Abstract
Genetically modified bone marrow derived mesenchymal stem cells (MSCs) have proven to be efficient cell carriers for local or systemic delivery of therapeutics as well as for growth factors to augment tissue formation. However, efficient non-viral gene transfer to these cells is limiting their applicability. Although most studies focus on designing more efficient condensation agents for DNA, our focus in this manuscript is to study the role of two extracellular matrix proteins on the ability of MSCs to become transfected, collagen I (Col I) and fibronectin (Fn). Here we report that plating MSCs on Col I-coated surfaces inhibits transfection, while plating MSCs on Fn-coated surfaces enhances transfection. The mechanism by which these ECM proteins affect non-viral gene transfer involves the endocytosis pathway used for polyplex uptake and intracellular tension. We found that Fn promoted internalization through clathrin-mediated endocytosis and that this pathway resulted in more efficient transfection than caveolae-mediated endocytosis and macropinocytosis. Further, the disruption of actin-myosin interactions resulted in an enhancement of gene transfer for cells plated on Fn coated surfaces, but not for cells plated on Col I. We believe that the cellular microenvironment can be engineered to enhance the ability of cells to become transfected and that through understanding the mechanisms by which the ECM affects non-viral gene transfer, better materials and transfection protocols can be realized.
Introduction
Gene delivery can be achieved with the use of modified viruses (viral delivery) or polymers (non-viral delivery) that encapsulate or condense plasmid DNA into particles that can transport the DNA inside the cell. Although viral delivery is generally more efficient than non-viral approaches, it has limitations due to its potential immunogenicity and insertion mutagenesis [1]. Because of the mentioned safety concerns, non-viral approaches have been investigated. The investigation of effective ways to deliver genes using a non-viral approach has focused on (1) the vector used to condense DNA into a nanoparticle, (2) the sustained release of DNA or DNA nanoparticles from scaffolds [2], (3) the design of the plasmid DNA used [3] and, more recently, (4) the engineering of the cell and the cellular microenvironment to enhance the process of gene transfer [4, 5]. Most of the effort has been focused on (1), the design of more sophisticated condensing agents for DNA that can more efficiently target desired cells and achieve enhanced internalization, intracellular trafficking and nuclear entry [6]. Among all the condensing agents, cationic polymers, such as poly(ethylene imine) (PEI), are widely utilized for non-viral gene delivery because they are able to condense DNA through electrostatic interactions between the positively charged amines in the cationic polymer and the negatively charged phosphates on the DNA. The condensation of DNA with PEI forms particles (polyplexes) in the range of 50 to 200 nm in diameter [7]. DNA/PEI polyplexes enter the cell through endocytosis and are believed to be able to escape the endosome through endosomal buffering (the proton sponge effect, [8, 9]). Aside from DNA condensation, the amines in PEI have been heavily modified with ligands, peptides, polymers to enhance targeting [10], endosomal escape [11], nuclear localization [11, 12] and stability [13]. After each iteration, a new condensing agent for DNA is realized and the field gets closer to an efficient vector for non-viral gene delivery. However, other aspects of the non-viral gene delivery process such as the role of the cellular microenvironment and the mechanistic pathways involved within the cell should be considered to arrive at an optimal solution for efficient and targeted gene transfer.
Although not as widely studied, the cellular microenvironment has been successfully engineered to enhance gene transfer to a variety of cell types. For example, the stiffness of the matrix where the cells are bound affects their ability to internalize and process DNA with stiffer substrates achieving higher polyplex internalization and overall gene transfer efficiency [14]. Further, several studies have found that the integrins through which the cells are bound and the ligand density also modulate the process of gene transfer. Cells plated on surfaces with different densities and nano-scale arrangements of the integrin binding peptide RGD, demonstrated that surfaces that contained the highest density of RGD and shortest distance between RGDs tested enhanced transgene expression [15]. Cationic lipid-mediated gene transfer to rat smooth muscle cells is enhanced when the cells are plated on surfaces that promote αvβ3 binding, with antibodies against αvβ3 and β3 decreasing the amount of gene transfer [16]. Fibronectin (Fn) has been found to enhance non-viral gene transfer to mesenchymal stem cells [17, 18]. Last, studies that compared gene transfer on different structural ECM proteins showed that gene transfer to NIH/3T3 fibroblast was enhanced when the cells were plated on Fn, when compared to cells plated on collagen I (Col I), laminin and BSA [4] and that gene transfer to PC12 cells was enhanced in collagen IV compared to Col I, laminin, Fn, and polylysine [19].
Although the mechanism by which the cellular microenvironment modulates non-viral gene transfer has not been thoroughly investigated, some studies begin to point to the role of the uptake pathways and cell cytoskeleton. Studies investigating the mechanism of PEI/DNA complex internalization and overall gene transfer efficiency into adherent cell concluded that actin polymerization into stress fibers, microtubule motor proteins, as well as protein kinase C (PKC) induced phosphorylation have a major role in polyplex internalization and trafficking [15, 17, 20, 21]. The Fn-induced enhancement of gene transfer to fibroblast was correlated to endocytosis [4], while for PC12 cells the enhancement on collagen IV was correlated to the relative projected nuclear area of the plated cells [5]. These studies suggest that the extracellular matrix environment can be engineered to modulate cell processes that enhance the process of non-viral gene transfer.
In this manuscript, we report that Col I and Fn can modulate the efficiency of non-viral gene transfer to mouse cloned mesenchymal stem cells (mMSCs), with Col I inhibiting non-viral gene transfer and Fn enhancing non-viral gene transfer. We also investigated the mechanism by which Col I and Fn modulate either the inhibition or enhancement of non-viral gene transfer to mMSCs. Because the cellular cytoskeleton and endocytosis pathway has been shown to affect the process of non-viral gene transfer for adherent cells, we investigated if the possible differences in non-viral gene transfer for mMSCs plated on either collagen I or Fn was due to differences in their cytoskeleton and internalization pathways. mMSCS were seeded on Col I or Fn coated dishes and transfected in the presence or absence of pharmacological inhibitors for cytoskeletal or endocydic components. mMSCs were used because they have potential applications in regenerative medicine and cell therapy. We believe that through understanding the role of the extracellular matrix (ECM) on the process of non-viral gene transfer, more efficient approaches for gene transfer will be elucidated that put the cell and the ECM as key parameters in the process.
Materials and Methods
Materials
pEGFP-luc plasmid was purchased from clontech (Palo Alto, CA) and expanded using a Giga Prep Kit from Qiagen following the manufacturer’s protocol. Linear poly-(ethylene imine) (25Kg/mol, PEI) was purchased from Polysciences (Warrington, PA). Various inhibitors namely Amiloride hydrochloride hydrate, Cytochalasin D, Nocodazole, and Butanedione monoxime (BD) were purchased from Sigma Aldrich (St Louis, MO). Endothelin I was purchased from Calbiochem (Gibbstown, NJ). Genistein, Chlorpromazine hydrochloride and chloroquine diphosphate salt were purchased from Fisher scientific. Fn was purchased from Sigma and Col I was purchased from R&D Systems (Minneapolis, MN). All other reagents were purchased from Fisher Scientific unless otherwise specified.
