Abstract
Hypoglycemia is common during development and is associated with the risk of neurodevelopmental deficits in human infants. The effects of hypoglycemia on the developing hippocampus are poorly understood. The sequential changes in energy substrates, amino acids and phosphocreatine were measured from the hippocampus during 180 min of insulin-induced hypoglycemia (blood glucose <2.5 mmol/l) in 14-day-old rats using in vivo 1H NMR spectroscopy. Hypoglycemia resulted in neuroglycopenia (brain glucose <0.5μmol/g). However, the phosphocreatine/creatine (PCr/Cr) ratio was maintained in the physiological range until approximately 150 min of hypoglycemia, indicating that energy supply was sufficient to meet the energy demands. Lactate concentration decreased soon after the onset of neuroglycopenia. Beyond 60 min, glutamine and glutamate became the major energy substrates. A precipitous decrease in the PCr/Cr ratio, indicative of impending energy failure occurred only after significant depletion of these amino acids. Once glutamate and glutamine were significantly exhausted, aspartate became the final energy source. N-acetylaspartate concentration remained unaltered, suggesting preservation of neuronal/mitochondrial integrity during hypoglycemia. Correction of hypoglycemia normalized the PCr/Cr ratio and partially restored the amino acids to pre-hypoglycemia levels. Compensatory neurochemical changes maintain energy homeostasis during prolonged hypoglycemia in the developing hippocampus.
Keywords: 1H NMR spectroscopy, hippocampus, hypoglycemia, neurochemistry, newborn, rat
Introduction
Hypoglycemia is a common metabolic condition in human infants. In spite of the potential for neuronal injury, the effects of hypoglycemia on the developing brain are incompletely understood (Cornblath et al. 2000). Even though glucose is its principal energy substrate, a lower energy requirement, secondary to decreased neuronal activity, combined with higher brain glycogen stores and ability to utilize alternate energy substrates is proposed to protect the developing brain during hypoglycemia (Nehlig and Pereira de Vasconcelos 1993; Vannucci and Vannucci 2001). Conversely, a proportionately higher demand for glucose, secondary to an increased brain-to-bodyweight ratio, combined with poor high-energy phosphate reserves and an unpredictable supply of alternate substrates, due to immaturity of the counterregulatory system, may predispose the developing brain to injury during hypoglycemia (McGowan and Perlman 2006; Burns et al. 2008).
When glucose supply is compromised, compensatory changes that maintain cerebral oxidative metabolism are initiated in the developed brain. The amino acids, glutamate and glutamine become the major sources of carbon for supporting cerebral tricarboxylic acid (TCA) cycle activity (Lewis et al. 1974a; Behar et al. 1985; Sutherland et al. 2008). Combined with an enhanced substrate delivery through increased cerebral blood flow (CBF) and mobilization of brain glycogen stores, these endogenous substrates maintain energy homeostasis and prevent neuronal injury (Choi et al. 2001; Choi et al. 2003; Sutherland et al. 2008).
The neurochemical changes in the developing brain during hypoglycemia are not well understood. Specifically, the role of cerebral amino acids in preserving energy sufficiency has yet to be determined. The objective of the present study was to evaluate the sequential changes in the neurochemical profile of the hippocampus during prolonged hypoglycemia using high-field in vivo 1H NMR spectroscopy in developing rats. Previous studies have demonstrated the suitability of this method for the simultaneous and longitudinal quantification of neurochemical markers of energy metabolism, neuronal and glial integrity, amino acids and neurotransmitters in the brain regions of developing rats (Tkac et al. 2003; Rao et al. 2007). In the present study, we focused on the hippocampus due to its vulnerability during hypoglycemia in human infants and developing rodents (Anderson et al. 1967; Yamada et al. 2004; Kim et al. 2005; Perantie et al. 2008).
Material and Methods
Animal preparation
Experiments were performed using postnatal day (P) 14 Sprague-Dawley rat pups (average body weight, 33 g). Hippocampal development in P14 rats is comparable to that in full term newborn human infants (Avishai-Eliner et al. 2002). Pregnant dams were purchased (Harlan Sprague Dawley, Indianapolis, IN, USA) and allowed to deliver spontaneously. The litter size was culled to 8 on P3. Rats were maintained on 12 h day-and-night cycle (lights out at 18:00 h) in a temperature and humidity controlled room. Pups were allowed to nurse ad libitum until the day of the experiment. Pain and distress were minimized using anesthesia during the procedures. The Institutional Animal Care and Use Committee approved all procedures.
Rat pups (N = 6, hypoglycemia; N = 2, control) underwent tracheotomy for respiratory assistance using a small animal ventilator (Kent Scientific, Litchfield, CT, USA). An indwelling intraperitoneal catheter (24G Jelco; Smiths Medical, Carlsbad, CA, USA) was placed for administering drugs and secured in place using glue (Loctite® Easy-Squeeze Super Glue Gel; Henkel Corporation, Rocky Hill, CT, USA). The ventilator settings were adjusted based on end tidal CO2 monitored continuously using a capnometer (Model SC-300; BCI International, Waukesha, WI, USA) (Choi et al. 2001). The rectal temperature was maintained at 36.4±1.0 °C using thermostat-regulated warm water circulating in tubes (Cole Palmer, Vernon Hills, IL, USA). Heart rate and respiratory rate were continuously monitored using a MR-compatible monitoring system (Model 1025; SA Instruments, Inc., Stony Brook, NY, USA).
Induction of hypoglycemia
Acute hypoglycemia was induced as previously described (Ennis et al. 2008). Briefly, pups were fasted for 4 hours by separating from the dam. Human regular insulin (Novo Nordisk Inc., Clayton, NC) was injected in a single dose of 6 IU/Kg i.p. The target plasma glucose concentration was <2.5 mmol/l, a value conventionally used to define hypoglycemia in human newborn infants (Cornblath et al. 2000; Burns et al. 2008). Equivalent volume of normal saline was injected to rats in the control group. Plasma glucose concentration was determined in blood samples from the tail before and at the conclusion of NMR spectroscopy in a glucose analyzer (Model GM7 Micro-stat; Analox Instruments, London, UK). The small blood volume of P14 rats and technical difficulties with blood collection inside the magnet precluded frequent plasma glucose measurements in these rats. Therefore, to confirm hypoglycemia during 1H NMR spectroscopy, littermates were subjected to hypoglycemia using the same insulin dose and monitored outside the magnet (N = 6). Plasma glucose was determined every 30 min, and β-hydroxybutyrate concentration was determined at 0 min, 120 min and 240 min (Precision Xtra™; Abbott Laboratories, Oxon, UK) after insulin administration in these rats. Hypoglycemia was terminated 240 min after insulin administration by injecting 0.2 ml of 10% dextrose, a dose that normalizes brain glucose concentration in hypoglycemic newborn rats (Vannucci and Vannucci 1978).
