Abstract
The rhizome of Curcuma longa (CL) has been commonly used in Asia as a potential candidate for the treatment of different diseases, including inflammatory disorders and cancers. The present study evaluated the anti-proliferative activities of the isolated compounds (3 curcuminoids and 2 turmerones) from CL, using human cancer cell lines HepG2, MCF-7 and MDA-MB-231. The immunomodulatory activities of turmerones (α and aromatic) isolated from CL were also examined using human peripheral blood mononuclear cells (PBMC). Our results showed that the curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) and α-turmerone significantly inhibited proliferation of cancer cells in dose-dependent manner. The IC50 values of these compounds in cancer cells ranged from 11.0–41.8 μg/ml. Alpha-turmerone induced MDA-MB-231 cells to undergo apoptosis, which was confirmed by annexin-V & propidium iodide staining, and DNA fragmentation assay. The caspase cascade was activated as shown by a significant decrease of procaspases-3, -8 and -9 in α-turmerone treated cells. Both α-turmerone and aromatic-turmerone showed stimulatory effects on PBMC proliferation and cytokine production. The anti-proliferative effect of α-turmerone and immunomodulatory activities of ar-turmerone were shown for the first time. The findings revealed the potential use of CL crude extract (containing curcuminoids and volatile oil including turmerones) as chemopreventive agent.
Keywords: Turmerone, Breast cancer, Apoptosis, Immunomodulatory, Peripheral blood mononuclear cells
1. Introduction
Various species of the perennial plant Curcuma (family Zingiberaceae) are found as common ingredients in many health supplements in Asia. Curcuma longa Linn. (CL) is a spice commonly used in Indian and Chinese cuisine and traditional medicine. In Ayurvedic medicine, the rhizome of CL was suggested to be used as a stimulant, tonic, stomachic and depurative. It was also used in combinations for sprains and bruises. According to the Ayurvedic Pharmacopoeia of India, essential oil from rhizome of CL was used as a carminative, stomachic and tonic (Ministry of Health & Family Welfare, Government of India, 2001). In ancient Chinese medicine, the use of the plant Curcuma firstly appeared in “Yao Xing Lun” during Tang dynasty (A.D.618–907) and was formally recorded in the Compendium of Materia Medica (Ben Cao Gang Mu) of Ming dynasty (A.D.1590). According to the Chinese Pharmacopoeia, CL was suggested to have the functions of eliminating blood stasis, promoting the flow of “qi”, stimulating menstrual discharge and relieving pain (Chinese Pharmacopoeia Commission, 2005).
In modern pharmacological studies, CL constituents, in particular curcumin had previously been shown to possess anti-inflammatory, antioxidant, and chemopreventive properties. Curcumin was known to suppress NF-κB activation (Singh and Aggarwal, 1995) and inhibited COX-2 through the arachidonic acid metabolism pathway which accounted for its anti-inflammatory activities (Plummer et al., 1999). Curcumin also suppressed tumor growth by blocking signal transduction pathways in the target cells (Mori et al., 2001). Recent reports suggested that curcumin down-regulated expression of cell proliferation and anti-apoptotic and metastatic gene products (Aggarwal et al., 2006) and potentiated antitumor activity of gemcitabine in pancreatic cancer model (Kunnumakkara et al., 2007). Furthermore, curcumin has been shown to inhibit proliferation and induce apoptosis in human leukemic cell lines (Anuchapreeda et al., 2008), human breast (Ramachandran et al., 2002, 2005; Simon et al., 1998), hepatoma (Cao et al., 2006, 2007; Chan et al., 2005), pancreatic (Kunnumakkara et al., 2007; Li et al., 2004) and colon (Cheng et al., 2007) cancer cells. On the other hand, curcumin has been suggested to modulate the proliferation and cellular response of various immune cell types, such as T cells, B cells, macrophages, neutrophils, NK cells and dendritic cells (Jagetia and Aggarwal, 2007; Bhaumik et al., 2000).
In addition to curcumin, other constituents of CL such as sesquiterpenoids, also possessed various biological activities. Turmeric oil was shown to offer protection against benzo(a)pyrene induced increase in micronuclei in human lymphocytes. It also decreased the number of micronucleated cells in oral submucous fibrosis patients (Hastak et al., 1997). Volatile oil isolated from CL had been reported to have antibacterial (Norajit et al., 2007) and antifungal activities (Roth et al., 1998), anti-proliferation in human leukemia (Shi et al., 2003) and lung cancer cells (Wang et al., 2005). Previous studies have shown that aromatic turmerone isolated from CL could induce apoptosis in human leukemia cells (Aratanechemuge et al., 2002) and murine leukemia cells (Ji et al., 2004). The aromatic turmerone also possessed antioxidant (Jayaprakasha et al., 2002) and antiplatelet activities (Lee, 2006).
There are only a few pharmacological studies regarding the extracts of CL and the individual curcuminoids of CL (other than curcumin) (Anuchapreeda et al., 2008; Funk et al., 2006; Deters et al., 2008). The present study aimed to isolate and characterize the bioactive components from CL using bioassay-guided fractionation and to evaluate the biological activities of such components using in vitro models. The anti-proliferative effects in human hepatoma cells and breast cancer cells, as well as the immunomodulatory activities in human peripheral blood mononuclear cells of the isolated compounds from CL prepared in our laboratory were evaluated.
2. Materials and Methods
2.1 Materials
Dried rhizome of Curcuma longa L. (CL) was purchased from a herbal supplier in Hong Kong with the source of origin in India. Organoleptic, microscopic and chemical authentication were accomplished in accordance with the Chinese Pharmacopoeia (State Pharmacopoeia Commission of People’s Republic of China, 2005). Authenticated voucher specimen (no. HK 40400) was deposited in the Hong Kong Herbarium of the Agriculture, Fisheries and Conservation Department of Hong Kong Special Administration Region, China.
The organic solvents, dichloromethane, 95% ethanol, ethyl-acetate, n-hexane and methanol were purchased from Lab-Scan (Thailand) and were either HPLC-grade or AR grade.
2.2 Preparation of crude extract and sub-fractions
One kilogram of CL rhizome was cut into small pieces and soaked in 1.0 L of 95% (v/v) ethanol for 60 minutes. It was then extracted twice with 1.5 L of 95% (v/v) ethanol under reflux for 90 minutes. Following filtration, the ethanolic extracts were combined and centrifuged at 5250×g for 10 minutes. About 2.0 L of supernatant was collected and evaporated under reduced pressure at 60°C to dryness (with a yield of 7.7% w/w).
The dried ethanolic extract was allowed to re-dissolve in 1.0 L of 10% (v/v) methanol in water, followed by partitioning twice with 400 ml of n-hexane. The hexane layers were combined and evaporated to dryness to give fraction 1 (F1, 34.8% w/w). The remaining aqueous layer was subjected to partition with firstly dichloromethane and then ethyl acetate successively by following the same procedure to give dried fraction F2 (37.8% w/w) and F3 (15.1% w/w), respectively. The remaining aqueous layer was collected and concentrated under reduced pressure at 50°C. The concentrated aqueous solution was subjected to lyophilization to give dried fraction 4 (F4, 11.8 % w/w). The crude ethanolic extract and its fractions (F1-F4) have been tested for their inhibitory and stimulatory activities in cancer cells and peripheral blood mononuclear cells (PBMC), respectively. Fraction F1 showed the most potent stimulatory effect on PBMC proliferation while fractions F2 and F3 exhibited potent inhibitory activities in cancer cells (data not shown). Therefore, fractions F1, F2 and F3 were subjected to further fractionation.