Cell culture
Mouse bone marrow cloned mesenchymal stem (D1, CRL12424) were purchased from ATCC (Manassas, VA, USA). Cells were maintained in Dulbecco’s modified eagle’s medium (Sigma-Aldrich) containing 10% bovine growth serum (BGS, Hyclone, Logan, Utah) and 1% penicillin/streptomycin antibiotics (Invitrogen, Grand Island, NY) and cultured at 37°C and 5% CO2.
Protein coating
Proteins were coated using a method described by Kruger et al. [22] with minor modifications. The stock solutions of various Fn and Col I were diluted in PBS to obtain the final densities used for coating. Protein solution at specific initial densities was added in each well of a tissue culture plate and the plate was incubated over night at 4°C in a humid environment, followed by incubation at 37°C for 2 hours. The solution was removed and wells were washed twice with PBS to remove unbound proteins. Wells were further incubated with BSA (1% in PBS) for 30 minutes at 37°C followed by two washings with PBS.
Imaging of coated proteins
The homogeneity of the immobilized proteins was qualitatively observed using the NanoOrange Protein Quantitation Kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions with minor modifications. The NanoOrange solution was added to ECM coated plates (96 well or 48 well tissue culture plates) coated prior to BSA blocking. The plate was then incubated at 95°C for 10 minutes and at room temperature for 20 minutes before reading the plate using a typhoon scanner. The scan was visualized for protein homogeneity and was quantified using gray scale intensity readings.
Transfection
mMSCs were seeded on 48-well plates that were previously modified with ECM proteins at cell densities of 20,000 cells/well respectively. The cells were allowed to attach and incubated on the surfaces for 16-hours before DNA/PEI polyplexes were added. DNA/PEI polyplexes were formed by mixing equal volumes of plasmid DNA with 25kDa-Linear PEI. For every 1 μg of DNA, 1.65 μg of PEI was added to the DNA solution to get N/P of 12, vortexed for 15 seconds and incubated at room temperature for 15 minutes. Polyplexes were added directly to the medium of the plated cells at a final DNA concentration of 0.5 μg for 48-well plates. Salt was added directly to the wells post addition of transfection solution to get a final concentration of 150mM NaCl. Transfection was quantified at 48 hrs post transfection using the Promega Luciferase Assay system using a Luminometer (Turner biosystems, modulus, Sunnyvale, CA) and an integration period of 10 seconds.
Cell proliferation
The Cell Titer 96®Aqueous One Solution Cell Proliferation Assay (Promega) was performed to determine the cytotoxicity and proliferation of the cells exposed to different conditions. The Aqueous One Solution (20 μL) was added in each well to be assayed and incubated for 2 hours. SDS (25 μl of a 10% solution) was added to each well after the incubation. The fluorescence was measured using a plate reader at 490 nm.
Internalization of polyplexes
Plasmid DNA (pGFPluc) and the fluorescent DNA-intercalator YOYO-1 were mixed at a ratio of 1 YOYO-1 molecule per 50 base pairs and were allowed to complex for 60 minutes at room temperature. YOYO-1 labeled DNA was then used to prepare PEI/DNA complexes at a N/P of 12 (as mentioned above) and bolus transfection was performed. Two hours after exposure to the polyplexes, cells were washed with PBS, trysinized with 50 μl of 0.25%trypsin-EDTA and finally suspended in 350 μl of 0.04% tryphan blue in 1% BGS (bovine growth serum) in PBS. Fluorescent cells were detected by flow cytometry with a FACScan X and data was analyzed with CELLQuest (Beckton Dickinson). Experiments were performed in triplicates analyzing 7000 total events per sample. For all samples, gating was done such that the negative control had 5% positive events (i.e. 5% of the negative control had internalized DNA).
Studying the role cytoskeleton in gene transfer
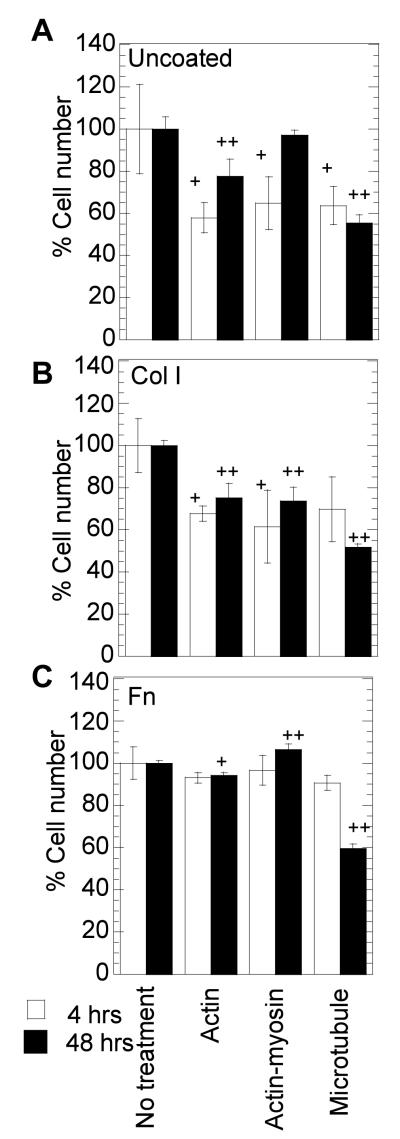
For treatment, the cells were allowed to attach for 14-15 hours before the media in the wells was replaced with media containing the inhibitor for 30 minutes. The media in the wells was changed with fresh complete medium after treatment prior to transfections. 10μM Nocodazole (Noc) treatment was given to depolymerize microtubules, 10mM Butanedione monoxime (BD) treatment was given to inhibit myosin ATPase and 20μM Cytochalasin D (CD) treatment was given to inhibit actin polymerization and the resultant tension. Immediately after treatment, cell morphology was analyzed. Transfections were done to study cell viability, internalization and gene expression after treatment, using previously mentioned methodologies. Cell viability was studied 4 and 48 hour post treatment and transfection.