In vivo 1H NMR spectroscopy
1H NMR spectroscopy was performed using a horizontal bore 9.4T/31 cm magnet (Varian/Magnex; Oxford, United Kingdom) equipped with a 15-cm internal diameter gradient coil insert (450 mT/m, 200 μs) and strong second-order shims (XZ = YZ = Z2 = 2000 Hz/cm2, XY = X2Y2 = 1000 Hz/cm2). The magnet was interfaced to a Varian INOVA console (Varian, Inc.; Palo Alto, CA).
In vivo 1H NMR spectra were collected using previously described protocol (Tkac et al. 2003). Briefly, all first- and second-order shims were adjusted using FASTMAP (Gruetter and Tkac 2000). Ultra-short echo-time STEAM (echo time TE = 2 ms, repetition time TR = 5 s) combined with outer volume suppression and VAPOR water suppression (Tkac et al. 1999) was used to acquire spectral data from 8 μL (2.5 × 1.3 × 2.5 mm3) volume of interest (VOI) centered in the left dorsal hippocampus (Tkac et al. 2003; Rao et al. 2007). Multi-slice coronal and sagittal RARE imaging technique (echo train length ETL = 8, echo spacing ESP = 15 ms, TE = 60 ms, matrix = 256 × 128, FOV = 25 mm × 25 mm, slice thickness = 1 mm) was used for selecting the VOI. 1H NMR spectra were acquired sequentially during the entire period of hypoglycemia. Acquiring 160 transients per spectrum with 5 s repetition time resulted in 13.5 min time resolution. The effect of 10% dextrose administration on the neurochemistry was also assessed in two rats subjected to hypoglycemia.
Quantification of metabolites
Metabolite concentrations were quantified using LCModel with macromolecule spectra included in the basis set (Provencher 1993; Tkac et al. 2003). Unsuppressed water signal was used as internal reference. A brain water content of 83% was used in the calculation, based on our previous study (Tkac et al. 2003). The concentrations of the following 17 metabolites were determined: alanine (Ala), ascorbate (Asc), aspartate (Asp), β-hydroxybutyrate (BHB), creatine (Cr), γ-aminobutyric acid (GABA), glucose (Glc), glutamate (Glu), glutamine (Gln), glutathione (GSH), lactate (Lac), myo-inositol (Ins), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphoethanolamine (PE) and taurine (Tau). The sum of glycerophosphocholine (GPC) and phosphocholine (PC) was determined, because of the inability to reliably differentiate the individual compounds due to their spectral similarity. The Glu/Gln and PCr/Cr ratios were calculated. Thus, the neurochemical profile consisted of 18 metabolites and two ratios.
Statistical Analysis
The changes in plasma glucose and blood β hydroxybutyrate were assessed using ANOVA. The effect of hypoglycemia on individual metabolites was determined using ANOVA and linear regression analysis as indicated. Within group differences were established using Bonferroni-adjusted independent t tests. The SPSS program (version-15; SPSS, Chicago, IL) was used. Data are presented as mean±SD. The statistical significance criterion was set at α = 0.05.
Results
Plasma glucose and blood β-hydroxybutyrate concentrations
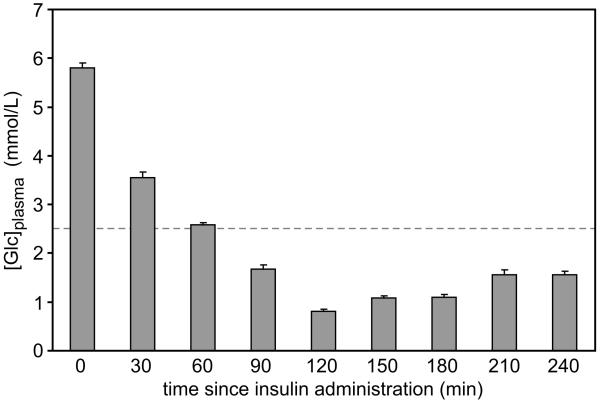
The plasma glucose concentrations were similar in the rats studied in the magnet and the littermates used for bench-top experiments. The target plasma glucose concentration (<2.5 mmol/l) was achieved 60 min after insulin administration and was maintained until dextrose administration at 240 min (Figure 1). The lowest glucose concentration (0.81±0.03 mmol/l) was observed at 120 min (Fig 1). The β-hydroxybutyrate concentration in blood decreased from 1.43±0.01 mmol/l prior to insulin injection (i.e. at time zero) to 0.60±0.02 mmol/l and 0.68±0.03 mmol/l at 120 min and 240 min, respectively (P<0.001, each). The β hydroxybutyrate concentration was 2.3±0.2 mmol/l at the conclusion of 1H NMR spectroscopy in the two rats in the control group.
Figure 1.
Plasma glucose concentration after insulin administration in postnatal day 14 rats. Values are mean ± SEM, N = 6-8 at each time. The dashed line represents 2.5 mmol/l, a value conventionally used to define hypoglycemia in newborn infants.
Neurochemical analysis
Sequential in vivo 1H NMR spectra (12-16 spectra per rat) were obtained, beginning at 44±11 min after insulin administration and continuing until 270±23 min (range: 247-306 min). Data from one rat in the hypoglycemia group was excluded due to the presence of irregular heart rate, persistent hypothermia and elevated lactate, suggesting unstable physiology during the study. Thus, the final analysis included data from 5 hypoglycemic and 2 control rats.
The heart rate (beats per min) increased from 375±15 to 410±14 within 60 min of insulin administration and was maintained at 445±19 thereafter. The heart rate rapidly decreased to 395±19 following 10% dextrose administration. The heart rate did not alter during the entire experimental period in the two control rats.
A representative in vivo 1H NMR spectrum obtained during hypoglycemia and the typical location of the VOI in hippocampus is shown in Figure 2. The water signal linewidth decreased from approximately 9 Hz to 7 Hz within 120 min after insulin administration in all rats. The concentrations of alanine, β-hydroxybutyrate and glucose decreased below detection threshold (0.5 μmol/g) within 120 min after insulin administration (i.e. during the initial 60 min of hypoglycemia, arbitrarily labeled Phase I). Lactate concentration decreased 50% during this period.
