Abstract
Frequent interictal spikes are a common finding in the electroencephalograms of children with epileptic encephalopathies. While it is well recognized that interictal spikes are a biological marker of seizures and can lead to transitory cognitive impairment, whether interictal spikes can result in long-standing adverse effects on learning and memory in children is not known. Here we investigated the consequences of interictal spikes in rat pups without seizures on long-term learning and memory. Rat pups were given a low dose of flurothyl for four hours for 10 days during continuous electroencephalographic monitoring. Rats developed interictal spikes without seizures while age-matched controls under similar testing conditions had few interictal spikes. When rats were tested as adults, there was impairment in reference memory in the probe test of the Morris water maze, reference memory impairment in the four-trial radial-arm water maze and impaired long-term potentiation. Early-life interictal spikes resulted in impaired new cell formation and decreased cell counts in the hippocampus but did not cause an increase in apoptosis. This study, for the first time demonstrates that interictal spikes in rat pups without seizures can result in long-standing spatial cognitive impairment. Our findings suggest that suppressing IIS may be as important as treating seizures during brain development.
Keywords: learning, memory, water maze, apoptosis, BrdU
Introduction
The epileptic encephalopathies consist of a group of epileptic conditions in which psychomotor deterioration appears to occur independently of the etiology of the seizures (Shields, 2000; Nabbout and Dulac, 2003) and these disorders constitute one of the most catastrophic conditions encountered in pediatric neurology (Holmes and Lenck-Santini, 2006; Galanopoulou and Moshe, 2009). The key concept of the epileptic encephalopathies is that the slowing or regression of development is primarily due to seizures, abnormal interictal spikes (IIS), or cortical dysrhythmia as reflected in the electroencephalogram (EEG), and not solely due to the underlying cause of the seizures (Nabbout and Dulac, 2003). While it can be reasonably argued that all of the epilepsy syndromes have an underlying etiology that could lead to the cognitive impairment, the observation that children with epileptic encephalopathies who are successfully treated with medications or surgery can regain normal cognitive function is a demonstration that it is the seizures or abnormal EEG, or both, that are responsible for the cognitive impairment (Asarnow et al., 1997; Matsuzaka et al., 2001; Zupanc, 2003; Besag, 2004).
Frequent and widespread IIS are a hallmark of the EEG features of the epileptic encephalopathies. IIS are transient (<70 milliseconds), focal neural discharges seen on EEG recordings. They are a result of synchronous, paroxysmal depolarizations of neurons producing a rapid succession of action potentials (Matsumoto and Ajmone-Marsan, 1964; Dichter and Spencer, 1968; Prince and Connors, 1986). IIS are closely related to the seizure focus (Engel, Jr., 1984) and are one of the most important factors in the diagnosis of epilepsy (Blume, 2001). It is also known that IIS can lead to transitory cognitive effects (Hutt et al., 1977; Aarts et al., 1984; Shewmon and Erwin, 1988a; Shewmon and Erwin, 1988b; Shewmon and Erwin, 1988c; Shewmon and Erwin, 1989; Krauss et al., 1997).
In addition to the transitory cognitive impairment, there is concern that IIS may contribute to long-standing cognitive impairment in children with epileptic encephalopathies (Holmes and Lenck-Santini, 2006). Activity-dependent mechanisms play an important role in brain development (Nelson et al., 1995; Moody and Bosma, 2005; Spitzer, 2006) and it possible that IIS through the nature of their aberrant electrical activity will interfere with critical activity-dependent developmental processes. While there is evidence that focal IIS can interfere with cortical development in the visual cortex (Crabtree et al., 1981; Ostrach et al., 1984), whether IIS during early brain development can cause long-standing cognitive impairment is not known.
In this study we wished to determine the consequences of IIS independent of behavioral and electroencephalographic seizures during early life on subsequent learning and development. We report here that IIS without seizures in rat pups have long-term adverse consequences on spatial cognition and synaptic plasticity.
Methods
Overview of experiments
To assess the effects of IIS independent of seizures in normal rat pups we developed a model in which an inhalant chemoconvulsant produced clear repetitive IIS in rat pups. Flurothyl, an inhalant convulsant (Gatt et al., 1993; Moshé et al., 1994) when administered at slow rates produces IIS without electroencephalographic or behavioral seizures. In pilot experiments we found that following flurothyl the IIS are widespread, involving multiple brain structures including the hippocampus and neocortex. Because movement in freely moving rat pups obscures the EEG recording, we administered low doses of isoflurane in conjunction with flurothyl. The combination of isoflurane and flurothyl allowed us to readily elicit and record IIS.
Seventy rats had an electrode implanted in the right CA3 at postnatal (P) day nine (Table 1A). At P12 all rats were divided into four groups and received different treatments for two weeks (five days a week separated by two days): flurothyl-induced IIS (IIS, n = 21); control (Cont, n = 18); isoflurane (Iso, n = 16) and flurothyl/isoflurane exposure without IIS (No IIS, n = 15). Each of the treatment groups had controls that were separated from their dams for the same duration as the experimental groups.
Table 1.
Studies performed and rat numbers. 1A. All rats in this group had electrodes placed at P9. In Table 1B rats were treated identically to those in Table 1A but did not have electrode placement at P9. Rats in 1B were placed in the same chamber and received identical amounts of flurothyl, isoflurane or both. Numbers listed indicated number of rats that completed the study.
| Groups | Surgery | Treatment | BrdU/ Tunel |
Morris Water Maze |
Four-Trial Radial Arm Water Maze |
Histology/ Cell Count |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Age | # | Age | # | Age | # | Age | # | Age | # | Age | |
| IIS | 21 | P9 | 21 | P12-23 | 8 | P23 | 8 | P60 | 8 | P68 | 8 | P80 |
| Controls | 18 | P9 | 18 | P12-23 | 10 | P23 | 8 | P60 | 8 | P68 | 8 | P80 |
| Isoflurane | 16 | P9 | 16 | P12-23 | 8 | P23 | 8 | P60 | 8 | P68 | 8 | P80 |
| No IIS | 15 | P9 | 15 | P12-23 | 7 | P23 | 7 | P60 | 7 | P68 | 7 | P80 |
| Table 1A | ||||||||||||
| Groups | Treatment | LTP | Histology/Electrode Placement |
|||
|---|---|---|---|---|---|---|
| # | Age | # | Age | # | Age | |
| IIS | 9 | P12-P23 | 7 | P120 | 7 | P130 |
| Controls | 9 | P12-P23 | 9 | P120 | 9 | P130 |
| Isoflurane | 4 | P12-P23 | 4 | P120 | 4 | P130 |
| No IIS | 8 | P12-P23 | 8 | P120 | 8 | P130 |
| Table 1B | ||||||
At P23, immediately after finishing the tenth day of treatment, a subset of rats from each group were perfused and the brains were stained with bromodeoxyuridine (BrdU) to assess new cell formation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) fluorescence staining to evaluate apoptosis. The remainder of the rats who had surgery at P9 were housed and underwent sequential testing in the Morris water maze (P60) and the four-trial radial-arm water maze (P68). After watermaze testing was finished, rats were sacrificed for histological evaluation which included cell counting.
An additional 30 rats were treated exactly as the rat pups in the four groups but did not have electrodes placed at P9 because of the concern that surgery could alter the long-term potentiation and paired-pulse inhibition studies (Table 1B). These rats were age-and treatment-matched with the animals in Table 1A. The rat pups were placed in the chamber next to rats with electrodes in place and received the identical dose of drugs as the pups listed in Table 1A. This subset of rats was evaluated for synaptic plasticity with LTP and paired -pulse facilitation/inhibition at P120.