2.3 Isolation of α-turmerone and ar-turmerone from lipophilic fraction (F1)
The fraction F1 was subjected to silica gel column chromatography eluting with dichloromethane and methanol (98:2). The eluents were monitored by thin layer chromatography (TLC) and similar fractions were combined to afford 5 fractions (F1-A, F1-B, F1-C, F1-D and F1-E). The inhibitory activities in cancer cells of the fractions had been tested and the fraction F1-B was shown to be most potent (data not shown). The fraction F1-B was further fractionated by silica column chromatography eluting with hexane and ethyl acetate (96:4) to afford 5 subfractions (F1-B1, F1-B2, F1-B3, F1-B4 and F1-B5). Subfractions F1-B3 and F1-B5 were characterized and identified as α-turmerone (0.03% w/w) and ar-turmerone (0.027% w/w), respectively, using 1H and 13C NMR spectral analysis and mass spectrometry.
2.4 Isolation of curcumin, demethoxycurcumin and bisdemethoxycurcumin from lipophilic fractions (F2 and F3)
The fractions F2 and F3 were combined as similar constituents were present in these fractions. The combined fraction was subjected to silica gel column chromatography eluting with dichloromethane and methanol (95:5). The eluents were monitored by thin layer chromatography (TLC) and similar fractions were combined to afford 6 fractions (F2&3-A, B, C, D, E and F). The solvents from elutes were concentrated under vacuum and recrystallized. Curcumin (0.35% w/w), demethoxycurcumin (0.08% w/w) and bisdemethoxycurcumin (0.04% w/w) were recrystallized with ethanol from F2&3-A, F2&3-C and F2&3-E, respectively. The purified compounds were characterized using 1H and 13C NMR spectral analysis and mass spectrometry.
2.5 Cell preparation
Cell line culture
The human hepatoma cell line HepG2, human breast cancer cell lines MCF-7 and MDA-MB-231, and human skin fibroblast cell line Hs-68 were obtained from American Type Culture Collection (MD, USA). The cancer cell lines were maintained in RPMI-1640 medium, while Hs-68 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. Media and supplements were obtained from Invitrogen GIBCO (NY, USA). The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and those cells in the exponential growth phase were used for experiments.
Preparation of human peripheral blood mononuclear cells (PBMC)
Fresh human buffy coat obtained from the Hong Kong Red Cross Blood Transfusion Service was diluted with phosphate-buffered saline (PBS) at a ratio of 1:1. The diluted buffy coat sample was layered on equal volume of Ficoll-Paque™ Plus solution (GE healthcare, UK) and then centrifuged at 800×g for 20 minutes at 18°C. The thin white middle PBMC layer was collected and the PBMC were washed with PBS twice and centrifuged at 100×g for 10 minutes at 18°C. The supernatant was discarded and the PBMC were resuspended in RPMI-1640 medium plus 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cell number was counted with a hematocytometer and the viability of the cells was checked by trypan blue exclusion assay. Only the isolated cells with 95% or above viability were resuspended to the target density, which was 4 × 106 cells/ml, for experiments. The PBMC were incubated at 37°C in a humidified atmosphere of 5% CO2.
2.6 Cell viability assay
The HepG2, MCF-7, MDA-MB-231 and Hs-68 cells (1 × 104/well) or PBMC (4× 105/well) were seeded in 96-well flat-bottom culture plates (Iwaki, Japan) and incubated with various concentrations (3.125–100 μg/ml) of isolated compounds from CL prepared in our laboratory (as mentioned in sections 2.3 and 2.4) for 48 hours. Plain medium was added to the control wells. The turmerones were diluted in absolute ethanol at 100 mg/ml and stored at −20°C. The lyophilized powder of curcuminoids was dissolved in DMSO at 100 mg/ml and stored at −20°C. For experiments, the final concentrations of the tested compounds were prepared by diluting the stock with culture medium. Control wells and drug-treated wells received the vehicle solvent (1% v/v ethanol or 0.5% v/v DMSO).
Following the incubation of cells with the extracts, the medium was discarded and 30 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma, USA) in PBS were added to each well and the plates were further incubated for 3 hours at 37°C. For the PBMC, the plates were centrifuged at 300×g for 10 minutes. The supernatant was then removed and 100 μl of DMSO was added to each well to dissolve the purple formazan crystals. The absorbance at a wavelength of 540 nm was measured spectrophotometrically with a BMG FLUOstarOptima microplate reader (BMG LABTECH GmbH, Germany). Results were expressed as the percentage of MTT absorbance with respect to vehicle-treated control cells.
2.7 Cell cycle analysis
Human breast cancer cells MDA-MB-231 (3 × 105/well) were seeded at 6-well culture plates and incubated overnight. The cells were treated with α-turmerone at various concentration for 24 hours. Cells were harvested after incubation, washed and fixed in 70% v/v ethanol at 4°C overnight. Before analysis, the cells were resuspended in PBS containing propidium iodide (PI, 20 μg/ml) and RNase A (10 μg/ml) at 37°C in the dark for 30 minutes. Cell cycle distribution was analyzed by flow cytometry (Becton Dickinson FACSCanto II, USA). Data from 10,000 cells per sample were collected and Modfit LT (Verity Software House, ME, USA) was used for cell cycle and apoptotic peak modeling.
2.8 Annexin V-FITC/PI staining assay
Human breast cancer cells MDA-MB-231 (3 × 105/well) were treated with α-turmerone at various concentration for 24 hours. Then, the cells were collected, washed and stained with Annexin-V FITC kit according to the manufacturer’s protocol (Trevigen, MD, USA). Briefly, cells were washed twice with 1X binding buffer and incubated in 100 μl labeling solution containing 1 μl annexin-V FITC conjugate and 10 μl PI in the dark for 15 min at room temperature. The fluorescence of the samples was detected by flow cytometry (Becton Dickinson FACSCanto II, USA).
2.9 DNA fragmentation assay
Human breast cancer cells MDA-MB-231 (1 × 106) were seeded in 100 mm culture dish and incubated for 24 hours to allow attachment. Various concentrations (40–80 μg/ml) of α-turmerone or 0.4% v/v ethanol (control) were added to the dishes and incubated for 48 hours. After treatment, attached and floating cells were harvested and washed twice with cold PBS. The cell pellets were then lysed with 200 μl DNA lysis buffer (200 mM Tris-HCl, 100mM EDTA, 1% SDS, pH 8.3) for 15 minutes at 37°C. The samples were centrifuged at 6000×g for 5 minutes to collect the DNA-containing supernatants. The supernatant was added with 50 μl of 5% SDS and 10 μl RNase A (0.4 mg/ml) at 56°C for 90 minutes. After incubation, 20 μl of proteinase K (1.5 mg/ml) was added into the mixture and incubated at 56°C for further 90 minutes. The DNA was precipitated with 30 μl of 3M sodium acetate and 750 μl of absolute ethanol. The resulting DNA was collected by centrifugation, rinsed with cold 75% v/v ethanol and then absolute ethanol. The DNA pellet was left to air dry and the dried pellet was dissolved in 30 μl TE buffer at 37°C for 30 minutes. The extracted DNA was subjected to electrophoresis through a 1.5% agarose gel containing ethidium bromide. The DNA bands were visualized under ultraviolet illumination.
2.10 Western blot analysis
Human breast cancer cells MDA-MB-231 (1 × 106) were seeded in 100 mm culture dish and incubated for 24 hours to allow attachment. Various concentrations (40–80 μg/ml) of α-turmerone or 0.4% v/v ethanol (control) were added to the dishes and incubated for 24 or 48 hours. After treatment, attached and floating cells were harvested and washed twice with PBS. The cell pellets were then lysed with whole cell extraction buffer (2% SDS, 10% glycerol, 625 mM Tris-HCl, pH6.8) for 15 minutes on ice. The samples were heated at 95°C for 10 minutes and then centrifuged at 14000×g for 20 minutes at 4°C. The supernatant proteins were resolved by 12% SDS-polyacrylamide gel and transferred to 0.45μm polyvinylidene fluoride (PVDF) membrane (Immobilon, Millipore, USA). The membrane was blocked with 5% non-fat milk in Tris buffered saline containing Tween-20 (20mM Tris-HCl, pH7.6, 150 mM NaCl, 0.1% Tween-20). The blots were incubated overnight with primary antibodies against human β-actin (1:2000, Sigma, USA), Bcl-2, procaspases-3, and -8 (1:100, Santa Cruz, USA) procaspase-9 and p21 (1:1000, Cell Signalling, USA) and cyclin D (1:200, BD Pharmingen, USA). After incubation with the secondary horseradish peroxidase-conjugated antibodies (1:2000, Invitrogen, USA) for 1 hour, detection was performed using enhanced chemiluminescence assay kit (GE Healthcare, USA).
2.11 Detection of mitochondrial transmembrane potential (Δψm)
The Δψm was analyzed by JC-1, a cationic carbocyanine fluorescent dye accumulated in mitochondria. JC-1 forms monomers and emits green fluorescence when Δψm is relatively low. Cells with high Δψm were indicated by red fluorescence as JC-1 aggregated. Human breast cancer cells MDA-MB-231 (3 × 105/well) were treated with α-turmerone at various concentrations for 24 hours. Then, the cells were collected, washed and incubated with 10 μM JC-1 for 30 min at 37°C in darkness. Then, changes in JC-1 signals were analyzed by flow cytometry (Becton Dickinson FACSCanto II, USA).
2.12 Cell proliferation assay of PBMC and collection of culture supernatant
The isolated PBMC were seeded in a 96-well flat bottom microplate and incubated with various concentrations (3.125–100 μg/ml) of turmeric extracts or isolated compounds for 24, 48 and 72 hours. To ensure the effects of Turmeric extracts on the PBMC were not due to the presence of LPS, polymyxin B (10 μg/ml), an antibiotic that can bind LPS (Duff and Atkins, 1982), were added to the extracts. The mitogen, phytohaemagglutinin (PHA), was added to target wells when appropriate at a final concentration of 10 μg/ml. Polymyxin B and PHA were purchased from Sigma (USA). Proliferation rates were estimated by (methyl-3H)-thymidine incorporation. After the incubation period, the microplates were centrifuged at 300×g for 10 minutes to obtain cell-free supernatant. The supernatant was collected and stored at −80°C until cytokine ELISA experiments.
The effects of Turmeric extracts on the proliferation of resting and mitogen (PHA)-activated PBMC were determined by (methyl-3H)-thymidine incorporation. Following incubation of cells with the extracts for 72 hours, tritiated thymidine (0.5 μCi/well; GE healthcare, UK) was added into each well and further incubated for 6 hours. Then the cells were harvested on glass fiber filters by cell harvester. Radioactivity in the filters was measured by Packard TopCount NXT™ Microplate Scintillation and Luminescence Counter (PerkinElmer Inc., USA).
For in vitro cell proliferation assay, the turmerones were dissolved in absolute ethanol. During experiment, all the drug-treated or control wells were added same concentrations of vehicle (0.5% DMSO or 1% ethanol) so that the only variable was the concentrations of test compounds. In fact, control wells without vehicle have also been set in each experiment and our data showed that the presence of vehicle (DMSO or ethanol) did not significantly change in proliferation or cytokine production of PBMC cultures (n=8, data not shown).
2.13 Determination of human IL-2, IFN-γ and TNF-α production by PBMC
The PBMC culture supernatants were subjected to test for the concentrations of cytokines such as IL-2 and IFN-γ by enzyme-linked immunosorbent assay (ELISA; BD Pharmingen, USA). The assay was carried out according to the procedures recommended in the ELISA kit manual.
2.14 Statistical analysis
Data were expressed as the mean ± standard deviation (S.D.). Statistical analysis and significance, as measured by the Student’s t-test for paired samples or one way analysis of variance (ANOVA) followed by Bonferroni post-test, as appropriate, were performed using GraphPad PRISM software version 3.0 (GraphPad Software, USA). In all comparisons, p < 0.05 was considered statistically significant.
3. Results
3.1 Isolation and characterization of α-turmerone, ar-turmerone, curcumin, demethoxycurcumin and bisdemethoxycurcumin from lipophilic fractions
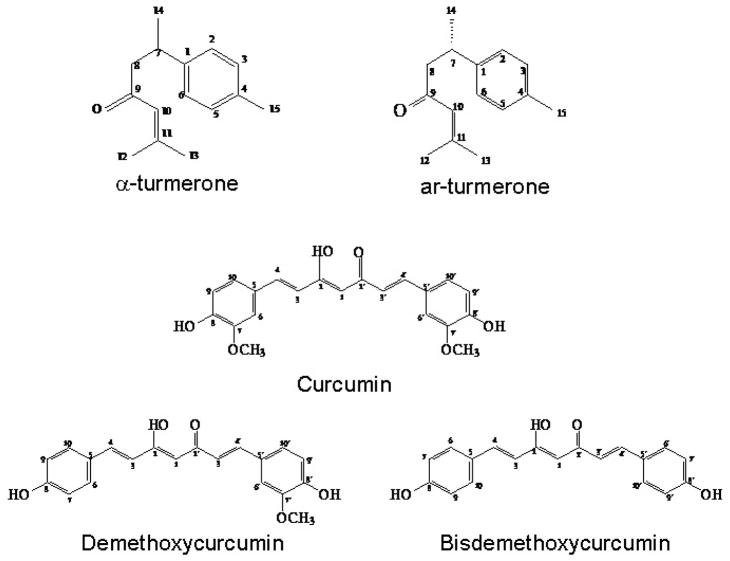
Compounds F1-B3 and F1-B5 were characterized and identified as α-turmerone and ar-turmerone, respectively, using 1H and 13C NMR spectral analysis and mass spectrometry (Table 1a). Chemical shifts of the turmerones were in accordance with the reported values (Golding and Pombovillar, 1992; Dhingra et al., 2007). The compounds F2&3-A, F2&3-C and F2&3-E were characterized and identified as curcumin, demethoxycurcumin and bisdemethoxycurcumin, respectively, using 1H NMR spectral analysis and mass spectrometry (Table 1b). Chemical shifts of the curcuminoids were in accordance with the reported values (Jayaprakasha et al., 2002; Park et al., 2005; Uehara et al., 1992). Their chemical structures are shown in Fig. 1.
