24) = 3.31, p = .054. Planned comparisons revealed a significant difference in the performance on the Stroop test between mice that received working memory training with overlapping cues and each of the remaining two groups (ps < .05), but no difference between the animals trained with no overlapping cues and animals not subject to any working memory training. These results indicate that the added selective attention load required to perform the working memory task when cues overlapped promoted superior performance on the subsequent test of selective attention. It is noted that the two forms of radial arm maze training (with overlapping or non-overlapping cues) made very different demands on the animals, and it is possible that the difficulty performing these tasks (rather than selective attention demands per se) may promote differences in performance in tests of selective attention. To address whether the two radial arm maze tasks varied in difficulty, we performed a factorial ANOVA on the acquisition data in the black maze for groups trained with overlapping or non-overlapping visual cues, and no difference between groups was observed, F(1, 19) = .79. Moreover, a t-test was performed to compare the performance of the two groups on the last trial of this training, and again, no difference was observed, t(19) = 1.43. These results suggest that the two tasks were similarly difficult, and thus that task difficulty was not a principal determinant of the observed improvement in selective attention.
Figure 2.
A. Animals were first trained to asymptote in two radial arm mazes, then during single trials, alternated choices in the two mazes (working memory training). One group of animals received training wherein the two mazes shared overlapping visual cues (which guide the animals’ search), and one group received training on mazes that were explicitly separated, sharing no overlapping visual cues. Plotted is the total number of errors committed on each trial (across the two mazes) during 12 such trials. A third group received only equivalent handling, but did not perform in the mazes. Brackets indicate standard errors. B. Animals performed an odor discrimination and a visual discrimination in each of two distinct contexts. At the time of six trials, the odor and visual cues were simultaneously presented in the context that signaled the odor discrimination or the visual discrimination. This constituted a mouse analog of the human Stroop test, where task-relevant distracters must be ignored in order for the animal to perform efficiently. The total errors across the six test trials are plotted as a function of group (i.e., working memory training with overlapping or no overlapping visual cues, or no working memory training). Prior working memory training facilitated selective attention, and that effect was accentuated if that training required animals to segregate information related to shared (overlapping) cues. C. From principal components analysis of all learning tasks, general learning ability (primary factor score) is plotted as a function of group. Brackets indicate standard errors. Prior working memory training promoted an improvement in general learning performance, and that effect was accentuated if that training required animals to segregate information related to shared (overlapping) cues. D. From principal components analysis of all learning tasks, general learning ability (primary factor score) is plotted as a function of group for both the top and bottom half of the distribution of general learning abilities. Working memory training supported improved general learning abilities in animals drawn from both halves of the distribution, and that effect was accentuated if that training required animals to segregate information related to shared (overlapping) cues. Brackets indicate standard errors.
Results from individual tasks in the learning battery are presented in supplementary material. In this experiment, a principal components analysis of learning performance extracted a single factor with an eiganvalue of 1.73, which accounted for 35% of the variance in performance of individual animals across all tasks. From this analysis, factor scores were extracted which represented the general learning performance of individual animals. As in the previous experiment, we verified the accuracy of these factor scores as descriptors of animals’ aggregate performance in the learning battery by assessing the correlation of these scores with animals’ average rank performance across all five learning tasks. The two measures were significantly correlated, r(25) = .84, p < .0001.
When factor scores from the three treatment conditions were compared, a main effect of treatment was observed (Figure 2C), F(2, 24) = 10.57, p < .001 (omega squared effect size = .54). Post-hoc comparisons of factor scores revealed significant differences between the group that received overlapping cues during working memory training and the group that received no overlapping cues during this training (p < .05) and animals that received no working memory training (p <.001). Lastly, a difference was observed between the group that received working memory training with no overlapping cues and the group that received no working memory training (p < .05).
