Abstract
The streptococcal collagen-like protein-1, Scl1, is widely expressed by the wellrecognized human pathogen group A Streptococcus(GAS). Screening of human ligands for binding to recombinant Scl1 identified cellular fibronectin and laminin as binding partners. Both ligands interacted with the globular domain of Scl1, which is also able to bind the low-density lipoprotein. Native Scl1 mediated GAS adherence to ligand-coated glass cover slips and promoted GAS internalization into HEp-2 cells. This work identifies new ligands of the Scl1 protein that are known to be important in GAS pathogenesis and suggests a novel ligandswitching mechanism between blood and tissue environments, thereby facilitating host colonization and GAS dissemination.
Keywords: Scl1, cellular fibronectin, laminin, GAS internalization
Introduction
Group A streptococci (GAS) typically colonize the human throat and skin causing superficial infections, such as pharyngitis and impetigo respectively. However, GAS infections may also lead to invasive diseases including necrotizing fasciitis and streptococcal toxic shock syndrome or may result in the postinfectious autoimmune sequelae acute rheumatic fever and acute glomerulonephritis (Cunningham 2000). Host colonization is accomplished through interactions between GAS cell-surface adhesins and host cellular receptors or extracellular matrix components (ECM). Depending on the strain, GAS may express multiple surface proteins, including the streptococcal collagen-like proteins Scl1 (Lukomski et al. 2000; Rasmussen et al., 2000) and Scl2 (Lukomski et al., 2001; Rasmussen and Bjorck 2001; Whatmore 2001).
Structurally, Scl1 and Scl2 proteins contain a signature central collagenlike (CL) region, which is composed of a repeating Gly-Xaa-Yaa sequence capable of adopting a stable triple helical structure similar to mammalian collagens (Xu et al., 2002). This structural mimicry by the CL region has been shown to support direct interactions with collagen-binding integrins (Humtsoe et al. 2005) and mediate GAS adherence to and internalization by human cells (Caswell et al., 2007).
The amino-terminal part of the Scl proteins, termed the variable (V) region, forms a globular domain which is protruded away from the GAS-cell surface by the CL region (Xu et al. 2002). The V-region sequences vary significantly between Scl1 and Scl2. In addition, the V-region sequence of each Scl protein is conserved in strains of the same M-type but differs considerably among Scls from strains of different M-types. Despite the observed sequence variation, two main ligands have been identified that bind different Scl1 variants via their Vregions. The Scl1.6 and Scl1.55 proteins of M6- and M55-type GAS respectively, bind human plasma glycoproteins factor H and the factor H-related protein 1 (Caswell et al. 2008). On the contrary, several other Scl1 variants bind the lowdensity lipoprotein (LDL) including Scl1 proteins of the M1-, M2-, M12-, M28-, and M41-type GAS (Han et al. 2006). The latter Scl1.41 protein also binds integrins α2β1 and α11β1 via direct interaction with the CL region (Caswell et al., 2008). This suggests specialization in ligand binding among Scl1 proteins and their importance as pathogenicity traits.
The binding of the extracellular matrix (ECM) components by pathogens is known to be a common strategy used to establish host colonization. Several GAS cell-surface molecules have been reported to initiate this interaction including several M proteins, F1/SfbI, F2, SOF, SfbII, Lbp and Shr(Hanski et al. 1992; Kreikemeyer et al., 1995; Jaffe et al., 1996; Molinari and Chhatwal 1998; Courtney et al,. 1999; Terao, et al., 2002; Fisher et al., 2008). Thus, in this work, we hypothesized that Scl proteins possess binding capacities to ECM components that, in turn, would facilitate bacterial adhesion to human extracellular matrix and internalization by host cells. It is known that Scl1 is expressed by virtually all GAS strains (Lukomski et al., 2000; Rasmussen et al. 2000); therefore, this work further supports the role of Scl1 protein as an important accessory, multifunctional surface adhesin of GAS.
Materials and methods
GAS strains and growth conditions
The M41-type MGAS 6183 wild-type and the scl1 mutant strain were used. The isogenic scl1mutant of MGAS 6183 was constructed by allelic replacement as described previously (Caswell et al., 2007). To prepare GAS cells for experiments, cultures were grown overnight on brain–heart infusion agar (BD Biosciences, Sparks, MD) at 37°C in an atmosphere of 5% CO2-20% O2. Overnight cultures were used to inoculate Todd-Hewitt broth (BD Biosciences) supplemented with 0.2% yeast extract and the cultures were incubated at 37°C until they reached logarithmic phase of growth (OD600 ~0.5). The bacteria were collected by centrifugation, washed and then suspended in phosphate-buffered saline (PBS) at the desired cell concentration, which was verified by plating on tryptic soy agar containing 5% sheep’s blood (Remel, Lenexa, KS).
Enzyme-linked immunosorbent assays (ELISA)
Recombinant Scl (rScl) proteins used in ELISA were expressed in E. coli and purified by affinity chromatography using the Strep-tag II system (IBA-GmbH, Goettingen, Germany) as described previously (Xu et al., 2002, Zwiefka et al., 2006). Briefly, the DNA fragments of several scl1 and scl2 alleles, encoding the extracellular portions of the Scl1 and Scl2 proteins, were amplified by polymerase chain reaction with Deep Vent Taq Polymerase (New England Biolabs, Beverly, MA) and cloned into the pASK-IBA2 vector designed for periplasmic expression.
