Abstract
Our previous work suggested a role for oligomerization in regulating dopamine transporter (DAT) internalization, with d-amphetamine dissociating DAT oligomers and monomers being endocytosed. This model was put to detailed testing in the present work with the use of DAT constructs differentially tagged with Myc or Flag, reversal of tags in coimmunoprecipitation and cross-linking assays, and application of antibodies against different tags in biotinylation experiments. Upon pairing wild-type (WT) DAT with W84L mutant, effects of d-amphetamine on oligomerization (decrease) but not surface DAT are observed. Internalization of W84L monomers appears to be slow as inferred from the inability of d-amphetamine to reduce surface Myc upon co-expressing Flag-WT with Myc-W84L but not Myc-WT with Flag-W84L, and from the sluggish Myc-W84L endocytosis rate (both with or without d-amphetamine). Results obtained for D313N, D345N, or D436N mutants can all be accommodated by a model in which d-amphetamine is unable to dissociate mutant protomers from oligomers (tetramers or higher-order assemblies) that contain them; this interpretation is confirmed in experiments with both tag reversal in co-expression and antibody reversal in Western blotting. Upon co-transfecting Myc-and Flag-tagged constructs, resulting tetramers can be calculated to be composed of different species (MycMycMycMyc, MycMycMycFlag, MycMycFlagFlag, MycFlagFlagFlag, and FlagFlagFlagFlag), but it is shown that outcomes predicted by models based on MycMycFlagFlag oligomers are not changed in a major way by the occurrence of the additional species.
Keywords: dopamine transporter, mutant transporters, oligomerization, coimmunoprecipitation, internalization, amphetamine
The dopamine (DA) transporter (DAT) clears DA released from neurons (Rothman and Baumann 2003; Rice and Cragg 2008). Early radiation inactivation studies indicate the existence of DAT assemblies containing at least two protomers (Berger et al. 1994; Milner et al. 1994), and more direct evidence for a quaternary organization of DAT has been advanced with cross-linking techniques by Javitch and colleagues (Hastrup et al. 2001; Hastrup et al. 2003) and ourselves (Chen and Reith 2008), in co-purification experiments with differentially tagged DATs (Torres et al. 2003; Chen and Reith 2008), and by FRET microscopy as well as coimmunoprecipitation by Sorkina et al. (2003). Supportive evidence has been advanced for the assembly of DAT dimers into dimers (i.e., tetramers) with two distinct symmetrical interfaces in transmembrane domain (TM) 4 and 6 (Hastrup et al. 2001; Hastrup et al. 2003). However, other interaction sites could be involved as well, with evidence pointing to TM2 (Torres et al. 2003). Crystallographic evidence collected regarding the oligomeric structure of a DAT homolog bacterial transporter, the leucine transporter (LeuT), conflicts with the above biochemical and imaging information for DAT, in that TMs 9 and 12 together with their symmetry partners form a four-helix bundle at the protomer-protomer interface (Yamashita et al. 2005). However, the latter may be mere crystal contacts rather than actual oligomers.
There is only sketchy information about the role of oligomerization in the function or regulation of DAT. Regarding function, there is some information for other transporters in the same SLC6 family that DAT belongs to. Differential sensitivity has been reported to inactivation by the sulfhydryl reagent MTSET for protomers in oligomers of the norepinephrine transporter (NET) and serotonin transporter (SERT) (Kilic and Rudnick; Kocabas et al. 2003). Furthermore, a concatemer of SERT and the GABA transporter (GAT) appears to consist of separate protomers involved in substrate influx and efflux (Seidel et al. 2005). Experiments with dominant-negative mutants of DAT point to both functional and regulatory effects: With some mutants (Y335A and D79G) DAT activity is reduced as a result of formation of non-functional oligomeric complexes at the cell surface, whereas with a mutant with replacements of leucine repeats in TM2 (TM24LA) the observed lack of oligomer formation may underlie also its inability to become glycosylated (Torres et al. 2003; however, see Ozaslan et al. 2003, for a somewhat different perspective on this issue in the case of SERT).
Our previous work (Chen and Reith 2008) suggested a role for oligomerization in regulating DA transport function, as indicated by the action of substrates. Thus, DA and d-amphetamine appeared to dissociate DAT oligomers, shifting the distribution of surface DAT towards a smaller ratio of oligomers to monomers as seen in both coimmunoprecipitation and cross-linking experiments. It was speculated that the substrate-induced reduction in surface DAT (which could be blocked by endocytosis inhibitors such as phenylarsine oxide or sucrose) was mediated by internalization of DAT monomers (Chen and Reith 2008). However, alternative mechanisms could be entertained: Oligomers (rather than monomers) of DAT could internalize, and this could be thought to be stimulated by substrate with a reduction in oligomer over monomer ratio as a result; in the latter scenario, blocking endocytosis would simply restore the oligomer over monomer ratio at the cell surface. In the present study, such models have been put to further testing with the use of DAT mutants and by monitoring differential responses to d-amphetamine, while correlating effects of d-amphetamine on oligomerization with effects on surface presence of DAT. Oligomerization was measured by coimmunopreciptation and by copper cross-linking, whereas surface presence of DAT was monitored by biotinylation. Specific hypotheses linking oligomerization and internalization were tested by reversing tags in coimmunoprecipitation assays and applying antibodies against different tags in biotinylation experiments. DAT constructs were chosen with mutations in different locations, W84L, D313N, D345N, D436N, known from previous work to display different DAT functionalities (Chen et al. 2001), and with conformational bias towards outward- (W84L, D313N) or inward-facing conformations (D345N, D436N) (Chen et al. 2004b; Chen et al. 2004a; and unpublished results for D436N).
Materials and methods
Chemicals
The pCIN4 vector and the pCIN4-synthetic human DAT construct (pCIN4-DAT) were generous gifts from Dr. Jonathan A. Javitch (Columbia University, New York, NY). DA, d-amphetamine, 3X Flag peptide, anti-Flag antibody and anti-Flag M2 affinity gel were from Sigma Chemical Co (St. Louis, MO). Sulfo-NHS-SS-Biotin, NeutrAvidin, Mammalian Protein Extraction Reagents, protein inhibitor cocktails, goat horseradish peroxidase (HRP)-conjugated secondary antibodies, and SuperSignal West Dura Extended Duration substrate were from Pierce (Rockford, IL). Rabbit anti-human DAT polyclonal antibody was from Chemicon (Temecula, CA). Anti-Myc antibody was from Santa Cruz Biotechnology, Santa Cruz, CA. Lipofectamine 2000 and NuPAGE were from Invitrogen Life Technologies Inc. (Carlsbad, CA). Other chemicals and molecular biology reagents were provided by commercial sources.
Generation of tagged DAT cDNA constructs, cell culture and transfection
Full-length DAT cDNA tagged with Myc or Flag epitope at the N-terminal (inserted immediately after the translational start sequence) was generated by inverse PCR mutagenesis as we described previously (Chen et al. 2004b; Chen and Reith 2008). The Myc- and Flag-sequences were EQKLISEEDL and DYKDDDDK, respectively. All tagged DATs used in this study displayed uptake and binding comparable to WT. Human embryonic kidney cells (HEK-293, ATCC CRL 1573) were grown in Dulbecco’s modified Eagle’s medium/Ham’s F12 medium supplemented with 10% bovine calf serum and 2 mM l-glutamine at 37°C and 5% CO2. For transient co-expression, cells were seeded into 10 cm dishes allowed to grow to 90% confluence. Then cells were co-transfected with two full-length DAT cDNA constructs, one tagged with the Myc epitope and the other with the Flag epitope, at 1:1 ratio (8 μg/dish each), or they were transfected individually with Myc-DAT or Flag-DAT cDNA (16 μg/dish). Functional assays and expression quantitation were performed approximately 48 hours after transfection.