Again we assessed whether working memory training differentially affected the general learning performance of animals drawn from the top or bottom half of the distribution of learning abilities (Figure 2D). In the top half of the distribution, there was a main effect of group, F(2, 10) = 8.47, p < .01. Planned comparisons revealed a significant difference between the group that received working memory training with overlapping cues and control animals that received no working memory training (p < .005). However, trends were seen towards differences between mice that received working memory training with overlapping cues relative to those that received this training with no overlapping cues (p = .09), as well as between mice that received working memory training with no overlapping cues and animals that had received no working memory training (p = .06). In the bottom half of the distribution of learning abilities, the same pattern of results was observed. There was a significant main effect of group, F(2, 10) = 13.97, p < .005. Planned comparisons revealed significant differences in general learning performance between mice that received working memory training with overlapping cues and animals that received no working memory training (p < .001), mice that received working memory training with no overlapping cues and those that did not receive working memory training (p < .05), and mice that received working memory training with overlapping or no overlapping cues (p < .05). Thus working memory training again promoted an improvement in general learning abilities in animals in both the upper and lower halves of the distribution of abilities. Importantly, working memory training without overlapping cues produced performances intermediate to training with overlapping cues and no training in both the upper and lower halves of the distribution.
This experiment provides a second demonstration that working memory training facilitates the implementation of selective attention and promotes a commensurate increase in general learning abilities. When the selective attention load of the complex radial arm maze task was reduced, the effect of working memory training on general learning abilities was similarly attenuated (although not eliminated). This diminished (yet persistent) influence of working memory training with a low selective attention load on general learning abilities can be accounted for in two ways. First, it is possible that working memory training by itself, regardless of selective attention load, is sufficient to promote an increase in general learning abilities. Consistent with this possibility, variations in working memory span have been determined to predict general learning abilities in mice as well as general intelligence in humans [10,17], although it does so to a lesser degree than tests of working memory which more heavily tax the selective attention system. Another possibility is that overlapping cues were simply reduced rather than eliminated, i.e., the animals may have used uncontrolled (i.e., undetected) aspects of the training context to guide their behavior. From the present data, we cannot determine which of these two, if either, accounts for the residual impact of working memory training with a low selective attention load on the aggregate general learning abilities of mice.
Discussion
The present set of experiments provides evidence that training regimens that “exercise” working memory promote an increase in the efficacy of selective attention and a commensurate increase in general learning abilities. We have extended previous results obtained with human subjects [12,18–22] by comparing a working memory training regimen that specifically taxes the selective attention system to one with a nominally reduced selective attention load and find that the selective attention load of this training is a critical determinant of the consequent improvement in general cognitive performance. This provides evidence that training regimens which heavily tax the selective attention system have a particularly potent capacity to increase general learning abilities in mice.
In humans, a diverse set of training methods have been established that promote aspects of attention and self-regulation [22]. In combination with the parameters established here that promote selective attention and general learning abilities, the capacity to modulate attention in humans has important implications for understanding intelligence and the modulation of this trait in humans. For instance, training programs that promote attention have been determined to have modest effects on children’s performance on tests considered to be indicative of fluid intelligence [20,21,23], and this effect is accentuated in children that express attention deficit disorders [18]. Qualitatively similar results have been observed in aged humans [24]. In combination, these results have begun to converge on the conclusion that the capacity for attention can be modified through structured training, and that this training may commensurately impact performance that reflects variations in intelligence.
One might ask if the beneficial effects of working memory training reflect a consequence of that training or simply reflect the experientially deprived state of the control mice that did not receive training outside of the home cage, i.e., would any experience outside of the home cage have similar beneficial effects on general learning abilities. Relatedly, it is possible that the manipulation of any correlate of general learning ability of mice might similarly impact those abilities. To assess these considerations, we have previously demonstrated that repeatedly exposing mice to novel environments (for an amount of time and days comparable to that used here for the implementation of working memory training) promotes an increase in the animals’ subsequent exploration of new environments. However, this exposure to novel environments and the resultant increase in exploratory behaviors had no impact on the animals’ general learning performance [15], despite the fact that native exploratory propensity has been repeatedly determined to strongly predict general learning abilities [13,25,26].
It is worth noting that in a paper currently under review, we report that the expression of a cluster of genes related to dopamine signaling in the prefrontal cortex is directly related to the aggregate performance of mice in the cognitive test battery described here, and the dopaminergic processes regulated by these genes have been demonstrated to contribute to the efficacy of working memory [27–29]. It is thus tempting to speculate that working memory training, like that imposed here, might up-regulate the expression of these same genes, providing a potential epigenetic mechanism for the impact of working memory training on general cognitive abilities.