Table 1a.
1H NMR and 13C NMR spectral data of α-turmerone and ar-turmerone
| 1H NMR (DMSO) | 13C NMR (CDCl3) | |||
|---|---|---|---|---|
| Carbon no. | α-turmerone | ar-turmerone | α-turmerone | ar-turmerone |
| 1 | 2.35–2.45, (1H, m) | 39.69 | 143.7 | |
| 2 | 5.82, (1H, dd, J=1.5, 10.2) | 7.28 (1H, s) | 129.1 | 126.6 |
| 3 | 6.32, (1H, dd, J=2.4, 10.2) | 7.28, (1H, s) | 126.6 | 129.1 |
| 4 | 133.8 | 135.5 | ||
| 5 | 4.91, (1H, br) | 7.28, (1H, s) | 120.1 | 129.0 |
| 6 | 2.35–2.45, (2H, m) | 7.28, (1H, s) | 25.7 | 126.6 |
| 7 | 2.35–2.45, (1H, m) | 3.45, (1H, m) | 34.0 | 35.3 |
| 8a | 2.63–2.72, (1H, m) | 2.86, (1H, dd, J=6.0, 15.6) | 48.0 | 52.7 |
| 8b | 2.35–2.45, (1H, m) | 2.76, (1H, dd, J=8.4, 15.6) | ||
| 9 | 199.2 | 199.8 | ||
| 10 | 6.22, (1H, br) | 6.18, (1H, br) | 124.1 | 124.1 |
| 11 | 155.1 | 155.0 | ||
| 12 | 2.29, (3H, s) | 2.26, (3H, s) | 27.7 | 20.7 |
| 13 | 2.04, (3H, s) | 2.00, (3H, s) | 20.7 | 27.6 |
| 14 | 1.03, (3H, d, J=6.4) | 1.40, (3H, d, J=6.9) | 17.0 | 22.0 |
| 15 | 2.03, (3H, s) | 2.46, (3H, s) | 20.9 | 20.9 |
Table 1b.
1H NMR and 13C NMR spectral data of curcumin, demethoxycurcumin and bisdemethoxycurcumin
| 1H NMR (DMSO) | 13C NMR (CDCl3) | |||||
|---|---|---|---|---|---|---|
| Carbon no. | Curcumin | Demethoxycurcumin | Bisdemethoxycurcumin | Curcumin | Demethoxycurcumin | Bisdemethoxycurcumin |
| 1 | 5.87 (1H, s) | 5.82 (s) | 5.81 (1H, s) | 101.2 | 101.2 | 101.7 |
| 2, 2′ | 183.3 | 183.4 | 184.5 | |||
| 3, 3′ | 7.55 (2H, d, J=15.9) | 7.55 (2H, dd, J=16.5Hz) | 6.48 (2H, d, J=15Hz) | 121.8 | 122.0 | 160.4 |
| 4, 4′ | 6.55 (2H, d, J=15.9) | 6.48 (2H, dd, J=16.5Hz) | 7.56 (2H, d, J=15Hz) | 140.5 | 140.6 | 140.97 |
| 5, 5′ | 127.7 | 127.8 | 127.7 | |||
| 6, 6′ | 7.05 (2H, d, J=1.8Hz) | (6) 6.86 (2H, d, J=9Hz), (6′) 6.90 (1H,d) | 7.41 (2H, d, J=9Hz) | 109.6 | 122.9 | 130.9 |
| 7, 7′ | 7.43 (2H, d, J=9Hz) | 6.83 (2H, d, J=9Hz) | 146.8 | 116.0 | 122.0 | |
| 7′-OCH3 | 3.90 (3H,s) | |||||
| 8, 8′-OH | 9.54 (br, s), 8.89 (br, s) | 147.9 | 147.8 | 116.7 | ||
| 9, 9′ | 6.88 (2H, d, J=8.1Hz) | (9) 7.43 (2H, d, J=9Hz) (9′) 6.86 (1H, d, J=9Hz) |
7.41 (2H, d, J=9Hz) | 114.8 | 116.0 | 122.0 |
| 10, 10′ | 7.06 (2H, dd, J=1.8, 8.4) | (10) 6.86 (2H, d, J=9Hz) (10′) 7.12 (1H, dd, J=1.8Hz) |
6.83 (2H, d, J=9Hz) | 122.9 | 122.9 | 130.9 |
Figure 1.
Chemical structures of α-turmerone, ar-turmerone, curcumin, demethoxycurcumin and bisdemethoxycurcumin.
3.2 Comparison of inhibitory activities on cell proliferation of HepG2, MCF-7 and MDA-MB-231 cells among different curcuminoids and turmerones
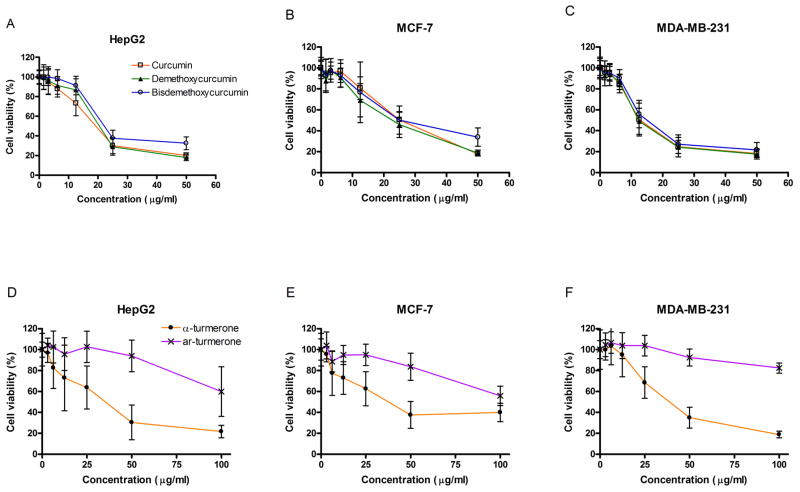
Assays for proliferative response in human hepatoma cells (HepG2) and human breast cancer cells (MCF-7 and MDA-MB-231) were performed to evaluate the anti-proliferative effects of 3 curcuminoids isolated from CL ethanolic extracts (Fig. 2). The cells were treated with a series of concentrations of curcuminoids from 1.6–50.0 μg/ml for 48 hours. As shown in Fig. 2, all three curcuminoids showed significant anti-proliferative activities in HepG2, MCF-7 cells and MDA-MB-231 cells in a concentration-dependent manner (Fig. 2A-2C). The concentrations producing 50% growth inhibition (IC50) of the curcuminoids on the three cell lines were listed in Table 2. Among the species tested, curcumin exhibited the most potent inhibitory effect on MDA-MB-231 cells proliferation (IC50 = 11.0 μg/ml). Demethoxycurcumin and bisdemethoxycurcumin could achieve 50% proliferation inhibition with a concentration of 11.4 and 12.1 μg/ml, respectively (Table 2).
Figure 2.