Coimmunoprecipitation combined with biotinylation and Western blotting
All procedures were as described in our previous study (Chen and Reith 2008). Briefly, cells transiently co-expressing two N-terminal tagged DATs were incubated with vehicle or d-amphetamine (2 or 20 μM) in phosphate-buffered saline (PBS, pH 8.0) at 37°C for 30 min. The pretreated cells were washed with cold PBS with 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-CM), incubated with Sulfo-NHS-SS-Biotin (1 mg/ml PBS-CM) and subsequently 100 mM glycine in PBS-CM, followed by extensive wash. After lysis in Mammalian Protein Extraction Reagents supplemented with protein inhibitor cocktail, samples were centrifuged, and the cleared cell lysates were divided into three portions, which were used for direct Western blotting, separation of biotinylated proteins with NeutrAvidin followed by Western blotting, and immunoprecipitation followed by Western blotting, respectively. The biotinylated proteins were separated with immobilized monomeric NeutrAvidin and eluted with SDS-PAGE sample buffer. For coimmunoprecipitation, cell lysates from co-transfected cells were incubated with anti-Flag M2-agarose affinity gel overnight. After washing, the precipitated DATs were eluted with 3X Flag peptide (200 ng/ml) in PBS. The total lysates, biotinylated proteins, and immunoprecipitation eluates were resolved on 8% reducing SDS-PAGE gels. In some experiments, proteins were separated on NuPAGE gels. Flag-DAT was probed with polyclonal rabbit anti-Flag antibody (1 μg/ml) followed by HRP-conjugated goat anti-rabbit antibody (20 ng/ml). Myc-DAT was probed with monoclonal anti-Myc antibody 9E10 (1 μg/ml) followed by HRP-conjugated goat anti-mouse antibody (20 ng/ml). MagicMarker XP Western Protein Standards (20–220 kDa) were run in parallel with the samples on gels. The transporter signal was visualized with SuperSignal West Dura Extended Duration chemiluminescence substrate solution, and images were quantified with the FluorChem 8800 Image System (Alpha Innotech). For comparison of data between control and drug group in cotransfected cells, the integrated density value (IDV) for the coimmunoprecipitated Myc-DATs was normalized with that for the corresponding precipitated Flag-DATs (normalized IDVMyc-DAT drug = IDVMyc-DAT drug × [IDVFlag-DAT control ÷ IDV Flag-DAT drug]), while the IDV for the biotinylated myc-DATs was normalized with that for the corresponding Myc-DATs in total lysates (normalized IDV biotinylated Myc-DAT drug = IDV biotinylated Myc-DAT drug × [IDV lysate Myc-DAT control ÷ IDV lysate Myc-DAT drug]). The final drug data were expressed as percentage of control data. Statistical differences between vehicle and d-amphetamine treatment were assessed with the two-tailed one-sample Student’s t-test (P ≤ 0.05 with vehicle at 100%).
Assessment of DAT Endocytosis by Reversible Biotinylation
Procedures were as described in our previous study (Li et al, 2004). The assay consisted of reaction of surface proteins with disulfide-cleavable biotin, sulfo-NHS-SS-biotin (Pierce) for 30 min at 4°C, incubation for 20 min at 37°C (with 20 μM d-amphetamine or vehicle) or 4°C (vehicle), and treatment with cell-impermeant reducing agent 2-mercaptoethanesulfonic acid (MESNA, 50 mM in PBS, pH 8.0) at 4°C for 20 min. The 4°C vehicle incubation group was additionally followed up by treatment under the same conditions without MESNA to provide the amount of surface DAT available for endocytosis. After solubilization in Mammalian Protein Extraction Reagents supplemented with protein inhibitor cocktail, biotinylated DATs were isolated using monomeric avidin beads. DATs internalized to the inside of the cell at the time of MESNA treatment were protected from reduction and could be subsequently isolated via monomeric avidin-agarose. Internalized DAT (represented by the MESNA-insensitive fraction) was quantified following Western analysis. Statistical differences between groups were assessed with the two-tailed Student’s t-test (P ≤ 0.05).
Cross-linking combined with biotinylation and Western blotting
For many of the conditions studied by coimmunoprecipitation in this work, cross-linking approaches were applied also. The methods were exactly as described previously by us (Chen and Reith 2008); in the present study, the copper reagent was CuSO4 (the Cu2+ condition in the previous paper). Cross-linked proteins were resolved on 4–12% non-reducing SDS-PAGE gels. DATs were probed with anti-hDAT antibody (1 μg/ml). DAT signals were quantified as described above, and cross-linking data were expressed as the ratio of dimer IDV to monomer IDV.
Results
Overall layout of experiments illustrated with co-expression of combinations of wild-type and mutant D436N DAT – effect of d-amphetamine
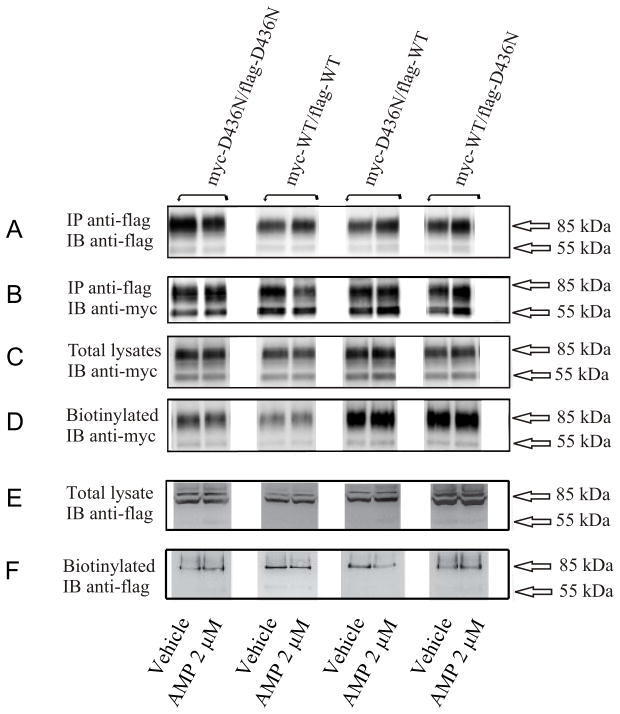
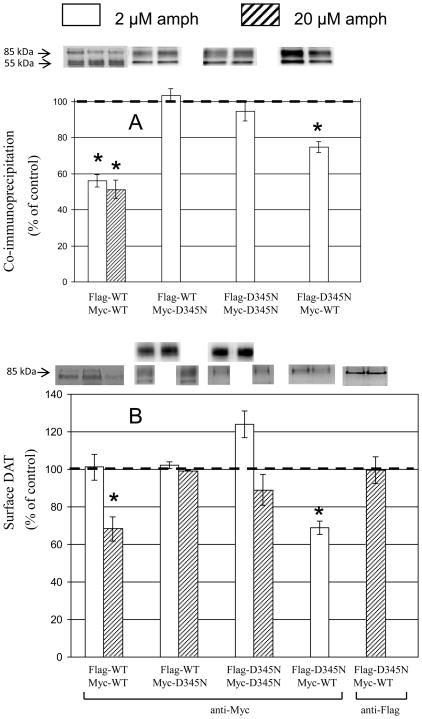
In the following experiments, cells were cotransfected with Myc- and Flag-DAT, immunoprecipitation was performed with anti-Flag antibody, and proteins were separated on 8% gels followed by Western blotting with anti-Myc antibody in order to reveal oligomerized DAT; additionally, biotinylated proteins were separated on 8 % gels followed by Western blotting with anti-Myc antibody (and in two cases also anti-Flag antibody) in order to reveal DATs present at the cell surface. The combination of wildtype (WT) DAT with a given mutant (Mu) DAT tagged with either Myc or Flag in a transient co-expression experiment yields four mixed-tag combinations: Myc-Mu/Flag-Mu, Myc-WT/Flag-WT, Myc-Mu/Flag-WT, and Myc-WT/Flag-Mu. An example of these combinations of WT with D436N is shown in Fig 1. When the various combinations of WT and D436N were subjected to immunoprecipitation with anti-Flag antibody and analysis on gels with immunoblotting with anti-Flag antibody, mainly mature 85 kDa DAT was observed (Fig. 1A - top panel), whereas immunoblotting with anti-Myc antibody (representing oligomers) revealed additional immature 55 kDa DAT for all DAT species (panel B) as shown in our previous work for WT (Chen and Reith 2008). It turned out that this was not related to the co-immunoprecipitation step but rather to the affinity of the different antibodies to the mature and immature forms of DAT, as total lysates monitored with anti-Flag antibody (panel E) also showed less immature DAT than total lysates monitored with anti-Myc antibody (panel C). The same can be inferred from comparing panels D and F, and, more clearly, Fig. S1 (compare anti-Flag gel bands in top panel with anti-Myc bands in bottom panel). Total lysates analyzed with anti-Myc immunoblotting antibody again showed both mature and immature DAT (Fig. 1, panel C), whereas surface DAT monitored with the biotinylation procedure mainly showed mature DAT (panel D). Co-transfection of WT with mutant seems to enhance surface residence of Myc-DATs (panel D) suggesting that the presence of D436N protomers retains more oligomers at the surface. However, it is important to recall here that proteins in the biotinylation procedure were not subjected to immunoprecipitation and therefore the surface protein fraction studied here does not contain only oligomerized DAT. The effect of d-amphetamine (2 μM for 30 min at 37 °C) in reducing coimmunoprecipitated Myc- with Flag-WT DAT (Fig. 1, panel B, note lack of effect of d-amphetamine detected with anti-Flag antibody in panel A) was reduced or obliterated with the co-presence of D436N (see patterns to the left and right of the WT/WT pair).
Fig. 1.
Various combinations of WT and D436N subjected to immunoprecipitation (IP) with anti-Flag antibody or directly worked up as total lysates or as biotinylated surface fractions, and to immunoblotting (IB) with either anti-Flag or anti-Myc antibody. Cells were treated for 30 min at 37°C with 2 μM d-amphetamine (AMP) or vehicle. Proteins in panels A to D were separated on 8% SDS-PAGE gels, and proteins in panels E and F were separated on 4–12% NuPAGE gels.
Averages from quantified multiple experiments are presented below for the coimmunoprecipitation and biotinylation procedure with anti-Myc immunoblotting antibody as shown in panels B and D in Fig. 1. For most mutants studied in this work, data on effects of d-amphetamine will be presented for all four mixed-tag combinations shown in subsequent figures in the sequence Flag-WT/Myc-WT, Flag-WT/Myc-Mu, Flag-Mu/Myc-Mu, and Flag-Mu/Myc-WT (please see previous paper for control experiments consisting of mixing cells expressing either Myc- or Flag-tagged DAT expressing cells, (Chen and Reith 2008)). First, results are presented for all pairs with always the Flag-tag on WT DAT. This is followed by a description of the consequence of reversing the Flag- and Myc-tag for the WT/Mu pair, and, finally, the consequence of reversing the antibody used for immunoblotting of biotinylated proteins (anti-Flag instead of anti-Myc).
Co-expression of combinations of wild-type DAT (tagged with Flag) and W84L DAT monitored with anti-Myc immunoblotting antibody
The question was examined whether tags themselves affected the expression of DAT. Standard curves for Myc-DAT and Flag-DAT were created with anti-Myc, anti-Flag, and anti-DAT antibody, and used to quantify the expression level of differently tagged DAT as shown in the Supplementary Information. These relationships allowed us to assess, in cells co-expressing Myc-and Flag-DAT, the amount of DAT expressed by Myc-DAT and Flag-DAT separately (Fig. S1, asterisks). The data show these co-expressions to be very similar.
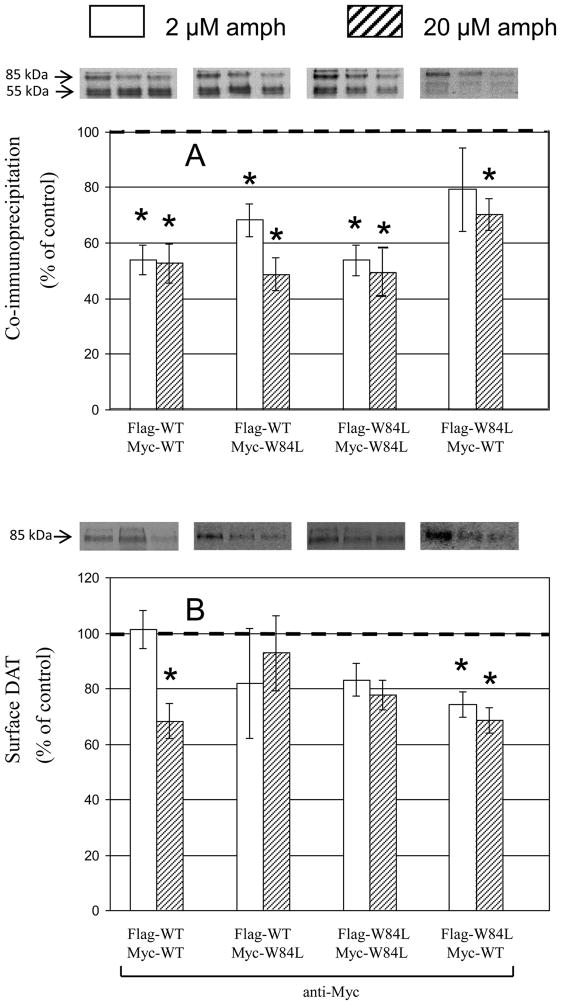
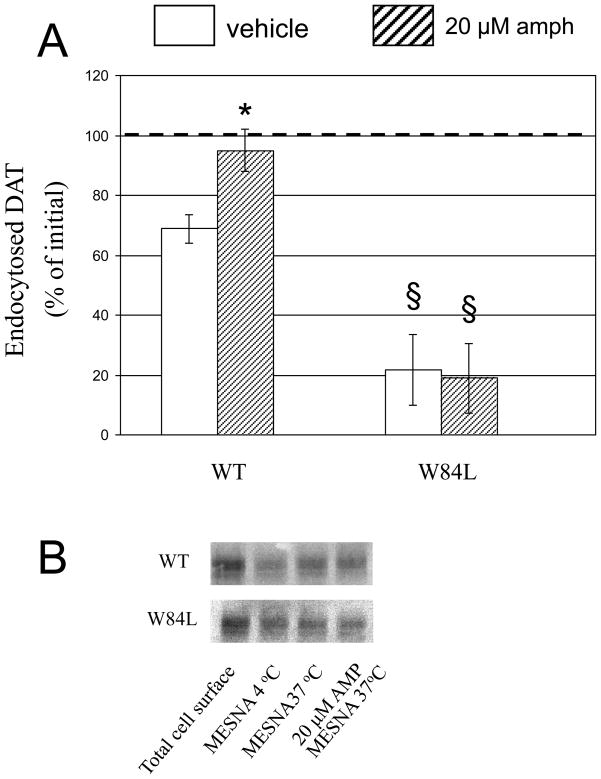
d-Amphetamine, at both 2 and 20 μM for 30 min at 37°C, appreciably decreased mature Myc-WT coimmunoprecipitated with Flag-WT extracted from cells co-expressing Flag- and Myc-tagged WT (Fig. 2 first set of bars in top panel). Surface Myc-WT was reduced only by the 20 μM concentration of d-amphetamine (bottom panel) as seen in our previous work (chen Reith 2008). Thus, WT oligomerization appears more sensitive to d-amphetamine than WT’s surface presence consonant with the idea that the oligomer-dissociating effect of d-amphetamine precedes its effect on surface presence (Chen and Reith 2008). Upon co-expressing Myc-W84L with Flag-WT the coimmunoprecipitation effect was retained (Fig. 2 second set of bars in top panel) but the surface effect was abolished (bottom panel); the same phenomena were seen with co-expression of Flag- and Myc-W84L (third set of bars in both panels). These findings indicated that in the presence of W84L, the ability of d-amphetamine to enhance endocytosis of DAT was reduced. This was tested directly by monitoring the effect of d-amphetamine (20 μM) on endocytosis of WT (in cells expressing WT only) or W84L (in cells expressing W84L only) for 20 min at 37°C (Fig. 3). With surface DAT at 4°C as a reference point (no endocytosis), 70% of WT was internalized in 20 min, but this was increased to 96% with d-amphetamine. In contrast, d-amphetamine had no effect at all on the endocytosis of W84L (Fig. 3, second set of bars). An additional finding was that the endocytosis of W84L was considerably less extensive (21% in 20 min) than that of WT (70%).
Fig. 2.
Effect of d-amphetamine (amph) on coimmunoprecipitation and surface expression of WT and W84L DAT. Treatment with d-amphetamine was for 30 min at 37°C at 2 μM (open bars) or 20 μM (striped bars) and results are expressed as % of those from vehicle treatment. Myc was coimmunoprecipitated with Flag (A), and surface DAT (B) was assessed with the antibody indicated at the bottom of the figure. The used combinations of differentially tagged constructs are indicated below the bars for each condition. Gel bands from representative experiments are positioned above the bars; for each co-expression condition, bands corresponding to drug treatments are lined up with bars, and band from vehicle treatment is placed to the left of those (there is no vehicle bar). Data are means ± SEM (vertical bar) of 3 – 4 experiments. * P < 0.05 compared with 100% (one sample two-tailed t-test).
Fig. 3.
Endocytosis of WT and W84L DAT in presence and absence of d-amphetamine (amph) (20 μM) for 20 min at 37°C. Control incubation for 20 min at 4°C (disallowing endocytosis) provided the amount of surface DAT available at the start of the endocytosis measurement, and endocytosed DAT was expressed as % of this initial amount (A). Representative gel bands are shown for the various incubation conditions applied to WT and W84L (B). Data are means ± SEM (vertical bar) of 3 experiments. * P < 0.05 compared with Vehicle and $ P < 0.05 compared with corresponding WT (two-tailed t-test).
Co-expression of combinations of wild-type DAT (tagged with Flag) and D313N, or D345N, or D436N DAT, monitored with anti-Myc immunoblotting antibody
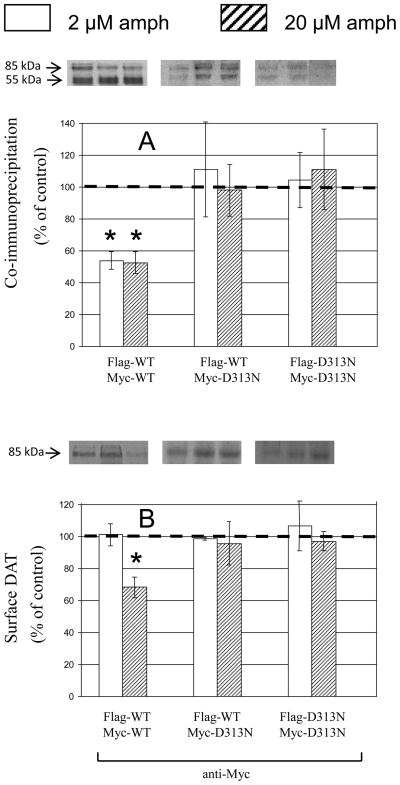
The findings for D313N differed from those for W84L in that, compared with WT control data, all effects of d-amphetamine were abolished (Fig. 4). This was true for both coimmunoprecipitation findings (top panel) and surface results (bottom panel).
Fig. 4.
Effect of d-amphetamine (amph) on coimmunoprecipitation and surface expression of WT and D313N DAT. Data are means ± SEM (vertical bar) of 3 – 4 experiments. All other details are as in Fig. 2.
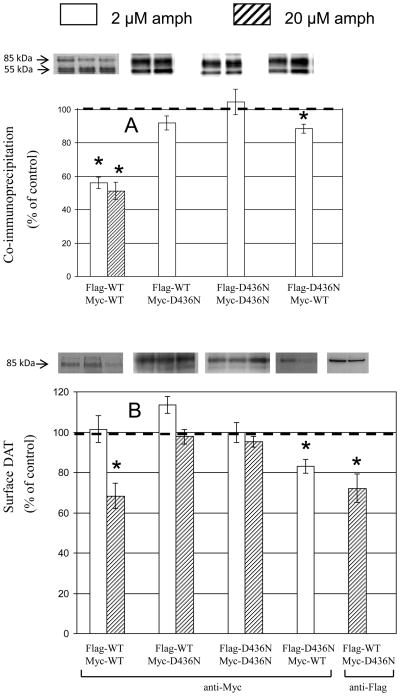
D345N (Fig. 5) and D436N (Fig. 6) behaved as D313N. In coimmunoprecipitation experiments, WT showed effects at the lowest concentration of d-amphetamine tested, 2 μM, and therefore only that concentration was tested for the mutants. For D345N and D436N the pattern of effects of 2 μM d-amphetamine in coimmunoprecipitation assays, and pattern of effects of 2 μM and 20 μM in biotinylation experiments, was identical to that for D313N (compare three first sets of bars in Figs. 5 and 6 with those in Fig. 4).
Fig. 5.
Effect of d-amphetamine (amph) on coimmunoprecipitation and surface expression of WT and D345N DAT. Because coimmunoprecipitation effects on WT were as strong with 2 μM as with 20 μM of amphetamine, subsequent combinations of DAT constructs were tested only at the lower 2 μM dose. Representative bands from different experiments are lined up horizontally. Data are means ± SEM (vertical bar) of 3 – 6 experiments. All other details are as in Fig. 2.
Fig. 6.
Effect of d-amphetamine (amph) on coimmunoprecipitation and surface expression of WT and D436N DAT. Data are means ± SEM (vertical bar) of 3 – 6 experiments. All other details are as in Fig. 5.
Tag reversal in coimmunoprecipitation experiments: co-expression of combinations of wild-type DAT (tagged with Myc) and mutant DAT (tagged with Flag) monitored with anti-Myc immunoblotting antibody
In the above coimmunoprecipitation and biotinylation experiments WT always carried the Flag tag, raising the question as to whether it mattered how the tags were distributed over WT and mutant. When the Flag tag was put on the mutant and Myc on WT, for W84L the reduction by d-amphetamine in the coimmunoprecipitation experiments was smaller but still significant at the 20 μM concentration (Fig. 2 top panel, 4th set of bars); surprisingly, the lack of effect on surface DAT with Flag-WT/Myc-W84L turned into a significant decrease with Flag-W84L/Myc-WT (Fig. 2 bottom panel compare 2nd and 4th set of bars).
Also surprisingly, upon tag reversal the previous lack of effect of d-amphetamine (2 μM) on oligomerization of Flag-WT/Myc-D345N (Fig. 5 top panel 2nd condition) or of Flag-WT/Myc-D436N (Fig. 6 top panel 2nd condition was turned into a significant decrease upon tag reversal (Figs. 5 and 6 top panel 4th condition); the same happened with the surface levels of DAT for these WT/mutant combinations (Figs. 5 and 6 bottom panel, compare 2nd and 4th condition).