Animals
Male Sprague–Dawley rats (total n = 100) from Charles River Laboratories were used throughout the study and were treated in accordance to the guidelines set by the National Institute of Health and the Animal Care and Use Committee of Dartmouth College for the humane treatment of animals.
Surgery
At P9, 70 rats had four intracranial electrodes (0.003 in) implanted. The electrodes were twisted together through a 5 mm long guiding tube. The tips of electrodes were 1 mm longer than the guiding tube and were cut diagonally with a difference of 1 mm among tips. Electrode implantations were done with isoflurane (1.5–2%) anesthesia and were implanted in the right CA3 area (2.0 mm anterior to inter-aural line, 3.1 mm lateral to midline, and 3.2 mm below dura) based on the stereotaxic atlas of the developing rat brain (Sherwood and Timiras, 1970). A referential electrode was placed in the occipital cortex and all recordings were done using a referential montage.
Interictal Spike Induction
Starting at P12, rats from the IIS group were placed in a plastic container located in an exhaust hood that allowed unidirectional flow of air from an inlet connected to an isoflurane and oxygen pump. A cotton swab was placed within the tubing just before the inlet of the container. Isoflurane (1%) and oxygen (100%) were infused at a rate of 1 L/min into the chamber.
The implanted intracranial electrodes were used for continuous EEG monitoring. Following 10 min of baseline EEG recordings liquid flurothyl was then injected through a 1 ml syringe into the cotton swab with a loading dose of 0.1 ml and subsequently at a rate of 0.02 ml per 4–5 min to maintain IIS every 2–10 sec on EEG. The animals were closely monitored for behavioral and EEG indications of ictal behavior. Rats were excluded from the study if they experienced a behavioral seizure or had an EEG seizure (operationally defined as rhythmic spikes occurring at a frequency of greater than 1 Hz that lasted 10 sec or longer). Flurothyl exposure was four hours daily with two two-hour sessions for 10 days (five days per week). A heating pad was put under the container to keep rats warm during sessions. Each two hour session was separated by two hours in which the pups returned to the cage with their mothers.
Rats from the Iso group were monitored for the same duration while receiving only isoflurane (1%) and oxygen (100%). Rats from the No IIS group were treated in the same setup except the isoflurane concentration was lower (0.5%) and flurothyl was administered with an infusion pump at a speed of 0.1–0.15 ml/hour for a total of 8–10 hours to ensure the same amount of flurothyl (1.2–2 ml/day) and isoflurane exposure as the IIS and Iso groups without inducing IIS. In the No IIS group rat pups were separated from their mother for two two-hour sessions, identical to what was done with the other groups. During the remainder of the exposure to isoflurane and oxygen the mothers were in the chamber with their pups. Thus in all of the animal groups, pups were separated from their dams for an identical time as the experimental groups, eliminating stress as a compounding factor in the experimental design.
The EEG recordings were performed using a differential AC amplifier with the data digitized at 500 Hz. A pre-amplifier was connected to the headset to eliminate movement artifact during recordings. Spectral analysis of EEG epochs was done at a sampling frequency of 500 Hz, using a Blackman window with a window length of 2048 and a spectral resolution of 0.244 Hz to determine the root mean square value of each spectral band. Only frequencies below 100 Hz were analyzed.
BrdU staining
BrdU is a synthetic nucleoside that is an analogue of thymidine incorporated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle (McCabe et al., 2001). At P22, 36 hours before completion of the 10 day treatment, a subset of rats from each group received a series of three intraperitoneal (IP) injections of BrdU (100 mg/kg, Sigma) every 12 hours to label mitotically active cells. Rats were sacrificed at P23, 36 hours after BrdU administration, when they finished the 10 day treatment.
After deep anesthesia with sodium pentobarbital (65 mg/kg), rats were perfused transcardially with normal saline followed by 4% paraformaldehyde (PFA). The brains were cut in half in the sagittal plane. One hemisphere was cut at 40 µm through the entire extent of the hippocampus on a cryostat and stored in phosphate buffered saline (PBS) until processed. BrdU double-labeling with the neuron-specific nuclear protein (NeuN) was performed. In brief, free floating slices were washed with PBS twice (five min each) then kept in 2N HCl for 30 min in room temperature followed by another four washes with PBS. Slices then stayed in blocking solution for one hour at 4° C then incubated in anti-NeuN (1:200) (mouse anti-neuronal nuclei monoclonal antibody, Chemicon International Inc. CA) and anti-BrdU (1:100) (monoclonal rat anti-BrdU, Accurate chemical corp. NY) solution overnight at 4° C. The second day, after another four washes in PBS, slices were incubated in Alexa 546 (1:750) and Alexa 488 (1:250) for two hours at 4° C. After another two washes, slices were mounted and put on a coverslip with fluoromount solution. Fluorescence microscopy was used to visualize BrdU cell. Based on anatomical landmarks, equivalent sections from each group were chosen (3.3 mm to 3.6 mm posterior to bregma, (Paxinos and Watson, 1998). Histological specimens obtained from the dorsal hippocampus were analyzed with the total number of labeled cells in the two blades of dentate gyrus counted manually by two examiners blinded to experimental group (GLH and OIK).
TUNEL staining
The TUNEL stain was used to detect DNA fragmentation that results from apoptotic signaling cascades (Negoescu et al., 1996; Negoescu et al., 1998) using previously described techniques (Tewari and Dixit, 1995). One hemisphere underwent paraffin embedded coronal sectioning at a thickness of 4 µm. Based on identical anatomical landmarks used for the BrdU studies, equivalent sections were chosen. A fluorometric TUNEL kit (Promega Corporation, Madison, WI) and its protocol were used for staining. In brief, slices were deparaffinized and rehydrated followed by washes with normal saline and PBS. Slices were incubated with Proteinase K for eight min. After rinses with PBS, slices were equilibrated with Equilibration Buffer for five min, followed by incubation with terminal deoxynucleotidyl transferase, recombinant incubation buffer at 37° C for one hour. The reaction was terminated by 2X SSC. After several washes, a drop of VECTASHIELD+PAPI was added to slides to stain nuclei and coverslips were placed. Fluorescence microscopy was used to visualize TUNEL positive cell co-labeled with DAPI. Cell counting was done manually by two examiners blinded to experimental group (GLH and OIK).
Morris water maze
To assess spatial memory function, we used the Morris water maze, a test of hippocampal-dependent spatial memory (Morris et al., 1982; Morris, 1984; Morris, 2007), the closest parallel to episodic memory in humans (Jeltsch et al., 2001; Spiers et al., 2001a; Spiers et al., 2001b). Rats underwent Morris water maze testing on P60 using techniques previously described in our laboratory (Dube et al., 2008; Lenck-Santini and Holmes, 2008; Karnam et al., 2009b).
Apparatus
A stainless-steel circular swimming pool (2 m in diameter, 50 cm high) was filled to a depth of 25 cm with water. Non-toxic white paint was added to make the water opaque and prevent the rats from seeing the platform. Room cues visible from the water surface were constant from day to day. Four points on the perimeter of the pool were designated north (N), east (E), south (S), and west (W), thus dividing the pool into four quadrants (NW, NE, SE, SW). A clear plexiglass escape platform 8 cm in diameter was positioned in the center of one of the quadrants, 2 cm below the water surface.
Behavioral procedures
All procedures were videotaped and analyzed using a computerized tracking system. On the first day each rat was placed in the pool for 60 seconds without the platform present; this free swim enabled the rat to become habituated to the training environment. Starting the second day the rats began the hidden escape platform portion of the test. The rats underwent six timed, hidden platform trials with the platform in the same quadrant across days, for four days (Days 2–5). The point of immersion into the pool varied between N, E, S and W in a random order for each trial, so that the rat was not able to predict the platform location from the point at which it was placed in the pool. The latency from immersion into the pool to escape onto the platform was recorded for each trial, and the observer also recorded the route taken by the rat to reach the platform. On mounting the platform, rats were given a 30-sec rest period, after which the next trial was started. If the rat did not find the platform in 120 sec, it was manually placed on the platform for a 30-sec rest. At the start of each trial, the rat was held facing the perimeter and dropped into the pool to ensure immersion.
On day six the platform was removed and animals swam in the maze for 60 sec. The number of seconds spent in the quadrant where the platform had previously been located was compared to time spent in the other three quadrants. The test began with the rat in the quadrant opposite to the trained platform location. The path and time spent in the quadrant where the platform had previously been placed was recorded. In this part of the water maze, termed the probe test, normal animals typically spend more time in the quadrant where the platform had been previously located than in the other quadrants. The testing procedure used during the four days of locating the hidden platform provides a measure of spatial reference memory, while the probe trial is a measure of the strength of spatial learning (Jeltsch et al., 2001).
Four-trial radial-arm water maze
After finishing the Morris water maze, the four-trial radial-arm water maze was used to measure spatial learning and memory (Sayin et al., 2004; Mortazavi et al., 2005; Cornejo et al., 2007). The test allows assessment of reference memory and requires a greater memory load than the Morris water maze (Hyde et al., 1998).
Apparatus
The radial-arm maze consisted of eight stainless steel arms (length – 50 cm, width – 15 cm) extending radially from a central area (diameter 40 cm) placed in a stainless-steel circular swimming pool filled with water. One arm was chosen as the target arm, in which a clear plexiglass escape platform was submerged at the end of the arm that allowed the rat to climb atop it, thus exiting the water, and rest. The escape platform remained in a constant position for the duration of the experiment. White paint was added to make the water opaque and prevent the rats from seeing the platform. Cue cards were distributed around the maze and remained constant throughout the experiment.
Behavioral procedures
Each rat was placed in the start arm and the trial continued until the escaped platform was found or until two 120 sec had elapsed. If the rat did not find the platform in two min, it was manually placed on the platform. Rats had a 30 sec rest on the platform before the next trial start. Rats were pre-trained in the maze for two days with five trials per day. The rats were then tested on four consecutive trials per day with four trials per day. The start arm for each trial was determined in a pseudorandom fashion with a given arm used once per day. The goal arm changed every day but remained the same throughout the testing day.
During the radial-arm water maze, each rat was assessed for latency to find the goal arm and memory errors (number of times the rat entered into an incorrect arm or number of times the rat re-entered into an arm during a trial) recorded.
Long-Term Potentiation (LTP)
Starting at P120, a total of 30 rats from the four groups that did not have surgery done at P9 were placed in a stereotaxic frame under urethane anesthesia (1.2 g/kg, ip). Both recording and stimulating electrodes were bipolar 125-µm twisted wires. A stimulating electrode was fixed in the ventral hippocampal commissure (0.14 cm posterior to bregma, 0.05 cm lateral to midline, and 0.38 cm below dura) and a recording electrode was placed in the CA1 subfield of right hippocampus (0.38 cm posterior to bregma, anterior to inter-aural line, 0.25 cm lateral to midline, and 0.2 cm below the dura). The final position for the recording electrode was adjusted based on the shape and amplitude of the field excitatory postsynaptic potential (fEPSP) to maximize amplitude and stability of the waveform.
The recording setup was the same as described earlier and records were analyzed with the Mini Analysis program (Synaptosoft Inc., Fort Lee, New Jersey). For each rat, an input/output curve was drawn based on the slope of the of the population spike. The baseline stimulus intensity was set to 70% of the intensity that had the maximum slope of the population spike. The EEG was recorded for 30 min (stimulation frequency - 0.05 Hz; stimulation pulse duration - 0.2 ms) as baseline. LTP was evoked by high frequency stimulation (HFS) consisting of three trains with 10 sec of inter-train intervals. Each train consisted of one sec of 200 Hz stimulations with a pulse duration of 0.3 ms and an intensity of 90% of the intensity that caused the maximum slope. Immediately after the HFS, the stimulation setting was changed back to the baseline parameters. Responses were stimulated and recorded for three more hours. Every 10 min, 30 normalized responses were averaged to represent one point on the figure. The rat was kept on a warming pad throughout the recording period.
Histology/Cell Counting
At the end of the experiment, rats were sacrificed with a lethal dose of sodium pentobarbital (65 mg/kg) and perfused transcardially with: 1) 200 ml of normal saline; and 2) 200 ml of 4% PFA. The brains were removed, postfixed in 4% PFA for 24 hours and placed in 30% sucrose for 24 hours or longer until the brains sank. Coronal sections along the entire extent of the hippocampus were cut at 20 µM on a freezing
Equivalent sections were chosen based on anatomical landmarks in the dorsal hippocampus (Paxinos and Watson, 1998) and sections selected for counting were done using systematic random sampling. Cell counting of the dentate gyrus, hilus, CA3 and CA1 were done manually and independently by two investigators (FM and GLH) blinded to the treatment groups. A 10 × 10 box grid was placed over the CA1 and CA3 region at 40× magnification. All cells in a block of 10 × 2 boxes were counted including cells that were partially in the box. Due to the high cell density in the dentate gyrus cell counting was done at 100× and cells within a 10 × 8 grid were counted. Only cells that were in focus within 5 µm of the initial focal plane were counted. In CA1, CA3 and the dentate two adjacent sections were counted. To count cells in the hilus an imaginary line was drawn between the two blades of the dentate gyrus and all cells counted within the hilus at 10×.
Statistical analysis
The Kolmogorov-Smirnov goodness-of-fit test was used to assess normality (Gaussian-shaped distribution) for all continuous variables. Data from the Morris water maze (swimming speed, mean escape latency to platform, time spent in the target quadrant), four-trial radial-arm maze (mean escape latency to platform, working and reference memory errors), LTP (comparison between slopes), paired-pulse excitation/inhibition (ratio of amplitude of the second population spike to the first population spike) and cell counts were compared using the repeated measures ANOVA with Tukey’s multiple comparison test to compare individual groups. Results are presented as means±standard errors and a p <0.05 was used to define statistical significance.
Results
Induction of IIS
Rat pups from the IIS group and isoflurane groups were mildly sedated during the two, two-hour sessions whereas pups from the control and No IIS groups were more active, moving occasionally around the cage. IIS were readily induced and recorded when the rats were sedated under isoflurane, appearing within one min of flurothyl administration. IIS were observed in all pups in the IIS group. The frequency of spikes was directly correlating with the amount of flurothyl used and the dose of flurothyl was frequently adjusted to maintain IIS at a rate of 6–30 IIS/min.
After each two-hour session, the container was aired in the hood for 10 min and during that time, the rats usually woke up and moved around. They were then put back with their mother for a two-hour break followed by a second two-hour session of treatment. All of the treatment groups recovered within 15 min of the treatment.
All of the rats tolerated the procedures without discernible morbidity or mortality. No significance in weight were noted in the four groups (F(3) = 2.681; p = 0.066). Five rats were excluded from the study because of seizure during the IIS induction period.
Electrodes were placed to record above and below the CA1 layer (Fig. 1A) and IIS were typically seen in all four of the electrodes. Some IIS had a phase reversal at the CA1 cell layer or stratum radiatum indication - a sink in this region whereas other spikes had a similar morphology in all four electrodes suggesting propagation from outside the hippocampus (Fig. 1A). The EEG in the controls, isoflurane and No IIS groups consisted of a mixture of delta, theta and faster frequencies with very rare spikes seen (Fig. 1B). The IIS occurred as isolated events as well as in brief paroxysmal bursts of 2–5 IIS within 1–4 sec. Fig. 1C is a typical example of 180 sec of recording from a rat treated with flurothyl.
Fig. 1.
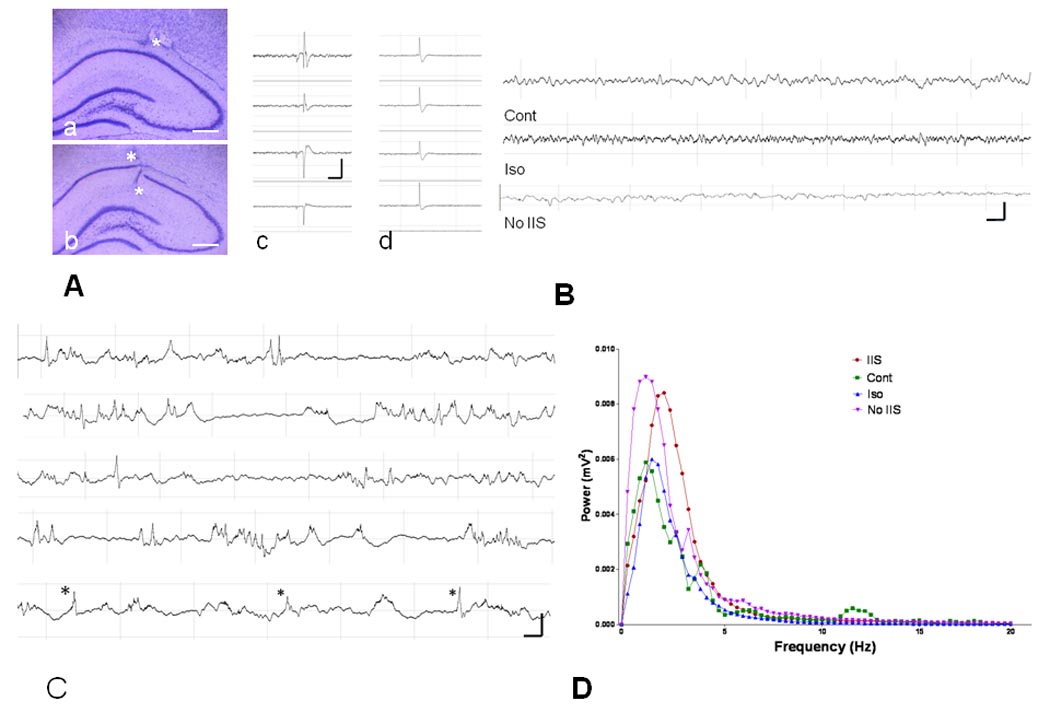
Effects of treatment on IIS and background activity. A. Histological examples of electrode placement. a. Electrode tip above CA1 (asterisk). b. Electrode tips above and below CA1 (asterisks). Calibration = 500 µm. c. Example of morphology of the spike from the four electrodes in the CA1 region of the hippocampus. There is a phase reversal of the spike and sharp wave across channels 3 and 4 suggesting a sink in the stratum radiatum or cell layer. In d the spike has a different amplitude but similar morphology in all four channels indicating propagation of the IIS from outside CA1. B. Examples of EEG from the Control, Isoflurane, and No IIS groups. Few IIS were seen in these three groups. C. Example of frequency and morphology of IIS from one animal. These traces came from 180 sec of continuous recording. In the bottom trace spikes are marked with an asterisk. Voltage/time calibration in A, B and C indicate one second and 0.5 mV. D. Spectral power from the four groups. The maximum peak for the IIS group was faster than the other groups. Note that the control animal had peaks at approximately 2, 4 and 12 Hz whereas in the other groups the majority of the power was in the delta bandwidths. Total power did not differ between groups.
The mean number of IIS in the four groups were: i) Control group: 0.02±0.01 IIS/min; ii) Isoflurane: 0.05±0.01 IIS/min; iii) IIS: 10.82±3.53 IIS/min; and iv) No IIS: 0.05±0.05 IIS/min (F(3) = 5.318; p = 0.003). Spikes disappeared from the EEG within 20 min after the flurothyl was discontinued and were always absent when recordings began the following day.
Spectral analyses of the EEGs during the four conditions are shown in Fig. 1D. In the controls, peaks were seen at approximately 2, 4, and 12 Hz whereas in the other groups the predominant frequencies were in the delta range (<4 Hz). The maximum power frequency differed in the four groups (IIS: 1.98±0.06 Hz; Iso: 1.66±0.06; Cont: 1.63±0.48; No IIS: 1.59±0.26; F(3) = 5.315; p = 0.002 with the IIS group demonstrating a higher maximum frequency than the other three groups). Total power in frequencies up to 100 Hz did not differ between groups.
Morris water maze
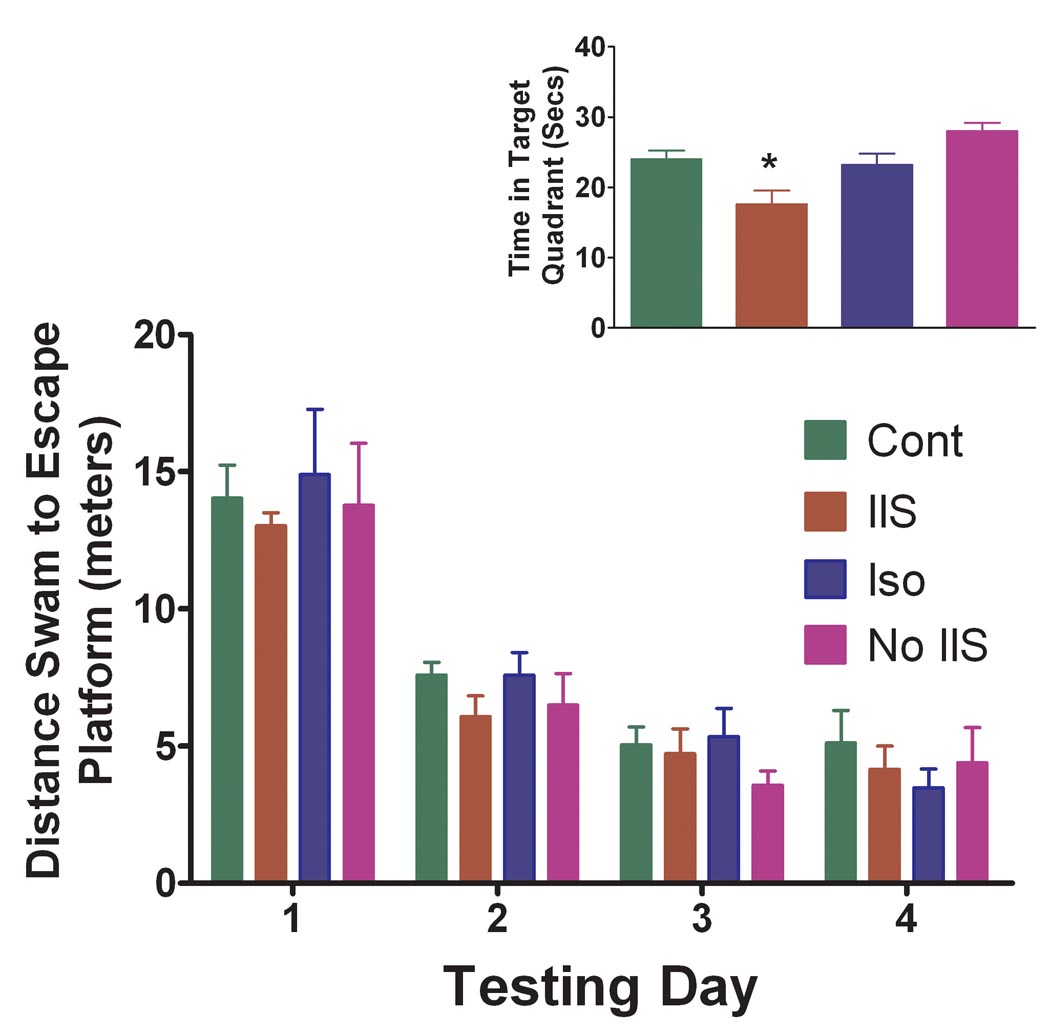
Distance swam to reach the hidden platform decreased for all four groups over the four days of testing (F(7,844) = 2.23, p = 0.039) indicating that all groups learned to find the platform over the four days of testing (Fig. 2). No significant differences were seen in the four groups in distance swam to the escape platform (F(3,84) = 0.18, p = 0.910). There were no differences noted in the four groups in swimming speed (F(3) = 2.773, p = 0.060). In the probe test (Fig. 2, insert), a measure of the strength of reference memory, there was a significant difference in groups (IIS -18.6±1.96 secs; Iso - 23.2±1.61; Cont.-24±1.23; No IIS – 28.0±1.20; F(3) = 3.463, p = 0.031) with the IIS group significantly different from the No IIS group (p <0.05). No significant differences were noted between the Controls, Isoflurane and No IIS groups. When these three groups were combined and compared to the IIS group there was a significant difference between the IIS group and the three other groups (t(29) = 2.123, p = 0.042).