Figure 2.
In vivo 1H NMR spectrum measured from the hippocampus of a postnatal day 14 rat. The inset shows the MRI with the volume of interest (VOI) from which the spectra were collected. STEAM, TE = 2 ms, TR = 5 s, number of transients NT = 160. See Materials and Methods for abbreviations.
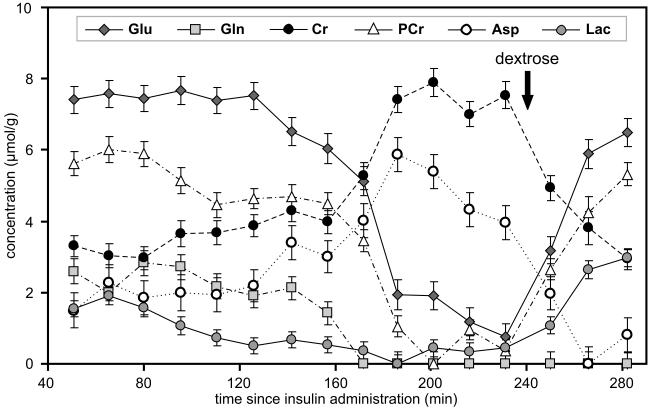
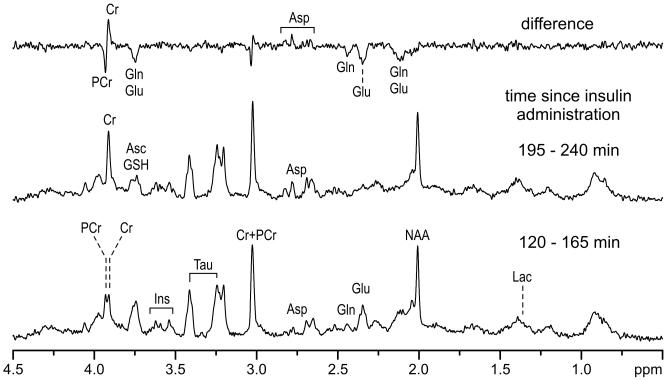
The sequential changes in Cr, PCr, aspartate, glutamate, glutamine and lactate concentrations over time in a rat are shown in Figure 3. During the initial 60 min of hypoglycemia (i.e. Phase I), other than a slight decrease in PCr and a corresponding increase in Cr concentration, the neurochemical profile was unaltered. Between 120 and 160 min after insulin administration (i.e. 60-100 min of hypoglycemia, Phase II), first glutamine and then glutamate concentrations decreased steadily with a corresponding increase in aspartate concentration. The PCr/Cr ratio was maintained between 1.1 and 1.2 during this period. During the subsequent, very brief period (160-180 min after insulin administration, Phase III), a precipitous decrease in PCr with an equally rapid reciprocal increase in Cr was observed that resulted in very low PCr/Cr ratio (≤0.14). The concentration of glutamine decreased below the detection threshold (0.5 μmol/g), glutamate decreased by 75%, and aspartate increased approximately 300% of the corresponding pre-hypoglycemia levels, respectively, during this phase (100-120 min of hypoglycemia). These dramatic changes were clearly visible in the 1H NMR spectra acquired during this period (Figure 4). During the subsequent period (180-240 min after insulin administration, i.e. 120-180 min of hypoglycemia, Phase IV), PCr/Cr ratio remained close to zero with further decrease in glutamate concentration to approximately 10% of pre-hypoglycemia level. Aspartate concentration also began to decrease during this period.
Figure 3.
Changes in the concentrations of creatine (Cr), phosphocreatine (PCr), glutamate (Glu), glutamine (Gln), aspartate (Asp) and lactate (Lac) in the hippocampus during hypoglycemia and following 10% dextrose administration in a postnatal day 14 rat. The arrow indicates the time of 10% dextrose administration.
Figure 4.
In vivo 1H NMR spectra from the hippocampus during early (120-165 min, bottom) and late (195-240 min, middle) phases of hypoglycemia in a postnatal day 14 rat (same as the one shown in Figure 3). The differences between the two spectra are shown in the top. STEAM, TE = 2 ms, TR = 5 s, number of transients NT = 480.
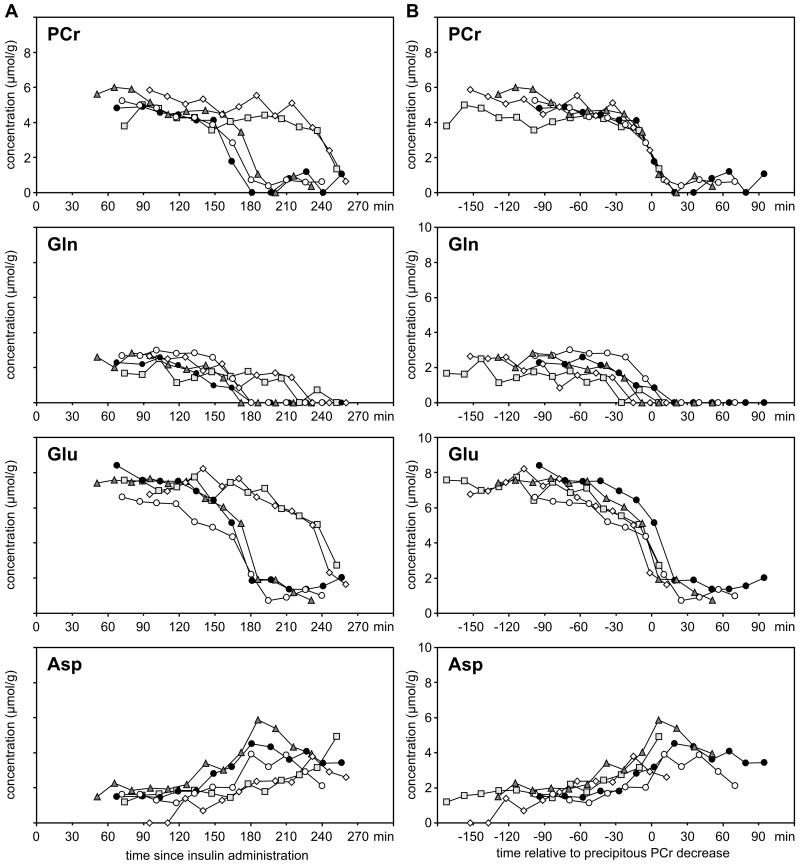
The patterns of neurochemical changes were similar in all 5 hypoglycemic rats (Figure 5). However, there were inter-animal variations in the time-course of PCr and amino acid changes (Figure 5A). Nevertheless, when the trajectories of the neurochemical changes were shifted along the time axis, such that the precipitous phase of PCr decrease in individual rats were aligned with each other, a robust consistency in the pattern of amino acid changes was observed (Figure 5B). There was a strong correlation between PCr/Cr ratio and glutamate+glutamine concentration (R2 = 0.86; P<0.001, Supplemental Figure). The rate of glutamate+glutamine decrease was always greater than the corresponding increase in aspartate concentration (Figure 6). Overall, substantial net consumption of amino acids (glutamate+glutamine+asparatate) occurred during hypoglycemia.
