Summary
Preterm and term infants are frequently exposed to high concentrations of oxygen for prolonged periods. In experimental models, high and prolonged oxygen exposures cause delayed alveolar septation and a bronchopulmonary dysplasia phenotype. Often, however, the oxygen exposure is tolerated in that the infants recover without severe lung or systemic injury. Multiple exposures change oxygen sensitivity in adult and newborn animals. Examples are antenatal corticosteroids, inflammatory mediators or preconditioning with oxygen, which will increase tolerance to oxygen injury. Intrauterine growth restriction or postnatal nutritional deficits will increase oxygen injury. Different infants probably have quite variable sensitivities to oxygen injury, but there are no biomarkers available to predict the risk of oxygen injury.
Keywords: Bronchopulmonary dysplasia, Injury, Lung, Prematurity
Introduction
Oxygen is the most frequently used effective and essential drug for the care of the newborn. However, recent research reviewed in this monograph demonstrates that surprisingly short exposures to oxygen can alter long term biochemical indicators of oxidant stress and may adversely affect outcomes.1 The use of oxygen thus has a risk to benefit equation just as for any other drug. Therefore, a balanced perspective of the scientific and clinical aspects of oxygen use is needed, especially for the preterm. Much of the data for oxidant injury and long term effects come from animal models, which inform us about what can happen, but do not establish what does happen to sick preterm infants. The focus of this review is on longer term effects of oxygen on the newborn rather than on the acute effects associated with oxygen use for resuscitation, which are reviewed in other papers in this monograph. Our goal is to summarize clinical and animal model observations to build the concept that the effects of oxygen exposure of the newborn are likely to be modulated by multiple mechanisms such that the risk of oxygen toxicity may differ for each infant, depending on antenatal history, age after birth, and care strategies.
Evidence of oxygen toxicity: BPD
Bronchopulmonary dysplasia (BPD) has been the poster child for oxygen toxicity in the preterm since the original description of the disease by Northway et al. in 1967.2 These authors also reported that 100% oxygen caused inflammation, fibrosis, and ‘emphysema’ in the lungs of newborn mice in 1976.3 The clinical contribution of oxygen to BPD has been less clear because sick infants generally require both ventilatory support and supplemental oxygen and the use of both therapies track in parallel during the weeks after birth. Certainly oxygen exposure does correlate with BPD defined as the use of supplemental oxygen at 36 weeks of gestation. Laughon et al.4 reported that 44% of 1340 infants with gestations <28 weeks had persistent oxygen requirements that averaged between 40% and 50% for the first 2 weeks of life, and 67% of this population developed BPD (Table 1). By contrast, of the 19% of infants who had minimal oxygen needs for 2 weeks, only 17% developed BPD. However, these infants were exposed to other variables that determined the BPD outcome, as has been reported extensively previously.5,6 For the population reported by Laughon et al.,4 antenatal corticosteroids and chorioamnionitis were not predictive of oxygen use at 14 days or BPD. The major predictors of oxygen use and BPD by multivariant analysis were gestational age, growth restriction, and mechanical ventilation. Although chronic oxygen exposure of preterm infants is a variable contributing to BPD, the response of the preterm population is not consistent. For example, of the infants exposed to high oxygen for the first 2 weeks of life, Laughon et al.4 found that 33% did not develop BPD whereas 50% of infants exposed to <30% oxygen for the first week of life developed BPD. These numbers are understandable given the other known contributors to BPD, but they confound the attribution of a cause–effect relationship of oxygen to BPD. A theme we develop below is that modulation of oxygen-mediated injury in preterm humans mitigates the relationship between high oxygen exposures and BPD in some infants.
Table 1.
Clinical variables that correlated with oxygen use at 14 days of age for 1340 preterm infants: 23–27+6 weeks of gestation
| O2 use (%) | |||
|---|---|---|---|
| Low | Increasing | Persistent | |
| Percentage oxygen at 14 days | 21 | 40 | 49 |
| Percentage of population | 20 | 38 | 43 |
| Antenatal exposures | |||
| Corticosteroids | 92 | 91 | 88 |
| Chorioamnionitis | 55 | 54 | 53 |
| Funicitis | 40 | 33 | 32 |
| Postnatal exposures | |||
| Surfactant | 78 | 89 | 97 |
| Presumed sepsis | 27 | 34 | 41 |
| Echo-diagnosed PDA | 36 | 48 | 57 |
| CPAP at 7 days | 50 | 30 | 10 |
| Conventional or high frequency ventilation at 7 days | 21 | 57 | 84 |
| BPD: oxygen/ventilation at 36 weeks | 17 | 51 | 67 |
Data from Laughon et al.4
PDA, patent ductus arteriosus; CPAP, continuous positive airway pressure; BPD, bronchopulmonary dysplasia.
Animal models of oxygen-mediated lung injury help frame the question. Northway et al. demonstrated that 100% oxygen caused a BPD-like lung injury in newborn mice.3 They also found that newborn mice survived 100% oxygen exposure for weeks whereas adult mice died within days – an important observation to which we return later. Many investigators have since used newborn rodents as models for lung oxidant injury and BPD.7,8 These models gained relevance with the recognition of the similar pathology of alveolar simplification and ablated microvascular development in both autopsy specimens from patients with BPD and in oxygen-exposed newborn rodents.9,10 However, a cautionary note is important. Supplemental oxygen is given to infants to maintain an adequate systemic PaO2, generally evaluated clinically by measuring O2 saturation. By contrast, oxygen exposure of normal newborn or adult animals will result in very high systemic arterial O2 pressure (PaO2) values, which can lead to generalized systemic toxicity and perhaps more lung vascular injury. Nevertheless, there is no question that chronic oxygen exposure with 85–100% oxygen causes an anatomic BPD phenotype in newborn mice and rats.11
The most compelling demonstrations in animals that oxygen can cause BPD are the studies with preterm ventilated baboons. De Lemos et al.12 reported that preterm baboons exposed to only enough oxygen to achieve adequate PaO2 values (<30% oxygen after day 3 of life) had minimal lung injury after 11 days of continued mechanical ventilation. By contrast, animals exposed to 100% oxygen for 11 days had severe acute lung injury (pulmonary interstitial emphysema, hemorrhage, pseudocysts) and a BPD anatomy. Because both groups were ventilated similarly, the oxygen exposure was the major cause of the lung injury in these preterm primates. Other preterm baboons were ventilated with only enough supplemental oxygen to maintain PaO2 for 21 days, while a comparison group received 100% oxygen for 7 days and 80% oxygen for 14 more days.13 Lungs of the animals were evaluated about 8 months later, and the high oxygen exposure resulted in enlarged alveoli and decreased alveolar numbers. There were minimal abnormalities in the low oxygen exposed, but ventilated lungs. The high oxygen exposure and acute lung injury progressed to a persistent loss of alveoli months later. These experiences in animal models do not address the clinical question of how much oxygen is needed to provide adequate oxygen while minimizing the potential for injury.
Chronic oxygen use in infants: BPD and retinopathy of prematurity (ROP)
Two large randomized and controlled trials have asked the questions: once an infant is oxygen dependent, does the oxygen saturation change growth, development, or the severity of ROP? The STOP-ROP trial randomized oxygen-dependent infants at about 35 weeks of gestation to 89–94% saturation or 96–99% saturation targets with the primary question related to progression of ROP14 (Table 2). There was no important effect on progression of ROP, but the higher oxygen exposure resulted in multiple indicators of persistence of lung injury as secondary outcomes. The infants targeted to the higher saturations had similar weight gains, but more pneumonia/BPD episodes, and at 3 months' corrected age the infants were more likely still to be hospitalized, on oxygen, and on diuretic therapy. The second trial asked whether oxygen targeting would change growth or neurodevelopment at 12 months' corrected age.15 The investigators used saturation monitors with calibration offsets that blinded the investigators to the actual oxygen saturations. The 178 oxygen-dependent infants that were targeted at 32 weeks of gestation to a saturation of 91–94% had equivalent growth and neurodevelopmental outcomes compared with the 180 infants randomized to a 95–98% target. However, the infants targeted to the higher saturations were significantly more likely to be dependent on oxygen at 36 weeks (more BPD), and to require oxygen after discharge at home for a longer period.
