Abstract
Self-report and behavioral data suggest that impulsivity may contribute to the development and maintenance of obesity. Neuroimaging studies implicate a widespread neural network in inhibitory control and suggest that impulsive individuals show hypoactivity in these regions during tasks requiring response inhibition. Yet, research has not directly tested whether body mass correlates inversely with activation of these regions during response inhibition tasks. The present study used functional magnetic resonance imaging (fMRI) to investigate neural activations during a food-specific go/no-go task in adolescent girls ranging from lean to obese. When required to inhibit prepotent responses to appetizing food, body mass index (BMI) correlated with response inhibition at both the behavioral and neural level, with more overweight adolescents showing greater behavioral evidence of impulsivity as well as reduced activation of frontal inhibitory regions, including superior frontal gyrus, middle frontal gyrus, ventrolateral prefrontal cortex, medial prefrontal cortex, and orbitofrontal cortex, than leaner individuals. As well, activation in food reward regions (e.g., temporal operculum/insula) in response to food images correlated positively with BMI. Results suggest that hypofunctioning of inhibitory control regions and increased response of food reward regions are related to elevated weight.
Keywords: obesity, BMI, go/no-go, response inhibition, impulsivity, fMRI
Mounting evidence suggests that deficits in inhibitory control are related to overeating and obesity. Self-reported impulsivity correlates positively with caloric intake (Guerrieri et al., 2007a; Guerrieri, Nederkoorn, & Jansen, 2007b), activation of reward circuitry in response to images of food (Beaver et al., 2006), and body mass index (BMI) scores (Braet, Claus, Verbeken, & Vlierberghe, 2007; Ryden et al., 2003) and negatively with weight loss during obesity treatment (Jonsson, