Abstract
Background:
Chronic consumption of ethanol (EtOH) is well recognized to lead to defective innate and adaptive immune responses and increase the severity of pulmonary infections. Our own studies have demonstrated that chronic EtOH consumption decreases CD8 T cell immunity to influenza virus infections (IAV) leading to severe infections and mortality. Interestingly, anti-viral treatment of influenza virus infections has been shown to be compromised in mice and humans that are immuno-deficient. It is known that EtOH can alter the pharmokinetics of anti-virals. Therefore the effectiveness of influenza anti-viral therapy during chronic ethanol consumption remains in question.
Methods:
BALB/c mice were placed on 18% (w/v) EtOH in their drinking water for 8 weeks. Chronic EtOH consuming and water controls were then treated with 10mg/kg oseltamivir orally and infected intranasally with influenza virus 4 hours post oseltamivir treatment. The mice were then treated with oseltamivir twice daily until day 7 post infection. Influenza disease severity was measured by morbidity and mortality, pulmonary viral titers and histology.
Results:
Chronic EtOH consuming mice infected with IAV and treated with oseltamivir have decreased morbidity and mortality, pulmonary viral titers and pulmonary pathology compared to untreated EtOH mice.
Conclusions:
Despite the severe immune defect seen in chronic EtOH mice as well as the potential for EtOH to inhibit the conversion of oseltamivir into an active form, treatment with oseltamivir reduces viral shedding as well as disease severity. These data suggest that the combination of a limited adaptive immune response plus the anti-IAV drug oseltamivir is sufficient to curb high mortality and mediate resolution of influenza virus infections in mice chronically consuming ethanol.
Keywords: Influenza virus, Oseltamivir, Chronic Ethanol Consumption
INTRODUCTION
Influenza A virus infections (IAV) result in significant morbidity and mortality within the industrialized world each year(Stohr, 2003). Coordinated responses by both the innate and adaptive arm of the immune system are required to mediate viral clearance. To this end, patients and mice that have compromised immune systems from genetic defects, bone marrow transplants or lymphoma display an increased susceptibility to IAV, prolonged viral shedding and increased chances of severe complications(Baz et al., 2006; Ison et al., 2006a; Ison et al., 2006b; Nichols et al., 2004).
Mice and humans chronically consuming EtOH have severe defects in both innate and adaptive immune responses(Brown et al., 2006; Cook, 1998; Cook et al., 2007; Meyerholz et al., 2008; Song et al., 2002). Further, chronic EtOH exposure has been shown to enhance susceptibility and severity of pulmonary infections(Happel and Nelson, 2005; Jerrells et al., 2007) as well as acute respiratory distress syndrome (ARDS)(Joshi and Guidot, 2007). We have previously demonstrated that mice chronically consuming ethanol (EtOH) have an increased susceptibility to IAV infections(Meyerholz et al., 2008). The mechanism for increased disease severity was correlated with a defect in the generation of an IAV-specific CD8 T cell response in the lungs. These mice were unable to efficiently eliminate the virus and as a result displayed increased pulmonary pathology as well as increased morbidity and mortality.
Currently there are multiple therapeutic agents directed against specific proteins of IAV that disrupt the spread of the virus within the host. The first anti-IAV drugs were inhibitors of the M2 ion channel protein (von Itzstein, 2007). Unfortunately many IAV subtypes, including some that currently circulate, have acquired mutations making them resistant to M2 inhibitors without affecting viral fitness(De Clercq, 2006). The other main class of anti-influenza drugs are the neuraminidase (NA) inhibitors oseltamivir (given orally as a pro-drug) and zanamivir (inhaled in the active form). These drugs block the function of NA, which is required for cleavage of sialic acid residues allowing the virus to bud off the cell surface and infect a new cell(De Clercq, 2006).
Interestingly mice with severe combined immunodeficiency disease (SCID, i.e. lacking B and T cells) that have been infected with IAV and treated with oseltamivir for the initial seven days p.i. still display increased morbidity and mortality after removal of oseltamivir treatment. Conversely, wild-type mice infected with IAV exhibit limited to no viral disease following removal of the drug(Ison et al., 2006b). Furthermore, SCID mice compared with wild type mice, also have prolonged viral shedding and have an increased percentage of IAV isolates that contain mutations conferring resistance to oseltamivir(Ison et al., 2006b). Similarly, patients who are immunocompromised because of hematopoietic stem cell transplantation (HSCT) therapy and treated with oseltamivir during IAV infections have prolonged viral shedding and an increased frequency of the development of NA inhibitor resistant strains when compared to healthy controls(Cohen-Daniel et al., 2009). All together these data suggest that in an immunocompromised host oseltamivir alone is not sufficient to clear the infection and that prolonged viral shedding likely increases the chances of developing NA inhibitor resistant strains.
