Abstract
Glucagon like peptide-1 (GLP-1) has been shown to be a potent stress-regulating neuropeptide in animal models, but little is known about whether genetic polymorphisms that influence this peptide influence stress responses in humans. We therefore explored whether a missense mutation (rs1042044) in the GLP-1 receptor was associated with morning and evening salivary cortisol levels in preschool aged children. Morning and evening saliva samples and individual buccal swabs for DNA extraction were collected from seventy-seven preschool aged children. Salivary cortisol was assayed using a time-resolved fluorescence immunoassay with fluorometric end-point detection (DELFIA), and the rs1042044 single nucleotide polymorphism (SNP) was genotyped using allele specific TaqMan probes. Children homozygous for the phenylalanine (C) substitution in GLP-1R gene had significantly higher morning salivary cortisol levels than children with other GLP-1R genotypes (p = 0.029). Additionally, children with one or two copies of the phenylalanine (C) allele had significantly higher morning cortisol levels compared to children homozygous for the leucine (A) allele (p = 0.008). Our results identify associations between a novel genetic variant of GLP-1R and hypothalamus-pituitary-adrenal (HPA) axis regulation. This polymorphism may have functional significance in stress-related psychiatric disorders.
Keywords: cortisol, GLP-1R, HPA axis, polymorphism, rs104204, stress
1. Introduction
Glucagon-Like Peptide-1 (GLP-1) is a peptide of thirty amino acids produced in the intestines, the pancreas, and the brain (Drucker and Asa, 1988). Functioning both as a hormone and a neuropeptide, GLP-1 has widespread systemic targets (Holst, 2007). The role of GLP-1 in glucose metabolism has been studied (Holz et al., 1993) and reviewed (Tolhurst et al., 2008), and a handful of animal studies have examined GLP-1 as a neuropeptide in the brain. Evidence that GLP-1 is a potent neuropeptide comes from various murine model studies demonstrating that intracerebroventricular (i.c.v) administration of GLP-1 modulates activity of the hypothalamus-pituitary-adrenal (HPA) axis. Larsen et al. (1997) showed GLP-1-dependent activation of the HPA axis through corticotrophin releasing factor (CRF) neurons in vivo, a finding replicated by Beak et al. (1998), who demonstrated GLP-1-dependent activation of hypothalamic GT-1 cells and neurons of the paraventricular nucleus (PVN). Molecular studies also indicate that GLP-1 receptor deficits lead to hyperactivation of the HPA axis, as indexed by increased plasma levels of CRF, vasopressin and corticosterone (MacLusky et al., 2000; Kinzig et al., 2003; Zhang et al., 2009).
Abnormalities in the HPA axis system marked by cortisol hypersecretion (i.e., hypercortisolemia) are hypothesized to play a role in the etiology and manifestation of various psychiatric conditions, such as major depressive disorder (Bhagwagar et al., 2005), bipolar disorder (Deshauer et al., 2003), and anxious traits (Jezova et al., 2004). HPA axis dysfunction appears to be more than a marker of state psychopathology; indeed, evidence for its etiological significance comes from studies showing that elevated morning cortisol predicts the onset of major depression in youths (Goodyer et al., 2000, 2009; Halligan et al., 2007) and adults (Harris et al., 2000). This observed HPA axis abnormality in morning cortisol is thought to have an underlying genetic basis. Family and twin studies support the heritability of HPA function, particularly morning cortisol, such that much of the variance in morning cortisol levels is attributable to heritable factors while cortisol assessed later in the day is not (Bartels et al., 2003; Kupper et al., 2005; Steptoe et al., 2009). Given the role of GLP-1 in HPA axis activity and the role of HPA axis activity in stress-related disorders, understanding whether genetic variants that influence the GLP-1 pathway are linked to early HPA axis function could further our understanding of the pathophysiology of stress-related disorders, and may help identify children at risk for mental health problems later in life.
As GLP-1 signalling is mediated through the GLP-1 receptor in the PVN (MacLusky et al., 2000; Kinzig et al., 2003; Zhang et al., 2009), we examined associations between morning and evening cortisol and polymorphic variants of the GLP-1 receptor gene (GLP-1R), located on chromosome 6 of the human genome (OMIM: 138032). In humans, a missense single-nucleotide polymorphism (SNP) (rs1042044) is located in exon 7 of the GLP-1R gene, and occurs at a minor allele frequency of greater than 5% in the Caucasian population. This SNP results in an adenine (A) to cytosine (C) change at the sequence level and leucine (Leu) to phenylalanine (Phe) at position 260 of the mature GLP-1R protein (Leu260Phe). As the role of GLP-1R in human stress pathway is unknown, this study is exploratory; however, due to the heritable nature of morning cortisol (Bartels et al., 2003; Kupper et al., 2005; Steptoe et al., 2009), we predicted that genetic variation of the GLP-1R gene would be associated with morning cortisol levels, but not with evening cortisol.
2. Methods
2.1. Participants
One hundred and sixty-six children were recruited from a larger community sample participating in a study of temperament and risk for depression. Families of young children were identified using a commercial mailing list. Children between the ages of 3 and 4 years (43.2 ± 2.4 months), with no significant medical or developmental disabilities, and who lived with at least one biological parent were eligible.
Of the 166 families asked to participate in the cortisol assessment study, 87 provided morning and evening salivary cortisol samples on the same day. Average times of morning and evening samplings were 0809h (SD = 47 min) and 2011h (SD = 59 min). To maintain population homogeneity, ten children of non-White and unknown ethnicity were excluded from final analysis to yield a final sample of 77 children (47 males). None of the children met criteria for a mood disorder as assessed using the Preschool-Age Psychiatric Assessment (PAPA; Egger et al., 1999). Children were of average cognitive ability as indexed by the Peabody Picture Vocabulary Test (M = 104.42, SD = 13.62; PPVT; Dunn and Dunn, 1997). The Committees on Research Involving Human Subjects at Stony Brook University approved and oversaw the study. A complete description of the study to parents was provided and written informed consent was obtained.
2.2. Saliva collection and cortisol quantification
Parents were given a cortisol collection kit at a laboratory visit. Saliva for cortisol analysis was collected and stored as described by Talge and colleagues (2005). Parents were instructed to collect a sample of their child’s saliva 30 minutes after wakening and 30 minutes before bed, and to avoid feeding their child any food/drink before sampling. Parents were also asked to record the sampling times. Although repeated sampling over several days yields more reliable estimates, and is therefore preferred (Gunnar & Talge, 2008), the current study collected salivary cortisol samples from children on a single occasion to reduce the burden placed on participants, who were participating in a large scale project with multiple waves of data collection. Nevertheless, moderate stability (r = 0.53) has been demonstrated for home basal cortisol levels (Goldberg et al., 2003).
Samples were assayed in duplicate using a time-resolved fluorescence immunoassay with fluorometric end-point detection (DELFIA). The inter- and intra-assay coefficients of variation were between 7.1%–9.0% and 4.0%–6.7%, respectively. Consistent with previous research (Talge et al., 2005), morning and evening cortisol values showed a positively skewed distribution (skewness = 1.31 and 3.03). We applied a log10 transformation to the data to yield unskewed values (skewness = .06 and .75). As evening cortisol values were generally less than 1.0 nmol/L, these values were negative after log10 transformation.
2.3. Genotyping
Individual genomic DNA was extracted from buccal swabs (Epicentre, Madison, USA) using the QIAGEN DNA MicroKit® (Valencia, CA) according to manufacturer’s instructions. DNA elutes were kept at +4°C while undergoing genotyping assays and kept at −80°C for long-term storage. The genotyping and cortisol assays were compiled by technicians at two different laboratories and kept blinded from corresponding data sets.
The SNP marker rs1042044 was chosen for this study as it one of the few non-synonymous variants that exists with a minor allele frequency (MAF) cut-off of greater than 5% in Caucasian population. The rs1042044 marker was genotyped using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) functionally tested by Applied Biosystems (C_2491143). TaqMan polymerase chain reactions (PCR) were performed using 1X ABI TaqMan Genotyping Master Mix, TaqMan 40X probe mix, and 25ng of DNA, for a 10µl total volume. The PCR conditions for the TaqMan SNP Genotype Assays were: enzyme activation step at 95.0 °C for 10 min, and 50 cycles of denaturation at 95.0 °C for 15 s, annealing and extension at 60.0 °C for 1 min and a 3 min final extension step. All PCR reactions and allelic discrimination reactions were performed on an ABI StepOne™ Real-Time PCR System (Applied Biosystems) and analyzed using SDS v3.0 software (Applied Biosystems). As a quality control measure, all samples were run in duplicates. Additionally, a random subset of the sample (~10%) was reanalyzed by a technician blind to initial genotyping results. All calls were consistent with initial results. Fourteen children were homozygous for the AA genotype, forty were heterozygous for the AC genotype, and twenty-three were homozygous for the CC genotype. The genotypic distribution is in Hardy-Weinberg equilibrium (X2 = 0.219, df = 1, p = 0.634).
2.4. Statistical analyses
Multiple group comparisons were performed using one-way analysis of variance (ANOVA) for quantitative outcomes or chi-square tests for categorical variables. The level of significance was set at .05 (two-tailed). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 16).
4. Results
GLP-1R SNP genotypes were not associated with child cognitive ability (F = 0.443, df = 2, 74, p = .646 or child gender (X2 = 0.276, df = 2, p = 0.871). Sampling time was not significantly associated with either morning cortisol (r = −0.155, p = 0.193) or evening cortisol levels (r = −0.140, p = 0.262). There was no association between child gender and morning (F = 2.075, df = 1, 75, p = 0.154) or evening cortisol levels (F = 0.856, df = 1, 75, p = 0.358).
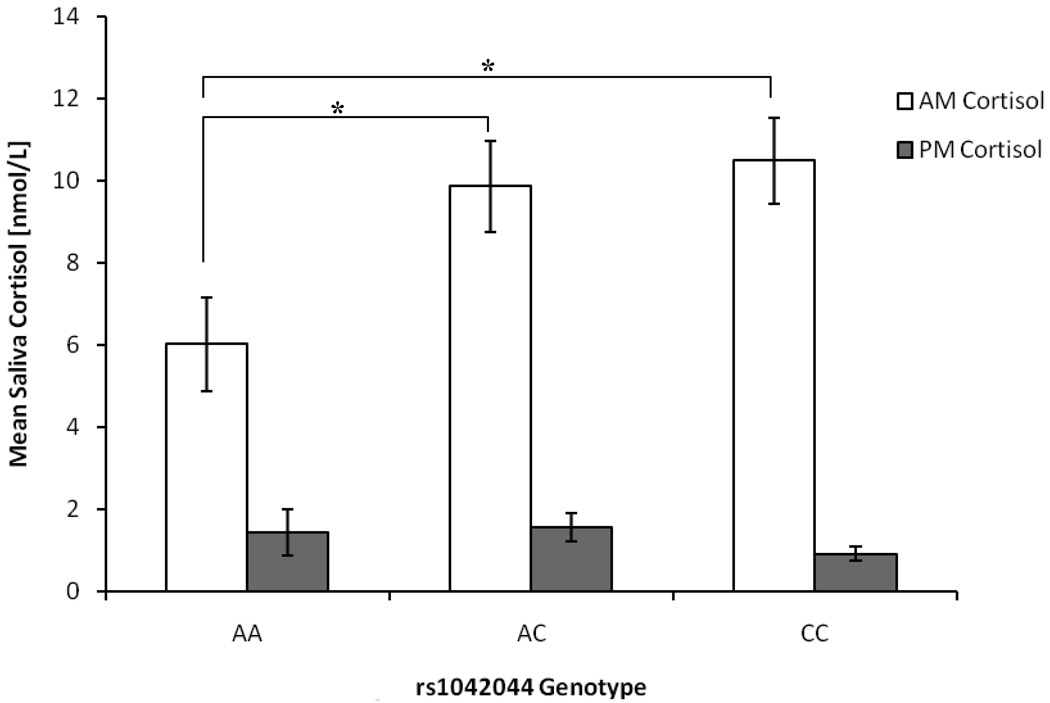
Analysis of variance (ANOVA) was used to examine group differences across the three rs1042044 SNP genotypes for morning and cortisol levels. GLP-1R SNP (rs1042044) genotypes were significantly associated with morning (F = 3.024, df = 2, 74, p = 0.029) but not with evening cortisol levels (F = 0.525, df = 2, 74, p = 0.594)1. As seen in Figure 1, post-hoc analysis (Tukey’s HSD) indicated that children homozygous for the AA genotype had significantly lower mean morning cortisol than those with the AC (mean difference = −0.142, p = 0.044) and CC genotypes (mean difference = −0.161, p = 0.034). In light of the genotypic association with morning cortisol levels, we wanted to see if the associations were allele specific. Indeed, morning cortisol levels were strongly associated with rs1042044, as children carrying either one or two copies of the phenylalanine (C) allele had significantly higher morning cortisol as compared to children homozygous for the leucine (A) allele (F = 7.374, df = 1, 75, p = 0.008).