Effects of curcuminoids and turmerones on cell viabilities of (A&D) HepG2, (B&E) MCF-7 and (C&F) MDA-MB-231 cells. Cells were treated with increasing concentrations of curcumin (open square), demethoxycurcumin (closed triangle), bisdemethoxycurcumin (open circle), α-turmerone (closed circle) and ar-turmerone (x) for 48 hours, and cell viability was determined by the MTT assay. Results are expressed as percentages of MTT absorbance with respect to the untreated vehicle control wells (mean ± S.D. of 5–6 independent experiments with 6 wells each).
Table 2.
Concentrations producing 50% growth inhibition (IC50) of curcuminoids and turmerones isolated from Curcuma longa on cancer cell lines and normal skin fibroblasts.
| IC50 (μg/ml) | ||||
|---|---|---|---|---|
| HepG2 | MCF-7 | MDA-MB-231 | Hs-68 | |
| Curcumin | 15.8 | 24.8 | 11.0 | >50 |
| Demethoxycurcumin | 18.1 | 22.1 | 11.4 | >50 |
| Bisdemethoxycurcumin | 19.5 | 28.2 | 12.1 | >100 |
| α-turmerone | 32.9 | 41.8 | 30.2 | >100 |
| ar-turmerone | >100 | >100 | >100 | >100 |
To evaluate the effects of turmerones isolated from CL on cell proliferation of cancer cells, cells were treated with a series of concentrations of turmerones from 3.125–100 μg/ml for 48 hours. In Fig. 2, α-turmerone at concentration of 50 μg/ml caused nearly 70% inhibition of cell proliferation compared to the control in all three cell lines. The inhibitory activities of α-turmerone (12.5–100 μg/ml) were significantly lowered than those of ar-turmerone. To inhibit cell proliferation of HepG2 and MDA-MB-231 cells at 50%, the concentrations of α-turmerone were lowered than that required for MCF-7 cells (Table 2).
3.3 Effects of curcuminoids and turmerones on cell viability of normal skin cells
To determine whether the inhibition of cell proliferation on these cancer cell lines was selective, human normal fibroblast (Hs-68) cells were used as a negative control. Hs-68 cells were incubated with the curcuminoids and turmerones under the same conditions as the cell viability assay of the cancer cells. The tested curcuminoids (3.125–50 μg/ml) induced no significant suppression on the proliferation of Hs-68 (Table 2). The maximum percentage of inhibition of cell proliferation of each tested curcuminoids (50 μg/ml) was ranged from 23.5% to 63.4% (n = 4). Besides, the tested turmerones (3.125–100 μg/ml) did not show significant suppression on the proliferation of Hs-68 (Table 2). When compared with the cancer cells, Hs-68 cells were much less susceptible to the anti-proliferative effects of the curcuminoids and turmerones at the concentration of 50 μg/ml, respectively.
3.4 Presence of apoptotic cell fraction in α-turmerone-treated MDA-MB-231 cells
Since the IC50 value of α-turmerone was found to be the smallest in MDA-MB-231 cells, further experiments were performed in MDA-MB-231 cells to elucidate the mode of cell death induced by α-turmerone. Cell cycle phases were detected by propidium iodide (PI) staining. As shown in Fig. 3A & 3B, the population of sub-G1 phase increased from 2.80% to 38.86% after 48 hours of α-turmerone exposure (30–45 μg/ml). Apoptotic cells, as judged from the appearance of a sub-G1 peak, were observed in α-turmerone-treated cells. However, there was no arrest at any phase of the cell cycle after treatment. In addition, DNA agarose gel electrophoresis experiment showed that DNA fragmentation, a marker of apoptosis, was detected in α-turmerone-treated MDA-MB-231 cells after 24 and 48 hours (Fig. 3C). However, the pattern was not detected in the solvent control (0 μg/ml).
Figure 3.
Effects of α-turmerone on apoptosis induction on MDA-MB-231 cells. (A) Cell cycle analysis of MDA-MB-231 cells treated with vehicle or α-turmerone for 48 hours. After treatment, cells were analyzed by flow cytometry (n=3). (B) The fractions of cells in each cell cycle phase were summarized in the table. (C) Agarose gel of electrophoresis of genomic DNA of MDA-MB-231 cells treated with different concentrations of α-turmerone for 24 or 48 hours (n=3). M represents 100 bp DNA marker.
3.5 α-turmerone treatment induced phosphatidylserine externalization in MDA-MB-231 cells
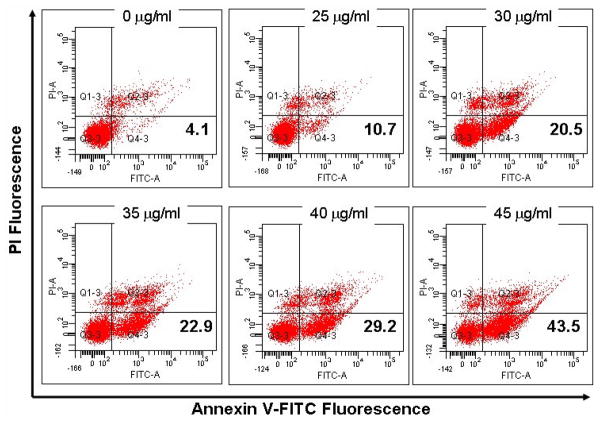
To further confirm whether α-turmerone caused apoptosis in MDA-MB-231 cells, annexin V-FITC/PI staining experiment was performed. The occurrence of phosphatidylserine (PS) externalization onto the cell surface is a characteristic of apoptotic cells. The results showed that the proportion of early apoptosis cells (Annexin V-FITC positive and PI negative) increased with the concentrations of α-turmerone applied (Fig. 4).
Figure 4.
α-turmerone-induced phosphatidylserine externalization on MDA-MB-231 cells. Cells were stained with Annexin-V FITC and PI and detected by flow cytometry. The lower right quadrant (Annexin-V+ PI−) represents early apoptosis, whereas upper right quadrant (Annexin-V+ PI+) represents late apoptosis. All results are representative of three independent experiments.
3.6 α-turmerone induced apoptosis via activation of caspase-dependent pathway
For elucidating the molecular mechanisms of action of α-turmerone in MDA-MB-231 cells, the apoptosis signal transduction was investigated. Western blotting (Fig. 5) showed that α-turmerone significantly decreased the expression of cyclin D1, which is involved in cancer cell proliferaton. Caspases play a crucial role in initiation and execution of apoptosis (Nunez et al., 1998). Caspases-8 and -9 are known as initiator caspases, whereas caspase-3 is considered as executioner caspase (Budihardjo et al., 1999). Two distinct and well-known initiator caspases, caspase-8 for the death receptor-mediated and caspase-9 for the mitochondria-mediated pathways, have been shown to initiate apoptosis. Therefore, the expression levels of caspases-3, -8 and -9 as well as PARP, the substrate of caspase-3, were detected by Western blot analysis. As shown in Fig. 5, α-turmerone treatment significantly decreased the expression levels of pro-caspases-3 and -8. Proteolytic cleavage of mitochondria-mediated apoptotic cell death initiator pro-caspase-9 was observed in α-turmerone-treated MDA-MB-231 cells and the smaller fragments p35 and p37 were shown. Cleaved PARP was also observed in α-turmerone-treated MDA-MB-231 cells.
Figure 5.
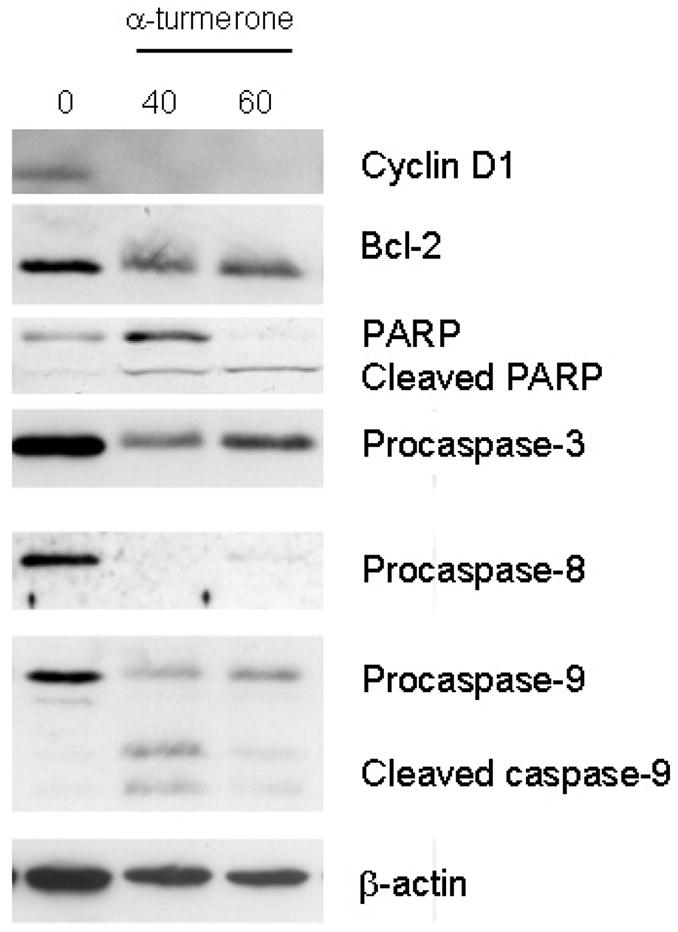
Effects of α-turmerone on cyclin D1, Bcl-2, PARP, procaspase-3, -8 and -9 expression levels in MDA-MB-231 cells. Total proteins of α-turmerone-treated (40 or 60μg/ml) cells was isolated for testing the expression by Western blotting. All results are representative of three independent experiments.
3.7 α-turmerone caused mitochondrial transmembrane depolarization in MDA-MB-231 cells and altered expression of Bcl-2 protein
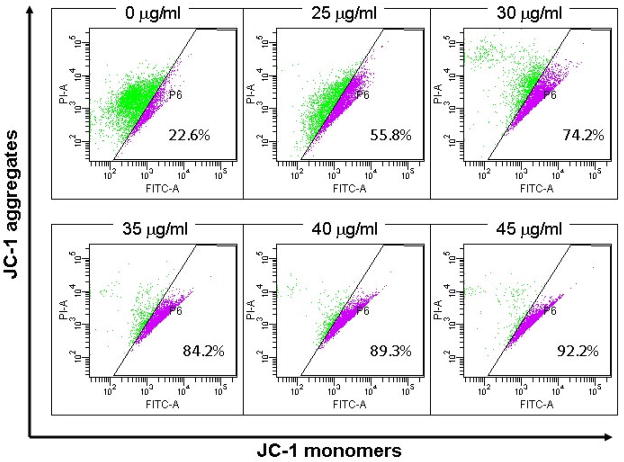
The mitochondria membrane potential change (Δψm) was measured by JC-1 staining. Mitochondria with normal Δψm concentrated JC-1 into aggregates (red fluorescence), while in depolarized mitochondria, JC-1 formed monomer (green fluorescene). The Δψm in cells treated with α-turmerone (25–45μg/ml) for 24hrs was decreased in a concentration-dependent manner. The percentage of depolarization cells was increased from 22.6% to 92.2% (Fig. 6). On the other hand, the Bcl-2 protein expression level in α-turmerone-treated cells was investigated. As shown in Fig. 5, treatment of MDA-MB-231 cells with α-turmerone slightly down-regulated Bcl-2 expression.
Figure 6.
α-turmerone caused mitochondrial transmembrane depolarization in MDA-MB-231 cells. Cells were stained with JC-1 fluoresence dye and changes in Δψm were determined by flow cytometry using green (FITC) and red (PI) channels. All results are representative of three independent experiments.
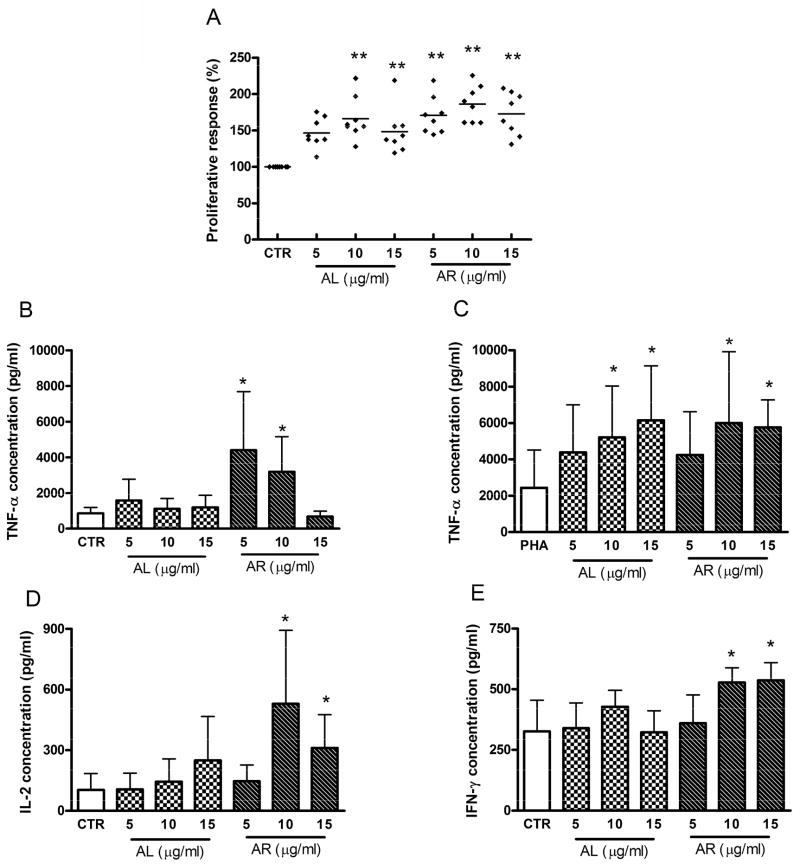
3.8 Effects of α-turmerone and ar-turmerone on cell proliferation and cytokine production of PBMC
Different concentrations of α-turmerone (AL) and ar-turmerone (AR) were tested in cultured PBMC to examine their effects on the proliferation of PBMC. The turmerones were added to PBMC with polymyxin B in order to rule out the possible contamination by the endotoxin. As shown in Fig. 7, α-turmerone (AL, 10 or 15 μg/ml) and ar-turmerone (AR, 5 -15 μg/ml) were found to significantly stimulate the proliferation of PHA-activated PBMC after 72 hours treatment. However, turmerones added at 5 -15 μg/ml did not alter the proliferative response of resting PBMC (without mitogen) (data not shown). When ar-turmerone was added to PHA-activated PBMC at 10 μg/ml, the resulting proliferation responses were slightly higher than those stimulated by α-turmerone (Fig. 7A). After incubating the PBMC with α-turmerone (AL) and ar-turmerone (AR), the culture supernatants were collected. The concentrations of TNF-α, IL-2 and IFN-γ were determined by ELISA. After 24 hours stimulation in PHA-activated PBMC, the TNF-α production was significantly increased by α-turmerone (AL) and ar-turmerone (AR) at 10 and 15 μg/ml (Fig. 7C). The productions of IL-2 and IFN-γ in PHA-activated PBMC were higher than those in resting PBMC (IL-2: 5867.2 ± 3185.4 vs 103.4 ± 81.2 pg/ml; IFN-γ: 6158.2 ± 1954.1 vs 325.7 ±135.1 pg/ml). However, neither α-turmerone (AL) nor ar-turmerone (AR) further altered the productions of IL-2 and IFN-γ in PHA-activated PBMC (data not shown). On the other hand, in resting PBMC, ar-turmerone (AR) significantly increased TNF-α, IL-2 and IFN-γ productions (Fig. 7B, 7D and 7E).