Table 2.
Saturation targets and outcomes of preterm infants who were oxygen dependent
| Saturation target | ||
|---|---|---|
| 89–94% | 96–99% | |
| N | 325 | 324 |
| Birth weight (g)a | 721 ± 160 | 731 ± 160 |
| Age at start of O2 target (weeks)a | 35.3 ± 2.6 | 35.4 ± 2.5 |
| Outcome after randomization | ||
| Weight gain over 2 weeks (g)a | 291 ± 137 | 278 ± 143 |
| Pneumonia or BPD events (number) | 25 | 38* |
| Outcomes at 3 months' corrected age (%) | ||
| Remained in hospital | 6.8 | 12.7* |
| Remained on oxygen | 38 | 47* |
| Treated with diuretics | 24 | 36* |
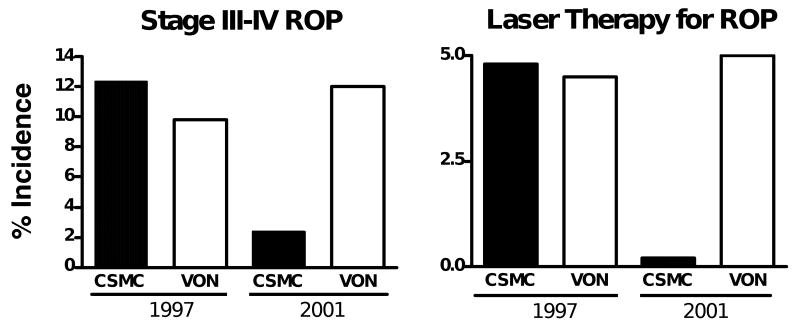
There are other clinical experiences that support the concept that chronic high oxygen saturations can adversely affect lung and eye outcomes of preterm infants. Tin et al.16 compared concurrent care strategies in the north of England that targeted higher saturations of 88–98% with lower targets of 70–90%. The infants exposed to the lower targets required less mechanical ventilation, a shorter period of supplemental oxygen, and had less ROP. Chow et al.17 evaluated whether a change in care practice from routines minimally focused on oxygen saturations to strict management to avoid saturations >93–95% would change the incidence of ROP. With strict management of O2 saturations, the incidences of ROP and the need for laser therapy decreased strikingly across all birth weight categories (Fig. 1). A similar experience with a change of neonatal intensive care unit practice from minimal focus on saturations to strict targeting of saturations to 85–93% resulted in a decrease in ROP and BPD.18 These reports in aggregate provide strong and biologically plausible information that preterm infants can be managed with saturations <95% and that oxidant-related adverse outcomes such as BPD and ROP can be decreased. Thus, preterm infants must be sensitive to chronic exposure to excessive oxygen even within the range that is normally considered to be therapeutic. These clinical outcomes result from oxygen exposures that are much less extreme than those used for the animal models. The conclusion is that supplemental oxygen should be given to preterm infants – both acutely and chronically – only in the amounts necessary. Trials are now in progress to define what the minimal oxygen saturation targets should be.
Figure 1.
Effect of careful O2 saturation monitoring on the incidence of retinopathy of prematurity (ROP) and need for laser therapy at Cedars Sinai Medical Center (CSMC, closed bars) relative to the Vermont–Oxford Neonatal database (VON, open bars). Data are for infants 500–1500 g for 1997 and 2001. A clinical emphasis on keeping O2 saturations >93–95% correlated with a large decrease in ROP at CSMC. Data from Chow et al.17
Oxygen sensitivity of the newborn
Although we have built the case with clinical and animal model information that supplemental oxygen can harm the newborn, it is also essential to use supplemental oxygen frequently, but carefully, for clinical management of the preterm. Therefore, it is useful to ask the question: How sensitive to oxygen is the newborn relative to the adult? The default answer of most neonatologists is that the newborn is more sensitive to oxidant injury because of immaturity and decreased oxidant protective mechanisms. That answer can be challenged by a large amount of experimental information and by clinical experience. Term infants with diseases such as meconium aspiration syndrome and pulmonary hypertension often are exposed to very high concentrations of supplemental oxygen for prolonged periods (days) with few apparent adverse effects. Similarly, of the preterms that required high oxygen for the first 2 weeks of life, 33% did not develop BPD.4 As with term infants, some preterm infants are exposed to very high supplemental oxygen concentrations for long periods and do well. By contrast, adult animals and the normal adult human are quite sensitive to exposure to 85–100% oxygen. Most adult animals die within 72 h of such oxygen exposures.19 Infant deaths are not attributed to oxygen exposure alone.