Cell morphology
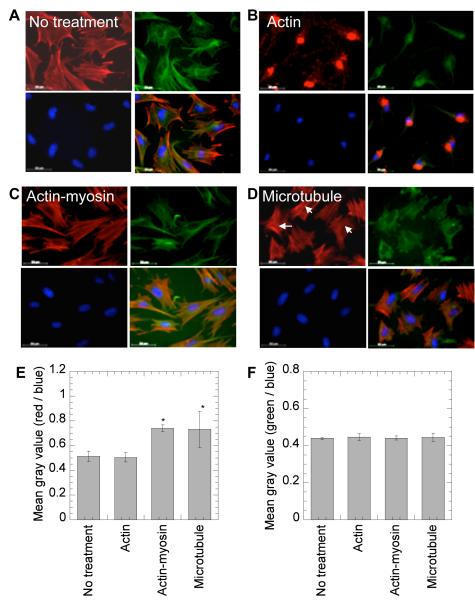
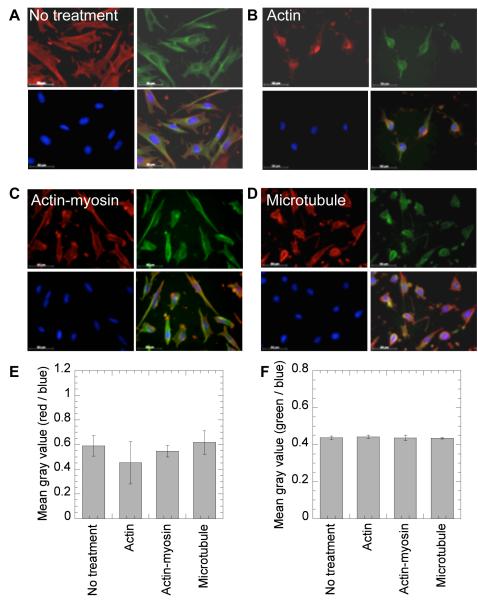
Cell morphology was analyzed to assess the effect of cytoskeltal inhibitors. Wells were placed on a plastic coverslip using an 8 well flexiperm and the assembly was placed inside a tissue culture sterile petridish. mMSCs cells (20,000 cells per 250 μl media) were seeded per well on the coverslip pre-coated with Fn or Col I and incubated for 16 hours before being treated with cytoskeletal inhibitors. Immediately after treatment, cells were fixed and stained for actin, microtubulin and nuclear DNA using rhodamine-phalloidin, Alexa488-microtubulin stain and HOECHT dye respectively, following the manufacturer’s instructions and standard staining protocols. Briefly, after two PBS washes the cells were fixed with 4% paraformaldehyde (PFA) for 15 minutes at room temperature. Cell membrane was weakened with 0.1% tritonX100 in 1x PBS for 3 minutes. The staining solution consisted phalloidin and Hoechst dyes. The staining solution was added in each well and left in dark for 30-60 minutes at room temperature followed by washings with 0.05% tween-20. The sample was observed with a fluorescence microscope. The cell nucleus was observed as a blue stain, the actin filaments were observed as a red stain, while the microtubulin was observed as a green stain. Images were taken with a Zeiss AxioObserver Z1 inverted microscope at 40 × magnification, keeping the same exposure for all images and analyzed using Image J software to obtain the mean gray-scale values.
Analyzing endocytotic pathways
Cells were treated with inhibitors for endocytosis 16 hours post cell attachment prior to transfection to determine the endocytotic pathways active in cells plated on Fn and Col I coated surfaces. Clathrin mediated endocytosis was inhibited by using 10μg/ml chlorpromazine, caveolae mediated endocytosis was inhibited using 200μM genistein and macropinocytosis was inhibited using 100μM Amiloride hydrochloride. A 30 minutes pretreatment was given followed by incubation with polyplexes for 4 hours in presence of inhibitors. The media was then replaced with fresh media. Gene expression and internalization were analyzed as described above. Cell proliferation after inhibitor treatment was analyzed 4 hour and 48 hours post transfection using above-mentioned method.
Statistics
All statistical analysis were performed using the computer program Instat (GraphPad, San Diego, CA). Experiments were statistically analyzed using the Tukey test, which compares all pairs of columns, Dunnet test which compares all columns versus a control column or the unpaired t-test (two tail p-value) which compares two columns, as mentioned in the figure legend. All test were done using a 95% confidence interval.
Results and Discussion
Col I and Fn modulate gene transfer to mMSCs
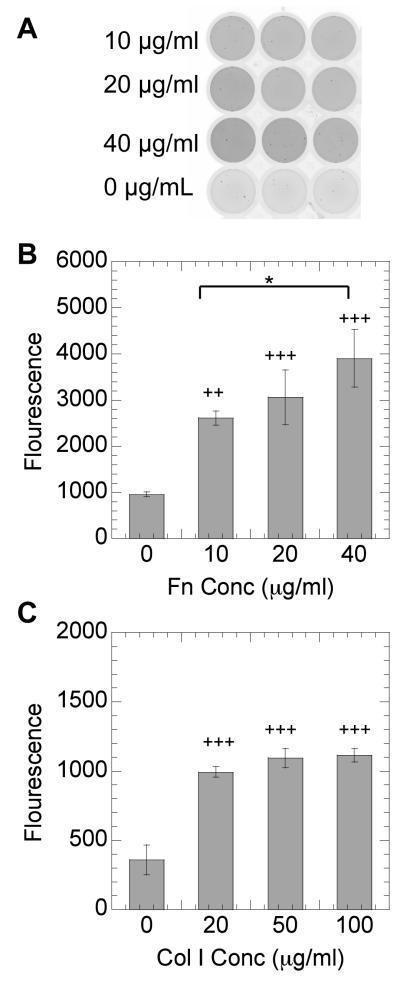
The extracellular matrix environment has been implicated on the modulation of transgene expression to a variety of cell types [4, 5]. Because of the potential therapeutic applications of the efficient transfection of MSCs [23], we wanted to investigate how extracellular matrix proteins that bind through different integrin receptors affect non-viral gene transfer to MSCs. The role of Col I and Fn on the efficiency of non-viral gene transfer with DNA/PEI to mMSCs was studied by plating mMSCs on surfaces that have been pre-coated with Col I and Fn and then performing a traditional bolus transfection. Protein coatings are reported as the solution concentration used to coat the surface. The solution concentrations used are those suggested by the manufacturer for the protein type. We kept the solutions concentrations within the same order of magnitude for both proteins being studied. However, for Col I higher coating densities are recommended (50 to 100 μg/mL) than for Fn (10 to 40 μg/mL). Increasing protein coating with increasing protein solution concentration was confirmed through staining the protein with a fluorescence protein quantification assay (Figure 1). The protein-modified surfaces were imaged through a typhoon scanner. The protein coating was found to be uniform (Figure 1A) with the fluorescence intensity increasing with increasing protein solution concentration (Figure 1B, C).
Figure 1.
NanoOrange staining of immobilized proteins. Picture of wells coated with Fn at an initial density of 0, 10, 20, and 40 μg/ml respectively (A). Mean NanoOrange fluorescence of wells coated with Fn (B) and Col I (C) respectively using a typhoon scanner. Statistical analysis was done using the Tukey-Kramer Multiple Comparison test. The symbol * represents a significant change in fluorescence between the two surfaces at a level of p < 0.05, while + and +++ represents a significant change with respect to an uncoated surface at a level of p<0.05 and p < 0.001 respectively.