Recombinant Scl proteins (0.5 µM) were immobilized onto Strep-Tactin-coated microplate wells for 1.5 hours at room temperature. Following overnight blocking with Tris-buffered saline (TBS) supplemented with 1% bovine serum albumin (BSA) at 4°C, 1 µg of each ligand that included plasma fibronectin (pFn), cellular fibronectin (cFn), laminin (Lm), bovine collagen types I and IV, decorin, heparin, and fibrinogen (all proteins were purchased from Sigma) was added to triplicate wells and the mixture was incubated at room temperature for 1 hour. rScl-bound ligands were detected with specific primary antibodies and appropriate secondary antibodies conjugated to horseradish peroxidase (HRP). The HRP reaction was developed with 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate and recorded at OD415 after 15 minutes of color development.
In the ligand competition experiments, purified cFn and Lm were used in a molar ratio 1:1. First, the primary ligands e.g., cFn or Lm, were added to triplicate wells immobilized with P176 and incubated for 1 h at room temperature. Following washes with TBS, secondary ligands were added to the appropriate wells e.g., Lm was added to wells containing Scl1-cFn complex and vice versa; samples were incubated for 1 h at room temperature. Subsequently, the ELISA proceeded as described above.
GAS whole-cell attachment assay to ECM proteins
To generate GFP-expressing GAS cells, the wild-type strain, the scl1-inactivated mutant, and mutant complemented in-trans for Scl1.41-protein expression (plasmid pSL230) (Caswell et al., 2007) were transformed with the plasmid pSB027 (Cramer et al., 2003). Glass cover slips were placed in the wells of 24-well tissue culture plates and coated with 2.5 µg of purified ECM proteins or BSA overnight at 4°C, and subsequently blocked with 1% BSA in TBS for 1 h. Approximately 1×107 CFU of fluorescent GAS cells were added to each well for 1 h at room temperature and unbound cells were removed by washing with PBS. ECM-bound GAS cells were fixed with 3% para-formaldehyde in PBS for 30 min. The cover slips were removed from the wells, air-dried, placed on microscope slides, and viewed by fluorescent microscopy using a 450–490 nm excitation channel at 400× and 1000× magnification. For quantification, GAS cells were counted in 10 random fields under 1000× magnification.
GAS internalization assays
An antibiotic protection assay was employed for GAS internalization as described previously (Caswell et al. 2007) with some modifications. Briefly, human HEp-2 cells were grown in 24-well tissue culture plates until semi-confluent. All co-culture experiments were performed in serum-free and ECM-free Delbeco’s Modified Eagle Medium (DMEM). For ECM treatment, 10 ml of 1×107 CFU/ml of each prepared GAS strain was pre-incubated with 15 µg of purified cFn or Lm for 1 hour at room temperature on an end-over-end rotator. Subsequently, ~1×106 CFU of ECM-treated or ECM-untreated wild type or scl1-inactivated mutant GAS were co-cultured with the HEp-2 cells (MOI 1:100) for 2 hours at 37°C. Cell layers were washed with PBS, and culture medium containing 100 µg·ml−1 gentamicin and 5 µg·ml−1 penicillin G was added to each well to kill extracellular bacteria. After 2 hours, the medium was removed and the cells were washed with PBS. To determine the level of GAS internalization, the epithelial cells were lysed in distilled water and serial dilutions were plated onto blood agar. The internalization level of ECM-untreated wild-type strain was considered 100%.
Statistical analysis
Statistical significance was determined using a two-tailed paired Student’s t-test. The results were considered statistically significant with P< 0.05(*), P< 0.01(**), and P< 0.001(***).
Results and discussion
M41-serotype strains of GAS have emerged as a major cause of streptococcal impetigo during 1950s and 1960s (Anthony 2000). They were isolated from skin infections in several geographical locations, including Minnesota (Top et al., 1967), Alabama (Dillon and Wannamaker 1971), and Trinidad (Dillon et al., 1974) with frequencies of 12–14% of all cases. The M41-type isolates were also reported in a recent GAS surveillance study of patients with invasive infections in the United States (O'Loughlin et al., 2007). Strain MGAS 6183 used here was cultured from a leg abscess during the epidemics of invasive GAS infections in Texas.