Antibody reversal in biotinylation experiments: co-expression of combinations of wild-type DAT (tagged with Flag) and mutant DAT (tagged with Myc) monitored with anti-Flag immunoblotting antibody
In above experiments surface DAT was always measured with anti-Myc antibody, raising the question as to whether the same results would be obtained with anti-Flag antibody. When D345N was tested in the Flag-D345N/Myc-WT pair (as in above tag reversal design), the effect of d-amphetamine (2 μM) observed with anti-Myc antibody in Western blotting (Fig. 5 bottom panel 4th condition), was lost for an even higher d-amphetamine concentration (20 μM) with the anti-Flag antibody (Fig. 5 bottom panel, 5th condition). Conversely, when D436N was tested with Flag on WT, compared with the lack of d-amphetamine (20 μM) effect with anti-Myc in western blotting (Fig. 6 bottom panel 2nd set of bars), d-amphetamine (20 μM) reduced surface DAT measured with anti-Flag antibody (Fig. 6 bottom panel 5th condition).
Cross-linking data from experiments with varying combinations of wild-type and mutant DAT
Effects of 2 and 20 μM d-amphetamine were monitored with both coimmunoprecipitation and cross-linking approaches for varying combinations of co-expressed WT and mutant DAT (total of 18, see Supplementary Information to this article, Table S1). The cross-linking agent was copper sulfate as described by us previously (Chen and Reith 2008), generating dimeric and higher-order assemblies of DAT (Chen and Reith 2008; Hastrup et al. 2003). Out of the 18 comparisons for the various conditions between coimmunoprecipitation and cross-linking, in 14 cases the effects or lack of effects of d-amphetamine (2 or 20 μM) were comparable, and, in the case of effects, in the same direction (Table S1). In four cases, cross-linking effects were more sensitive to d-amphetamine (i.e., cross-linking picked up effects not seen with coimmunoprecipitation). Intriguingly, these four cases involve coimmunoprecipitation of Myc-tagged D313N, D345N, or D436N with Flag-tagged WT: Upon reversal of these tags (i.e. Flag on mutant and Myc on WT) in the coimmunoprecipitation, the lack of correspondence between coimmunoprecipitation and cross-linking data turned into a correspondence (Table S1).
Comparison of effect of dopamine and d-amphetamine on WT, D345N, or D436N
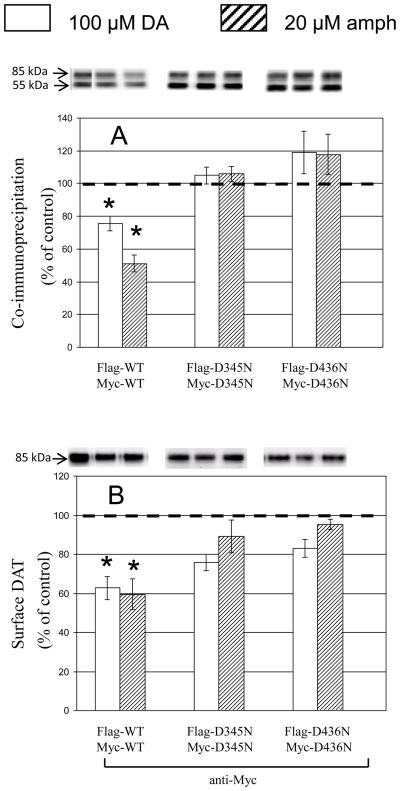
Effects of dopamine (100 μM) and d-amphetamine (20 μM) (30 min at 37°C) were compared in parallel experiments (Fig. 7). There was a striking parallel between the two compounds in reductions observed for WT, and lack of effect for D345N or D436N.
Fig. 7.
Effect of dopamine (DA) and d-amphetamine (amph) on coimmunoprecipitation and surface expression of WT, D345N, and D436N DAT. Treatment with DA (100 μM, open bars) or amph (20 μM, striped bars) was for 30 min at 37°C. Data are means ± SEM (vertical bar) of 4 – 6 experiments. All other details are as in Fig. 2.
Discussion
Models for role of oligomerization of DAT in its endocytosis
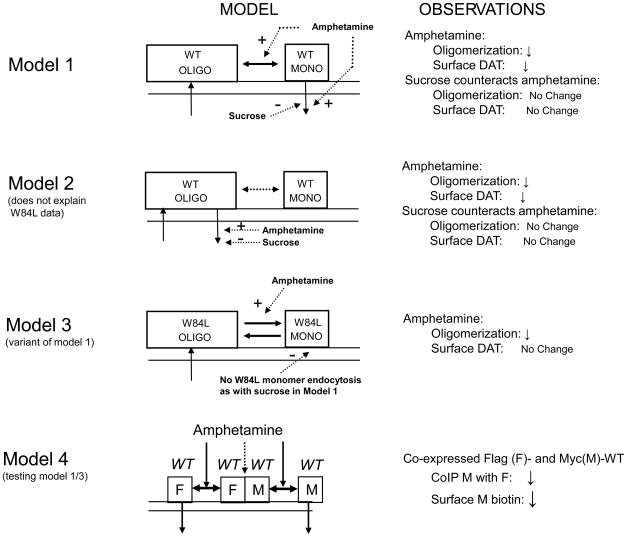
In our previous work (Chen and Reith 2008) we speculated that substrate-induced reduction in surface DAT was mediated by internalization of DAT monomers. Thus, DA and d-amphetamine appeared to dissociate DAT oligomers, shifting the distribution of surface DAT towards a smaller ratio of oligomers to monomers. Under these conditions, crosslinking results with copper phenanthroline indicated that the amount of monomers at the surface stayed the same, whereas oligomers at the surface decline as monomers liberated from them were being internalized; as a result, both the oligomer/monomer ratio and the total amount of DAT at the surface were reduced (see Fig. 8 model 1). In this scenario, endocytosis inhibitors such as phenylarsine oxide and sucrose given prior to amphetamine, kept the monomers at the surface, which then re-formed oligomers through rapid equilibration. Inhibition of endocytosis by itself (without d-amphetamine) did not enhance surface DAT as recycling is too slow over the time frame of the experiment.
Fig. 8.
Models for wild-type DAT (WT) (Models 1, 2, 4) and W84L (Model 3). See Discussion section for description of the models, their predicted outcomes, and experimental observations. OLIGO is oligomer, MONO is monomer, F stands for Flag, and M is Myc. Upon co-expressing Flag- with Myc-DAT, Myc was coimmunoprecipitated with Flag (CoIP M with F).
The same observations could be explained by an alternative model in which d-amphetamine enhances the internalization of oligomers, not monomers; the oligomer/monomer ratio at the surface would go down in this scenario if the equilibrium between oligomers and monomers were slow as compared with the internalization such that re-formation of oligomers could not keep up with endocytosis in the time frame of the experiment (see model 2 in Fig. 8). In addition, this would result in a decrease in total DAT at the surface as observed. In such a model, an endocytosis inhibitor such as sucrose would be expected to counteract both of these effects of d-amphetamine. In both model 1 and 2, in the absence of d-amphetamine, a steady state is contemplated (Fig. 8) consisting of internalization on the one hand, and delivery of DAT to the plasma membrane on the other hand. After synthesis and assembly of DAT into oligomers (the latter in endoplasmic reticulum), it is generally thought that DAT is inserted at the surface in its quaternary form (Torres et al. 2003; Sitte et al. 2004); it is reasonable to assume the same insertion mechanism during recycling from endosomes. In the cartoon, all inserted DAT (from synthesis and recycling) is shown to be in oligomer assembly.