Figure 5.
(A) The time course of changes in phosphocreatine (PCr), glutamine (Gln), glutamate (Glu), aspartate (Asp) concentrations in the hippocampus of individual postnatal day 14 rats during hypoglycemia. (B) The trajectories of the neurochemical changes are shifted along the time axis, such that the precipitous phase of PCr decrease in individual rats is aligned with each other, demonstrating robust consistency in the pattern of amino acid changes among the rats (N = 5).
Figure 6.
Rate of change in the concentration of glutamine (Gln), glutamate (Glu) and aspartate (Asp) in the hippocampus during different phases of hypoglycemia and following 10% dextrose administration in postnatal day 14 rats (N = 5).
In addition to the changes in amino acids, a modest, an approximately linear change in the concentrations of the following neurochemicals were observed during hypoglycemia: ascorbate (rate of decrease, 0.17 μmol g−1 hr−1), GABA (0.10 μmol g−1 hr−1), N-acetylaspartylglutamate (0.14 μmol g−1 hr−1), taurine (0.36 μmol g−1 hr−1) and Cr+PCr (0.26 μmol g−1 hr−1) concentrations decreased (P<0.01, each; Supplemental Table). The concentration of GPC+PC increased (0.05 μmol g−1 hr−1, P = 0.015; Supplemental Table). Myo-inositol, GSH, phosphoethanolamine and NAA concentrations were not altered during hypoglycemia.
Following 10% dextrose administration, the brain glucose concentration increased to 1.63±0.20 and 2.52±0.37 μmol/g in the two hypoglycemic rats. Lactate concentration increased above the pre-hypoglycemia level (2.99±0.69 μmol/g vs. 1.73±0.78 μmol/g). PCr/Cr ratio was fully restored and glutamate concentration was restored to 88% of the corresponding pre-hypoglycemia levels, respectively. However, there was no recovery of glutamine during the 50 min of observation. The substantially decreased total amino acid concentration was only partially restored ([Glu + Gln + Asp]: 5.8±0.5 μmol/g vs. 10.5±0.2 μmol/g during the initial 120 min after insulin administration, P<0.001) following dextrose administration.
In the two control rats, sequential spectra were obtained for 115 min (9 individual blocks) and 269 min (16 individual blocks), starting at 24 min and 39 min after normal saline injection, respectively. Brain glucose concentration was maintained at 2.88±0.18 μmol/g during the entire period of 1H NMR spectroscopy. None of the 18 simultaneously measured metabolites was altered in these rats (data not shown).
Discussion
This study presents the sequence of neurochemical changes resulting from insufficient glucose supply to the developing rat hippocampus. Simultaneous detection of changes in 18 metabolites, enabled by in vivo 1H NMR spectroscopy at 9.4T, revealed sequential phases of hypoglycemia characterized by utilization of various substrates as endogenous fuel supplies. The results demonstrate that the duration of hypoglycemia is a critical factor that determines the neurological sequelae, because the ability to maintain energy homeostasis is dependent on the availability of alternate substrates, whose supplies are limited. Nevertheless, these compensatory changes preserved energy sufficiency for extended periods of time and may explain the relative resistance of the developing hippocampus to injury in this model (Ennis et al. 2008; Rao et al. 2009).
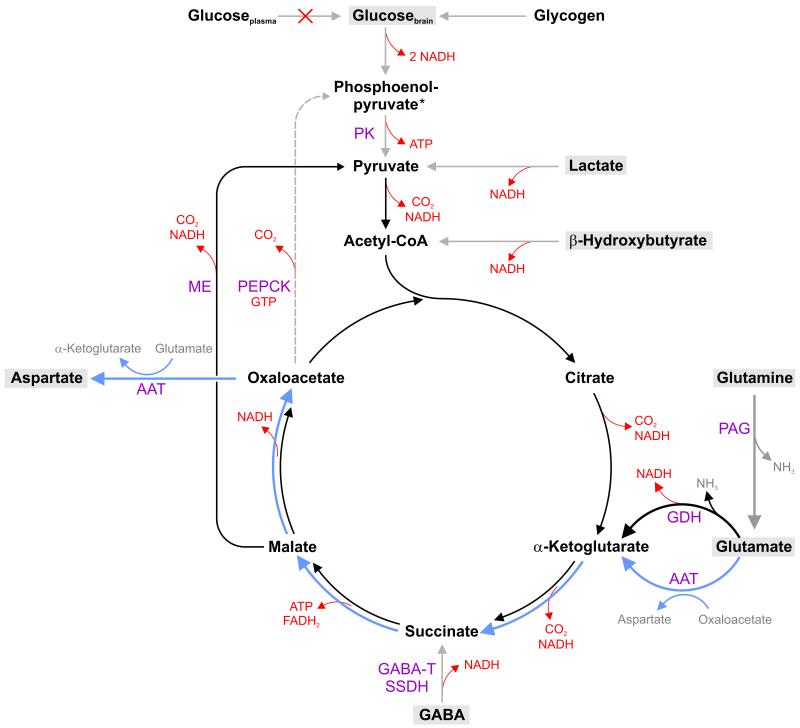
During cerebral glycopenia, CBF is increased to enhance substrate delivery (Mujsce et al. 1989; Choi et al. 2001). Brain glycogen is mobilized to supply glucose for energy production (Vannucci and Vannucci 1978; Choi et al. 2003). When these measures become inadequate, available endogenous substrates replenish TCA cycle intermediates through anaplerotic reactions (Figure 7). This simplified schema also includes information on the generation of ATP, NADH or FADH2 and highlights the reaction steps of the intermediary metabolism contributing to the energy production during neuroglycopenia.