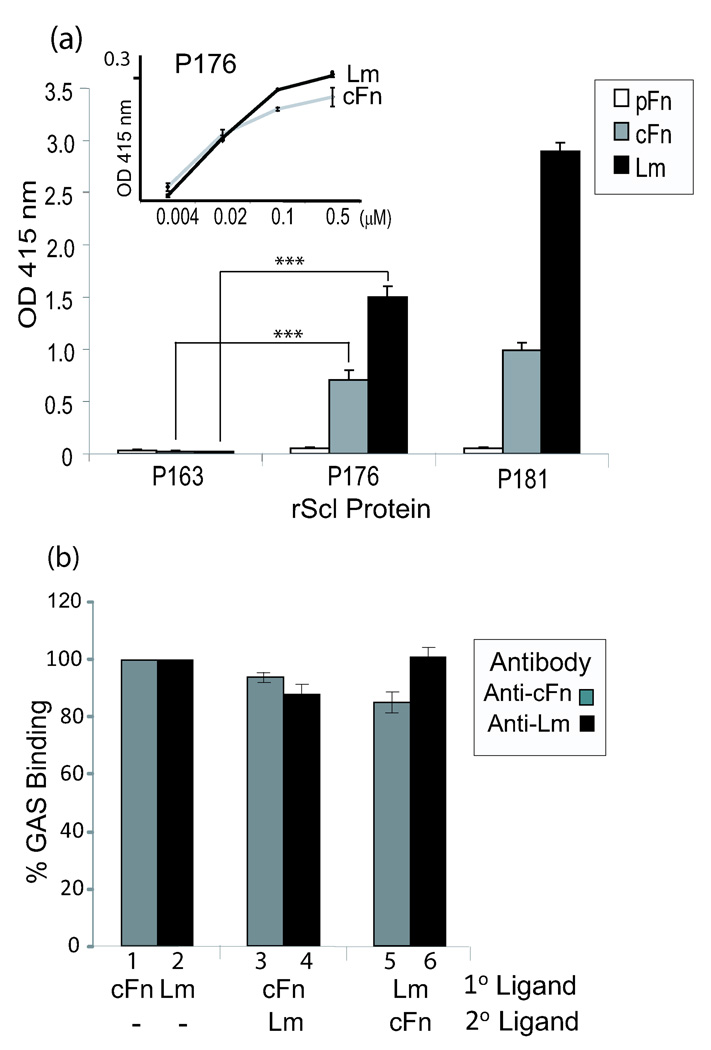
We have previously reported that the recombinant Scl1.41 protein, designated P176, bound human collagen receptors via its collagen-like (CL) region and LDL via the variable (V) region (Han et al., 2006; Caswell et al., 2008). Here, we evaluated the binding of an array of potential human ligands, including several ECM proteins, to the recombinant P176 by ELISA (Fig. 1a). We also used recombinant construct P163, derived from Scl2 protein of M28-type GAS, for which no ligands have been identified to date. None of the ligands tested here bound to the recombinant protein P163. No significant binding to P176 was detected for fibrinogen, decorin, heparin, and collagens I and IV (data not shown). Remarkably, P176 bound cellular fibronectin (cFn) but not plasma fibronectin (pFn). The observation that Scl1 binds to cFn but not pFn is novel and very intriguing. Various forms of Fn are products of alternatively spliced mRNA transcript of a single gene containing about 50 exons (Alberts et al. 1994). The pFn form is predominantly produced by hepatocytes and circulates in plasma as a covalently linked dimer. More heterogeneous cFn is produced by many cell types and forms large aggregated structures known as fibronectin fibrils. The cFn comprises a large group of isoforms produced from splicing events that may or may not include the type III repeats called extra domains, EDA and EDB, lacking in pFn (Pankov et al., 2002). It is currently not known whether the presence of the extra domains or supra structural organization is responsible for the selective binding of cFn to Scl1 protein.
Fig. 1.
Characterization of ECM binding to recombinant Scl1.41 protein. (a) Recombinant Scl1.41 protein (P176) binds cellular fibronectin and laminin. Recombinant proteins P176, P163, and P181 containing portions of P176 and P163 were tested by ELISA for binding to purified plasma fibronectin (pFn), cellular fibronectin (cFn), or laminin (Lm). Graph bars represent the mean OD415±SD of triplicate wells. (Inset): Concentration-dependent binding of cFn and Lm to P176. (b) Competition between cFN and Lm for binding to P176. Immobilized recombinant protein P176 was pre-incubated with either cFN prior to incubation with Lm and vice versa. Scl1-ECM complexes were immunoreacted with anti-cFn and anti-Lm Abs. Immunoreactivity of the same amounts of P176-cFn and P176- Lm were considered as 100% binding. Data is from a single experiment that is representative of three independent experiments.
In addition to cFn, P176 also bound laminin (Lm). The cFn and Lm binding to P176 was concentration-dependent indicating binding specificity (Fig. 1a, inset). The laminins comprise a family of A, B1 and B2 heterotrimeric glycoproteins that are constituents of basal lamina and are found in virtually all human tissues (Alberts et al., 1994). Various isoforms of laminin exist that are associated with characteristic tissue distribution. Early studies by Switalski et al. described GAS binding to Lm, although the GAS product responsible for this binding was not identified (Switalski et al., 1984). Terao et al. identified a GAS Lm-binding protein, designated Lbp (Terao et al., 2002), which was recently characterized as primarily a zinc-binding protein with capacity to bind Lm (Linke et al. 2009). GAS interactions with Lm were also attributed to another streptococcal protein Shr that primarily binds human plasma hemoproteins (Fisher et al., 2008). Thus, unrelated surface proteins of GAS possess binding capacities towards extracellular matrix components Fn and Lm.
Since both cFn and Lm contain the collagen-binding domains (Alberts, et al., 1994), we could not exclude a possibility that the CL region of Scl1 was responsible for ECM binding. Therefore, we constructed a chimeric recombinant protein by domain swapping consisting of the V-region of P176 and the CL-region of the ECM-binding-negative protein P163. The resulting construct P181 bound cFn and Lm indicating that ECM-binding is mediated by the P176-V region (Fig. 1a). We next devised a competition assay to investigate whether cFn and Lm binding is localized to the same site within P176 V-region (Fig. 1b). First, immobilized P176 was incubated with one of the primary ECM ligands, cFn or Lm, and then incubated with an alternate secondary ECM ligand. Sets of triplicate wells were immunoreacted with antibodies specific for both ECM ligands to assess the presence of cFn and Lm attached to P176. Immunoreactivity of the same amounts of P176-cFn and P176-Lm were considered as 100% binding (Fig. 1b; bars 1–2). Pre-incubation of P176 with cFn did not prevent Lm binding (bar 4) nor Lm displaced the cFn from P176 (bar 3). Likewise, pre-incubation with Lm did not prevent cFn binding to P176 (bar 5) nor cFn was able to displace the Lm from P176 (bar 6). Our data suggest that under these experimental conditions, the cFn and Lm did not compete for binding to P176.