Figure 7.
Proliferative response and cytokine productions of α-turmerone (AL) and ar-turmerone (AR) treated PBMC. (A) PHA-activated PBMC were seeded in 96-well plates and incubated with 5, 10 or 15 μg/ml of α-turmerone (AL) or ar-turmerone (AR) in culture medium for 72h, and expressed as the mean % ratio of count per minute in treated and untreated control cells of 8 individual blood donors. The resting PBMC (B, D, E) and PHA-activated PBMC (C) were seeded in 96-well plates and incubated with 5, 10 or 15 μg/ml of α-turmerone (AL) or ar-turmerone (AR) for 24 hours. Culture supernatants were collected and the cytokine concentrations were specifically determined by ELISA. Results were expressed as mean concentration + SD of 8 blood samples with four wells each. Differences between the treated and untreated control group were determined by Student’s unpaired t-test. * P < 0.05, **P < 0.01 as compared to the control group.
4. Discussion
The use of traditional medicine has expanded and health supplement consist of different types of herbal medicines have become very popular in Asia in recent years. Curcuma longa (CL) and its extracts, curcumin, are widely consumed as food additive and medicine, which are believed to possess anti-inflammatory, anti-oxidant, anti-cancer and immunomodulatory properties. However, scientific investigations to compare their pharmacological effects are seldom reported. In the present study, the anti-proliferative activities on human cancer cells and immunomodulating activities in human PBMC of the CL extracts were evaluated in vitro. All the extract fractions and isolated compounds were prepared by bioassay-guided fractionation process.
The present study demonstrated that the curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) and α-turmerone isolated form CL lipophilic fraction significantly inhibited proliferation of cancer cells in a dose-dependent manner, with curcumin being the most potent. Dose-response curves were obtained and demonstrated IC50 values of the curcuminoids ranged from 11.0 to 28.2 μg/ml in three cancer cell lines. On the contrary, the tested compounds (demethoxycurcumin, bisdemethoxycurcumin, α-turmerone and ar-turmerone, 3.125–25 μg/ml) did not induce any significant inhibition of cell proliferation in human normal skin fibroblasts, illustrating their selective cytotoxic effects on cancer cell lines (Table 2).
The mechanisms of action of the cytotoxic effects of curcumin in hepatoma cells (Cao et al., 2006, 2007) and breast cancer cells (Ramachandran et al., 2002, 2005; Mehta et al., 1997; Shao et al, 2002) have been demonstrated. They suggested that curcumin induced mitochrondrial and nuclear DNA damage in HepG2 cells and apoptosis in MCF-7 and MDA-MB-231 cells. These results were consistent with our present findings that all three tested curcuminoids significantly inhibited proliferation of HepG2, MCF-7 and MDA-MB-231 cells in a dose-dependent manner (Fig. 2). The anti-inflammatory activities (Funk et al., 2006; Lantz et al., 2005) and inhibitory activities on WT1 gene expression in leukemic cells (Anuchapreeda et al., 2008) of demethoxycurcumin and bisdemethoxycurcumin have been reported and compared with the activities of curcumin. Their results suggested that curcumin showed higher efficacy. However, a recent study demonstrated that demethoxycurcumin and bisdemethoxycurcumin showed higher antimetastasis potency than curcumin by the differentially down-regulation of extracellular matrix degradation enzymes (Yodkeeree et al., 2009). Our results also demonstrated that the inhibitory activities on cancer cells proliferation among curcuminoids have no significant difference. Moreover, the effects of α-turmerone and ar-turmerone on cancer cell proliferation were compared with the curcuminoids in the present study; the activities of the turmerones on cancer cells could be manifested.
The cell line MCF-7 is prototypes for estrogen-sensitive breast cancer cells, representative of relative early stages in breast cancer development. Contrary to MCF-7 cells, MDA-MB-231 cells are prototypes for estrogen-insensitive, highly-invasive breast cancer cells, representative of relative late stages in breast cancer development (Toillon et al., 2002). The anti-invasive effects of curcumin in estrogen receptor-negative MDA-MB-231 cells have been demonstrated by Shao et al. (Shao et al., 2002), nevertheless, the suppressive activities of other constituents of CL, such as α-turmerone, on this cell type are worth attention. Besides, apoptosis induced by ar-turmerone in leukemia cells has been reported (Aratanechemuge et al., 2002; Ji et al., 2004). To date, the molecular mechanisms of action of α-turmerone and its effects on breast cancer cells are not fully understood. Therefore, mechanistic study of α-turmerone on breast cancer cells were performed in the present study. Cell cycle analysis was performed to evaluate the effect of α-turmerone. The results showed the accumulation of sub-G1 cell population in a dose-dependent manner (Fig. 3A). DNA fragmentation and apoptotic cell population were shown in DNA laddering assay and Annexin V/PI staining assay, respectively. These implied that apoptosis occurred in the α-turmerone-treated MDA-MB-231 cells. The role of mitochondria in the intrinsic pathway of apoptosis was examined by detecting changes of fluorescent probe JC-1. Disruption of mitochondria membrane potential was observed in α-turmerone-treated cells (Fig. 6). This further activated the procaspase-9 cleavage (Fig. 5), which then activated the procaspase-3 and finally triggered apoptosis.
The inhibition of cell proliferation in both breast cancer cell lines by curcuminoids and α-turmerone was demonstrated in this study, implying that the potential use of turmeric oil and curcuminoids as health supplement for breast cancer treatment at different stages could be justified. Meanwhile, Lantz et al. suggested that the fraction containing curcuminoids and turmeric oils was more effective than the fraction of curcuminoids alone at inhibiting PGE2 production (Lantz et al., 2005).