Fig. 2.
Results of the Morris water maze. All groups learned the location of the platform across the four days of testing. No differences between groups were noted in distance to escape platform. The insert shows the results of the probe test. In the probe test, a measure of the strength of reference memory, there was a significant difference in groups (F(3) = 3.463, p = 0.031) with the IIS group significantly different from the No IIS group (p<0.05; asterisk).
These results demonstrate that there is a mild impairment in reference memory in adult rats subjected to IIS during early life.
Four-trial radial-arm water maze
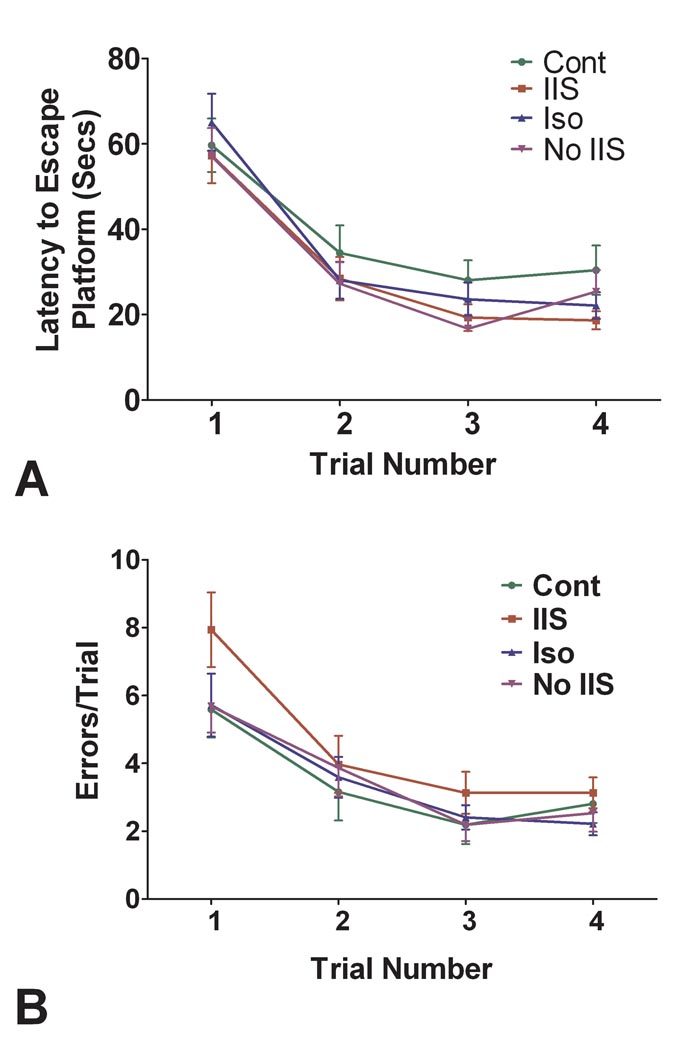
In the four-trial water maze all the rats except one completed the test without difficulty. One rat did not swim and was excluded from the study. There were no significant differences in latencies to the escape platform (Fig. 3A). However, using a repeated measures ANOVA there were significant differences between groups in errors (F(3) = 2.695, p = 0.046) with the IIS group differing statistically from each of the other groups (Fig. 3B). All of the groups reduced errors across trials with a highly significant effect of trial number on number of errors (F(3,372) = 22.48; p<0.001). In a comparison of errors versus day of testing there was a group difference (F(3.72) = 3.39, p = 0.022) without a significant relationship between day of testing and error rate. These results are interpreted as showing rat pups subjected to IIS have impaired reference memory when tested as adults.
Fig. 3.
Comparison of the four groups in four-trial radial-arm test. A. Latency to escape platform. The groups did not differ in latencies to the escape platform. B. Errors in the four-trial water maze tests. There was a significant differences in groups (F(3) = 2.695, p = 0.046) with rats in the IIS group having more errors than the other groups.
LTP
Thirty rats were used in the LTP study. Two rats were excluded from the study because of poor reproducibility of the fEPSP and population spike. Figure 4A shows an example of a fEPSP and population spike and the method by which the slope and the amplitude were calculated. As shown in Figure 4B, following HFS there was an increase in the slope and amplitude of the population spike following HFS. Figure 4C shows the results of the LTP. There was a significant effect of time on slope of the fEPSP (F(1,153) = 181.0; p <0.0001) and a time-group interaction (F(8,153) = 41.04; p<0.0001). There were significant differences between the rats with IIS versus the other groups (F(8,153) = 25.25; p <0.001) with the IIS group differing significantly from each of the other three groups (p <0.05). The findings indicate that early-life IIS result in impaired synaptic plasticity and impairment of feedback inhibition in adults.
Fig. 4.
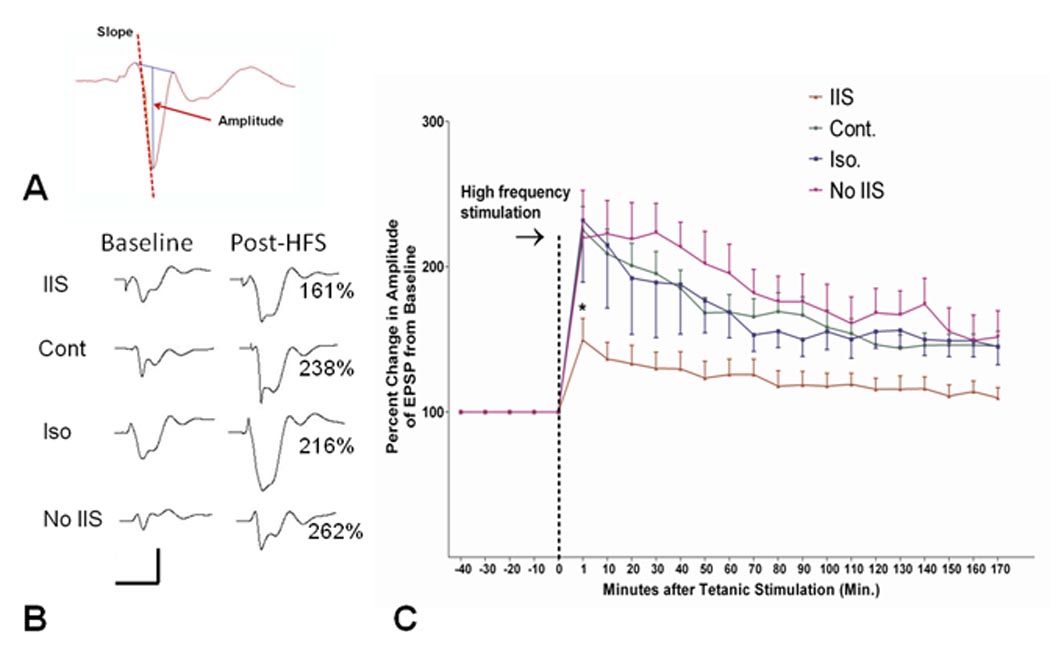
LTP following IIS. A. Example of fEPSP with population spike. The amplitude was measured by drawing a line between beginning and end of the population spike and measuring the distance between this line and the peak of the population spike. The slope of the population spike was calculated from the initial downward slope. B. Examples of population spikes before and after HFS. Note the increases in slope in the controls, isoflurane and No IIS groups compared to the IIS group. Percentage of increase of the slope is provided at the right of the post-HFS waveforms. C. Comparison of the four groups in LTP. Change in the slope of the fEPSP following high frequency stimulation is presented for the four groups. Rats with IIS had impaired LTP compared to the other control groups (p <0.001).