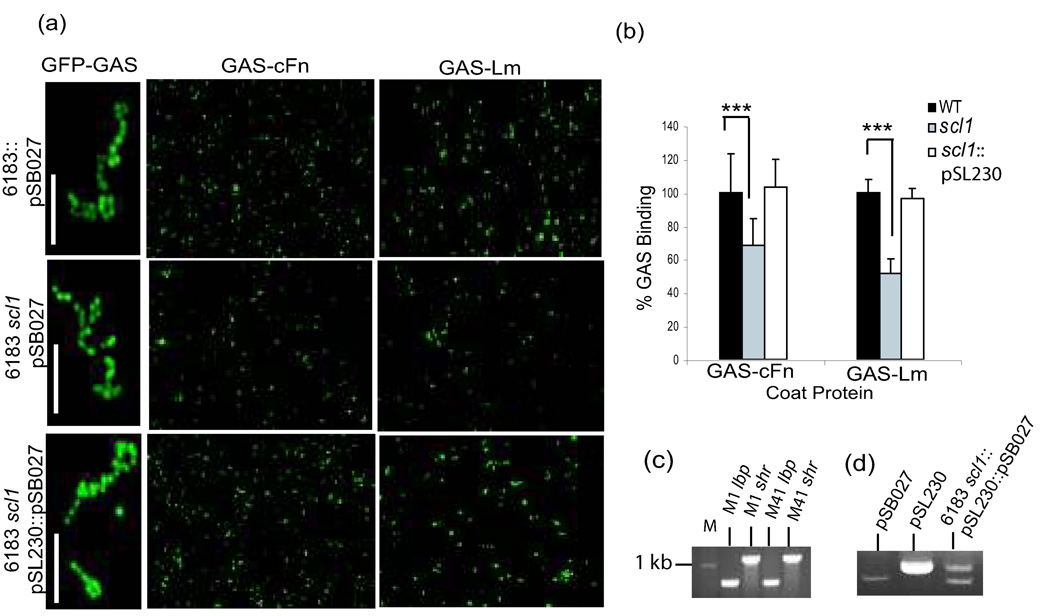
Binding between rScl1.41 construct P176 and ECM proteins may not translate into binding between GAS-expressed Scl1.41 protein and ECM components. Therefore, a whole-cell binding assay (Fig. 2) was carried out using the wild-type MGAS 6183 strain, the scl1-inactivated isogenic mutant, and the mutant complemented with plasmid pSL230 expressing in-trans the Scl1.41 protein (Caswell et al., 2007). All three strains were first transformed with the plasmid pSB027 to generate GFP-expressing cells (Fig. 2a, images at left). The stability of two plasmids pSL230 and pSB027 within the complemented mutant strain was confirmed by isolating total DNA from these cells (Fig. 2d). Fluorescent GAS strains were next tested for binding to ECM-coated glass cover slips (Fig. 2a, images at middle and right columns). More fluorescent wild-type cells were seen attached to the cover slips coated with cFn and Lm, as compared with scl1 mutant GAS. Furthermore, complementation of the scl1 mutant with pSL230 considerably increased cell binding to both ECMs. Quantitative analysis by counting the numbers of GAS cells in random fields fully supported visual observations (Fig. 2b). The scl1-inactivated mutant bound 30% and 45% less to cFn and Lm, respectively, compared to the wild-type strain. Importantly, the complementation of mutant for Scl1.41 expression restored the wild-type levels of binding to both cFn and Lm, indicating that this phenotype was due to the lack of Scl1 expression. Residual cFn binding by the Scl1 mutant could be attributed to the presence of the prtf2 gene in this strain (Caswell et al., 2007) encoding an additional Fn-binding protein, F2 (Jaffe et al., 1996). Similarly, the observed binding of the Scl1-deficient mutant to Lm could be attributed to Lbp and Shr expression; however, the M41-type GAS was not included in the studies that characterized these ECM-binding proteins (Terao et al., 2002; Fisher, et al., 2008). Since lbp and shr genes are conserved among GAS strains of various M types, we used PCR to demonstrate the presence of both genes in M41-type strain MGAS 6183 (Fig. 2c). Altogether, our results demonstrate that Scl1.41 protein is an important surface adhesin that selectively binds to human cellular fibronectin and laminin and significantly contributes to ECM-GAS interactions.
Fig. 2.