In addition to the detrimental effects of EtOH on the immune system, studies have also shown that EtOH exposure can effect the conversion of pro-drugs into their active forms. For example a recent report demonstrates that EtOH exposure inhibits hydrolysis by carboxylesterases(Laizure et al., 2003), a critical step in converting the inactive oseltamivir pro-drug into the active oseltamivir carboxylate(Shi et al., 2006; Zhu and Markowitz, 2009).
Given that chronic EtOH consumption; 1) increases the length of viral shedding as well as reducing the anti-viral adaptive immune response and 2) could potentially change the pharmokinetics of oseltamivir, we sought to determine if anti-influenza therapeutics would reverse the increased disease severity and outcomes associated with chronic EtOH consumption during IAV infections. Interestingly, despite these potential limitations our results show that chronic EtOH exposed mice treated with oseltamivir are protected from increased morbidity and mortality as well as have a reduction in influenza viral titers. Furthermore, these mice also have decreased pulmonary pathology during IAV infections. All together these data suggest that the NA inhibitor oseltamivir together with the limited adaptive immune response observed in chronic EtOH mice, is sufficient to allow clearance of IAV infections resulting in protection from severe disease and mortality.
RESULTS
Oseltamivir reduces morbidity and mortality in chronic EtOH consuming mice
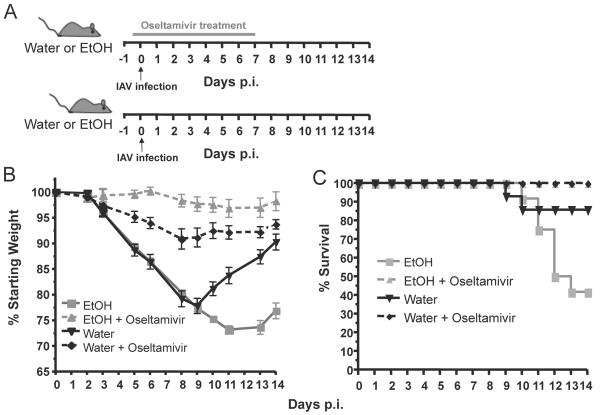
In order to determine the efficacy of anti-influenza virus treatment under conditions of chronic EtOH consumption, we utilized the Meadows-Cook model of chronic alcohol intake to mimic the immune defects seen in chronic alcoholics(Cook et al., 2007; Cook et al., 2004; Gurung et al., 2009; Meyerholz et al., 2008; Ness et al., 2008; Song et al., 2002). Briefly, BALB/c mice were placed on chronic EtOH, or water as a control, for eight weeks. These mice were then treated orally with the influenza NA inhibitor oseltamivir and four hours post treatment infected i.n. with IAV. Infected mice were treated with oseltamivir twice daily for seven days after which treatment was halted (Fig 1A). Importantly, given the severe immune defects in chronic EtOH mice, studies have demonstrated that oseltamivir treatment does not inhibit the adaptive immune response and therefore should not lead to enhanced immunodeficiency(Burger et al., 2000). Similar to our previous influenza virus based studies (Meyerholz et al., 2008), both untreated water and chronic EtOH mice exhibited a similar morbidity until day 9 p.i. where water mice began to recover and chronic EtOH mice continued to decline (Fig 1B). Further, untreated chronic EtOH mice also had a higher rate of mortality (~60%) compared to untreated water controls (~15%) (Fig 1C). However both chronic EtOH and water control mice treated with oseltamivir exhibited little to no morbidity (Fig. 1B) and no mortality (Fig. 1C) during IAV infection. In fact, contrary to a hypothesized decrease in the conversion of pro-oseltamivir to its active form in EtOH mice, oseltamivir treated ETOH mice had significantly higher weights than the corresponding water treated controls, suggesting that oseltamivir undergoes proper or even enhanced conversion into an active form in the chronic EtOH environment. In fact EtOH has been demonstrated to enhance the induction of multiple enzymes following exposure(Anderson et al., 1995; Lieber, 2005). All together these data suggest that oseltamivir treatment is able prevent the enhanced morbidity and mortality seen in chronic EtOH consuming mice. Surprisingly, after treatment was halted on day 7 p.i. chronic EtOH exposed mice maintained weight similar to water controls (Fig 1B). This result is in contrast to previous results observed in mice under immunosuppressive conditions/treatment where cessation of oseltamivir treatment leads to enhanced morbidity(Ison et al., 2006b). Given our previous results demonstrating that the influenza-specific adaptive immune response generated in chronic EtOH mice can only mediate limited reduction of viral titers between days six and 8 post IAV infections(Meyerholz et al., 2008) our current data suggest that despite the severe CD8 T cell defects and the inhibited immune response generated, this limited response together with the addition of oseltamivir treatment may be sufficient to prevent enhanced viral shedding and outgrowth of oseltamivir resistant viruses.
Fig. 1.
Oseltamivir treatment reduces the enhanced morbidity associated with IAV infection in chronic EtOH exposed mice. A.) Experimental model. Mice exposed to water or EtOH for 8 weeks were treated by oral gavage with 10mg/kg oseltamivir 4 hours prior to infection and then twice daily for 7 days (upper diagram). Control water/EtOH mice were infected and left untreated (lower diagram). B.) Morbidity (i.e. weight loss) was measured daily and expressed as percent starting weight loss. Significant differences were seen between; untreated EtOH and oseltamivir treated EtOH mice between days 5-13 p.i. p=0.0001, between untreated EtOH and water control mice on days 10-14 p.i. p≤ 0.001, between untreated water and oseltamivir treated water mice on days 5-10 p≤ 0.0005 and 11-14 p≤ 0.05 and treated water and EtOH mice on days 5-11 p≤ 0.025. C.) Untreated EtOH mice exhibited a significant increase in mortality compared to treated EtOH mice and water controls during IAV infections, p≤ 0.025. Treated water mice displayed similar levels of mortality compared to untreated water mice, p≤ 0.15. Data are representative of 2 independent experiments with 5-10 mice per group.
Oseltamivir reduces pulmonary viral titers in chronic EtOH consuming mice
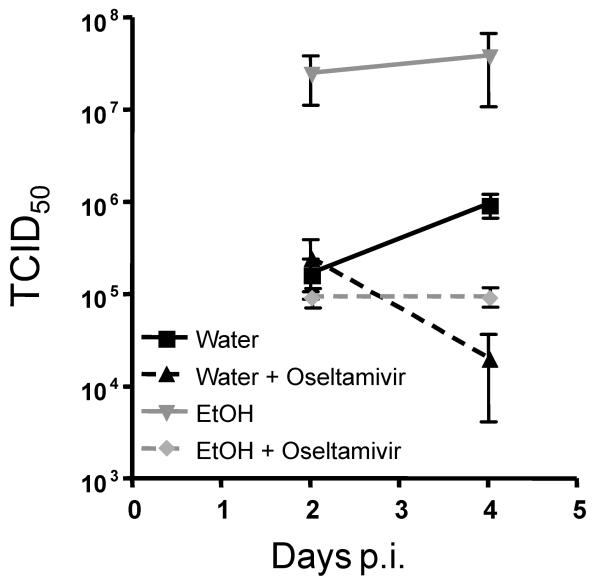
To determine if oseltamivir treatment is able to decrease viral shedding in chronic EtOH consuming mice, BALB/c mice were treated and infected as described in Fig. 1A and the level of virus within the lungs on day 2 and day 4 post infection was determined. Similar to previous results from our laboratory(Meyerholz et al., 2008), untreated chronic EtOH exposed mice had higher pulmonary viral loads than water controls on both days 2 and 4 post infection (Fig. 2). However, chronic EtOH exposed mice that were also treated with oseltamivir on days 0-4 p.i. had decreased viral titers on day 2 and significantly reduced titers on day 4 p.i. compared to untreated EtOH mice (Fig. 2). Importantly during IAV infections chronic EtOH consuming mice on oseltamivir treatment had pulmonary viral titers similar to untreated water controls (Fig. 2). As expected oseltamivir treatment significantly reduced viral titers in water mice (Fig. 2, day 4 p.i.). These data suggest that oseltamivir treatment is able to inhibit viral shedding in chronic EtOH consuming mice similar to controls. Importantly these data also support the idea that there is likely limited/no defect in the conversion of oseltamivir into its active form during chronic EtOH consumption.
Fig. 2.
Oseltamivir treatment reduces pulmonary virus titers in chronic EtOH exposed and control water mice. Mice were infected and treated with oseltamivir as described in Fig. 1A. On day 2 and day 4 p.i. pulmonary virus was titered by endpoint dilution assay in MDCK cells. On day 4 p.i. there was a significant reduction of viral titers between treated EtOH and untreated EtOH mice p≤0.05 and treated and untreated water mice, p≤0.0001. Data are representative of 1 experiment for day 2 and 2 experiments for day 4 with 3-5 mice per group.
Chronic EtOH consuming mice treated with oseltamivir have decreased lung pathology and neutrophilia during IAV infections
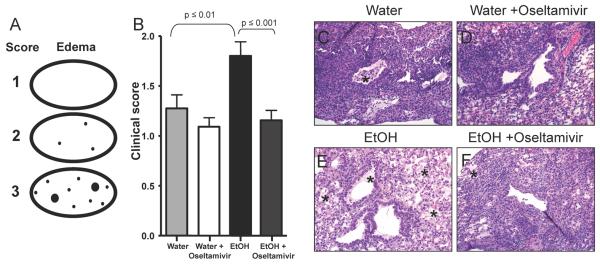
Given that oseltamivir treatment of chronic EtOH consuming mice is capable of reducing morbidity, mortality as well as viral titers during IAV infections it was important to determine if there was a similar decrease in pulmonary pathology associated with IAV-infection of EtOH mice in our previous studies (Meyerholz et al., 2008). Therefore, chronic EtOH mice and water controls were treated with oseltamivir and infected with IAV as described in Fig 1A. On day 14 p.i. lungs were examined by histology. Fig. 3A depicts a model of our clinical scoring system. Similar to what we have reported previously(Meyerholz et al., 2008), chronic EtOH mice had increased levels of pulmonary edema compared to water controls (Fig. 3B,C,E). Interestingly, oseltamivir treatment resulted in a significant decrease in pulmonary edema in chronic EtOH mice (Fig. 3B,D,F). Oseltamivir treatment additionally decreased both necrotizing bronchiolitis and atelectasis in chronic EtOH and water control mice compared to untreated controls (data not shown). These data suggest that, despite the defect in the adaptive immune response, oseltamivir treatment is able to rescue chronic EtOH mice from increased pulmonary disease.
Fig. 3.
Pulmonary pathology is decreased in chronic EtOH exposed mice receiving oseltamivir treatment. A.) Schematic of scoring for edema in low magnification lung sections (ovals). Black dots represent lesion distribution with increased dot size representing increased severity (see Materials and Methods for further description of the scores). B.) Mice were infected and treated with oseltamivir as described in Fig. 1A. On day 14 p.i. pulmonary edema was measured. Values represent the clinical score assigned by a blinded Veterinary Pathologist. Data are pooled from 2 independent experiments with 5-9 mice per group. C.) Representative sample of a control water mice receiving no treatment showing minimal levels of pulmonary edema. D.) Representative sample of an untreated EtOH mice with enhanced pulmonary edema. E.) Representative sample of a Water mice receiving oseltamivir treatment showing reduced levels of pulmonary edema. F.) Representative sample of an EtOH mice receiving oseltamivir treatment showing a significant reduction in pulmonary edema compared to untreated EtOH mice. * Represents areas of pulmonary edema. All images shown are at 20x magnification. Data are representative of 2 independent experiments with 5-9 mice per group.
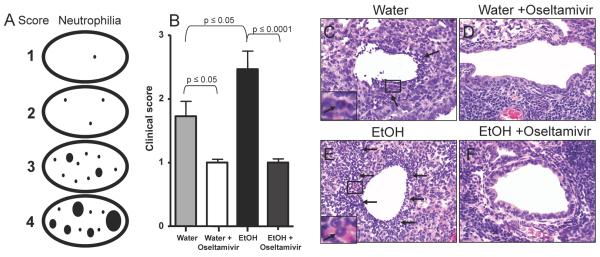
Severe IAV infections are often associated increases in neutrophilic infiltrate (Perrone et al., 2008; Tumpey et al., 2005) and results from our laboratory have demonstrated that chronic EtOH mice have increased neutrophilic recruitment during IAV-infection(Meyerholz et al., 2008). Therefore we next determined if oseltamivir treatment decreases the enhanced neutrophilia associated with IAV infections in chronic EtOH mice using the scoring system shown in Fig. 4A. Similar to our previous results(Meyerholz et al., 2008), chronic EtOH mice have increased neutrophilia compared to water controls (Fig. 4B,C,E). However, oseltamivir treatment significantly reduces neutrophilia in both chronic EtOH and water mice (Fig 4B-F). All together our results suggest that inhibition of viral replication by oseltamivir in EtOH consuming mice leads to protection from pathology and severe pulmonary disease.
Fig. 4.
IAV induced neutrophilia is decreased in oseltamivir treated chronic EtOH exposed mice. A.) Schematic of scoring for neutrophilia in low magnification lung sections (ovals). Black dots represent lesion distribution with increased dot size representing increased severity (see Materials and Methods for further description of the scores). B.) Mice were infected and treated with oseltamivir as described in Fig. 1A. On day 14 p.i. pulmonary neutrophilia was measured. Values represent the clinical score assigned by a blinded Veterinary Pathologist. Data are pooled from 2 independent experiments with 5-9 mice per group. C.) Representative sample of a control water mouse receiving no treatment showing minimal levels of neutrophilic infiltration. D.) Representative sample of an untreated EtOH mouse with enhanced neutrophilic foci in the airway. E.) Representative sample of a water mouse receiving oseltamivir treatment showing reduced levels of neutrophilic infiltration. F.) Representative sample of an EtOH mouse receiving oseltamivir treatment showing a significant reduction in airway neutrophilia compared to untreated EtOH mice. Arrows indicate areas of neutrophilic infiltration. All images shown are at 40x magnification. Data are representative of 2 independent experiments with 5-9 mice per group.
DISCUSSION
Previously we have demonstrated that mice chronically consuming EtOH have severe defects in IAV-specific CD8 T cell immunity as well as enhanced morbidity, mortality, viral titers and pulmonary pathology(Meyerholz et al., 2008). In this report we demonstrate that despite the severe immune defects seen in chronic EtOH mice, treatment with the NA inhibitor oseltamivir during the first seven days of IAV infection is capable of inhibiting the increased morbidity and mortality as well as pulmonary viral load and disease observed in EtOH mice.
Interestingly the ability of oseltamivir to reduce enhanced disease during IAV infection in chronic EtOH consuming mice was not predicted, as mice and patients who are immunocompromised can have prolonged viral shedding, outgrowth of oseltamivir resistant viruses and ultimately an inability to resolve the infection(Baz et al., 2006; Cohen-Daniel et al., 2009; Ison et al., 2006a; Ison et al., 2006b). However there are reports demonstrating successful elimination of IAV infection in immunocompromised patients(Ison et al., 2008). Our previous results demonstrate that although EtOH mice have up to a 70% reduction in IAV-specific CD8 T cells, these mice are still capable of reducing viral titers albeit at less effectively than water mice(Meyerholz et al., 2008). This decrease in viral titers coupled with the addition of oseltamivir is sufficient to mediate a substantial decrease of early pulmonary viral titers (Fig 2). This early decrease in pulmonary viral load likely leads to the decrease in overall disease severity (Fig. 1B-C) and appears to negate the enhanced pulmonary distress seen in chronic EtOH mice (Fig. 3 and 4). Importantly, our experiments were performed with oseltamivir treatment given prophylactically proceeding IAV infection. Therefore in the future it will be important to determine if therapeutic treatment (i.e. during the narrow 24 hours p.i. when this drug is effective) results in a similar reduction in disease severity and likewise prevents the outgrowth of oseltamivir resistant viruses. Recent data have demonstrated that immunocompromised patients treated either prophylactically or after onset of symptoms can develop NA inhibitor resistant virus as well as exhibit continued morbidity despite treatment(Baz et al., 2006; Ison et al., 2006a) a result not observed in our prophylactic studies. These findings suggest that therapeutic treatment may likewise protect from severe disease and outcomes.
Despite the possibility that EtOH is thought to inhibit carboxylesterases(Laizure et al., 2003), which are required for the conversion of oseltamivir into its active form, treatment of IAV infected chronic EtOH consuming mice still permitted drug mediated reduction of pulmonary viral titers (Fig. 2) and disease severity (Figs. 1B-C). These data suggest the possibility that EtOH consumption has minimal effects on the conversion of pro-oseltamivir phosphate to the active oseltamivir carboxylate and may only inhibit a fraction of the carboxylasterases. In fact EtOH consumption has been demonstrated to increase permeability within the both the lung and the gut(Amin et al., 2009; Bird and Kovacs, 2008; Bode and Bode, 2005; Brown et al., 2004; Joshi and Guidot, 2007; Keshavarzian et al., 2009; Nagata et al., 2007; Tang et al., 2009) suggesting the possibility that during chronic EtOH exposure oseltamivir may exit the gut and enter the lungs more efficiently-a result that would be consistent with our observed increased protection from morbidity in treated EtOH relative to treated water controls.
Together, the data from this report demonstrate that oseltamivir is capable protecting chronic EtOH consuming mice from increased disease severity and mortality during IAV infections. Importantly, EtOH treatment did not appear to inhibit the conversion of oseltamivir into its active form, as chronic EtOH mice had significantly reduced viral titers. Finally, IAV infected chronic EtOH mice treated with oseltamivir exhibited reduced morbidity, mortality and pulmonary pathology even after treatment was halted suggesting that oseltamivir is capable of eliminating the viral infection early in these mice and may represent a potent treatment option for chronic alcoholic patients during seasonal influenza outbreaks.
MATERIALS AND METHODS
Mice
Six to eight week old BALB/c mice were purchased from the National Cancer Institute (Fredrick, MD). All animals were housed and maintained in the animal care facility at the University of Iowa. All experiments using animals were performed in accordance with federal guidelines and approved by the Animal Care and Use Committee at The University of Iowa.
EtOH Administration
Following a one-week acclimation time, mice were separated and placed on either water or phased onto Ethanol (10% (w/v) for days 1-2, 15% for days 3-8, then to 18% for an additional 8 weeks) in their drinking water as the only source of water. Mice had access to laboratory chow ad libitum. Mice were maintained on 18% (w/v) EtOH throughout the course of the experiments as previously described(Coleman et al., 2008; Cook et al., 2007; Cook et al., 2004; Meyerholz et al., 2008).
Influenza virus infection
Control water and chronic EtOH exposed BALB/c mice were anesthetized by isofluorane and infected i.n. with mouse-adapted A/PuertoRico/8/34 (H1N1) in 50μl of Iscoves media. Viruses were grown and stored as previously described(Meyerholz et al., 2008). Morbidity was measured by weight loss daily.
Oseltamivir treatment
Control water and chronic EtOH exposed mice were treated orally using a 20 ga plastic feeding tube (Solomon scientific, San Antonio, TX) with 10 mg/kg oseltamivir resuspended in sterile water. Oseltamivir treatment was given 4 hours pre-infection, 6 hours post-infection and then twice daily for 6 days.
Histopathology examination
Lungs from infected mice were inflated and fixed with PBS containing 10% formalin on day 14 p.i. Paraffin sections were then deparafinized and rehydrated. Finally, slides were counterstained with Hematoxylin and Eosin and then coverslipped. Lung sections were scored as follows: Pulmonary Edema – 1, Absent; 2, Detectable seroproteinaceous fluid in one to a few alveoli; 3, seroproteinaceous fluid filling alveoli in a multifocal to coalescing pattern in lung. Neutrophilic infiltration – 1, Absent to rare solitary neutrophils; 2, detectable extravasated neutrophils seen as small loose cellular aggregates in one to a few airways and/or alveoli; 3, detectable extravasated neutrophils seen as loose to compact cellular aggregates in multiple to coalescing airway and/or alveoli with some effacement of lung architecture; 4, detectable extravasated neutrophils seen as compact cellular aggregates effacing most adjacent lung architecture.
Pulmonary virus titer
Lungs from infected mice were removed and quickly homogenized and viral titers were determined as previously described (Meyerholz et al., 2008) by end point dilution assay and expressed as 50% tissue culture infectious dose (TCID50). Briefly, 10-fold serial dilutions of lung homogenates from influenza virus-infected mice were mixed with 5×105 Madin-Darby canine kidney (MDCK) cells in DMEM and incubated at 37°C for 24 h. Culture supernatants were removed and DMEM containing 0.0002% L-1-(tosylamido-2-phenyl)ethyl chloromethyl ketone-treated trypsin (Worthington Diagnostics) and penicillin (100U/ml)/streptomycin (100 mg/ml) was added to each well. Following 4 days of incubation at 37°C, supernatants were mixed with an equal volume of 0.5% chicken RBC, the agglutination pattern was read, and the TCID50 values were calculated using Reed-Muench accumulative method.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant AA-019437 to K.L.L., AA-019438 to T.J.W. and AA-014405 to R.T.C. as well as support from the Department of Pathology at the University of Iowa.
REFERENCES
- Amin PB, Diebel LN, Liberati DM. Dose-dependent effects of ethanol and E. coli on gut permeability and cytokine production. J Surg Res. 2009;157(2):187–92. doi: 10.1016/j.jss.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Chhabra SK, Nerurkar PV, Souliotis VL, Kyrtopoulos SA. Alcohol-related cancer risk: a toxicokinetic hypothesis. Alcohol. 1995;12(2):97–104. doi: 10.1016/0741-8329(94)00089-1. [DOI] [PubMed] [Google Scholar]
- Baz M, Abed Y, McDonald J, Boivin G. Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin Infect Dis. 2006;43(12):1555–61. doi: 10.1086/508777. [DOI] [PubMed] [Google Scholar]
- Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukoc Biol. 2008;84(3):607–13. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29(11 Suppl):166S–71S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30(9):1624–31. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33(3):191–7. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Burger RA, Billingsley JL, Huffman JH, Bailey KW, Kim CU, Sidwell RW. Immunological effects of the orally administered neuraminidase inhibitor oseltamivir in influenza virus-infected and uninfected mice. Immunopharmacology. 2000;47(1):45–52. doi: 10.1016/s0162-3109(99)00184-8. [DOI] [PubMed] [Google Scholar]
- Cohen-Daniel L, Zakay-Rones Z, Resnick IB, Shapira MY, Dorozhko M, Mador N, Greenbaum E, Wolf DG. Emergence of oseltamivir-resistant influenza A/H3N2 virus with altered hemagglutination pattern in a hematopoietic stem cell transplant recipient. J Clin Virol. 2009;44(2):138–40. doi: 10.1016/j.jcv.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Young BM, Turner LE, Cook RT. A practical method of chronic ethanol administration in mice. Methods Mol Biol. 2008;447:49–59. doi: 10.1007/978-1-59745-242-7_4. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22(9):1927–42. [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31(10):1746–58. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, LaBrecque DR, Cook BL. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33(3):175–81. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral agents active against influenza A viruses. Nat Rev Drug Discov. 2006;5(12):1015–25. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen-specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J Leukoc Biol. 2009;85(1):34–43. doi: 10.1189/jlb.0208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–32. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis. 2006a;193(6):760–4. doi: 10.1086/500465. [DOI] [PubMed] [Google Scholar]
- Ison MG, Mishin VP, Braciale TJ, Hayden FG, Gubareva LV. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J Infect Dis. 2006b;193(6):765–72. doi: 10.1086/500464. [DOI] [PubMed] [Google Scholar]
- Ison MG, Sharma A, Shepard JA, Wain JC, Ginns LC. Outcome of influenza infection managed with oseltamivir in lung transplant recipients. J Heart Lung Transplant. 2008;27(3):282–8. doi: 10.1016/j.healun.2007.11.575. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, Wyatt TA. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41(5):357–69. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L813–23. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50(3):538–47. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laizure SC, Mandrell T, Gades NM, Parker RB. Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases. Drug Metab Dispos. 2003;31(1):16–20. doi: 10.1124/dmd.31.1.16. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9(1):1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181(1):641–8. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32(5):453–68. doi: 10.2131/jts.32.453. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32(4):657–68. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–6. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Yang J, Yang D, LeCluyse EL, Black C, You L, Akhlaghi F, Yan B. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319(3):1477–84. doi: 10.1124/jpet.106.111807. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72(6):1109–16. [PubMed] [Google Scholar]
- Stohr K. Preventing and treating influenza. BMJ. 2003;326(7401):1223–4. doi: 10.1136/bmj.326.7401.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33(7):1220–30. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–44. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov. 2007;6(12):967–74. doi: 10.1038/nrd2400. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Markowitz JS. Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants. Drug Metab Dispos. 2009;37(2):264–7. doi: 10.1124/dmd.108.024943. [DOI] [PubMed] [Google Scholar]