The experimental data demonstrating that the newborn is resistant to hyperoxia are quite compelling.3 Bonikos et al. reported in 1975 that 75% of term newborn mice survived in 100% oxygen for 7 days. Whereas newborn mice will survive for weeks in 100% oxygen, most adult mice die within 3–7 days. This initial observation in mice has been replicated in newborn rats and rabbits. However, newborn guinea-pigs and hamsters are as oxygen sensitive as adult animals.19 Unlike term newborn rabbits, preterm rabbits develop more lung injury and do not increase antioxidant enzymes with oxygen exposure.20 The oxygen exposures used by Coalson et al. to achieve a BPD phenotype in preterm ventilated baboons were 7 days in 100% oxygen followed by 14 days in 85% oxygen.13 This experience with primates and the clinical experience with oxygen use in term and preterm infants strongly indicate that newborn humans are relatively oxygen resistant to death caused by high supplemental oxygen.
Modulation of oxygen sensitivity
There is no information to calibrate the relative sensitivity of preterm humans to supplemental oxygen relative to term humans because oxygen is only used for clinical need. Further complicating any unified assessments of oxygen sensitivity are the multiple clinically relevant variables that profoundly modulate oxygen sensitivity. The concept of decreased oxygen sensitivity or oxygen tolerance has been developed in animal models and cannot ethically be evaluated in humans. We will first briefly review the variables that can modulate oxygen sensitivity and then speculate on the relevance to clinical neonatology.
As noted above, the newborn rat is less oxygen sensitive than the adult. In rats the oxygen resistance to death with 100% oxygen exposure is present from birth to about 1 month of age.21 Older animals develop a sensitivity to oxygen. However, tolerance to oxygen can be induced in adult rodents by prior exposure to oxygen. For example, rats exposed to 100% oxygen for 48 h and then to air for 24 h before a second 100% oxygen exposure will survive 7 days in 100% oxygen (Table 3).22 Multiple protocols for brief oxygen exposures to induce prolonged tolerance to 80–100% oxygen have been developed for a variety of experimental models,23 demonstrating that the adult lung can be ‘conditioned’ to tolerate prolonged exposures to high oxygen concentrations. Oxygen exposure probably represents just one of many stimuli that can modulate both tolerance to hyperoxia and hypoxia. For example, normobaric 95% hyperoxia induces tolerance to ischemic challenge of the adult rat brain.24 Similarly, heat and cold also can modulate oxygen-sensitive genes such as HIF-1α and heat shock proteins and also induce protection against hypoxia or hyperoic brain injury.25 Multiple ‘stressors’ seem to induce a variety of tolerance responses such as oxygen resistance in the adult. Most of this research has focused on brain injury and has not been extended to the newborn or the lung.
Table 3.
Protective effects of oxygen pre-exposure on oxygen tolerance in adult rats
| Period of exposure | |||
|---|---|---|---|
| Group | Death by 72 h | Death by 7 days | 72 h: pleural fluid |
| Air exposed | 0 | 0 | <0.10 ml |
| >95% oxygen | 79% | 100% | 4.9 ± 2.6 ml |
| >95% oxygen pre-exposed* | 0 | 0 | 0.12 ± 05 ml |
Data from Frank et al.22
*>95% O2 for 48 h, then air for 24 h before re-exposure to >95% O2.