According to model 2, a reduced oligomer/monomer ratio results from oligomer endocytosis and is therefore necessarily linked with a reduction in surface DAT (the rate of recycling, i.e. delivery to surface is considered constitutive and not agonist-sensitive, see Koenig and Edwardson (1997). However, the present results with W84L demonstrate the possibility to modify DAT such that d-amphetamine reduces its propensity to be oligomerized (Fig. 2A third pair of bars) without significantly affecting its surface presence (Fig. 2B third pair of bars). Apparently d-amphetamine is able to affect the distribution between oligomers and monomers without affecting the endocytosis of any form of DAT. This is not consonant with model 2. The W84L data could be explained by model 1 with d-amphetamine pushing the oligomer-monomer equilibrium to the right and the mutant DAT monomer not being able to internalize (for support for the latter see Fig. 3). Under these conditions, the degree of oligomerization is reduced but the total amount of surface DAT is not affected. This is similar to the situation for WT pretreated with sucrose prior to d-amphetamine (model 1) with one difference: for WT, but not W84L, the degree of oligomerization is at control level (vehicle instead of d-amphetamine) (Chen and Reith 2008 and current data). This can be accommodated by postulating that d-amphetamine preferentially enhances the dissociation rate constant of oligomers (see model 3 in Fig. 8). Thus, as monomers are kept at the surface, reformation of oligomers is still overshadowed by a more rapid dissociation into monomers. The W84L data suggest d-amphetamine acts at the level of the distribution between oligomers and monomers. This is consonant with the idea that d-amphetamine’s effects on oligomerization precede those on internalization, and indeed for WT d-amphetamine at 2 μM is observed to enhance oligomer dissociation but not yet internalization which requires a higher d-amphetamine concentration (20 μM) (Chen and Reith 2008 and current results, see Figs. 2, 4–6). With d-amphetamine acting on the distribution equilibrium, it then follows that in order for some linkage to occur between d-amphetamine’s effect on DAT oligomerization and internalization, the oligomers themselves are not endocytosed in a self-contained cycle which includes oligomer insertion as shown in model 2 (Fig. 8) (in this context, one can view the DAT oligomers with FRET signal observed by Sorkina et al. (2003) in early and recycling endosomes as the result of reassembly of monomers into oligomers). In the present work, therefore, we set out to test model 1, or similar model 3, entailing endocytosis of monomers.
Testing of model
In order to test the above model, differentially tagged DATs were co-transfected and studied by coimmunoprecipitation (please see Chen and Reith 2008 for controls with cells separately transfected with differentially tagged DAT, then mixed, and worked up as co-transfected cells). Model 4 (Fig. 8) is a short-hand depiction of models 1 and 3, visualized here for WT. DAT is depicted as a tetramer based on observations reported by Hastrup et al. (2003), but model 4 will also work for higher-order assemblies. Binding forces between protomers are thought to be other than disulfide bridges, non-covalent in nature, and susceptible to SDS (Hastrup et al. 2001; Chen and Reith 2008). According to the model, d-amphetamine promotes partial dissociation of WT DAT oligomers, with some units still non-covalently attached, as with full dissociation over the time window of experiment there would be no material left to be coimmunoprecipitated (which is not what was observed). Accordingly, Myc-tagged DAT coimmunoprecipitated with Flag-tagged DAT was reduced along with an observed decrease in surface Myc-tagged DAT.
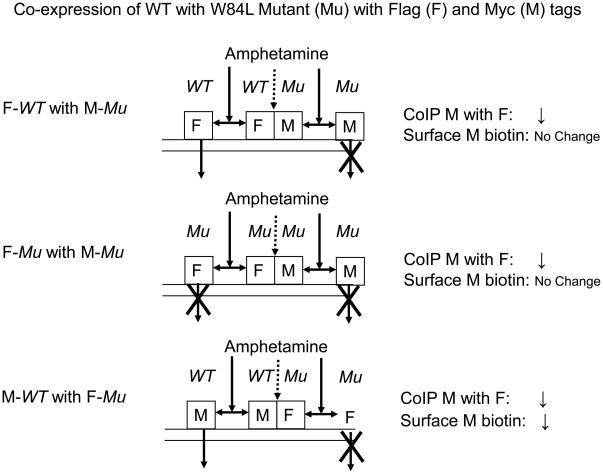
As depicted in Fig. 8 model 3, W84L co-expressed with itself caused a reduction in oligomerization as detected with coimmunoprecipitation without a change in surface DAT, consonant with a partial dissociation of oligomers without ability of mutant monomers to internalize (see middle of Fig. 8 for short-hand depiction with tags). Application of the same model to co-expression of Flag-WT with Myc-W84L, leads one to predict that d-amphetamine will cause a reduction in coimmunoprecipitation of Myc- with Flag-tag; because Myc is located on the non-endocytosable mutant, the model predicts no change in surface Myc (Fig. 9 top) as in fact was observed (Fig. 2B). The model also predicts that tag reversal will not change the reduction in coimmunoprecipitation of Myc with Flag, but the model strikingly foresees a decrease in surface Myc as this tag is now located on endocytosable WT monomers (Fig. 9 bottom). The actual observations (Fig. 2A) were in agreement with this. Further support for the model for W84L comes from the observation of severely reduced endocytosis for this mutant compared with WT (Fig. 3). In this context it can be noted that there is no evidence that the Myc- or Flag-tags themselves differentially affect DAT endocytosis. In separate experiments associated with our previous work with this approach (Chen and Reith, 2008), the same phenomena were observed for surface DAT monitored with anti-Myc or anti-DAT antibody.
Fig. 9.
Models for differentially tagged wild-type (WT) and W84L mutant (Mu). Mu containing oligomers can be dissociated just as purely WT containing oligomers but Mu monomers cannot internalize. Other details are as in Fig. 8.
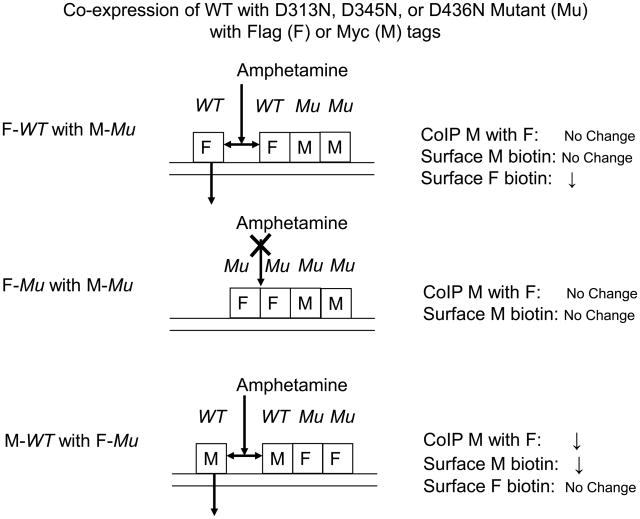
The results obtained for D313N, D345N, or D436N can all be accommodated by a model in which d-amphetamine is unable to dissociate mutant protomers from oligomers that contain them (Fig. 10). For the pair of Flag-WT with Myc-mutant, coimmunoprecipitated Myc with Flag is as expected not altered by d-amphetamine whereas surface Myc likewise is not affected (Figs. 4–6). However, the model predicts that surface Flag is reduced under these conditions, and this was actually observed. Strong support for the model comes from the observations with tag reversal that was applied to D345N and D436N. Tag reversal according to the model would change the effects of d-amphetamine on coimmunoprecipitated Myc with Flag, surface Myc, and surface Flag (compare top and bottom in Fig. 10). This was exactly what was observed (Figs. 5 and 6). For mutant co-expressed with itself, d-amphetamine is unable to dissociate oligomers preventing any coimmunoprecipitation or surface effects (middle of Fig. 10 and Figs. 4–6). In the tag reversal coimmunoprecipitation experiments with the 345 and 436 mutants there was less decrease in oligomerization by 2 μM d-amphetamine for the Flag-mutant/Myc-WT than Flag-WT/Myc-WT pair (compare 1st and 4th condition in top panel of Fig. 5 or 6): This suggests that there was somewhat greater difficulty for d-amphetamine in dissociating a WT protomer from oligomers containing 345 or 436 mutant protomers.