Scl1.41-mediated binding of GAS cells to ECM proteins. (a) Attachment of GAS cells expressing GFP marker on pSB027 to ECM-coated cover slips. Bound wild-type strain (6183::pSB027), the scl1-inactivated mutant (6183 scl1::pSB027), and the scl1 mutant complemented with plasmid pSL230 to express Scl1.41 (6183 scl1::pSL230::pSB027) were examined under the fluorescent microscope at 400× and 1000× magnification. Scale bars represent 10µm. (b) Quantification of ECM-bound GAS. GAS cells attached to ECM-coated cover slips were counted at 1000× magnification. Binding of the wild-type GAS was considered 100%. Statistical significance was determined at a level of P< 0.001 (***). (c) Presence of the lbp and shr genes in M1- and M41-type GAS. Genomic DNA from MGAS 5005 (M1) and MGAS 6183 (M41) were used as templates for PCR using primer sets: lbpFor (ACCGTCTGTAAATGATGTGG) and lbpRev (CATATGATGCTTACCAAGTTG), and shrFor (GTGCGTTTGTGCAATATCTG) and shrRev (AGCGTATAGGTTCCTTCTGTG). PCR products were detected by ethidium bromide agarose gel electrophoresis. M; 1-kb DNA marker. (d) Stability of pSB027 and pSL230 in MGAS 6183. The presence of Scl1.41-expressing plasmid pSL230 and GFP-expressing plasmid pSB027 in the complemented scl1 mutant strain was tested. GAS genomic DNA was isolated and compared with pSL230 and pSB027 plasmid markers in ethidium bromide agarose gel.
GAS interactions with ECM components have been exhaustively reported in the literature and much effort has been directed toward understanding its function in GAS adherence and internalization pertaining to human disease (Cue et al., 2000). The bulk of that work focuses on Fn, although the effect of exogenous cFn on GAS internalization was not specifically investigated. Far less is known about the contribution of Lm to GAS adherence and internalization. Recently, the Lm-binding protein Lbp of the M1-type strain was shown to facilitate the adherence to and internalization by HEp-2 cells; however, the observed decrease in internalization of the lbp mutant was not statistically significant compared with the wild-type strain (Terao et al., 2002). In addition, a mutant strain of the M49-type GAS deficient in surface protein Shr, which binds both soluble Fn and Lm, showed decreased adherence to HEp-2 cells compared with the parental strain (Fisher et al., 2008).
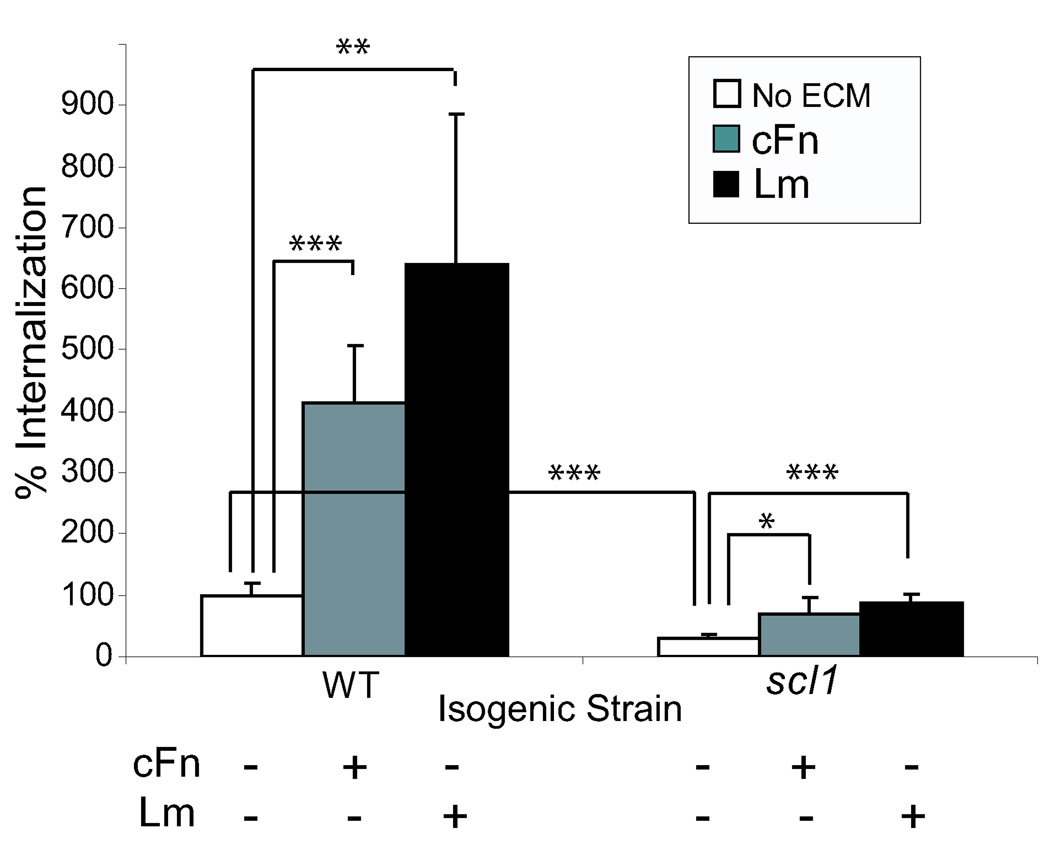
In our current studies, the HEp-2 cells were co-cultured with the wild type or the isogenic scl1-inactivated mutant GAS that were either treated or untreated with cFn or Lm. Following internalization, the numbers of surviving intracellular bacteria were determined. The Scl1-deficient GAS cells were internalized significantly less than the wild type strain in ECM-free medium (Fig. 3). Following pre-incubation with cFn and Lm, the wild type strain exhibited about a 4- and 6.5- fold increase in internalization, respectively, compared to ECM-untreated cells. The scl1-inactivated strain pre-incubated with cFn and Lm also showed about 2.2- and 2.8-fold increase in internalization compared to the ECM-untreated mutant cells; however, the overall levels of mutant internalization were lower compared to wild-type strain under each corresponding experimental conditions, emphasizing the contribution of Scl1 to cell invasion by GAS. It should be noted that the in vivo relevance of GAS internalization by human cells mediated by ECM binding has been debated in recent years. In spite of this, recent investigations using NMR spectroscopy, circular dichroism analyses, and experiments with monoclonal antibodies identified structural changes exerted by fibronectin upon binding to bacterial proteins that result in an enhanced Fn recognition by integrins (Bingham et al., 2008; Margarit et al., 2009). It is tempting to speculate that Scl1 binding to cFn and Lm may exert similar biological effects.