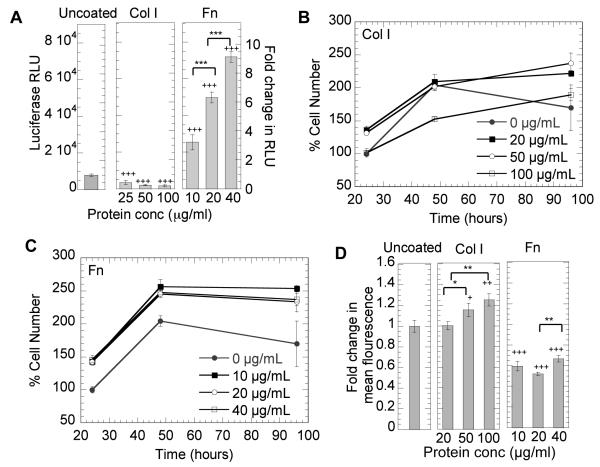
Polyplexes prepared at a N/P ratio of 12 were used to transfect cells plated on uncoated surfaces or surfaces precoated with Fn and Col I. It was observed from initial studies using different N/P ratio to analyze transgene expression and toxicity that as the N/P ratio increased, transfection efficiency also increased but so did the toxicity. N/P ratio of 12 was chosen as it was the highest N/P ratio with minimal toxicity, whereas with higher N/P ratios significant toxicity was observed. We observed that both Col I and Fn affected the efficiency of non-viral gene transfer to mMSCs. Col I significantly inhibited transgene expression, while Fn significantly enhanced transgene expression when compared to cells plated on uncoated surfaces, the traditional surface used for non-viral gene transfer studies (p < 0.001, Figure 2A). The efficiency of transgene expression was further modulated by the amount of protein being immobilized on the surface. Although not statistically significant, for Col I increasing concentration resulted in a dose response inhibition of transgene expression with gene transfer to mMSCs plated on 100 μg/mL Col I surface resulting in less transgene expression than mMSCs cultured on 25 μg/mL Col I coated surfaces (Figure 2A). On the contrary, for Fn increasing protein surface concentration resulted in a dose response enhancement of transgene expression with gene transfer to mMSCs plated on 20 μg/mL Fn coated surfaces resulting significantly less transgene expression than for mMSCs cultured on 40 μg/mL Fn coated surfaces (p < 0.001). The dose response for Fn coated surfaces agrees with previous reports that showed an enhancement in gene transfer for NIH/3T3 cells cultured on Fn [4]. However, the dose response inhibition of non-viral gene transfer for mMSCs cultured on Col I coated surfaces was not previously reported.
Figure 2.
Effect of Fn and Col I density on DNA/PEI transgene expression, proliferation and internalization. mMSCs were plated on uncoated, Col I coated or Fn coated surfaces for 16 hours prior to being transfected with a luciferase encoding plasmid. Transgene expression was analyzed at 48 hours (A), and cell proliferation was analyzed at 24, 48, and 96 hours for Col I coated (B) or Fn coated (C) surfaces, To analyze internalization (D), cells were transfected with transfected with YOYO-1 labeled polyplexes. After 2 hours cells were collected with trypsin and polyplex internalization analyzed by flowcytometry (D). A total of 7000 events were analyzed per sample. Gating was done such that the negative control had 5% positive events. Fold increase in transgene expression and internalization was calculated with respect to the uncoated control. Statistical analysis was done using the Tukey-Kramer Multiple Comparison test. The symbols *, ** and *** represent a significant change in gene expression or internalization between protein surfaces at different concentrations to the level of p < 0.05, p < 0.01 and p < 0.001, respectively. The symbol +, ++ and +++ represents a significant change in gene expression and internalization on protein coated surface with respect to that observed on uncoated to a level of p <0.05, p<0.01 and p < 0.001 respectively.
Proliferation does not fully explain the modulation of gene transfer by Col I and Fn
Our first inclination to explain the differences in gene transfer for cells plated on Col I and Fn coated surfaces was that the proliferation rate for mMSCs plated on the different surfaces was affected, which in turned affected the efficiency of gene transfer as has been previously reported [24]. Cells that divide faster have been shown to be easier to transfect than cells that are non-dividing [25, 26]. Cell proliferation was significantly enhanced for cells plated on Fn coated surfaces for all concentrations of Fn (p < 0.001) when compared to uncoated surfaces (Figure 2C) but not when compared to Col I coated surfaces (Figure 2B). For Col I 24 hours after cell plating proliferation was also enhanced when compared to uncoated surfaces (p at least < 0.05) except for cells plated on 100 μg/mL coated surfaces, which had the same level of proliferation of as uncoated surfaces. After 48 hours there was no difference between Col I and uncoated surfaces except for the highest concentration of Col I tested, which had significantly less cell proliferation. Although cell proliferation can explain the enhancement of gene transfer on Fn coated surfaces compared to uncoated surfaces, it does not explain why gene transfer was inhibited for cells plated on Col I or the dose response in gene transfer observed on Fn coated surfaces. Thus, to begin to understand the reason for the observed differences in gene transfer for mMSCs plated on Col I and Fn we investigated the internalization rate through flow-cytometry and the cellular cytoskeleton and endocytosis pathways through pharmacological inhibitors.
Polyplex internalization is enhanced in Col I coated surfaces
To determine if the internalization rate of DNA/PEI polyplexes by mMSCs was affected by the protein coat, flow-cytometry was performed with fluorescently labeled DNA. Cells were plated on Col I or Fn coated surfaces and fluorescently labeled polyplexes were added and incubated with the cells for 2 hours. We expected that the internalization rate on Fn coated surfaces to be significantly higher than that on Col I coated surfaces since the gene transfer efficiency on Fn surfaces was higher. However, we found the opposite. Polyplexes internalization on Fn coated surfaces was significantly less than that observed for cells plated on uncoated surfaces and cells plated on Col I coated surfaces for all concentrations of Fn tested (p < 0.001, Figure 2D). Further, internalization on Col I was enhanced when compared to internalization on uncoated surfaces (p < 0.05) even though gene transfer was inhibited (Figure 2D). To further confirm this finding, we performed internalization studies with a lower incubation time of 20 minutes. We found the same trend. Internalization on Col I was enhanced and internalization on Fn was decreased (data not shown). Since the endocytosis rate did not explain why mMSCs plated on Fn coated surfaces had enhanced gene transfer over uncoated and Col I coated surfaces we turned to determine if the specific endocytosis pathway used for uncoated, Col I coated an Fn were different.
Clathrin mediated endocytosis mediates efficient gene transfer to mMSCs
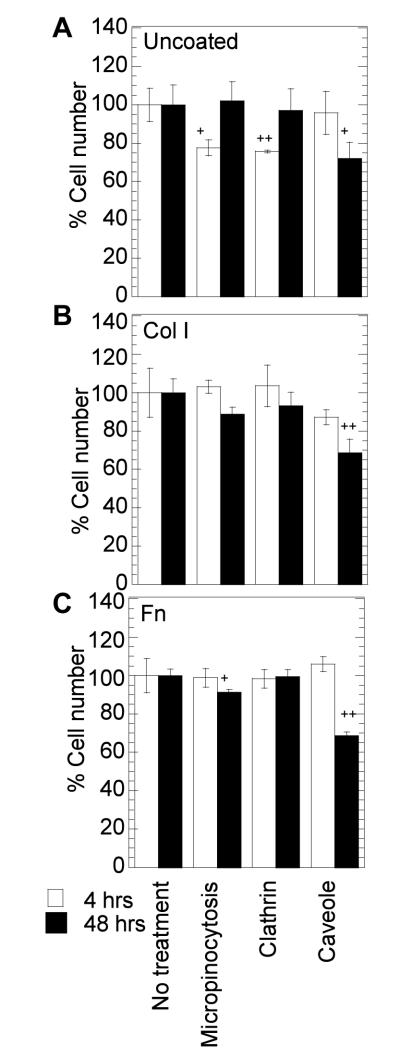
To determine if differences in polyplex endosomal route could explain the differences observed in transgene expression and polyplex internalization between cells plated on uncoated surfaces, Col I and Fn coated surfaces, the endosomal pathways used for polyplex internalization were studied. Because the reagents used to block specific endosomal pathways require the use of pharmacological inhibitors, we wanted to ensure that they were not toxic to the cells. We considered 65% percent viability an acceptable viability to conduct our assay. We found that the treatment with all endosomal inhibitors and polyplexes had at least 65% percent viability (Figure 3) compared to untreated cells.