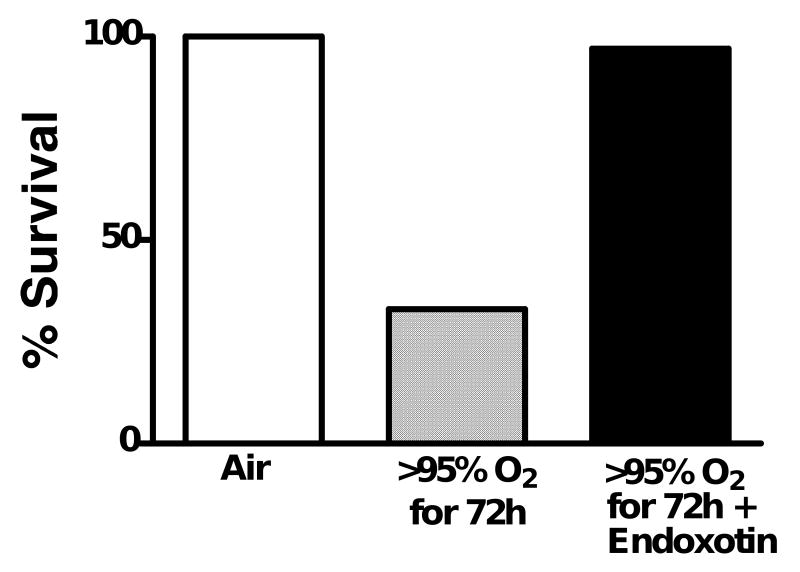
Another mediator of oxygen tolerance is counterintuitive: the observation that endotoxin from Gram-negative bacteria will protect adult rats from oxygen (Fig. 2).26 While not experimentally demonstrated, this observation with endotoxin probably can be generalized to the conclusion that sublethal inflammation may protect the organism from acute oxygen exposure. Castration of young male rats also induces oxygen tolerance and treatment of castrated rats with testosterone prevents the oxygen tolerance.27 Dexamethasone treatment of adult rats given lethal doses of intraperitoneal endotoxin prevents death even in >95% oxygen.28 These results demonstrate that oxygen tolerance can be induced in the adult lung by multiple stimuli.
Figure 2.
Intraperitoneal endotoxin protects adult rats from death caused by >95% oxygen for 72 h. Rats received doses of 0.1–0.5 mg endotoxin/day or control injections. Endotoxin protected the animals from oxygen. Data from Frank and Roberts.26
Modulators of oxygen toxicity in the developing lung
If we accept that the newborn is less sensitive to oxygen injury than the adult, there are multiple modulators of oxygen resistance that have been investigated in experimental models. Much of the earlier research focused on changes in antioxidant enzyme levels, although oxygen tolerance is mechanistically much more complicated than simply changes in antioxidant enzyme levels.29 Nevertheless, the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxide increase with increasing gestational age in multiple species. The nutritional state of the newborn also modulates oxygen sensitivity.30 Intrauterine growth-restriction caused by nutritional deprivation increased oxygen sensitivity, and premature weaning of young rats increasing oxygen tolerance.20,31 Sosenko and Frank30 have described the potential for nutrition to modulate oxygen injury and the clinical outcome of BPD. In the fetal rat and the fetal sheep, antenatal corticosteroids cause large increases in antioxidant enzymes in parallel with surfactant, effects that should translate into increased oxygen tolerance.32,33 However, oxygen exposure or corticosteroid exposure did not increase antioxidant enzyme activity in preterm rabbits.20 Vento et al.34 recently reported that very preterm infants exposed to antenatal corticosteroids had increased antioxidant enzymes and decreased indicators of oxidative stress relative to infants not exposed to antenatal corticosteroids. These clinical data imply that the corticosteroid-exposed preterm infant should be more resistant to oxygen.