Figure 7.
Simplified schema of metabolic pathways during acute hypoglycemia in the developing hippocampus. Substrates that support energy metabolism via the tricarboxylic acid (TCA) cycle during neuroglycopenia are highlighted. The figure also includes information on the generation of ATP, NADH or FADH2. Once glycogen, lactate and β-hydroxybutyrate are significantly depleted, glutamine and glutamate become the major sources of carbon for supporting TCA cycle. In neurons, glutamine is readily converted by the phosphate-activated glutaminase (PAG) to glutamate, which then enters the TCA cycle. Glutamate generates α-ketoglutarate from two pathways: via aspartate aminotransferase (ATT) pathway (blue) or glutamate dehydrogenase (GDH) pathway (black). The former pathway produces aspartate in a stoichiometric amount. The latter pathway allows complete oxidation of glutamate. However, this reaction requires a continuous supply of pyruvate. The conversion of malate into pyruvate through the malic enzyme (ME) is probably the primary pyruvate recycling pathway. Pyruvate recycling from oxaloacetate via phosphoenolpyruvate carboxykinase (PEPCK) reaction is less likely under the compromised energy status during hypoglycemia. Even though GABA can support energy production by producing succinate via GABA transaminase (GABA-T) and succinate-semialdehyde dehydrogenase (SSDH) reactions, the contribution is likely to be minor.
The glucose concentration in the hippocampus was <0.5 μmol/g within 60 min of the onset of hypoglycemia. Nonetheless, the PCr/Cr ratio was maintained in the physiological range, indicating that energy production was sufficient to meet the energy demands. Decreased glucose demand, resulting from suppressed neuronal activity in the anesthetized immature brain may be partly responsible for the energy balance. Additionally, other neuroprotective changes were initiated. A compensatory increase in CBF could be deduced in the present study from the narrowing of the spectral linewidth soon after the onset of neuroglycopenia. An increased oxyhemoglobin/deoxyhemoglobin ratio, secondary to enhanced oxygen delivery is likely responsible for this finding (Choi et al. 2001). The increased heart rate, mediated by catecholamines also potentially augmented CBF. Ketone bodies, such as β-hydroxybutyrate and lactate could be responsible for energy production during the phase I of hypoglycemia (Hernandez et al. 1980; Nehlig and Pereira de Vasconcelos 1993). The role of β-hydroxybutyrate was likely limited in the present study, since ketogenesis was suppressed following insulin administration. On the other hand, the decreased lactate concentration suggests that it likely contributed to energy production. Lactate uptake and utilization increases during hypoglycemia in the developing brain (Hernandez et al. 1980; Thurston et al. 1983). The shift of the redox state during hypoglycemia is conducive for the efficient oxidation of lactate (Lewis et al. 1974a; Norberg and Siesio 1976). Although changes in brain glycogen were not measured in the present study, a previous study demonstrated that glycogenolysis commences during this period of hypoglycemia in newborn rats (Vannucci and Vannucci 1978).
When these initial compensatory mechanisms were unable to maintain energy homeostasis, cerebral amino acids probably became important energy fuels (Figure 7), similar to the mature brain (Behar et al. 1985; Sutherland et al. 2008), The pyruvate carboxylase reaction, the most important anaplerotic pathway to replenish the TCA cycle intermediates, is unlikely to be functional during prolonged hypoglycemia, secondary to insufficient production of pyruvate. Therefore, as in the mature brain, the “emergency” anaplerotic reactions involving glutamate and glutamine, likely became the primary sources of the carbon skeleton for the TCA cycle during the phase II of hypoglycemia (Tews et al. 1965; Norberg and Siesio 1976; Behar et al. 1985; Erecinska et al. 1988; Yudkoff et al. 1994; Waagepetersen et al. 2005; McKenna 2007; Olstad et al. 2007a; Sutherland et al. 2008).
In neurons, glutamine is readily deaminated by the action of phosphate-activated glutaminase (PAG) to glutamate, which then enters the TCA cycle. Glutamate can generate α-ketoglutarate via two pathways: via aspartate aminotransferase (AAT) reaction and glutamate dehydrogenase (GDH) reaction (Figure 7) (see McKenna 2007 and the references therein). There is 3-5 fold increase in PAG, ATT and GDH activities in the rat hippocampus between birth and P14 (Rothe et al. 1983; Schunzel et al. 1986; Wolf et al. 1988), attesting to its ability to efficiently use glutamine and glutamate for energy production.
Our data indicate that a substantial flux of glutamate to α-ketoglutarate was through the GDH pathway (Figure 7, black arrows), since the increase in aspartate concentration was not proportional to the decrease of glutamate+glutamine. This is dissimilar to the data from adult rats, where a ≥1:1 relationship between aspartate production and glutamate consumption is seen (Lewis et al. 1974b; Yudkoff et al. 1994; McKenna 2007; Sutherland et al. 2008), suggesting that the ATT pathway predominates in the mature brain (Figure 7, blue arrows). While this “truncated” TCA cycle involving the ATT pathway yields the urgently needed energy, the five-carbon skeleton of glutamate is only partially oxidized, producing the four-carbon skeleton amino acid, aspartate. Conversely, the GDH pathway permits complete oxidation of glutamate. However, oxidation of α-ketoglutarate in the TCA cycle cannot continue without a constant supply of the acetyl-CoA. Hence, full oxidation of glutamate, entering the TCA cycle via the GDH pathway, would be possible if pyruvate recycling is active (Olstad et al. 2007b). Cell culture studies have demonstrated that pyruvate recycling is suppressed during severe hypoglycemia, possibly due to lack of high-energy phosphates and reducing equivalents (Bakken et al. 1998b; Bakken et al. 1998a). Therefore, pyruvate recycling from oxaloacetate via the combined actions of phosphoenolpyruvate carboxykinase (PEPCK) and pyruvate kinase (PK) may be suppressed in the present study, since the first step in the reaction requires GTP. However, the conversion of malate into pyruvate through the malic enzyme (ME) reaction is possible, since this reaction does not require any energy. The generated pyruvate reenters the TCA cycle and enables total oxidation of five-carbon skeleton through the complete TCA cycle (Figure 7).