Figure 3.
Cell viability after endocytosis inhibitor treatment. Cells were plated on uncoated (A), or Col I coated (B) or Fn coated (C) surfaces. Cells were pretreated with the inhibitors for 30 minutes followed by incubation with the DNA/PEI polyplexes for 4 hours in presence of the inhibitors. Cell viability was analyzed at 4 and 48 hours post addition of the polyplexes using the MTT assay. Statistical analysis was done using Dunnett multiple comparison test, which compares each treatment condition to the control. The symbol + and ++ represents the significant change to the level of p < 0.05 and p < 0.01 respectively.
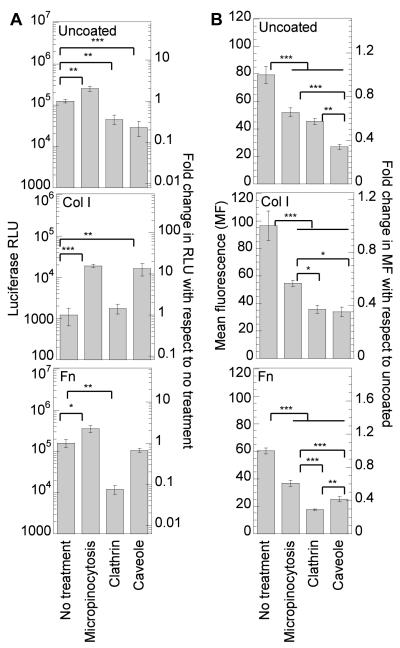
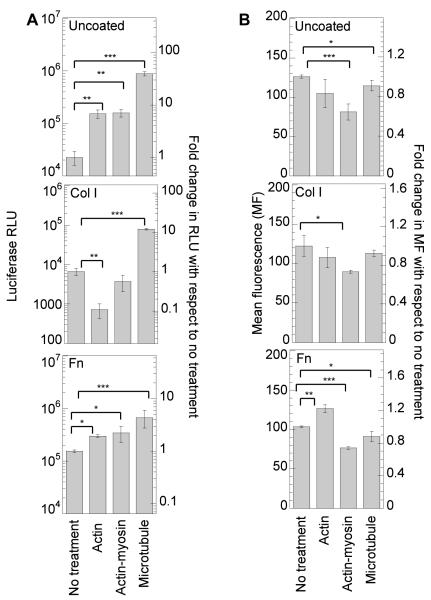
Inhibition of macropinocytosis, caveolae-mediated endocytosis and clathrin-mediated endocytosis in mMSCs, played a role in the internalization and the overall gene transfer efficiency of PEI/DNA complexes. To be able to compare the effect of the inhibitors directly, the data was normalized to make the no treatment condition (NT) for each surface coating 1. Inhibition of macropinocytosis resulted in a statistical (p<0.001) increase in gene expression on all surfaces, indicating that directing internalization via other pathways results in more efficient intracellular trafficking (Figure 4A). The increase in transgene expression was highest for cells plated on Col I coated surfaces (10-fold increase), indicating that for cells plated on Col I more polyplexes are actively internalized and routed using this endosomal pathway. Inhibition of macropinocytosis resulted in a statistical decrease of internalized polyplexes for all the surfaces (p <0.001), confirming that all surfaces promote internalization through this pathway (Figure 4B).
Figure 4.
Effect of endocytotic pathways in transgene expression and polyplex internalization. Cells were plated on uncoated, Col I (50μg/mL) coated or Fn (40 μg/mL) coated surfaces for 14-16 hours prior to treating the cells with amileroide, chlorpromazine and genistein to inhibit macropinocytosis, clathrin and caveole mediated endocytosis respectively. Cells were pretreated for 30 minutes following which bolus transfection was done with our without YOYO-1 labeled polyplexes for 2 or 4 hours in presence of inhibitors. Medium was replaced 2 or 4 hours after transfection with treatment. Transgene expression was analyzed 48 hours post transfection using luciferase assay (A) and internalization was assessed 2 hours post transfection using flowcytometry (B). 7000 total events were analyzed per sample. Gating was done such that the negative control had 5% positive events. The transgene expression obtained after treatment with specific inhibitor was compared to untreated sample using the unpaired t-test (two tail p value). Statistical analysis for internalization was done using Tukey-Kramer Multiple Comparisons, which compares all pairs within the same protein coat. The symbols *, **, *** represents the significant change to the level of p < 0.05, p < 0.01, and p < 0.001 respectively.
Inhibition of clathrin-mediated endocytosis resulted in a statistical decrease in gene expression for cells plated on uncoated and Fn coated surfaces (p at least < 0.01) and no change in gene expression for cells plated on Col I coated plates (Figure 4A). Internalization of polyplexes after clathrin-mediated endocytosis inhibition resulted in a significant decrease in polyplex internalization for all the surfaces tested (p <0.001, Figure 4B). Since gene transfer was not affected after clathrin inhibition on Col I, this indicates that clathrin is not an effective route for gene transfer on Col I. On the other hand, the significant decrease in gene transfer after clathrin-mediated endocytosis inhibition (92% decrease) on Fn coated surfaces, suggest that this pathway is critical for efficient gene transfer to cells plated on Fn.
Inhibition of caveolae-mediated endocytosis resulted in a significant decrease in transgene expression for cells plated on uncoated surfaces (p < 0.001), a 13.2-fold increase for cells plated on Col I coated plates, and no significant change for cells plated on Fn coated plates, indicating that caveolae-mediated endocytosis is activated to different extends for mMSCs seeded on the different surfaces (Figure 4A). Internalization of polyplexes after caveolae-mediated inhibition resulted in a significant decrease in polyplex internalization for all the surfaces tested (p <0.001, Figure 4B). Interestingly, although caveolae mediated endocytosis plays a role in internalization on Fn, it does not affect transgene expression, further suggesting that this internalization pathway is not an effective route for gene transfer to mMSCs plated on Fn. Overall, our results show that for mMSCs plated on protein coated surfaces, polyplexes internalized through caveolae-mediated endocytosis do not result in efficient gene transfer, while polyplexes internalized through clathrin-mediated endocytosis result in efficient gene transfer.