BrdU staining
Following 10 days of treatment a subset of eight rats from each group were sacrificed at P23 (36 hours after BrdU administration) and the brains stained with BrdU. BrdU positive cells were most densely distributed in the two blades of the dentate gyrus. As shown in Fig. 5, there was a significant decrease in BrdU labeling in the IIS group (F(3,43) = 6.982; p <0.001) with statistical differences between the IIS and the other groups (p <0.05).
Fig. 5.
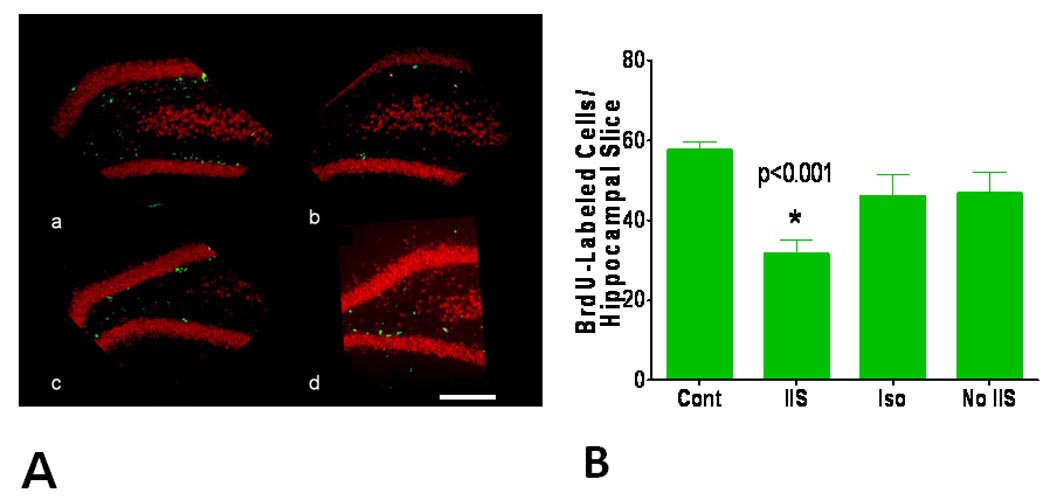
Effect of IIS on neurogenesis. A. Examples of BrdU staining from (a) control, (b) IIS, (c) Isoflurane and (d) No IIS groups. Calibration = 500 µm. B. Quantification of BrdU-labeled cells in the four study groups. Rats subjected to IIS had a significantly reduced number of labeled cells compared to the other groups (asterisk = p<0.001).
TUNEL staining
TUNEL staining on P23 revealed few positive cells (IIS – 2.87±0.37; Cont - 1.99±0.52; Iso - 3.67±0.52; No IIS - 3.75±0.64) with no significant difference among the four groups (F(3,40) = 2.723; p = 0.057).
Histological Analysis
Cell counts were obtained from CA1, CA3, dentate gyrus and the hilus (Fig. 6). There was a significant difference in cell counts in CA1 (F(3) = 12.53; p <0.0001), CA3 (F(3) = 18.56, p < 0.0001) and dentate F(3) = 33.69, p <0.0001) with the cell counts lower in the IIS than in the other three groups. While the mean number of cells in the hilus was lower than the other groups (Cont - 247.2 ±12.36 per field; IIS – 183.3±13.75; Iso – 233.3±21.54; No IIS – 242.5±18.80), these differences did not reach statistical significance (F(3) = 2.674; p = 0.072).
Fig. 6.
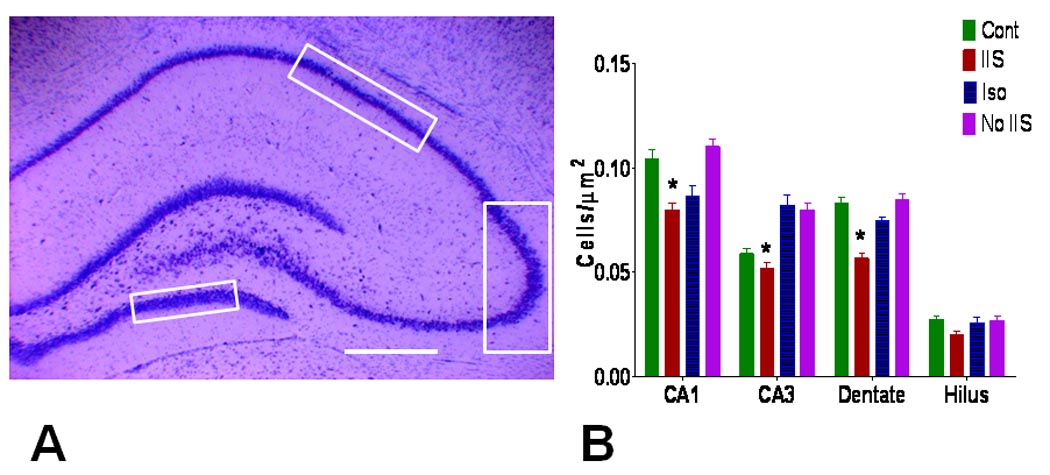
Cell loss in animals with IIS. A. Histological areas where cell counts were obtained. Cell counts were obtained in the dorsal hippocampus in CA1, CA3 and dentate as demarcated by the boxes. Cells in the entire hilus between the two blades of dentate (dotted line) were counted. Calibration = 500 µm. B. Number of cells per µm2. There were significant reductions in cell counts in the IIS group in CA1, CA3, and dentate compared to the other groups (asterisk = p<0.05). While the number of cells in the hilus was lower in the IIS group, the results did not reach statistical significance.
Discussion
In this study we found that rat pups with IIS and without seizures had behavioral, electrophysiological and anatomical deficits when compared to age-matched controls without IIS. Adult rats subjected to IIS as rat pups had significant hippocampus-dependent memory impairment compared to age-matched controls as measured by performance in the Morris water maze and four-trial radial-arm water maze. These behavioral deficits were paralleled by significant impairment in LTP and cell loss in the hippocampus.
The model we used was found to be quite effective and reliable in producing IIS at a frequency that would mimic those seen in children with epileptic encephalopathies. The IIS were frequent and widespread although limited to four hours a day over 10 days. By continuous on-line monitoring of behavior and the EEG, we could control the frequency of the IIS without eliciting behavioral or electroencephalographic seizures. Since the IIS ceased shortly after the end of the flurothyl inhalation, we had precise control of the IIS load. Using flurothyl in combination with isoflurane we were able to obtain EEG recordings which were free of artifact, allowing us to accurately quantify IIS. Since we used normal rats in this study, we were also able to tease out the effects of IIS independent of brain pathologies that elicit seizures.
The major disadvantage of this model is that we used low-dose isoflurane to produce mild sedation. Isoflurane has been shown to be proconvulsant by some authors (Veronesi et al., 2008), but anticonvulsant by others (Isaeva, 2008; Isaeva, 2009). In addition to altering excitability, isoflurane has been shown to result in apoptosis in rat pups (Nikizad et al., 2007; Wang et al., 2009). In this study, we did not find an increase rate of apoptosis following isoflurane, either administered alone or in combination with flurothyl. In addition, the group of rats exposed to isoflurane alone did not result in any cognitive deficits, a finding similar to that of others (Li et al., 2007).
In all groups besides the controls there was an absence of alpha range frequencies on the spectral analysis, indicating that isoflurane resulted in a reduction of alpha range frequencies. However, only the animals that had IIS demonstrated spatial cognitive deficits, decreased neurogenesis, cell loss and impaired LTP, suggesting that changes in electroencephalographic background frequencies were not responsible for the deficits seen in IIS animals.
This study demonstrates that IIS, occurring over a course of four hours a day for 10 days can have significant effects on cognitive function. Rats subjected to recurrent IIS had mild deficits in reference memory, as measured by the results in the probe test of the Morris water maze and the error rate in the four-trial radial-arm water maze. These findings parallel studies in which recurrent seizures in early life lead to long-standing deficits in learning and memory (Holmes et al., 1998; Huang et al., 1999; Karnam et al., 2009a; Karnam et al., 2009b). The deficits in the Morris water maze were relatively modest with only a mild impairment in the probe test of the water maze. The four- trial radial-arm maze also showed the animals had deficits in reference memory. Taken together, our findings indicate that IIS lead to deficits in hippocampal-dependent memory.
In addition to studying spatial cognition, we used LTP as a measure of synaptic plasticity (Muller et al., 2002; Miyamoto, 2006). Whereas there is not an exact relationship between LTP and spatial learning (Holscher, 1997; Holscher, 1999), the electrophysiological measure provides reliable information regarding synaptic transmission. The results were striking showing that IIS can have long-standing deleterious effects on synaptic signaling. Other studies have shown that repetitive seizures in the neonatal period using kainic acid (Cornejo et al., 2007) and flurothyl (Karnam et al., 2009b) result in reduced LTP. Changes in both inhibitory and excitatory neurotransmission could contribute to the IIS-induced changes in LTP (Lopantsev et al., 2009). Increased phosphorylation of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor (Rakhade et al., 2008), reductions in the membrane pool of the AMPA receptor subunit (GluR1), decreases in the total amount of N-methyl-Daspartate subreceptors (NR2A) and increases in the post-synaptic density protein 95 (PSD-95), the primary subsynaptic scaffold protein (Cornejo et al., 2007) following neonatal seizures, all of which would alter excitatory neurotransmission. In addition, long-standing reductions in GABAergic transmission occur following neonatal seizures has been observed (Isaeva et al., 2006; Isaeva et al., 2009). Whether the mechanisms responsible for the impaired LTP following IIS are similar to that following early-life seizures is not yet known. However, our findings indicate that long-standing impaired LTP following seizures is not restricted to neonatal seizures but also extends to IIS.
Other investigators have found that IIS can interfere with normal brain development. In elegant studies of the effects of IIS on striate cortex in rabbits, IIS were elicited by either penicillin (Baumbach and Chow, 1981; Crabtree et al., 1981) or bicuculline (Ostrach et al., 1984; Campbell et al., 1984) epidural focal application in the striate cortex. IIS were elicited for 6–12 hours following each drug application and drug applications were given daily from P8-9 to up to P24-30. In single-unit recordings from the lateral geniculate nucleus, superior colliculus and occipital cortex ipsilateral to the hemisphere with IIS there was an abnormal distribution of receptive field types whereas normal recording were found from the contralateral hemisphere. This finding was age-dependent: adult rabbits with similarly induced IIS had normal development of cells. These studies showed that disruption of orderly neuronal activity in the geniculostriate system during critical developmental periods have a detrimental effect on the development of receptive fields in this occipital cortex.
Because we found significant reductions in cell counts in the dentate, CA3 and CA1 we examined the brains for both apoptosis and new cell formation. Apoptosis occurs in experimental models of epilepsy (Repici et al., 2007; Henshall, 2007; Henshall and Murphy, 2008) and has been found in an eight month old girl with severe focal epilepsy with secondary generalization (Cherian et al., 2009). Reductions in neurogenesis have been found after repeated seizures in rat pups (McCabe et al., 2001; Liu et al., 2003; Porter, 2008; Rakhade and Jensen, 2009). While we did not find an increase in apoptosis, there was a significant decrease in new cell formation in the rats with early-life IIS, suggesting that the cell loss seen in the IIS was secondary to a lack of new cell formation as opposed to IIS-induced apoptosis. However, it should be noted that we examined only one time point for both apoptosis and new cell formation. In addition, while it is highly likely that the reduction in BrU-labeled cells represent a decrease in neurogenesis, we did not specifically identify whether the Brdu-labeled cells were neurons or glia. Future studies will need to examine multiple time points to determine the full scope of the effects of IIS on neurogenesis and apoptosis as well as determining the cell type involved in both processes.
Our findings support and extend this work, showing that recurrent IIS during early development can result in impairment of hippocampal function as demonstrated by the behavioral and electrophysiological deficits. Excessive and aberrant electrical activity generated by IIS likely perturbs the developing nervous system. While the behavioral deficits seen following the IIS were modest, it should be noted that IIS were elicited for a rather short period of time, a total of only four hours administered over 10 days. Compared to a child with IIS occurring 24 hours a day over many years the frequency of IIS in this study was rather modest.
Because our emphasis was on learning and memory, we examined only hippocampal-mediated function. Flurothyl produces widespread IIS and it is likely that other brain areas will be impaired by the IIS. Impairment in other brain areas, such as the prefrontal cortex may have contributed to some of the impairment in spatial cognition. Assessing the effects of IIS on other brain areas will be important in future studies.
While extrapolating results from rats to humans is difficult, our results suggest that IIS can no longer be considered to be only a biomarker of seizures, rather these findings suggest that IIS independent of behavioral and electroencephalographic seizures can contribute to brain dysfunction. Developing strategies to eliminate IIS as well as seizures during early brain development is critical.