A precipitous decrease in PCr/Cr ratio was observed during the phase III of hypoglycemia. This implies compromised energy metabolism, since PCr concentration below 0.5 μmol/g is associated with decreased ATP production (Vannucci and Vannucci 1978; Behar et al. 1985). The rapid rate of decrease of glutamate and glutamine during this phase suggest that significant alterations in these amino acids are the harbingers of impending energy failure during hypoglycemia. This extreme metabolic deviation from homeostasis was clearly discernible in the 1H NMR spectra obtained during this phase (Figure 4). Observed metabolite changes indicated that glutamate remained the only source feeding the TCA cycle during this phase of hypoglycemia (Figure 7).
During the phase IV of hypoglycemia, when the glutamate and glutamine pools were nearly exhausted, accumulated aspartate became the final source of carbon for energy production (Figure 7). Complete oxidation of aspartate in the TCA cycle is possible only if there is active pyruvate recycling (Olstad et al. 2007b). Depletion of all amino acids is most likely the terminal stage of hypoglycemia, when the rate of ATP production is no longer able to support the minimal energy demands of the brain and leads to neuronal injury (Sutherland et al. 2008).
Additional neuroprotective mechanisms were likely operative during hypoglycemia in the present study. The decreased GABA concentration may suggest its participation in energy production through GABA succinic acid pathway (Lewis et al. 1974b; Tillakaratne et al. 1995) (Figure 7). However, the contribution is likely to be minor, since as α-ketoglutarate produced from glutamate increases, oxidative metabolism supported by GABA decreases (Davis et al. 1970). Furthermore, decreased GABA concentration may be a reflection of decreased availability of its precursor, glutamate, during hypoglycemia (Madl and Royer 2000).
To our knowledge, alterations in ascorbate concentration during hypoglycemia have not been previously described. Due its role as an antioxidant and free radical scavenger (Rice et al. 2002), decreased ascorbate may reflect the presence of oxidative stress during hypoglycemia (McGowan et al. 2006; Suh et al. 2008). An approximately 10% decrease in taurine concentration was observed during hypoglycemia. Taurine is released into the extracellular space, presumably as an osmoregulatory response to brain edema and osmotic changes during hypoglycemia (Gisselsson et al. 1998; Pasantes-Morales et al. 2002; Sandberg et al. 1986; Silverstein et al. 1990). The extracellular release of taurine is considered a feedback mechanism for preventing excessive calcium influx during glutamate- and aspartate-mediated excitotoxicity during hypoglycemia (Sandberg et al. 1986; Silverstein et al. 1990; Chen et al. 2001). The observed small decrease in taurine concentration can be explained by its rapid efflux from the interstitial space of the brain to circulating blood by the efficient taurine transport system at the blood-brain barrier (Lee and Kang 2004). Of note, the effect of hypoglycemia on taurine appears to be age-related, being demonstrated in the developing brain and not in the mature brain (Wong and Tyce 1983; Engelsen et al. 1986; Petroff et al. 1988). The membrane phospholipid precursors, GPC+PC increased, potentially reflecting membrane breakdown or impaired repair during hypoglycemia (Agardh and Siesjo 1981; Sutherland et al. 2008). However, no changes in NAA were observed, suggesting the neuronal/mitochondrial integrity was intact. This is consistent with the previous studies in adult rats (Behar et al. 1985; Sutherland et al. 2008) and corroborates the absence of neuronal injury in the hippocampus during moderate hypoglycemia (Yamada et al. 2004; Tkacs et al. 2005; Ennis et al. 2008; Rao et al. 2009; Haces et al.).
Even though administration of 10% dextrose corrected neuroglycopenia and normalized PCr, the neurochemical profile remained abnormal, similar to the mature brain (Tews et al. 1965; Behar et al. 1985; Sutherland et al. 2008). An accelerated rate of glycolysis or inability to oxidize pyruvate, secondary to impaired pyruvate dehydrogenase activity potentially explains the increased lactate during recovery (Behar et al. 1985; Sutherland et al. 2008). A limited supply of branched chain amino acids, such as leucine may be responsible for the incomplete recovery of glutamate, and suboptimal energy production posthypoglycema may explain the nonrecovery of glutamine, since its synthesis by glutamine synthetase requires ATP (Davis et al. 1970; Sutherland et al. 2008). The altered amino acids may also imply an impaired glutamate-glutamine cycle posthypoglycemia (Sutherland et al. 2008). On a functional level, due to its central role in neurotransmission and synaptogenesis, incomplete recovery of glutamate may adversely impact hippocampal development and function (Yamada et al. 2004).
As we did not monitor the electrical activity of the brain, we are unable to correlate the neurochemical changes with the functional effects of hypoglycemia. However, previous studies in developing animals have demonstrated that hypoglycemia of comparable severity is not associated with EEG isoelectricity or convulsions (Petroff et al. 1988; Yamada et al. 2004). This limitation of our study also precludes direct comparison with previous studies in adult rats, which typically have induced a more severe hypoglycemia (plasma glucose <1.0 mmol/l) that is associated with EEG isoelectricity (Lewis et al. 1974b; Agardh et al. 1978; Butterworth et al. 1982; Behar et al. 1985; Sutherland et al. 2008). These studies have demonstrated that energy failure and significant depletion of amino acids, as observed during the phase III in the present study, occurs only after the onset of isoelectric EEG traces or convulsions. We are unaware of previous in vivo studies that monitored the sequential neurochemical changes in the hippocampus during hypoglycemia of comparable severity and duration in adult rats. A previous in vivo 13C and 1H NMR spectroscopy study of brain glycogen mobilization during moderate hypoglycemia (plasma glucose <2 mmol/l for 2 hr) reported minor changes in energy markers and amino acids in the VOI encompassing the cerebral cortex, hippocampus and striatum regions in adult rats (Choi et al. 2003). Collectively, these results suggest that the neurochemical changes may differ in the developing and mature brains during hypoglycemia. Future studies of hypoglycemia of equivalent severity and duration are necessary to confirm this hypothesis and to establish its role in the age- and region-specific vulnerability to hypoglycemia-induced injury (Tkacs et al. 2005; Ennis et al. 2008; Rao et al. 2009; Haces et al. 2010).
The results of the study may have clinical implications for human infants at risk of hypoglycemia. Currently, the management of hypoglycemia is based on plasma glucose measurements (Cornblath et al. 2000). The study demonstrates the poor relationship between plasma glucose and cerebral energy metabolism during hypoglycemia. The prediction of CNS injury based solely on plasma glucose becomes problematic under these circumstances. The sensitivity of the NMR method also attests to its potential for monitoring the effects of hypoglycemia in human infants and children.