Our results also showed that both α-turmerone and ar-turmerone exhibited stimulating effects on PBMC proliferation and cytokine production (Fig. 7). Since the activities of the turmerones were not affected by the addition of polymyxin B (10 μg/ml) (data not shown), the observed mitogenic effect was unlikely to be mediated by the endotoxin (lipopolysaccharide), which is a potent activator of B cells. Besides, α-turmerone and ar-turmerone were isolated from the lipophilic fraction (F1) of Curcuma longa crude ethanolic extract. The effects of crude ethanolic extract and the fractions (F1-F4, section 2.2) have been tested in PBMC and fraction F1 exhibited the most potent stimulatory effect on PBMC proliferation (data not shown) and this fraction was subjected to further column chromatography. Among the subfractions of F1, only the isolated compounds α-turmerone and ar-turmerone showed stimulating activities in PBMC. Therefore, the response of PBMC may be specific to such turmerones instead of other contaminants. On the other hand, the compounds curcumin, demethoxycurcumin and bisdemethoxycurcumin, which were isolated from F2 and F3, have not been tested in PBMC because fraction F2 and F3 showed neither stimulatory nor inhibitory effect on PBMC proliferation (data not shown). Moreover, a previous study has demonstrated that in fact curcumin inhibited PHA-induced proliferation and IL-2 production in PBMC (Yadav et al., 2005).
Along with these results, α-turmerone and ar-turmerone were shown to have immunostimulatory effects. Researches on turmeric oil have focused on the anti-inflammatory effects using macrophages. Nevertheless, little information is available about the immunomodulatory activities in other cell types of the immune system. Alpha-turmerone and ar-turmerone were shown for the first time to exert modulatory activities in human PBMC. Last but not least, the multiple targets abilities of CL extracts (including curcuminoids and turmerones) could be illustrated in the present study.
In conclusion, bioassay-guided fractionation of the rhizome of Curcuma longa led to the isolation of the curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin), α-turmerone and ar-turmerone. Curcuminoids and α-turmerone significantly inhibited proliferation of cancer cells in a dose-dependent manner. Both α-turmerone and ar-turmerone stimulated PBMC proliferation and cytokine production in vitro. This is possibly the first report on the anti-proliferative effect of α-turmerone and immunomodulatory activity exerted by ar-turmerone. These findings revealed the potential use of Curcuma longa extracts (including curcuminoids and volatile oil) as chemopreventive/antitumor agent.
Acknowledgments
This project was supported by Grant Number 5 P50 AT002779-02 from the National Institutes of Health of USA and the Botanical Research Center at Memorial Sloan-Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Anuchapreeda S, Tima S, Duangrat C, Limtrakul P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother Pharmacol. 2008;62:585–594. doi: 10.1007/s00280-007-0642-1. [DOI] [PubMed] [Google Scholar]
- Aratanechemuge Y, Komiya T, Moteki H, Katsuzaki H, Imai K, Hibasami H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int J Mol Med. 2002;9:481–484. [PubMed] [Google Scholar]
- Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Cao J, Jia L, Zhou HM, Liu Y, Zhong LF. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol Sci. 2006;91:476–483. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu Y, Jia L, Zhou HM, Kong Y, Yang G. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic Biol Med. 2007;43:968–975. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann N Y Acad Sci. 2005;1042:372–378. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Kozubek A, Ohlsson L, Sternby B, Duan RD. Curcumin decreases acid sphingomyelinase activity in colon cancer Caco-2 cells. Planta Med. 2007;73:725–730. doi: 10.1055/s-2007-981540. [DOI] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia. Shanghai: Commerical Press; 2005. pp. 260–261. [Google Scholar]
- Deters M, Knochenwefel H, Lindhorst D, Koal T, Meyer HH, Hansel W. Different curcuminoids inhibit T-lymphocyte proliferation independently of their radical scavenging activities. Pharm Res. 2008;25:1822–1827. doi: 10.1007/s11095-008-9579-2. [DOI] [PubMed] [Google Scholar]
- Dhingra OD, Jham GN, Barcelos RC, Mendonca FA, Ghiviriga I. Isolation and identification of the principal fungitoxic component of turmeric essential oil. Journal of Essential Oil Research. 2007;19:387–391. [Google Scholar]
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding BT, Pombovillar E. Structures of Alpha-Turmerone and Beta-Turmerone. Journal of the Chemical Society-Perkin Transactions. 1992;1:1519–1524. [Google Scholar]
- Hastak K, Lubri N, Jakhi SD, More C, John A, Ghaisas SD. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997;116:265–269. doi: 10.1016/s0304-3835(97)00205-x. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- Ji M, Choi J, Lee J, Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14:253–256. [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005;12:445–452. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Lee HS. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol. 2006;97:1372–1376. doi: 10.1016/j.biortech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- Mehta K, Pantazis P, McQueen T, Aggarwal BB. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs. 1997;8:470–481. doi: 10.1097/00001813-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Ministry of Health & Family Welfare, Government of India. The Ayurvedic Pharmacopoeia of India. New Delhi: The Controller of Publications Civil Lines; 2001. pp. 45–46. [Google Scholar]
- Mori H, Niwa K, Zheng Q, Yamada Y, Sakata K, Yoshimi N. Cell proliferation in cancer prevention; effects of preventive agents on estrogen-related endometrial carcinogenesis model and on an in vitro model in human colorectal cells. Mutat Res. 2001;480–481:201–207. doi: 10.1016/s0027-5107(01)00200-7. [DOI] [PubMed] [Google Scholar]
- Norajit K, Laohakunjit N, Kerdchoechuen O. Antibacterial effect of five Zingiberaceae essential oils. Molecules. 2007;12:2047–2060. doi: 10.3390/12082047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- Park BS, Kim JG, Kim MR, Lee SE, Takeoka GR, Oh KB. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. J Agric Food Chem. 2005;53:9005–9009. doi: 10.1021/jf051765z. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Fonseca HB, Jhabvala P, Escalon EA, Melnick SJ. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/s0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran C, Rodriguez S, Ramachandran R, Raveendran PK, Fonseca HB, Khatib Z. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25:3293–3302. [PubMed] [Google Scholar]
- Roth GN, Chandra A, Nair MG. Novel bioactivities of Curcuma longa constituents. J Nat Prod. 1998;61:542–545. doi: 10.1021/np970459f. [DOI] [PubMed] [Google Scholar]
- Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–240. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- Shi XY, Ku K, Tan R. Mechanistic study of anti-tumor effect of turmeric volatile oil. Pharmacology and Clinics of Chinese Materia Medica. 2003;19:15–16. [Google Scholar]
- Simon A, Allais DP, Duroux JL, Basly JP, Durand-Fontanier S, Delage C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998;129:111–116. doi: 10.1016/s0304-3835(98)00092-5. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Toillon RA, Descamps S, Adriaenssens E, Ricort JM, Bernard D, Boilly B. Normal breast epithelial cells induce apoptosis of breast cancer cells via Fas signaling. Exp Cell Res. 2002;275:31–43. doi: 10.1006/excr.2002.5490. [DOI] [PubMed] [Google Scholar]
- Uehara S, Yasuda I, Takeya K, Itokawa H. Terpenoids and curcuminoids of the rhizoma of Curcuma xanthorrhiza Roxb. Yakugaku Zasshi. 1992;112:817–823. doi: 10.1248/yakushi1947.112.11_817. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Yang HP. Effects of turmeric volatile oil on the morphology of human lung adenocarcinoma A549 cells. Acta Academiae Medicinae Militaris Tertiae. 2005;27:220–221. [Google Scholar]
- Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J Nutr Biochem. 2009;20:87–95. doi: 10.1016/j.jnutbio.2007.12.003. [DOI] [PubMed] [Google Scholar]