Figure 1.
Morning and evening mean saliva cortisol concentrations (±SE) in nmol/L of GLP-1R rs1042044 SNP genotype groups.
Note: GLP-1R = Glucagon-like peptide-1 receptor; A = Adenine; C = Cytosine.
* p < 0.05. Raw mean saliva cortisol concentrations in nmol/L are presented in the figure; however, due to the positive skewness of the data, statistical analyses were performed on log10 transformed mean cortisol values.
5. Discussion
Our results indicate that GLP-1 is associated with HPA-axis function in humans. To our knowledge, this is the first study to link a novel genetic variant of GLP-1R gene to HPA axis functioning in humans. Children homozygous for the phenylalanine (C) allele showed significantly higher morning salivary cortisol levels than children homozygous for the leucine (A) allele. These results are consistent with the extant animal model literature where GLP-1 has been shown to influence HPA axis activity (Beak et al., 1998; MacLusky et al., 2000; Kinzig et al., 2003; Zhang et al., 2009). Our findings also extend the existing literature on the heritability of morning cortisol levels (Bartels et al., 2003; Kupper et al., 2005; Steptoe et al., 2009) by identifying a novel genetic marker that may contribute to this phenotype. Although the functionality of rs1042044 is unknown, its position within the gene and mature translated protein indicate that it may be affecting downstream GLP-1 signalling through its receptor GLP-1R. It is also possible that this SNP variant may be capturing functional effects of a flanking linked SNP, and variation at these loci may influence survival and plasticity of stress-related neurons. Further research is needed to test these hypotheses specifically.
Our study had a few limitations. Our sample size was small, which limited the statistical power of this study. Second, we obtained only a single sample of morning and evening cortisol levels. However, when collecting cortisol samples from young children, caution is needed to avoid compliance issues (Gunnar and Talge, 2008), which can potentially result in a biased pattern of missing data (e.g., if child compliance is associated with risk for maladjustment). Furthermore, we did not use electronic devices to monitor protocol adherence, which may be a limitation of our study. Finally, we assessed children’s morning cortisol levels 30 minutes after waking, but did not assess the cortisol awakening response (CAR), which is the degree of increase in cortisol during the first 30–40 minutes post-awakening. However, we were primarily interested in cortisol activity as an index of vulnerability to depression and anxiety, and it is unclear whether the CAR is the optimal index of cortisol activity for this purpose, as there is relatively limited evidence supporting an association between CAR and psychopathology (Jessop and Tuner-Cobb, 2008; Chida and Steptoe, 2009). Recently, Adam and colleagues (2010) reported that the CAR is a predictor of depression in adolescents; however, more research is needed to confirm this finding. In contrast, a number of reports provide evidence that morning cortisol is a reliable prospective predictor of depressive pathology (Goodyer et al., 2000; 2009; Harris et al., 2000; Halligan et al., 2007). Peak cortisol secretion 30 minutes after awakening is a reliable index of adrenocortical activity in children and adults (Pruessner et al., 1997) and has greater test-retest stability than sampling based on fixed times (Wust et al., 2000).
The HPA axis has been implicated in the pathophysiology of a number of stress-related disorders (Deshauer et al., 2003; Jezova et al., 2004; Bhagwagar et al., 2005), but only a handful of studies have identified genetic variants that influence HPA axis functioning and morning cortisol levels in particular (Chen et al., 2009). Although our sample was small, we propose that this GLP-1R gene variant, rs1042044, may be a good candidate for future psychiatric studies of genetic markers that contribute to stress sensitivity.