In summary, acute hypoglycemia was associated with compensatory neurochemical changes in the developing rat hippocampus. These changes correlated with the duration of hypoglycemia and may represent compensatory mechanisms for preserving energy homeostasis and preventing neuronal injury. While these alterations appear to be beneficial in the short-term, their long-term impact on hippocampal structure and function has yet to be established. A thorough understanding of the effect of hypoglycemia on regional neurochemistry would aid in the development of optimum preventive and therapeutic strategies for at-risk human infants and children.
Supplementary Material
Acknowledgments
The assistance of Dee Koski and Christopher Nelson in animal preparation is gratefully acknowledged. Supported in part by grants from the National Institutes of Health (K08 HD47276, P41 RR008079, P30 NS057091), University Pediatrics Foundation, Viking Children’s Fund and the Graduate School, University of Minnesota. The 9.4T magnet is funded in part by the W. M. Keck Foundation.
Abbreviations
- Ala
alanine
- Asc
ascorbate
- Asp
aspartate
- AAT
aspartate aminotransferase
- BHB
β-hydroxybutyrate
- CBF
cerebral blood flow
- Cr
creatine
- GPC
glycerophosphocholine
- Glc
glucose
- Gln
glutamine
- Glu
glutamate
- GDH
glutamate dehydrogenase
- Lac
lactate
- ME
malic enzyme
- Ins
myo-inositol
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- PAG
phosphate-activated glutaminase
- PC
phosphocholine
- PCr
phosphocreatine
- PE
phosphoethanolamine
- PEPCK
phosphoenolpyruvate carboxykinase
- P
postnatal day
- PK
pyruvate kinase
- TCA cycle
tricarboxylic acid cycle
- Tau
taurine
- VOI
volume of interest.
Footnotes
Disclosure: Authors do not have conflicts of interest.
References
- Agardh CD, Siesjo BK. Hypoglycemic brain injury: phospholipids, free fatty acids, and cyclic nucleotides in the cerebellum of the rat after 30 and 60 minutes of severe insulin-induced hypoglycemia. J Cereb Blood Flow Metab. 1981;1:267–275. doi: 10.1038/jcbfm.1981.31. [DOI] [PubMed] [Google Scholar]
- Agardh CD, Folbergrova J, Siesjo BK. Cerebral metabolic changes in profound, insulin-induced hypoglycemia, and in the recovery period following glucose administration. J Neurochem. 1978;31:1135–1142. doi: 10.1111/j.1471-4159.1978.tb06236.x. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Milner RD, Strich SJ. Effects of neonatal hypoglycaemia on the nervous system: a pathological study. J Neurol Neurosurg Psychiatry. 1967;30:295–310. doi: 10.1136/jnnp.30.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken IJ, White LR, Unsgard G, Aasly J, Sonnewald U. [U-13C]glutamate metabolism in astrocytes during hypoglycemia and hypoxia. J Neurosci Res. 1998a;51:636–645. doi: 10.1002/(SICI)1097-4547(19980301)51:5<636::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bakken IJ, White LR, Aasly J, Unsgard G, Sonnewald U. [U-13C] aspartate metabolism in cultured cortical astrocytes and cerebellar granule neurons studied by NMR spectroscopy. Glia. 1998b;23:271–277. doi: 10.1002/(sici)1098-1136(199807)23:3<271::aid-glia9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Behar KL, den Hollander JA, Petroff OA, Hetherington HP, Prichard JW, Shulman RG. Effect of hypoglycemic encephalopathy upon amino acids, high-energy phosphates, and pHi in the rat brain in vivo: detection by sequential 1H and 31P NMR spectroscopy. J Neurochem. 1985;44:1045–1055. doi: 10.1111/j.1471-4159.1985.tb08723.x. [DOI] [PubMed] [Google Scholar]
- Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122:65–74. doi: 10.1542/peds.2007-2822. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Merkel AD, Landreville F. Regional amino acid distribution in relation to function in insulin hypoglycaemia. J Neurochem. 1982;38:1483–1489. doi: 10.1111/j.1471-4159.1982.tb07929.x. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY. Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J Neurosci Res. 2001;66:612–619. doi: 10.1002/jnr.10027. [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, Kalhan SC. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141–1145. doi: 10.1542/peds.105.5.1141. [DOI] [PubMed] [Google Scholar]
- Davis JM, Himwich WA, Pederson VC. Hypoglycemia and developmental changes in free amino acids of rat brain. J Appl Physiol. 1970;29:219–222. doi: 10.1152/jappl.1970.29.2.219. [DOI] [PubMed] [Google Scholar]
- Engelsen B, Westerberg E, Fonnum F, Wieloch T. Effect of insulin-induced hypoglycemia on the concentrations of glutamate and related amino acids and energy metabolites in the intact and decorticated rat neostriatum. J Neurochem. 1986;47:1634–1641. doi: 10.1111/j.1471-4159.1986.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Ennis K, Tran PV, Seaquist ER, Rao R. Postnatal age influences hypoglycemia-induced neuronal injury in the rat brain. Brain Res. 2008;1224:119–126. doi: 10.1016/j.brainres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Zaleska MM, Nissim I, Nelson D, Dagani F, Yudkoff M. Glucose and synaptosomal glutamate metabolism: studies with [15N]glutamate. J Neurochem. 1988;51:892–902. doi: 10.1111/j.1471-4159.1988.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Gisselsson L, Smith ML, Siesjo BK. Influence of hypoglycemic coma on brain water and osmolality. Exp Brain Res. 1998;120:461–469. doi: 10.1007/s002210050419. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Haces ML, Montiel T, Massieu L. Selective vulnerability of brain regions to oxidative stress in a non-coma model of insulin-induced hypoglycemia. Neuroscience. 2010;165:28–38. doi: 10.1016/j.neuroscience.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Hernandez MJ, Vannucci RC, Salcedo A, Brennan RW. Cerebral blood flow and metabolism during hypoglycemia in newborn dogs. J Neurochem. 1980;35:622–628. doi: 10.1111/j.1471-4159.1980.tb03701.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Yu ZX, Fredholm BB, Rivkees SA. Susceptibility of the developing brain to acute hypoglycemia involving A1 adenosine receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E562–569. doi: 10.1152/ajpendo.00112.2005. [DOI] [PubMed] [Google Scholar]