Although genistein is widely used as a caveolae-mediated endocytosis inhibitor, it is a Tyrosine kinase inhibitor and has been reported to effect cell growth and proliferation [27]. Thus the observed, changes in internalization and overall gene transfer efficiency could be due to changes in proliferation. Likewise treatment with chlorpromazine does not specifically block clathrin endocytosis. It is also a antipsychotic and acts as an antagonist (blocking agent) on different postsynaptic receptors [28]. However, since all the different conditions were treated the same, we believe that differences between protein coatings are beyond changes in proliferation.
Clathrin-mediated endocytosis has been previously reported to result in more efficient transgene expression than macropinocytosis and caveolae-mediated endocytosis for histidylated polylysine/DNA polyplexes [29] and alginate-chitosan particles [30]. The efficacy of the clathrin pathway in transfection has been attributed to complexes being trafficked to the endosome where they are released by the proton-sponge effect, while caveolae mediated endocytosis can lead to vesicle entrapped complexes [30]. In addition, a recent study has shown that the effective mode of endocytosis depends on the cell type along with the type of PEI used [31]. In COS-7 cells, the clathrin dependent pathway was observed as the main contributor to efficient gene transfer [31]. In HUH-7 cells, gene transfer by linear PEI polyplexes occurred mainly via the clathrin dependent route, whereas both pathways mediated transfection by branched PEI polyplexes [31]. In HeLa cells, both pathways were observed to mediate successful transfection, with the caveolae pathway being more efficient [31]. Our results indicate that the effective route of internalization can be modulated by the cellular microenvironment.
Reduction of cytoskeletal tension and microtubule polymerization enhances gene transfer to mMSCs
To further investigate the mechanism by which the extracellular matrix environment affects nonviral gene transfer to mMSCs, the role of the cellular cytoskeleton in cells plated on Fn was investigated. In particular, we investigated the role of actin polymerization, myosin-actin interactions and the microtubular network because they have been previously implicated in the modulation of non-viral gene transfer or endocytosis. To visualize and quantify the changes in the cytoskeleton network of cells after inhibitor treatment, cells were plated on Fn and Col I coated surfaces, treated with the inhibitor, fixed and stained for actin, microtubules and the nucleus. Images were taken at 40× magnification, and analyzed to obtain the grey scale values. After treatment with cytochalasin D (CD), an actin destabilizing agent, the cellular actin network was severely compromised for cells plated on Fn as well as Col I (Figure 5B and 6B). However, no change was observed in the actin (Figure 5E and 6E) and microtubulin (Figure 5F and 6F) fluorescence intensity. After treatment with nocodazole (Noc), a microtubule polymerization-destabilizing agent, no significant change was observed for microtubule fluorescence (Figure 5F and 6F). However, the cellular morphology was significantly affected on both Col I and Fn coated surfaces with cell becoming rounder and less spread (Figure 5D and 6D). For cells plated on Fn coated surfaces a significant increase in actin stress fibers was observed after Noc treatment (Figure 5D and E, p < 0.05). After treatment with butanedione monoxime (BD), an inhibitor of the actin-myosin interaction, a significant increase in actin stress fibers was observed for cells plated on Fn coated surfaces (Figure 5E, p < 0.05), but not for cells plated on Col I coated surfaces (Figure 6E, p > 0.05).
Figure 5.
Effect of treatment with cytoskeletal inhibitors on actin and microtubular networks on Fn. Cells were seeded on Fn coated surfaces and 14 hours post cell seeding treatment with no inhibitor (A) or inhibitors against the actin network (B), actin-myosin interactions (C) and the microtubular network (D) were given. Immediately post inhibitor treatment, changes in cell morphology were visualized through staining for actin using rhodamine-phalloidin, microtubulin using Alexa488 conjugated anti α-tubulin stain and for DNA using Hoechst dye. Images were taken with a Zeiss AxioObserver Z1 inverted microscope at 40 × magnification, and analyzed with Image J to obtain mean gray scale value for the actin (red), tubulin (green) and DNA (blue). The average of mean gray scale values for actin and tubulin staining was normalized with mean gray value for DNA staining (n=4 images) and plotted for each treatment (E and F, respectively). Statistical analysis was done using Tukey-Kramer Multiple Comparisons, which compares all pairs within the same protein coat. The symbols * represents the significant change to the level of p < 0.05.
Figure 6.
Effect of treatment with cytoskeletal inhibitors on actin and microtubular networks on Col I. Cells were seeded surfaces precoated with Col I and 14 hours post cell seeding no treatment (A) or treatment with inhibitors against the actin network (B), actin-myosin interactions (C) and the microtubular network (D) were given. Immediately post inhibitor treatment, changes in cell morphology were visualized through staining for actin using rhodamine-phalloidin, microtubulin using Alexa488 conjugated anti α-tubulin stain and for DNA using Hoechst dye. Images were taken with a Zeiss AxioObserver Z1 inverted microscope at 40 x magnification, and analyzed with Image J to obtain mean gray scale value for the actin (red), tubulin (green) and DNA (blue). The average of mean gray scale values for actin and tubulin staining was normalized with mean gray value for DNA staining (n=4 images) and plotted for each treatment (E and F, respectively).
As with the endocytosis inhibitors, we wanted to ensure that the cells plated on Col I coated, Fn coated and uncoated surfaces and treated with cytoskeleton inhibitors were viable before conducting our transfection studies. We considered 65 % viability an acceptable viability to conduct our assay using cytoskeletal inhibitors. We found that the treatment with CD and BD inhibitors had at least 65% percent viability (Figure 7) compared to untreated cells. However, treatment with Noc had 50% viability at 48 hours. This is likely due to the prevention of cell division since the microtubules are key contributors of mitosis. Nevertheless, Noc has been previously used in gene transfer studies [21, 32-34] and was used here to test the role of the microtubular network.
Figure 7.
Cell viability after cytoskeleton inhibitor treatment. Cells were plated on uncoated (A), or Col I (50μg/ml) coated (B) or Fn (40μg/ml) coated (C) surfaces. Cells were treated for 30 minutes, after which media was replaced and polyplexes added. Cell viability was analyzed 4 and 48 hours post addition of polyplexes using MTT assay. Statistical analysis was done using Dunnett multiple comparison test, which compares each treatment condition to the control. The symbol + and ++ represents a significant change to the level of p < 0.05 and p < 0.01 respectively.