Acknowledgements
The authors would like to thank Bonnie Donzella and Megan R. Gunnar (University of Minnesota) for consultation on cortisol data collection procedures; Andrea Gierens and the neuroendocrine laboratory at the University of Trier, Germany for assaying the salivary cortisol samples.
This work was supported by a Canadian Institutes of Health Research grants MOP 86458 (EPH) and MOP 89876 (SMS); National Institute of Mental Health grants RO1 MH069942 (DNK) and F31 MH075484-01A2 (LRD), the Society for a Science of Clinical Psychology Dissertation Grant Award (LRD), the Emeritus Faculty Dissertation Award (LRD), and a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources.
Abbreviations
- CRF
Corticotrophin releasing factor
- DELFIA
Dissociation-Enhanced Lanthanide Fluorescent Immunoassay
- GLP-1
Glucagon-Like Peptide-1
- GLP-1R
Glucagon-Like Peptide-1 receptor
- HPA
Hypothalamic-pituitary-adrenal
- I.C.V.
Intracerebroventricular
- OMIM
Online Mendelian inheritance in man
- PVN
Paraventricular nucleus
- PCR
Polymerase chain reaction
- PPVT
Peabody Picture Vocabulary Test
- PAPA
Preschool Age Psychiatric Assessment
- SNP
Single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To control for sampling time differences, we also tabulated the slope of diurnal cortisol rhythm (morning cortisol levels - evening cortisol levels). Using the slope of diurnal cortisol rhythm as a dependent variable, a linear regression model showed that rs1042044 genotypes was also significantly associated with the slope of diurnal cortisol levels, slope (β= −0.290, SE = 0.901, t(75) = −2.626, p = 0.010).
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010 Jan 13; doi: 10.1016/j.psyneuen.2009.12.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak SA, Heath MM, Small CJ, Morgan DGA, Ghatei MA, Taylor AD, et al. Glucagon-like peptide-1 stimulates luteinizing hormone releasing hormone secretion in a rodent hypothalamic neuronal cell line (GT1–7) J Clin Invest. 1998;101:1334–1341. doi: 10.1172/JCI610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, de Geus EJC, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behav Genet. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joorman J, Hallmayer J, Gotlin IH. Serotonin transporter polymorphism predicts waking cortisol in girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Deshauer D, Duffy A, Alda M, Grof E, Albuquerque J, Grof P. The cortisol awakening response in bipolar illness: a pilot study. Can J Psychiatry. 2003;48:462–466. doi: 10.1177/070674370304800706. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1998;263:13475–13478. [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed. Circle Pines, Minnesota: American Guidance Service; 1997. [Google Scholar]
- Egger HL, Ascher BH, Angold A. The Preschool Age Psychiatric Assessment (PAPA) Durham, NC: Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 1999. Unpublished manuscript. [Google Scholar]
- Gotlib I, Joormann J, Minor K, Hallmayer J. HPA axis reactivity: A mechanism underlying associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB. Cortisol concentrations in 12 to 18-month-old infants: Stability over time, location, and stressor. Biol Psychiatry. 2003;54:719–726. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Bacon A, Ban M, Croudace T, Herbert J. Serotonin transporter genotype, morning cortisol and subsequent depression in adolescents. Br J Psychiatry. 2009;195:39–45. doi: 10.1192/bjp.bp.108.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham P. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiat. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Developmental Psychophysiology: Theory, Systems, and Methods. In: Schmidt LA, Segalowitz SJ, editors. Neuroendocrine measures in developmental research. New York, NY: Cambridge University Press; 2008. pp. 343–366. [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts cortisol in adolescent offspring. Biol Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari K, Stanford K, Cleary SE, Shiers HM, et al. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiat. 2000;180:24–28. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The Physiology of Glucagon-like Peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: A focus on health and disease in children. Stress: The International Journal on the Biology of Stress. 2008;11:1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, et al. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology. 2000;141:752–762. doi: 10.1210/endo.141.2.7326. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, van Jaarsveld CH, Semmler C, Plomin R, Wardle J. Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology. 2009;34:273–280. doi: 10.1016/j.psyneuen.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Grierens A, Gunnar MR. It’s not that bad : Error introduced by oral stimulants in salivary cortisol research. Dev Psychobiol. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2008;15:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Packard BA, Tauchi M, D'Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA. 2009;106:5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]