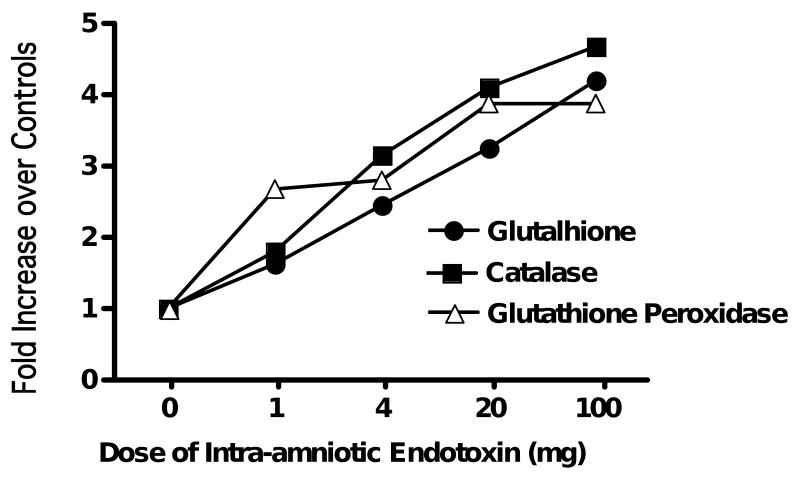
The striking effect of endotoxin on survival of oxygen-exposed adult rats also has a parallel in the newborn. Whereas hyperoxia did not increase antioxidant enzymes in preterm rabbits, sublethal endotoxin did increase antioxidant enzymes.20 Newborn rats treated with endotoxin survived a 14 day exposure to 95% oxygen as well as control rats, but significantly more of the endotoxin-treated rats tolerated a transition back to room air.35 The lungs of the endotoxin-exposed animals had less alveolar simplification and a more normal pulmonary microvasculature. Chorioamnionitis in fetal sheep induced with intra-amniotic endotoxin caused lung inflammation and a time- and dose-dependent increase in the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase (Fig. 3).36 This induction of oxidant-protective enzymes did not occur when intra-amniotic IL-1 was the pro-inflammatory agonist, demonstrating differences between IL-1- and endotoxin-mediated lung inflammation.37
Figure 3.
Dose response of antioxidant enzymes to intra-amniotic endotoxin in the lungs of fetal sheep. The fetuses were exposed to different doses of endotoxin by intra-amniotic injection administered 7 days before preterm delivery at 125 days of gestation. The endotoxin exposure increased antioxidant enzymes. Data from Sosenko and Jobe.36
Artwork: Fig. 3. In key, black circles should be labelled ‘Glutathione’ (t for l)
Late effects of neonatal hyperoxia
The lungs of patients with BPD are abnormal into early adulthood. However, those abnormalities need not result primarily from the prolonged oxygen exposure because of the multifactorial nature of BPD. However, the abnormal lungs of 8-month-old baboons caused by oxygen exposure clearly demonstrates the potential for persistence of the structural abnormalities initiated by oxygen in the newborn period.13 Oxygen exposure of newborn rodents with lungs in the saccular stage of development caused a decrease in alveolar number and an increase in alveolar size in the adult rodent.11,38 This structural alteration was associated with changes in elastin distribution at sites of alveolar septation.39 The lungs of 8-week-old mice exposed to oxygen on postnatal days 1–4 had fewer alveoli, fewer alveolar type II cells, and a persistently abnormal distribution of elastin within alveolar walls.38 Nevertheless, the mice grew normally and had reasonably normal lung function. However, when challenged with Influenza A virus at 8 weeks of age, the mice that had been oxygen-exposed as newborns had more lung inflammation and a higher mortality.40 This experiment in mice is reminiscent of the susceptibility of infants with RDS to developing severe RSV infection and respiratory failure.