Acknowledgements
Supported by grant support from the National Institute of Health (NINDS) NS27984 and NS044295.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107(Pt 1):293–308. doi: 10.1093/brain/107.1.293. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, LoPresti C, Guthrie D, Elliott T, Cynn V, Shields WD, Shewmon DA, Sankar R, Peacock WJ. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol. 1997;39:430–440. doi: 10.1111/j.1469-8749.1997.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Baumbach HD, Chow KL. Visuocortical epileptiform discharges in rabbits: differential effects on neuronal development in the lateral geniculate nucleus and superior colliculus. Brain Res. 1981;209:61–76. doi: 10.1016/0006-8993(81)91172-0. [DOI] [PubMed] [Google Scholar]
- Besag FM. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 2004;5 Suppl 1:S3–S13. doi: 10.1016/j.yebeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Blume WT. Current trends in electroencephalography. Curr Opin Neurol. 2001;14:193–197. doi: 10.1097/00019052-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Campbell BG, Ostrach LH, Crabtree JW, Chow KL. Characterization of penicillin- and bicuculline-induced epileptiform discharges during development of striate cortex in rabbits. Brain Res. 1984;317:125–128. doi: 10.1016/0165-3806(84)90147-0. [DOI] [PubMed] [Google Scholar]
- Cherian KA, Weidenheim K, Legatt AD, Shifteh K, Abbott IR, Moshe SL. Extensive apoptosis in a case of intractable infantile status epilepticus. Epilepsy Res. 2009;85:305–310. doi: 10.1016/j.eplepsyres.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Chow KL, Ostrach LH, Baumbach HD. Development of receptive field properties in the visual cortex of rabbits subjected to early epileptiform cortical discharges. Brain Res. 1981;227:269–281. doi: 10.1016/0165-3806(81)90113-9. [DOI] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Hippocampal penicillin "spike" discharge: epileptic neuron or epileptic aggregate? Neurology. 1968;18:282–283. [PubMed] [Google Scholar]
- Dube CM, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, McIntyre K, Nalcioglu O, Shatskih T, Baram TZ, Holmes GL. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2008;215:167–177. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr A practical guide for routine EEG studies in epilepsy. J Clin Neurophysiol. 1984;1:109–142. doi: 10.1097/00004691-198404000-00001. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Moshe SL. The epileptic hypothesis: developmentally related arguments based on animal models. Epilepsia. 2009;50 Suppl 7:37–42. doi: 10.1111/j.1528-1167.2009.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt G, Veliskova J, Liu Z, Moshé SL, Holmes GL. Ontogeny of flurothyl-induced seizures: A behavioral and EEG electroencephalographic analysis. Epilepsia. 1993;34 Suppl. 6:63. [Google Scholar]
- Henshall DC. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans. 2007;35:421–423. doi: 10.1042/BST0350421. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008;8:75–81. doi: 10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Holscher C. Long-term potentiation: a good model for learning and memory? Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:47–68. doi: 10.1016/s0278-5846(96)00159-5. [DOI] [PubMed] [Google Scholar]
- Holscher C. Synaptic plasticity and learning and memory: LTP and beyond. J Neurosci Res. 1999;58:62–75. [PubMed] [Google Scholar]
- Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, Holmes GL. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev Brain Res. 1999;118:99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Hutt SJ, Newton J, Fairweather H. Choice reaction time and EEG activity in children with epilepsy. Neuropsychologia. 1977;15:257–267. doi: 10.1016/0028-3932(77)90034-3. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23:1559–1566. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Long-term suppression of GABAergic activity by neonatal seizures in rat somatosensory cortex. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva EV. Effects of isoflurane on hippocampal seizures at immature rats in vivo. Fiziol Zh. 2008;54:40–45. [PubMed] [Google Scholar]
- Isaeva EV. Mechanism of antiseizure effect of isoflurane in the immature rat hippocampus. Fiziol Zh. 2009;55:57–60. [PubMed] [Google Scholar]
- Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Karnam HB, Zhao Q, Shatskikh T, Holmes GL. Effect of age on cognitive sequelae following early life seizures in rats. Epilepsy Res. 2009a;85:221–230. doi: 10.1016/j.eplepsyres.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009b;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss GL, Summerfield M, Brandt J, Breiter S, Ruchkin D. Mesial temporal spikes interfere with working memory. Neurology. 1997;49:975–980. doi: 10.1212/wnl.49.4.975. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Holmes GL. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J Neurosci. 2008;28:5053–5062. doi: 10.1523/JNEUROSCI.5024-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. 2007;53:942–950. doi: 10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exper Neruol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Both M, Draguhn A. Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges. Eur J Neurosci. 2009;29:1153–1164. doi: 10.1111/j.1460-9568.2009.06663.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;80:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Baba H, Matsuo A, Tsuru A, Moriuchi H, Tanaka S, Kawasaki C. Developmental assessment-based surgical intervention for intractable epilepsies in infants and young children. Epilepsia. 2001;42 Suppl 6:9–12. [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- Morris R. Theories of hippocampal function. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford: Oxford University Press; 2007. pp. 581–713. [Google Scholar]
- Morris R. Development of a water maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mortazavi F, Ericson M, Story D, Hulce VD, Dunbar GL. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005;7:629–638. doi: 10.1016/j.yebeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Velísek L, Holmes GL. Developmental aspects of experimental generalized seizures induced by pentylenetetrazol, bicuculline and flurothyl. In: Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R, editors. Idiopathic Generalized Epilepsies. London: John Libbey; 1994. pp. 51–64. [Google Scholar]
- Muller D, Nikonenko I, Jourdain P, Alberi SLTP. memory and structural plasticity. Curr Mol Med. 2002;2:605–611. doi: 10.2174/1566524023362041. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Dulac O. Epileptic encephalopathies: a brief overview. J Clin Neurophysiol. 2003;20:393–397. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Guillermet C, Lorimier P, Brambilla E, Labat-Moleur F. Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed Pharmacother. 1998;52:252–258. doi: 10.1016/S0753-3322(98)80010-3. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, Brambilla C, Brambilla E. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- Nelson PG, Fields RD, Liu Y. Neural activity, neuron-glia relationships, and synapse development. Perspect Dev Neurobiol. 1995;2:399–407. [PubMed] [Google Scholar]
- Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82. doi: 10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- Ostrach LH, Crabtree JW, Campbell BG, Chow KL. Effects of bicuculline-induced epileptiform activity on development of receptive field properties in striate cortex and lateral geniculate nucleus of the rabbit. Brain Res. 1984;317:113–123. doi: 10.1016/0165-3806(84)90146-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Porter BE. Neurogenesis and epilepsy in the developing brain. Epilepsia. 2008;49 Suppl 5:50–54. doi: 10.1111/j.1528-1167.2008.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Connors BW. Mechanisms of Interictal Epileptogenesis. In: Delgado-Escueta AV, Ward AA Jr, Woodbury DM, Porter RJ, editors. Advances in Neurology, Vol. 44. Basic Mechanisms of the Epilepsies. Molecular and Cellular Approaches. New York: Raven Press; 1986. pp. 275–299. [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repici M, Mariani J, Borsello T. Neuronal death and neuroprotection: a review. Methods Mol Biol. 2007;399:1–14. doi: 10.1007/978-1-59745-504-6_1. [DOI] [PubMed] [Google Scholar]
- Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkely, California: University of California Press; 1970. [Google Scholar]
- Shewmon DA, Erwin RJ. Focal spike-induced cerebral dysfunction is related to the after-coming slow wave. Ann Neurol. 1988a;23:131–137. doi: 10.1002/ana.410230205. [DOI] [PubMed] [Google Scholar]
- Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. I. General considerations. Electroencephalogr Clin Neurophysiol. 1988b;69:319–337. doi: 10.1016/0013-4694(88)90004-1. [DOI] [PubMed] [Google Scholar]
- Shewmon DA, Erwin RJ. The effect of focal interictal spikes on perception and reaction time. II. Neuroanatomic specificity. Electroencephalogr Clin Neurophysiol. 1988c;69:338–352. doi: 10.1016/0013-4694(88)90005-3. [DOI] [PubMed] [Google Scholar]
- Shewmon DA, Erwin RJ. Transient impairment of visual perception induced by single interictal occipital spikes. J Clin Exp Neuropsychol. 1989;11:675–691. doi: 10.1080/01688638908400924. [DOI] [PubMed] [Google Scholar]
- Shields WD. Catastrophic epilepsy in childhood. Epilepsia. 2000;41 Suppl 2:S2–S6. doi: 10.1111/j.1528-1157.2000.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001a;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001b;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Tewari M, Dixit VM. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Veronesi MC, Kubek DJ, Kubek MJ. Isoflurane exacerbates electrically evoked seizures in amygdala-kindled rats during recovery. Epilepsy Res. 2008;82:15–20. doi: 10.1016/j.eplepsyres.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res. 2009;66:435–440. doi: 10.1203/PDR.0b013e3181b3381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc ML. Infantile spasms. Expert Opin Pharmacother. 2003;4:2039–2048. doi: 10.1517/14656566.4.11.2039. [DOI] [PubMed] [Google Scholar]