Fig. 3.
Scl1-ECM interactions mediate GAS internalization by human epithelial cells. HEp-2 cells were co-cultured for two hours in ECM-free media with the wild-type (WT) and the scl1-inactivated (scl1) GAS cells that were either pretreated or untreated with cFn or Lm. Following antibiotic treatment, the numbers of intracellular bacteria were determined by plating the eukaryotic-cell lysates on blood agar. The results obtained with ECM-untreated wild-type GAS were considered 100%. All means (graph bars) and standard deviations (error bars) are combined values from three independent experiments. Statistical significance was determined at a level of P< 0.05 (*), P< 0.01 (**), and P< 0.001 (***).
It was shown previously by our group that Scl1 from M41-type GAS binds the human collagen integrin receptors, which mediates GAS internalization by host cells (Caswell et al., 2007; Caswell et al., 2008). Integrins bind directly the GLPGER sequence within the Scl1-CL region. Here, we show the V region of the same Scl1.41 protein binds to cFn and Lm, which also increases GAS internalization by HEp-2 cells. We think it is unlikely that cFn and Lm binding to the globular V domain affects Scl1-CL region binding to α2β1 and α11β1; however, we cannot fully exclude such a possibility. The HEp-2 cells express the α2,α3,α5, and β1 integrin subunits (Caswell et al., 2007), thus are capable of producing the α2β1, α3β1, and α5β1 heterodimers with ability to bind collagen, laminin, and fibronectin, respectively (Watt 2002)The α11β1 integrin expression is restricted to fibroblasts (Popova et al., 2007) and, thus, may not be present on the surface of HEp-2 cells. Therefore, Scl1 may be contributing to internalization of M41-type GAS by HEp-2 cells by two mechanisms: direct binding to the α2β1 integrin, and ECM-bridging mechanism via integrins α3β1 and α5β1.
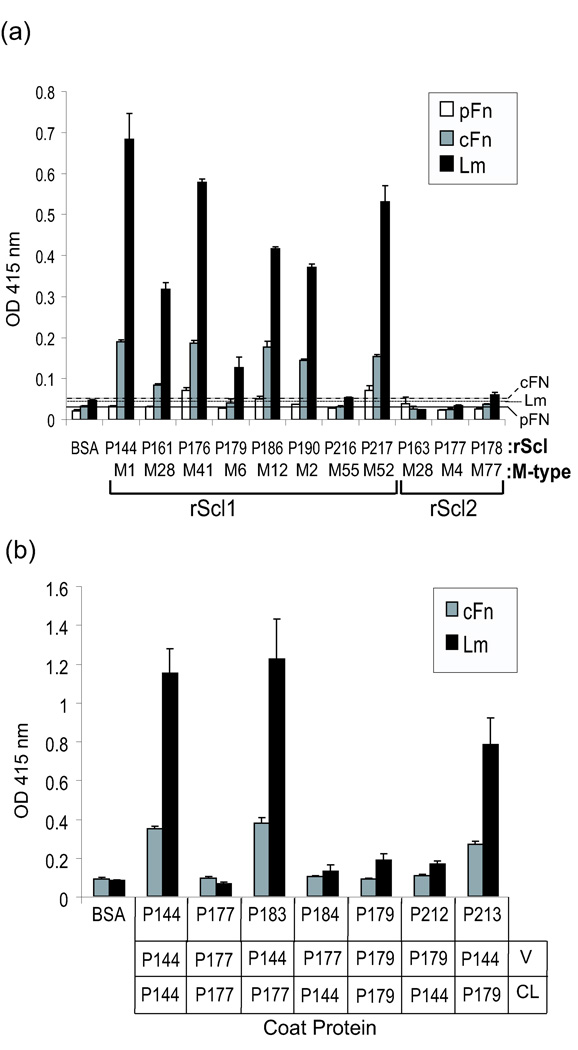
In a final series of experiments we tested several available rScl1 and rScl2 constructs for binding to the same panel of human ligands employing an ELISA-based assay. Similar to P176, no significant binding by any rScl protein was detected for fibrinogen, decorin, heparin, collagens type I and IV (data not shown). In general, the recombinant rScl1 constructs, derived from Scl1 proteins, bound cFn and Lm (Fig. 4a); while the Scl2-protein-based constructs P163, P177, and P178 were ECM-binding negative. Furthermore, none of the rScl1 proteins tested bound pFn, which is in agreement with our previous reports showing that those rScl1 proteins did not bind pFn from human plasma by affinity chromatography (Han et al. 2006; Caswell et al., 2008). All LDL-binding constructs derived from Scl1 proteins of the M1-, M28-, M41-, M12-, M2-, and M52-type GAS (Han et al., 2006) showed ECM binding, although to varying degrees. However, the CFH/CFHR-1-binding rScl1s originating from the M6- and M55-type GAS (Caswell et al., 2008) did not show any significant binding to ECM ligands. In order to determine the region of Scl1 responsible for binding to ECM proteins, an ELISA was performed using chimeric rScl constructs generated by domain-swapping (Fig. 4b). We employed two types of chimeric molecules: (i) derived from the ECM-binding positive (+) construct P144 (Scl1.1 of M1-type GAS) and the ECM-binding negative (−) construct P177 (Scl2 of M4-type GAS); and (ii) constructs derived from the ECM-binding positive P144 and the ECM-binding negative P179 (Scl1 of M6-type GAS). The rScl1 (+)-rScl2 (−) chimeric construct P183 (P144V/P177CL) but not P184 (P177V/P144CL) bound cFn and Lm. Likewise, the rScl1 (+)-rScl1 (−) chimeric construct P213 (P144V/P179CL) but not P212 (P179V/P144CL) bound cFn and Lm. These data strongly indicate that, indeed, the Scl1-V region is responsible for mediating interactions with ECM proteins.