Clinical correlates to research with oxygen toxicity
Inevitably, there is minimal clinical information about how toxic oxygen might be for the preterm or term infant. The normal preterm infant probably is inherently more oxygen tolerant than the adult. Preterm or term infants who receive significant oxygen therapy are not normal as they are receiving oxygen therapy for a reason. Most preterm infants also will have been exposed to antenatal corticosteroids, and at least half of the very low birth weight population will have chorioamnionitis associated with the preterm delivery. These two variables will increase oxygen tolerance based on the experimental data (Table 4). The exposure of the newborn to variable concentrations of oxygen has the potential to ‘precondition’ the lung to increase oxygen tolerance. However, fetal and postnatal nutritional deficits may sensitize the lung and promote oxygen injury. Other fetal exposures may modulate oxygen sensitivity in the newborn based on observations in animal models; these range from hypoxic episodes and hyperthermia to hypothermia. The net response to oxygen of one infant may be quite different from that of another infant with different antenatal and postnatal exposures. We suspect that there is a wide range of susceptibilities to oxygen injury for infants – and there is no information about how to predict which infant is at high or low risk to oxygen injury. All would agree that clinical care should emphasize the minimal supplemental oxygen exposure necessary. However, that practice needs to be tempered by the recognition that newborns in general are quite oxygen tolerant and should receive the oxygen they need to optimize their outcomes. In clinical practice, mechanical ventilation can be adjusted to decrease the supplemental oxygen need by increasing the mean or peak airway pressure – but is that the optimal trade-off for the best long-term outcome? Mechanical ventilation is likely to be more injurious to the lung than is oxygen.41 A lesson from the ventilation trials for acute respiratory distress syndrome was that higher pressures and tidal volumes increased the PaO2 but were associated with increased mortality.42 One goal for the future is to identify biomarkers of oxygen toxicity in preterm newborns that might be useful to the selection of care strategies.
Table 4.
Clinically relevant exposures and situations that modify oxygen sensitivity in animal models
| Models | Effect of oxygen exposure |
|---|---|
| Adult animals | |
| Preconditioning with oxygen | ↑ Oxygen tolerance |
| Endotoxin exposure | ↑ Oxygen tolerance |
| Castration | ↑ Oxygen tolerance |
| Fetal or newborn animals | ↑ Oxygen sensitivity |
| Intrauterine growth restriction | ↑ Oxygen sensitivity |
| Postnatal nutritional deficit | ↑ Oxygen tolerance |
| Early weaning | ↑ Oxygen tolerance |
| Antenatal corticosteroids | ↑ Oxygen tolerance |
| Endotoxin |
Practice points
Infants may be more oxygen tolerant than adults.
Clinical variables such as antenatal corticosteroids, chorioamnionitis, and nutrition may modulate oxygen sensitivity.
There are no biomarkers available to predict oxygen sensitivity in patients.
Research directions
Identification of biomarkers of oxygen sensitivity.
Develop treatments to induce oxygen tolerance in newborns.
Acknowledgments
Funding sources: There has been no participation from any funding sources in the preparation of this article.
Footnotes
Conflicts of interest: The authors have grants from the US National Institutes of Health and Human services to study fetal inflammation and lung injury: D-57869 to S.K. The authors also participate in studies of the causes of lung injury with the initiation of ventilation funded in part by Fisher & Paykel, New Zealand.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e49. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 2.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–68. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 3.Bonikos DS, Bensch KG, Northway WH., Jr Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am J Pathol. 1976;85:623–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Laughon M, Allred EN, Bose C, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123:1124–31. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–6. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 6.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8:63–71. doi: 10.1016/s1084-2756(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 7.Yee M, Chess PR, McGrath-Morrow SA, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–L9. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–17. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 9.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH. The New BPD: an arrest of lung development. Pediatr Res. 1999;46:641–3. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res. 2009;65:33–8. doi: 10.1203/PDR.0b013e31818a1d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delemos RA, Coalson JJ, Gerstmann DR, Kuehl TJ, Null DM., Jr Oxygen toxicity in the premature baboon with hyaline membrane disease. Am Rev Respir Dis. 1987;136:677–82. doi: 10.1164/ajrccm/136.3.677. [DOI] [PubMed] [Google Scholar]