It has been long recognized that the processing components of the working memory system such as working memory capacity and/or selective attention are strongly correlated with human’s performance on tests indicative of intelligence [30]. “Higher cognitive functions” (such as reasoning, comprehension, and learning) are the hallmark of contemporary intelligence test batteries, and form common colloquial descriptions of “intelligence”. Thus it is not surprising that working memory (or at least some of its subcomponents) have come to be viewed by some as the potential latent factor which underlies general (fluid) cognitive abilities, i.e., intelligence [17,31]. Accordingly, variations in working memory efficacy have been proposed to regulate individual differences in intelligence. While theoretically compelling, evidence for this causal relationship has been difficult to attain [10,11]. The results presented here, wherein it was demonstrated that working memory training has a direct commensurate influence on general learning abilities, provides tentative support for a causal relationship between the process (working memory) and the trait (general cognitive ability).
Experimental Procedures
Experiment 1 Subjects
Twenty nine outbred CD-1 mice were obtained at 55 days of age, an age typical of previous experiments in our laboratory. They were housed individually and maintained on ad libitum food and water unless noted otherwise in a temperature-controlled vivarium on a 12-hour light/dark cycle. They were acclimated to the vivarium for one week and were subsequently handled (removed from the home cage and held by the experimenter for 60s/day) for one week prior to the start of training. Owing to a scheduling error, three animals could not complete the experiment, resulting in final group sizes of 13.
Experiment 1 Design
This experiment was a two-group, between subjects design. Native exploratory tendencies are a good predictor of general learning abilities [13,26], and so animals were assigned to one of two groups based on their performance on a pre-test for the animals’ propensity for exploration in an elevated plus maze (see supplemental materials). Working memory training was administered to one group, while the other was equivalently handled. Training and testing were identical to that used previously in our laboratory [15,25] and is described in detail in the supplementary online material. In brief, mice were trained to asymptote on two distinct radial arm mazes, where the animals collected food at the end of each of eight arms. The mazes were located in the same room such that they shared common extra-maze visual cues (patterns of lights, pictures, and architectural details that are used by the animal to guide its search). After performance in each of the two mazes stabilized, animals then were tested on each of the two mazes each day (3 hr ITI) for 12 days (the order of testing in the two mazes alternated across days). Subsequent to this initial training, the animals then performed concurrently on both mazes once a day for twelve days (constituting working memory training). During this training, mice alternated choices in the two mazes, and consequently, were required to maintain a memory of the choices in each maze, and to segregate those memories despite the overlapping extramaze visual cues. Thus this training taxed both the maintenance of information as well as working memory capacity and selective attention.
Four days after working memory training (at 112 days of age), mice began testing on a battery of five learning tasks (Lashley Maze, passive avoidance, spatial water maze, odor discrimination, associative fear conditioning), which has been previously described [13,15,26]. One week after completion of the learning battery, mice were tested for selective attention abilities in a mouse-adapted version of the Stroop test [14]. In brief, mice were trained to find food by choosing a correct odor (from three possible) in one context and a correct visual stimulus (from three possible) in a second context. In the critical test, mice were tested separately in the odor and visual contexts; during the test trial, both sets of cues were presented simultaneously. To perform efficiently during this test trial, mice must ignore those cues that were relevant to the context other than the current test context, and in doing so, discriminate between cues relevant only to performance in the current test context (e.g., visual cues should be ignored when tested in the odor discrimination context). As we describe in detail elsewhere [9], while no test is process-pure, this test makes no nominal demands on working memory span or duration, but instead, requires animals to focus on task-specific cues while ignoring task-relevant distracters, and is thus conceptually consistent with contemporary descriptions of selective attention [32,33].
Experiment 2 Subjects
Thirty mice arrived in our laboratory at 45 days of age, and were maintained and tested as in Experiment 1. Owing to a training error, three animals could not complete the experiment, resulting in final group sizes of nine (working memory training with overlapping cues), nine (working memory training with no overlapping cues), and nine (no working memory training).