Fig. 4.
Characterization of ECM binding to recombinant Scl1 proteins. (a) Binding of pFn, cFn, and Lm to rScl proteins by ELISA. The designation of rScl constructs, as well as the GAS M-type from which each construct was derived (M-type), are indicated. The distinction between Scl1-derived (rScl1) and Scl2-derived (rScl2) proteins is shown. rScl- or BSA-bound ECM proteins were detected with specific antibodies. Graph bars represent the mean OD415 ±SD of triplicate wells from a single experiment that is representative of three independent experiments. The solid, dotted, and spaced lines represent binding of pFn, cFn, and Lm, respectively to the BSA-coated wells with average values plus 3 SD as negative controls. (b) Identification of the Scl1 domain responsible for ECM binding. Chimeric rScl proteins, which were constructed by domain swapping between ECM-binding-positive and ECM-binding-negative rScls identified in panel (a), were tested for binding to cFn and Lm by ELISA. Domain composition of each chimeric molecule is shown below the graph. Graph bars represent the mean OD415 ±SD of triplicate wells from a single experiment that is representative of three independent experiments.
The present and previous results underscore the functional diversity of the Scl1-V region. Of particular interest to us is the emergence of two main binding patterns among Scl1 variants. The more common pattern includes binding of plasma LDL and extracellular matrix components cFn and Lm, which may represent an intriguing adaptation of Scl1 to either the blood or tissue environment. Our previous molecular evolutionary genetic analysis identified an elevated constraint of the Scl1-V region sequence suggesting that this region responds to selective pressure (Lukomski et al., 2000). Inasmuch as the amino acid sequence in the V region differs between Scl1 proteins of different M-types, the prediction of two a-helices (Rasmussen et., 2000; Han et a., 2006) and the globular structure of the Scl1-V domain (Xu et al., 2002; Han et al., 2006) seem to be conserved among all Scl1 proteins. The present work provides a platform for future investigations that will determine the Scl1-ECM binding affinities and identify the specific amino acid sequences or structural motifs of Scl1 variants that constitute the molecular basis for the Scl1-ligand (ECM, LDL, and CFH) recognition.
Acknowledgements
We thank S. Beres for providing plasmid pSB027. This work was supported by the National Institutes of Health Grant AI50666 and by a research grant from the West Virginia University Health Science Center, Office of Research and Graduate Education (to S.L). H. Oliver-Kozup was supported by a grant from the West Virginia Graduate Student Fellowships in Science, Technology, Engineering and Mathematics (STEM) program.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. New York, N.Y: Garland Publishing; 1994. [Google Scholar]
- Anthony BF. Streptococcal pyoderma. In: Stevens DL, Kaplan EL, editors. Streptococcal infections. New York, NY: Oxford University Press; 2000. pp. 144–151. [Google Scholar]
- Bingham R, Rudiño-Piñera E, Meenan NA, Schwarz-Linek U, Turkenburg JP, Höök M, Garman EF, Potts JR. Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci USA. 2008;105:12254–12258. doi: 10.1073/pnas.0803556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell CC, Han R, Hovis K, Ciborowski P, Keene D, Marconi R, Lukomski S. The Scl1 protein of M6-type group A Streptococcus binds the human complement regulatory protein, factor H, and inhibits the alternative pathway of complement. Mol Microbiol. 2008;67:584–596. doi: 10.1111/j.1365-2958.2007.06067.x. [DOI] [PubMed] [Google Scholar]
- Caswell CC, Lukomska E, Seo N, Höök M, Lukomski S. Scl1-dependent internalization of group A Streptococcus via direct interactions with the α2β1 integrin enhances pathogen survival and re-emergence. Mol Microbiol. 2007;64:1319–1431. doi: 10.1111/j.1365-2958.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- Caswell CC, Barczyk M, Keene DR, Lukomska E, Gullberg DE, Lukomski S. Identification of the first prokaryotic collagen sequence motif that mediates binding to human collagen receptors, integrins α2β1 and α11β1. J Biol Chem. 2008;283:36168–36175. doi: 10.1074/jbc.M806865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney HS, Chiang HC, Thacker JL, Dale JB. Serum opacity factor is a major fibronectin-binding protein and a virulence determinant of M type 2 Streptococcus pyogenes. Mol Microbiol. 1999;32:89–98. doi: 10.1046/j.1365-2958.1999.01328.x. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cue D, Dombeck PE, Cleary PP. Intracellular invasion by Streptococcus pyogenes: invasins, host receptors, and relevance to human disease. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram-positive pathogens. Washington, D.C: ASM Press; 2000. pp. 27–33. [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon HC, Derrick CW, Dillon MS. M-Antigens Common To Pyoderma And Acute Glomerulonephritis. J Infect Dis. 1974;130:257–267. doi: 10.1093/infdis/130.3.257. [DOI] [PubMed] [Google Scholar]