Disruption of actin stress fibers statistically increased the transgene expression for cells plated on uncoated and Fn coated surfaces (p<0.05), while the expression on Col I was severely inhibited (p<0.01, Figure 8A). Internalization of polyplexes after actin stress fiber disruption was enhanced for cells plated on Fn (p < 0.01), but no significant difference was observed for cells plated on uncoated or Col I coated surfaces (p > 0.05, Figure 8B). This indicates that for cells seeded on Fn coated surfaces, which had poor internalization (Figure 2D), the disruption of the actin network enhances endocytosis. Since the enhancement in endocytosis resulted in enhanced gene transfer, it is likely that this internalization was through the clathrin pathway, since this pathway showed efficient gene transfer for cells seeded on Fn coated surfaces. Further, for cells seeded on Col I, the actin network appears critical for gene transfer. The already inhibited gene transfer to mMSCs seeded on Col I coated surfaces was further inhibited when the actin stress fibers were disrupted. Together, these results suggest that cells seeded on Fn have a more significant actin network than cells seeded on Col I, and that the amount of actin stress fibers to achieve efficient gene transfer goes through a maximum where too much actin reduces gene uptake (Fn case) and too little actin reduces effective intracellular transfer (Col I case).
Figure 8.
Role of cell cytoskeleton in transgene expression and polyplex internalization. Cells were plated on uncoated, Col I (50 μg/mL) coated or Fn (40μg/mL) coated surfaces for 14-16 hours prior to treating the cells with CD, BDM and Noc for 30 minutes to inhibit actin polymerization, myosin ATPase and microtubule polymerization, respectively. Immediately after treatment with inhibitor, the medium was replaced and bolus transfection was done with our without YOYO-1 labeled polyplexes. Transgene expression was analyzed 48 hours post transfection using luciferase assay (A) and internalization was assessed 2 hours post transfection using flowcytometry (B). A total of 7000 events were analyzed per sample. Gating was done such that the negative control had 5% positive events. The transgene expression and internalization obtained after specific inhibitor treatment were statistically compared with untreated sample using the unpaired t-test (two tail p value). The symbols *, **, and *** represents a significant change to the level of p < 0.05, p < 0.01, and p < 0.001 respectively.
Disruption of actin-myosin interactions statistically increased transgene expression for cells seeded on uncoated and Fn coated surfaces (p < 0.05), while the expression on Col I was unchanged (Figure 8A). However, the internalization of polyplexes was significantly decreased after disruption of the actin-myosin interactions on all surfaces (Figure 8B). These results suggest that for cells plated on Fn coated surfaces decreasing the contractility of the cell increases intracellular trafficking of the polyplexes toward the nucleus. Further, the results suggest that cellular contractility, a result of actin-myosin interactions, is low for cells seeded on Col I coated surfaces since there is no effect when they are disrupted, while actin-myosin interactions are high for cell seeded on Fn coated surfaces since when they are disrupted enhanced gene transfer occurs.
Disruption of microtubule polymerization significantly increased transgene expression on uncoated, Col I coated and Fn coated surfaces (p < 0.001, Figure 8A), while the polyplex internalization significantly decreased on Fn and uncoated surfaces (p<0.05, Figure 8B). The microtubular network has been previously implicated with caveole-mediated endocytosis [35] and lysosomal trafficking of endosomes [36]. Thus, the increase in gene transfer on all surfaces can be attributed to reduced trafficking to the lysosomes and reduced DNA degradation intracellularly. Since the enhancement in gene transfer was observed for all the surface coatings, the effect appears independent of protein surface coating.
As with the inhibitors for the endocyotic pathways, the action of the cytoskeletal inhibitors is not solely specific to cell cytoskeleton and affect the related pathways. Nocodazole interferes with microtubule polymerization affecting related processes such as mitotic spindle formation [37]. This explains the significant decrease in proliferation observed 48 hrs post nocodazole treatment (Figure 7). Cytochalasin D which is a potent inhibitor of actin polymerization, has also been reported to arrest the cell cycle at G1-S transition by activating the p53-dependent pathways [38]. However, the concentration or time period for the inhibitor treatments used in our investigation have been widely used to study cell cytoskeleton specifically [39, 40], and are different than those used for related actions such as cell cycle arrest mentioned before [37, 38]. 2,3-Butanedione monoxime which is widely used as a skeletal myosin II inhibitor has been shown be a cytoprotectant [41] as well as effects multiple mechanisms such as muscle contraction, ionic current flow and synaptic transmission [42]. As with the endocytotic inhibitors, all the different conditions were treated the same and we believe that differences between protein coatings are beyond changes in proliferation.
These studies indicate that the structural ECM constituents affect the efficiency of non-viral gene transfer by modulating cell cytoskeletal dynamics, uptake mechanisms and the resultant intracellular trafficking. We believe that an in depth understanding of the underlying mechanisms of gene transfer in cells provides cues to design cell pretreatments, develop scaffolds and gene delivery protocols able to enact efficient gene transfer.
In conclusion, we found that non-viral gene transfer to mMSCs plated on surfaces coated with Col I or Fn resulted in different levels of gene expression and polyplex internalization. Our analysis suggests that the differences observed are due to differences in the endosomal pathways used for polyplex uptake and differences in the cellular cytoskeleton of the cell. In particular, we found that efficient gene transfer to mMSCs plated on protein-coated surfaces is achieved when polyplexes are internalized through clathrin-mediated endocytosis and that Fn promotes clathrin-mediated endocytosis. Further, overall transgene expression for mMSCs plated on Col I coated surfaces was inhibited and this inhibition was attributed to the promotion of internalization through caveolae-mediated endocytosis and reduced actin polymerization. Last, we found that the amount of cellular tension affects non-viral gene transfer with decreased actin-myosin interactions resulting in decreased internalization on all surfaces. The understanding of how the cellular microenvironment affects the efficiency of non-viral gene transfer to MSCs can aid in the design of effective strategies to genetically modify these cells and achieve their therapeutic potential.