Fig. 10.
Models for differentially tagged wild-type (WT) and D313N, D345N, or D436N mutant (Mu). d-Amphetamine is unable to dissociate Mu protomers from oligomers that contain them. Other details are as in Fig. 8.
Comparison of coimmunprecipitation and cross-linking data
There is a remarkable agreement between coimmunoprecipitation and cross-linking data (see Supplementary Information Table S1). The four cases where cross-linking picks up an effect (decrease) and coimmunoprecipitation does not, represent WT paired with D313N, D345N, or D436N. In the model for those combinations (Fig. 10), d-amphetamine does dissociate these oligomers, but the tag used to monitor coimmunoprecipitation (Myc) happens to be on the part of the oligomer (on the mutant protomer) that is not dissociated; indeed, tag reversal (Myc on dissociatable WT) reveals the correspondence between coimmunoprecipitation and cross-linking results (Supplementary Information, Table S1). Cross-linking, according to the model, would be less sensitive to where the tag is used to monitor DAT: No matter where the tag is located in the oligomer, the tagged portion will become smaller with d-amphetamine upon dissociation of the oligomer (Fig. 10). With anti-DAT antibody in cross-linking, one expects to see a decrease by d-amphetamine in the ratio of oligomeric over monomeric DAT.
It can be seen in Fig. 8 (Model 4) and Fig. 9 that for experiments with WT and W84L it does not matter at all where the tag used to monitor cross-linking is located.
Protomer composition upon co-transfection
It should be pointed out that differently tagged populations of oligomers will occur according to chance upon co-tranfection with differentially tagged DAT. Table 1 shows the calculated tag distributions over tetramers upon co-expression of equal amounts of Flag- and Myc-tagged DAT according to chance (MacKinnon 1991). Although the bulk (37.5%) is represented by tetramers with two Flag- and two Myc-tags, as depicted in the models in Figs. 8–10, a substantial portion (25%) exists as tetramers with three Flag-tagged protomers, with another 25% carrying three Myc tags, 6.25% consisting of all Flag and 6.25% carrying all Myc. This prompted an analysis of predicted effects according to our models for all these differently tagged tetramers. Here one example of such an analysis will be given in detail, followed by a summary of the impact of tag distributions on the interpretation of the experimental results based on the models in Figs. 8–10. Detailed information on the impact tag distributions for all pairs of WT and mutant can be found in the Supplementary Information to this article (Figs. S2–7).
Table 1.
Chance of finding tetramers composed of Flag- and Myc-tagged DAT upon co-expressing equal amounts of Flag- and Myc-DAT.
| Percent chance | |
|---|---|
| Flag-Flag-Flag-Flag | 6.25 |
| Flag-Flag-Flag-Myc | 25.00 |
| Flag-Flag-Myc-Myc | 37.50 |
| Flag-Myc-Myc-Myc | 25.0 |
| Myc-Myc-Myc-Myc | 6.25 |
in which Fi is the fraction of oligomers (with a total of n DATs) that is i-type (i.e. has i Flag-DATs), f Myc and F Flag are the fractions of expressed Myc- and Flag-tagged DATs (in analogy with the analysis of MacKinnon (1991)) for n subunits of either wild-type or mutant channel protein). In the above, f Myc = 0.5 and F Flag = 0.5 under the assumption that equal amounts of transfected cDNA for each tag resulted in equal amounts of expressed DAT; furthermore, (ni ) = n!/[i!(n-i)!].
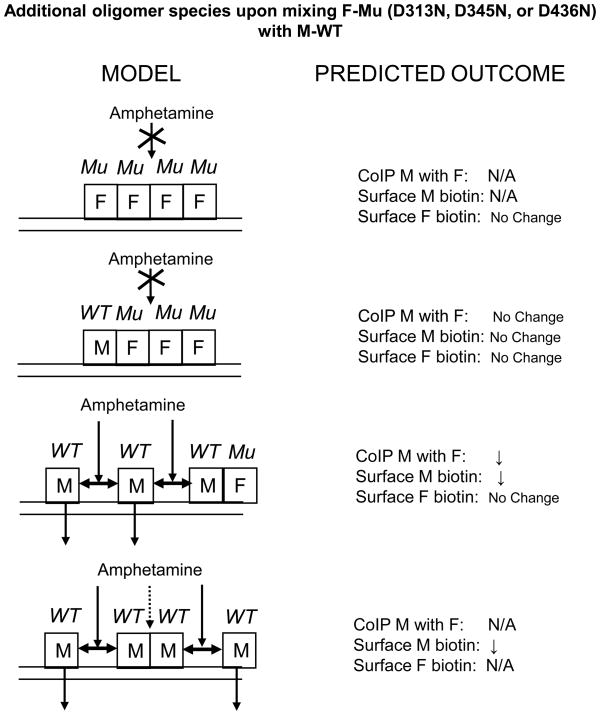
Fig. 11 shows all combinations for the pair Myc-WT/Flag-D345N that can occur in addition to the MMFF combo shown in Fig. 10 (bottom): FFFF, MFFF, MMMF, and MMMM, along with their predicted outcomes for coimmunoprecipitation and surface effects of d-amphetamine. For coimmunoprecipitation of Myc with Flag, there are two species (FFFF and MMMM) that obviously do not contribute to the measurement, one species (MFFF) that according to the model would not show any effect (as opposed to the reduction as observed, and modeled with MMFF, see Fig. 5A right bar), and one species (MMMF) that would show a reduction. Overall, then, the coimmunoprecipitation effect for all oligomer species would be in the same direction as modeled for MMFF but somewhat smaller because one species (25% of all present, Table 1) shows no effect in conjunction with two other species (37.5% plus 25% of all present) that do show the effect. For the effect of d-amphetamine on surface Myc (Fig. 11), one species (FFFF) obviously does not contribute to the measurement, one species (MFFF) would not show an effect, and two species (MMMF and MMMM) would show a reduction as modeled with MMFF (an effect in the direction as observed, see Fig. 5B 4th condition). The overall surface Myc effect is therefore similar to the effect modeled for MMFF with just one out of 4 species contributing to the measurement not displaying any effect. For surface Flag, it can be easily seen that one species does not contribute to the measurement, whereas according to the model all other species do not display an effect, just as observed (Fig. 5B right bar), and modeled for MMFF (Fig. 10).
Fig. 11.
Models for Myc-tagged wild-type (WT) and Flag-tagged D345N mutant (Mu). Models are shown for tetramers with composition other than fifty-fifty composition as shown in Fig. 9 (i.e. other than Myc-Myc-Flag-Flag). d-Amphetamine is unable to dissociate Mu protomers from oligomers that contain them. N/A: this species does not contribute to measurement as it cannot be detected with the particular combination of tags and antibodies used. Other details are as in Fig. 8.
Similar reasoning for all other WT/mutant pairs (See Supplementary Information) show that the presence of other oligomer species with tag distributions different from MMFF, does not alter in a major way the effects predicted by the models based on the MMFF composition.