- Dillon HC, Wannamaker LW. Skin infections and acute glomerulonephritis, report of a symposium. Military Medicine. 1971;136:122–127. [PubMed] [Google Scholar]
- Fisher M, Huang YS, Li X, McIver KS, Toukoki C, Eichenbaum Z. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A Streptococcus. Infect Immun. 2008;76:5006–5015. doi: 10.1128/IAI.00300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Caswell CC, Lukomska E, Keene DR, Pawlowski M, Bujnicki JM, Kim JK, Lukomski S. ) Binding of the low-density lipoprotein by streptococcal collagen-like protein Scl1 of Streptococcus pyogenes. Mol. Microbiol. 2006;61:351–367. doi: 10.1111/j.1365-2958.2006.05237.x. [DOI] [PubMed] [Google Scholar]
- Han R, Zwiefka A, Caswell CC, Xu Y, Keene DR, Lukomska E, Zhao Z, Höök M, Lukomski S. Assessment of prokaryotic collagen-like sequences derived from streptococcal Scl1 and Scl2 proteins as a source of recombinant GXY polymers. Appl Microbiol Biot. 2006;72:109–115. doi: 10.1007/s00253-006-0387-5. [DOI] [PubMed] [Google Scholar]
- Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humtsoe JO, Kim JK, Xu Y, Keene DR, Höök M, Lukomski S, Wary KK. A streptococcal collagen-like protein interacts with the α2β1 integrin and induces intracellular signaling. J. Biol. Chem. 2005;280:13848–13857. doi: 10.1074/jbc.M410605200. [DOI] [PubMed] [Google Scholar]
- Jaffe J, Natanson-Yaron S, Caparon MG, Hanski E. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two domains. Mol Microbiol. 1996;21:373–384. doi: 10.1046/j.1365-2958.1996.6331356.x. [DOI] [PubMed] [Google Scholar]
- Kreikemeyer B, Talay SR, Chhatwal GS. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- Linke C, Caradoc-Davies TT, Young PG, Proft T, Baker EN. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc-receptor. J Bacteriol. 2009;191:5814–5823. doi: 10.1128/JB.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Ireland RM, Reid SD, Adams GG, Musser JM. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect Immun. 2000;68:6542–6553. doi: 10.1128/iai.68.12.6542-6553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Shelvin BJ, Graviss EA, Musser JM. Identification and characterization of a second extracellular collagen-like protein made by group A Streptococcus: control of production at the level of translation. Infect Immun. 2001;69:1729–1738. doi: 10.1128/IAI.69.3.1729-1738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margarit I, Bonacci S, Pietrocola G, Rindi S, Ghezzo C, Bombaci M, Nardi-Dei V, Grifantini R, Speziale P, Grandi G. Capturing host-pathogen interactions by protein microarrays: identification of novel streptococcal proteins binding to human fibronectin, fibrinogen, and C4BP. FASEB J. 2009;723:3100–3112. doi: 10.1096/fj.09-131458. [DOI] [PubMed] [Google Scholar]
- Molinari G, Chhatwal GS. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J Infect Dis. 1998;177:1600–1607. doi: 10.1086/515310. [DOI] [PubMed] [Google Scholar]
- O'Loughlin R, Roberson A, Cieslak P, Lynfield R, Gershman K, Craig A, Albanese B, Farley M, Barrett N, Spina N, Beal B, Harrison L, Reingold A, Van Beneden C Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Popova SN, E Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiol. 2007;190:179–187. doi: 10.1111/j.1748-1716.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Björck L. Unique regulation of SclB-a novel collagen-like surface protein of Streptococcus pyogenes. Infect Immun. 2001;40:1427–1438. doi: 10.1046/j.1365-2958.2001.02493.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Edén A, Björck L. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect Immun. 2000;68:6370–6377. doi: 10.1128/iai.68.11.6370-6377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski LM, Speziale P, Höök M, Wadstrom T, Timpl R. Binding of Streptococcus pyogenes to laminin. J Bio Chem. 1984;259:3734–3738. [PubMed] [Google Scholar]
- Terao Y, Kawabata S, Kunitomo E, Nakagawa I, Hamada S. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect Immun. 2002;70:993–997. doi: 10.1128/iai.70.2.993-997.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top FH, Jr, Wannamaker LW, Maxted WR, Anthony BF. M antigens among group A streptococci isolated from skin lesions. J Exp Med. 1967;126:667–685. doi: 10.1084/jem.126.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth, and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmore AM. Streptococcus pyogenes sclB encodes a putative hypervariable surface protein with a collagen-like repetitive structure. Microbiology. 2001;147:419–429. doi: 10.1099/00221287-147-2-419. [DOI] [PubMed] [Google Scholar]
- Xu Y, Keene DR, Bujnicki JM, Höök M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]