- 13.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152:640–6. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 14.STOP. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 15.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–67. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 16.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–10. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J Perinatol. 2006;26:700–5. doi: 10.1038/sj.jp.7211608. [DOI] [PubMed] [Google Scholar]
- 19.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol. 1978;45:699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- 20.Frank L, Sosenko IR. Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res. 1991;29:292–6. doi: 10.1203/00006450-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Yam J, Frank L, Roberts RJ. Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res. 1978;12:115–9. doi: 10.1203/00006450-197802000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Frank L, Iqbal J, Hass M, Massaro D. New “rest period” protocol for inducing tolerance to high O2 exposure in adult rats. Am J Physiol. 1989;257:L226–31. doi: 10.1152/ajplung.1989.257.4.L226. [DOI] [PubMed] [Google Scholar]
- 23.Clark JM, Lambertsen CJ, Gelfand R, Troxel AB. Optimization of oxygen tolerance extension in rats by intermittent exposure. J Appl Physiol. 2006;100:869–79. doi: 10.1152/japplphysiol.00047.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bigdeli MR. Preconditioning with prolonged normobaric hyperoxia induces ischemic tolerance partly by upregulation of antioxidant enzymes in rat brain tissue. Brain Res. 2009;1260:47–54. doi: 10.1016/j.brainres.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz M. Heat acclimation and cross-tolerance against novel stressors: genomic–physiological linkage. Prog Brain Res. 2007;162:373–92. doi: 10.1016/S0079-6123(06)62018-9. [DOI] [PubMed] [Google Scholar]
- 26.Frank L, Roberts RJ. Endotoxin protection against oxygen-induced acute and chronic lung injury. J Appl Physiol. 1979;47:577–81. doi: 10.1152/jappl.1979.47.3.577. [DOI] [PubMed] [Google Scholar]
- 27.Neriishi K, Frank L. Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am J Physiol. 1984;247:R475–81. doi: 10.1152/ajpregu.1984.247.3.R475. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi M, Frank L, Massaro D. Dexamethasone protects against high-dose endotoxin without loss of tolerance to oxygen. J Appl Physiol. 1986;60:1209–12. doi: 10.1152/jappl.1986.60.4.1209. [DOI] [PubMed] [Google Scholar]
- 29.Wright CJ, Dennery PA. Manipulation of gene expression by oxygen: a primer from bedside to bench. Pediatr Res. 2009;66:3–10. doi: 10.1203/PDR.0b013e3181a2c184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosenko IR, Frank L. Nutritional influences on lung development and protection against chronic lung disease. Semin Perinatol. 1991;15:462–8. [PubMed] [Google Scholar]
- 31.Frank L, Lewis PA, Garcia-Pons T. Intrauterine growth-retarded rat pups show increased susceptibility to pulmonary O2 toxicity. Pediatr Res. 1985;19:281–6. doi: 10.1203/00006450-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Frank L, Roberts RJ. Effects of low-dose prenatal corticosteroid administration on the premature rat. Biol Neonate. 1979;36:1–9. doi: 10.1159/000241200. [DOI] [PubMed] [Google Scholar]
- 33.Walther FJ, Jobe AH, Ikegami M. Repetitive prenatal glucocorticoid therapy reduces oxidative stress in the lungs of preterm lambs. J Appl Physiol. 1998;85:273–8. doi: 10.1152/jappl.1998.85.1.273. [DOI] [PubMed] [Google Scholar]
- 34.Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. 2009;11:2945–55. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 35.Frank L. Oxygen toxicity in neonatal rats: the effect of endotoxin treatment on survival during and post-O2 exposure. Pediatr Res. 1987;21:109–15. doi: 10.1203/00006450-198702000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Sosenko IR, Jobe AH. Intra-amniotic endotoxin increases lung antioxidant enzyme activity in preterm lambs. Pediatr Res. 2003;53:679–83. doi: 10.1203/01.PDR.0000055769.19891.C4. [DOI] [PubMed] [Google Scholar]
- 37.Sosenko IR, Kallapur SG, Nitsos I, et al. IL-1a causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res. 2006;60:294–8. doi: 10.1203/01.pdr.0000233115.51309.d3. [DOI] [PubMed] [Google Scholar]
- 38.Yee M, Chess PR, McGrath-Morrow SA, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–9. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bland RD, Mokres LM, Ertsey R, et al. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177:1103–10. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Marter LJ, Allred EN, Pagano M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? Pediatrics. 2000;105:1194–201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 42.Davidson WJ, Dorscheid D, Spragg R, Schulzer M, Mak E, Ayas NT. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]