Acknowledgements
We sincerely appreciate the co-operation and valuable advice rendered by all the members of the Segura Lab especially Yuguo Lei, Quinn Ng, Sean Anderson, Talar Tokatlian and Shiva Gojggini. We would also like to thank undergraduate researchers Eleana Manousiouthakis, as well as high school student Caleb Shield for their time and effort during this project. This work was supported by National Institutes of Health grant 1R21EB009516-01A1. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics. 2003 May;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 2.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007 Nov;28(31):4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Mairhofer J, Grabherr R. Rational vector design for efficient non-viral gene delivery: challenges facing the use of plasmid DNA. Molecular biotechnology. 2008 Jun;39(2):97–104. doi: 10.1007/s12033-008-9046-7. [DOI] [PubMed] [Google Scholar]
- 4.Bengali Z, Rea JC, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. The journal of gene medicine. 2007 Aug;9(8):668–678. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchimura E, Yamada S, Uebersax L, Yoshikawa T, Matsumoto K, Kishi M, et al. On-chip transfection of PC12 cells based on the rational understanding of the role of ECM molecules: efficient, non-viral transfection of PC12 cells using collagen IV. Neuroscience Letters. 2005;378(1):40–43. doi: 10.1016/j.neulet.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. The AAPS journal. 2007;9(1):E92–104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 2005 Jul;60(2):247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo - Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995 AUG 1;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005 MAY;7(5):657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Gao X, Hu K, Pang Z, Cai J, Li J, et al. Galactose-poly(ethylene glycol)-polyethylenimine for improved lung gene transfer. Biochemical and biophysical research communications. 2008 Oct 24;375(3):378–383. doi: 10.1016/j.bbrc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Advanced drug delivery reviews. 2001 Dec 31;53(3):341–358. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 12.Bae Y Mi, Choi H, Lee S, Kang S Ho, Kim Y Tae, Nam K, et al. Dexamethasoneconjugated low molecular weight polyethylenimine as a nucleus-targeting lipopolymer gene carrier. Bioconjugate chemistry. 2007 Nov-Dec;18(6):2029–2036. doi: 10.1021/bc070012a. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Pan S, Feng M, Wen Y, Zhang W. Stability of poly(ethylene glycol)-graft-polyethylenimine copolymer/DNA complexes: influences of PEG molecular weight and PEGylation degree. Journal of materials science. 2009 Oct 17; doi: 10.1007/s10856-009-3903-1. [DOI] [PubMed] [Google Scholar]
- 14.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005 Jun;4(6):460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 15.Kong HJ, Hsiong S, Mooney DJ. Nanoscale cell adhesion ligand presentation regulates nonviral gene delivery and expression. Nano Lett. 2007 Jan;7(1):161–166. doi: 10.1021/nl062485g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, et al. DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: mechanisms of enhanced transfection. Gene Ther. 2003 Aug;10(17):1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa T, Uchimura E, Kishi M, Funeriu DP, Miyake M, Miyake J. Transfection microarray of human mesenchymal stem cells and on-chip siRNA gene knockdown. J Control Release. 2004 Apr 28;96(2):227–232. doi: 10.1016/j.jconrel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki A, Jo JI, Tabata Y. A Reverse Transfection Technology to Genetically Engineer Adult Stem Cells. Tissue Eng. 2006 Dec 1; doi: 10.1089/ten.2006.0185. [DOI] [PubMed] [Google Scholar]
- 19.Uchimura E, Yamada S, Uebersax L, Yoshikawa T, Matsumoto K, Kishi M, et al. On-chip transfection of PC12 cells based on the rational understanding of the role of ECM molecules: efficient, non-viral transfection of PC12 cells using collagen IV. Neurosci Lett. 2005 Apr 11;378(1):40–43. doi: 10.1016/j.neulet.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med. 2004 Jul;6(7):769–776. doi: 10.1002/jgm.558. [DOI] [PubMed] [Google Scholar]
- 21.Suh J, Wirtz D, Hanes J. Efficient active transport of gene nanocarriers to the cell nucleus. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruger-Krasagakes S, Grutzkau A, Krasagakis K, Hoffmann S, Henz BM. Adhesion of human mast cells to extracellular matrix provides a co-stimulatory signal for cytokine production. Immunology. 1999 Oct;98(2):253–257. doi: 10.1046/j.1365-2567.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene therapy. 2008 May;15(10):711–715. doi: 10.1038/gt.2008.35. [DOI] [PubMed] [Google Scholar]
- 24.Wilke M, Fortunati E, van den Broek M, Hoogeveen AT, Scholte BJ. Efficacy of a peptide-based gene delivery system depends on mitotic activity. Gene therapy. 1996 Dec;3(12):1133–1142. [PubMed] [Google Scholar]
- 25.Mortimer I, Tam P, MacLachlan I, Graham RW, Saravolac EG, Joshi PB. Cationic lipid-mediated transfection of cells in culture requires mitotic activity. Gene therapy. 1999 Mar;6(3):403–411. doi: 10.1038/sj.gt.3300837. [DOI] [PubMed] [Google Scholar]
- 26.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene therapy. 2000 Mar;7(5):401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 27.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989 Sep 15;49(18):5111–5117. [PubMed] [Google Scholar]
- 28.Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980 Dec;137(12):1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- 29.Goncalves C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol Ther. 2004 Aug;10(2):373–385. doi: 10.1016/j.ymthe.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Douglas KL, Piccirillo CA, Tabrizian M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68(3):676–687. doi: 10.1016/j.ejpb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.von Gersdorff K, Sanders NN, Vandenbroucke R, De Smedt SC, Wagner E, Ogris M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol Ther. 2006 Nov;14(5):745–753. doi: 10.1016/j.ymthe.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Brisson M, Tseng WC, Almonte C, Watkins S, Huang L. Subcellular trafficking of the cytoplasmic expression system. Human gene therapy. 1999 Nov 1;10(16):2601–2613. doi: 10.1089/10430349950016645. [DOI] [PubMed] [Google Scholar]
- 33.Grosse S, Aron Y, Thevenot G, Monsigny M, Fajac I. Cytoskeletal involvement in the cellular trafficking of plasmid/PEI derivative complexes. J Control Release. 2007 Sep 11;122(1):111–117. doi: 10.1016/j.jconrel.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg J, Fernandez MA, Ropp JD, Hamm-Alvarez SF. Nocodazole treatment of CV-1 cells enhances nuclear/perinuclear accumulation of lipid-DNA complexes and increases gene expression. Pharmaceutical research. 2001 Feb;18(2):246–249. doi: 10.1023/a:1011001022570. [DOI] [PubMed] [Google Scholar]
- 35.Wong AW, Scales SJ, Reilly DE. DNA internalized via caveolae requires microtubule-dependent, Rab7-independent transport to the late endocytic pathway for delivery to the nucleus. The Journal of biological chemistry. 2007 Aug 3;282(31):22953–22963. doi: 10.1074/jbc.M611015200. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Li P, Li G, Wang J, Wang E. The effect of nocodazole on the transfection efficiency of lipid-bilayer coated gold nanoparticles. Biomaterials. 2009 Mar;30(7):1382–1388. doi: 10.1016/j.biomaterials.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Hsu SL, Yu CT, Yin SC, Tang MJ, Tien AC, Wu YM, et al. Caspase 3, periodically expressed and activated at G2/M transition, is required for nocodazole-induced mitotic checkpoint. Apoptosis. 2006 May;11(5):765–771. doi: 10.1007/s10495-006-5880-x. [DOI] [PubMed] [Google Scholar]
- 38.Rubtsova SN, Kondratov RV, Kopnin PB, Chumakov PM, Kopnin BP, Vasiliev JM. Disruption of actin microfilaments by cytochalasin D leads to activation of p53. FEBS Lett. 1998 Jul 3;430(3):353–357. doi: 10.1016/s0014-5793(98)00692-9. [DOI] [PubMed] [Google Scholar]
- 39.Bhadriraju K, Yang M, Ruiz S Alom, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Experimental Cell Research. 2007;313(16):3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. American journal of physiology. 2004 Mar;286(3):C518–528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 41.Thum T, Borlak J. Butanedione monoxime increases the viability and yield of adult cardiomyocytes in primary cultures. Cardiovasc Toxicol. 2001;1(1):61–72. doi: 10.1385/ct:1:1:61. [DOI] [PubMed] [Google Scholar]
- 42.Sellin LC, McArdle JJ. Multiple effects of 2,3-butanedione monoxime. Pharmacol Toxicol. 1994 Jun;74(6):305–313. doi: 10.1111/j.1600-0773.1994.tb01365.x. [DOI] [PubMed] [Google Scholar]