Experiment 2 Design
As in Experiment 1, mice were all assessed in the elevated plus maze at approximately 80 days of age. They were then assigned to one of three groups, balanced for exploration in the elevated plus maze. One group received radial arm maze training as described in Experiment 1. A second group received radial arm maze training as described in Experiment 1 although a floor-to-ceiling opaque curtain with a different set of visual cues on each side was placed between the two radial arm mazes. This curtain effectively split the room in half, leaving each maze in what was essentially a distinct room with distinct extramaze cues. The relevant effect of this alteration to the training of the second group was to have mice trained on the same two radial arm mazes used for training the first group, although in this case, the mazes shared no common cues. Since the animals trained under these latter conditions needn’t segregate common information according to the relevant maze, this training imposed reduced demands on selective attention. A third group received no working memory exercise. Mice in this group received equivalent food deprivation and food rewards (delivered in their home cages), as well as handling equivalent to that administered to the working memory training groups.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Aging (AG022698) and the Busch Foundation (to L.D.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material
Supplemental data includes the results of individual tests of learning. In addition, a detailed description of the behavioral procedures and apparatus is provided.
References
- 1.Colom R, Rebollo I, Palacios A, Jaun-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- 2.Conway AR, Cowan N, Bunting M, Therriault DJ, Scott RB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–183. [Google Scholar]
- 3.Engle RW, Tuholski SW, Laughlin W, Conway AR. Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 4.Jarrod C, Towse JN. Individual differences in working memory. Neurosci. 2008;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Sub HM, Oberauer K, Wittman WW, Wilhelm O, Schulze R. Working memory capacity explains reasoning ability-and a bit more. Intelligence. 2002;30:261–288. [Google Scholar]
- 6.Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7:522–547. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Kyllonen PC, Christal RE. Reasoning ability is (little more than) working memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- 8.Kolata S, Light K, Townsend DA, Hale G, Grossman H, Matzel LD. Variations in working memory capacity predict individual differences in general learning abilities among genetically diverse mice. Neurobio. Learn. Mem. 2005;84:242–246. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Kolata S, Matzel LD, Light K. Selective attention is the primary determinant of the relationship between working memory and general learning abilities. Learn. Mem. 2007;14:22–28. doi: 10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neuroscience and Biobehavioral Reviews. 2010;34:23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychol. Bull. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- 12.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a "general" learning ability in mice. J. Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolata S, Light K, Matzel LD. Domain-specific and domain-general learning factors are expressed in genetically heterogeneous CD-1 mice. Intelligence. 2008;36:619–629. doi: 10.1016/j.intell.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Light K, Kolata S, Townsend DA, Grossman H, Hale G, Matzel LD. Up-regulation of exploratory tendencies does not enhance general learning abilities in juvenile or young-adult outbred mice. Neurobio. Learn. Mem. 2008;90:317–329. doi: 10.1016/j.nlm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Conway AR, Engle RW. Working memory and retrieval: a resource-dependent inhibition model. J. Exp. Psychol. Gen. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- 17.Unsworth N, Engle RW. Simple and complex memory spans and their relation to fluid abilities: Evidence from list length effects. Journal of Memory and Language. 2006;54:68–80. [Google Scholar]
- 18.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J. Clin. Exp. Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 19.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 20.Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YY, Posner MI. Attention training and attention state training. Trends Cogn Sci. 2009;13:222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Karbach J, Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science. 2009;10:1479. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- 24.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol. Aging. 2008;23:765–777. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman H, Hale G, Light K, Kolata S, Matzel LD. Pharmacological modulation of stress reactivity dissociates its role in the determination of the relationship of exploration and general cognitive abilitiies. Behav Neurosci. 2007;121:949–964. doi: 10.1037/0735-7044.121.5.949. [DOI] [PubMed] [Google Scholar]
- 26.Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobio. Learn. Mem. 2006;86:228–240. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 28.Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog. Brain Res. 1993;99:227–235. doi: 10.1016/s0079-6123(08)61349-7. [DOI] [PubMed] [Google Scholar]
- 29.Thurley K, Senn W, Luscher HR. Dopamine increases the gain of the input-output response of rat prefrontal pyramidal neurons. J. Neurophysiol. 2008;99:2985–2997. doi: 10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- 30.Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- 31.Mackintosh NJ. IQ and Human Intelligence. Oxford: Oxford University Press; 1998. [Google Scholar]
- 32.Baddeley AD. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 33.Baddeley AD, Logie RH. Working memory: The multiple component model. In: Miyake A, Priti S, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge: Cambridge University Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.