- Lee NY, Kang YS. The brain-to-blood efflux transport of taurine and changes in the blood-brain barrier transport system by tumor necrosis factor-alpha. Brain Res. 2004;1023:141–147. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Ljunggren B, Norberg K, Siesjo B. Changes in carbohydrate substrates, amino acids and ammonia in the brain during insulin-induced hypoglycemia. J Neurochem. 1974a;23:659–671. doi: 10.1111/j.1471-4159.1974.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Ljunggren B, Norberg K, Siesjo BK. Changes in carbohydrate substrates, amino acids and ammonia in the brain during insulin-induced hypoglycemia. J Neurochem. 1974b;23:659–671. doi: 10.1111/j.1471-4159.1974.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Madl JE, Royer SM. Glutamate dependence of GABA levels in neurons of hypoxic and hypoglycemic rat hippocampal slices. Neuroscience. 2000;96:657–664. doi: 10.1016/s0306-4522(99)00548-5. [DOI] [PubMed] [Google Scholar]
- McGowan JE, Perlman JM. Glucose management during and after intensive delivery room resuscitation. Clin Perinatol. 2006;33:183–196. x. doi: 10.1016/j.clp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006;399:111–114. doi: 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Mujsce DJ, Christensen MA, Vannucci RC. Regional cerebral blood flow and glucose utilization during hypoglycemia in newborn dogs. Am J Physiol. 1989;256:H1659–1666. doi: 10.1152/ajpheart.1989.256.6.H1659. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A. Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol. 1993;40:163–221. doi: 10.1016/0301-0082(93)90022-k. [DOI] [PubMed] [Google Scholar]
- Norberg K, Siesio BK. Oxidative metabolism of the cerebral cortex of the rat in severe insulin-induced hypoglycaemia. J Neurochem. 1976;26:345–352. doi: 10.1111/j.1471-4159.1976.tb04487.x. [DOI] [PubMed] [Google Scholar]
- Olstad E, Qu H, Sonnewald U. Glutamate is preferred over glutamine for intermediary metabolism in cultured cerebellar neurons. J Cereb Blood Flow Metab. 2007a;27:811–820. doi: 10.1038/sj.jcbfm.9600400. [DOI] [PubMed] [Google Scholar]
- Olstad E, Olsen GM, Qu H, Sonnewald U. Pyruvate recycling in cultured neurons from cerebellum. J Neurosci Res. 2007b;85:3318–3325. doi: 10.1002/jnr.21208. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ochoa L, Ordaz B. Osmosensitive release of neurotransmitter amino acids: relevance and mechanisms. Neurochem Res. 2002;27:59–65. doi: 10.1023/a:1014850505400. [DOI] [PubMed] [Google Scholar]
- Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Young RS, Cowan BE, Novotny EJ., Jr. 1H nuclear magnetic resonance spectroscopy study of neonatal hypoglycemia. Pediatr Neurol. 1988;4:31–34. doi: 10.1016/0887-8994(88)90021-5. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rao R, Sperr D, Ennis K, Tran P. Postnatal Age Influences Hypoglycemia-induced Poly(ADP-ribose) Polymerase-1 Activation in the Brain Regions of Rats. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181bbce69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:729–740. doi: 10.1038/sj.jcbfm.9600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Forman RE, Chen BT, Avshalumov MV, Cragg SJ, Drew KL. Brain antioxidant regulation in mammals and anoxia-tolerant reptiles: balanced for neuroprotection and neuromodulation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:515–525. doi: 10.1016/s1532-0456(02)00116-3. [DOI] [PubMed] [Google Scholar]
- Rothe F, Schmidt W, Wolf G. Postnatal changes in the activity of glutamate dehydrogenase and aspartate aminotransferase in the rat nervous system with special reference to the glutamate transmitter metabolism. Brain Res. 1983;313:67–74. doi: 10.1016/0165-3806(83)90202-x. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Butcher SP, Hagberg H. Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: in vivo dialysis of the rat hippocampus. J Neurochem. 1986;47:178–184. doi: 10.1111/j.1471-4159.1986.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Schunzel G, Wolf G, Rothe F, Seidler E. Histophotometric evaluation of glutamate dehydrogenase activity of the rat hippocampal formation during postnatal development, with special reference to the glutamate transmitter metabolism. Cell Mol Neurobiol. 1986;6:31–42. doi: 10.1007/BF00742974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein FS, Simpson J, Gordon KE. Hypoglycemia alters striatal amino acid efflux in perinatal rats: an in vivo microdialysis study. Ann Neurol. 1990;28:516–521. doi: 10.1002/ana.410280408. [DOI] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Tyson RL, Auer RN. Truncation of the krebs cycle during hypoglycemic coma. Med Chem. 2008;4:379–385. doi: 10.2174/157340608784872235. [DOI] [PubMed] [Google Scholar]
- Tews JK, Carter SH, Stone WE. Chemical changes in the brain during insulin hypoglycaemia and recovery. J Neurochem. 1965;12:679–693. doi: 10.1111/j.1471-4159.1965.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Hauhart RE, Schiro JA. Lactate reverses insulin-induced hypoglycemic stupor in suckling-weanling mice: biochemical correlates in blood, liver, and brain. J Cereb Blood Flow Metab. 1983;3:498–506. doi: 10.1038/jcbfm.1983.77. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. gamma-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247–263. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of the rat brain at 1ms echo time. Magnetic Resonance in Medicine. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Tkacs NC, Pan Y, Raghupathi R, Dunn-Meynell AA, Levin BE. Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. J Cereb Blood Flow Metab. 2005;25:1645–1655. doi: 10.1038/sj.jcbfm.9600152. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Cerebral carbohydrate metabolism during hypoglycemia and anoxia in newborn rats. Ann Neurol. 1978;4:73–79. doi: 10.1002/ana.410040114. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Hypoglycemic brain injury. Semin Neonatol. 2001;6:147–155. doi: 10.1053/siny.2001.0044. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Sonnewald U, Shimamoto K, Schousboe A. Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons. Neurochem Int. 2005;47:92–102. doi: 10.1016/j.neuint.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wolf G, Richter K, Schunzel G, Schopp W. Histochemically demonstrable activity of phosphate-activated glutaminase in the postnatally developing rat hippocampus. Brain Res. 1988;469:101–108. doi: 10.1016/0165-3806(88)90173-3. [DOI] [PubMed] [Google Scholar]
- Wong KL, Tyce GM. Glucose and amino acid metabolism in rat brain during sustained hypoglycemia. Neurochem Res. 1983;8:401–415. doi: 10.1007/BF00965097. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.