Role of mutated residues
Our original hypothesis was that d-amphetamine needs to act on outward-facing DATs to dissociate oligomers and therefore would do so more readily when the bias is towards outward-(W84L, D313N) than inward-facing conformations (W345N, D436N). The results, however, did not support this hypothesis as the data contrasted W84L, on the one hand, against D313N, W345N, and D436N, on the other hand. Rather, the present data can be interpreted in terms of the location of the residues in relation to the symmetrical interface of transmembrane (TM) 4 and of TM6 thought to be involved in the assembly of DAT oligomers (Hastrup et al. 2001; Hastrup et al. 2003). Thus, W84 at the top of TM1 is too far removed from TM4 and 6 to influence the noncovalent bonds holding the oligomers together, and therefore d-amphetamine can dissociate oligomers containing W84L the same way as purely WT oligomers. In contrast, mutation of D313, at the top of TM6, could influence the TM6 symmetrical interface (in this case, making it more tight rendering d-amphetamine powerless). D345 is in the intracellular loop 3 connected to TM6 and with its known impact on conformational changes of DAT (Chen et al. 2000) can be thought to also affect the TM6 interface. There is evidence implicating D436 in an intracellular network related to the internal gate (Kniazeff et al. 2008): A salt bridge between D436 and R60 is postulated to be stabilized by a cation-π interaction between R60 and Y335 at the end of TM6. Indirectly, therefore, mutation of D436 could impact TM6. In this light, mutation of D313, D345, or D436 could all impact the TM6 oligomer interface blocking d-amphetamine’s effect in dissociating DAT oligomers as opposed to mutation of W84 allowing this effect.
DAT mutations W84L, D313N, D345N, and D436N clearly blocked the effect of d-amphetamine in dissociating DAT oliogomers. A parallel presents itself between this phenomenon and the transport of dopamine by these mutants. As shown by us previously (Chen et al., 2001), the Vmax of dopamine transport is severely reduced for W84L, D313N, D345N, and D436N; only in D436N this effect is opposed by a similar reduction in Km which would nullify the Vmax effect at low [dopamine]s (i.e., below Km). With the location of the mutations far removed from the substrate binding site according to LeuT (Yamashita et al., 2005), transport of d-amphetamine in these mutants could well parallel that of DA. Thus, the data suggest that it is quite possible that poor transport of d-amphetamine by the mutants plays a role in the blockade of the effects of d-amphetamine on oligomer dissociation.
Literature context for functional role of DAT oligomers and for oligomerization in general in protein trafficking
What is the difference between the functional activity of DAT oligomers and that of single DAT molecules? In previous work we showed weakened potency of DAT blockers (but not substrates) at higher levels of DAT expression (Chen and Reith, 2007), as observed previously for SERT blockers and SERT by Ramsey and DeFelice (2002). One speculation that can be advanced to explain the results of both studies is that oligomerization increases at higher expression level and that oligomerized transporter has a lower affinity for blockers (not substrates). In addition, we are collecting data on [3H]CFT binding suggesting an impact of oligomerization beyond mere addition of binding properties of individual protomers (manuscript in preparation).
The literature linking receptor oligomerization and agonist-induced internalization is growing. Heteromers with differential internalization responses to agonist have been described upon pairing GABAA and GABAB receptors (Balasubramanian et al. 2004), α1a- and α1b-adrenergic receptors (Stanasila et al. 2003), DA D1 and glutamate receptors (Fiorentini et al. 2003; Missale et al. 2006), adenosine A2A and DA D2 receptors (Genedani et al. 2005), DA D1 and D3 receptors (Fiorentini et al. 2008), and μ-opioid and α2A-adrenergic receptors (Tan et al. 2009). To our knowledge, for transporters, our previous work (Chen and Reith 2008) and the present results are the first in linking oligomerization with internalization phenomena for this class of proteins. It is intriguing that all available receptor data linking the two processes have been collected for heteromers rather than homomers. It is likely that this is due to the greater ease of measuring the impact of oligomer composition on agonist effects if there are protomers in an oligomer with differential agonist selectivity, as is the case with an heteromer composed of two pharmacologically different protomers. It will be crucial to demonstrate the functional significance of homomers vs. dissociated monomers for both receptors and transporters.
Supplementary Material
Acknowledgments
The study was supported by National Institute on Drug Abuse Grant DA019676.
The abbreviations used are
- DA
dopamine
- DAT
dopamine transporter
- GABA
γ-aminobutyric acid
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- IDV
integrated density value
- NE
norepinephrine
- NEM
N-ethylmaleimide
- NET
NE transporter
- PBS
phosphate-buffered saline
- PMA
phorbol 12-myristate 13-acetate
- SERT
serotonin transporter
References
- Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J Biol Chem. 2004;279:18840–18850. doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- Berger SP, Farrell K, Conant D, Kempner ES, Paul SM. Radiation inactivation studies of the dopamine reuptake transporter protein. Mol Pharmacol. 1994;46:726–731. [PubMed] [Google Scholar]
- Chen N, Ferrer JV, Javitch JA, Justice JB., Jr Transport-dependent accessibility of a cytoplasmic loop cysteine in the human dopamine transporter. J Biol Chem. 2000;275:1608–1614. doi: 10.1074/jbc.275.3.1608. [DOI] [PubMed] [Google Scholar]
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem. 2007;101:377–388. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Reith MEA. Substrates dissociate dopamine transporter oligomers. J Neurochem. 2008;105:910–920. doi: 10.1111/j.1471-4159.2007.05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Rickey J, Berfield JL, Reith MEA. Aspartate 345 of the dopamine transporter is critical for conformational changes in substrate translocation and cocaine binding. J Biol Chem. 2004a;279:5508–5519. doi: 10.1074/jbc.M306294200. [DOI] [PubMed] [Google Scholar]
- Chen N, Vaughan RA, Reith MEA. The role of conserved tryptophan and acidic residues in the human dopamine transporter as characterized by site-directed mutagenesis. J Neurochem. 2001;77:1116–1127. doi: 10.1046/j.1471-4159.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhen J, Reith MEA. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR 12909 and benztropine as opposed to cocaine. J Neurochem. 2004b;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Gardoni F, Spano P, Di LM, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- Genedani S, Guidolin D, Leo G, Filaferro M, Torvinen M, Woods AS, Fuxe K, Ferre S, Agnati LF. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J Mol Neurosci. 2005;26:177–184. doi: 10.1385/JMN:26:2-3:177. [DOI] [PubMed] [Google Scholar]
- Hastrup H, Karlin A, Javitch JA. Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc Natl Acad Sci U S A. 2001;98:10055–10060. doi: 10.1073/pnas.181344298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastrup H, Sen N, Javitch JA. The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem. 2003;278:45045–45048. doi: 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- Kilic F, Rudnick G. Oligomerization of serotonin transporter and its functional consequences. Proc Natl Acad Sci U S A. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem. 2008;283:17691–17701. doi: 10.1074/jbc.M800475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabas AM, Rudnick G, Kilic F. Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters. J Neurochem. 2003;85:1513–1520. doi: 10.1046/j.1471-4159.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JA, Edwardson JM. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith MEA. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Milner HE, Beliveau R, Jarvis SM. The in situ size of the dopamine transporter is a tetramer as estimated by radiation inactivation. Biochim Biophys Acta. 1994;1190:185–187. doi: 10.1016/0005-2736(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Ozaslan D, Wang S, Ahmed BA, Kocabas AM, McCastlain JC, Bene A, Kilic F. Glycosyl modification facilitates homo- and hetero-oligomerization of the serotonin transporter. A specific role for sialic acid residues. J Biol Chem. 2003;278:43991–44000. doi: 10.1074/jbc.M306360200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ramsey IS, DeFelice LJ. Serotonin transporter function and pharmacology are sensitive to expression level: evidence for an endogenous regulatory factor. J Biol Chem. 2002;277:14475–14482. doi: 10.1074/jbc.M110783200. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, Holy M, Koppatz K, Krivanek P, Freissmuth M, Sitte HH. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Farhan H, Javitch JA. Sodium-Dependent Neurotransmitter TRANSPORTERS: OLIGOMERIZATION as a Determinant of Transporter Function and Trafficking. Mol Intervent. 2004;4:38–47. doi: 10.1124/mi.4.1.38. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J Biol Chem. 2003;278:28274–28283. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- Stanasila L, Perez JB, Vogel H, Cotecchia S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J Biol Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie CW. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A-adrenergic receptors in neurons. J Biol Chem. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.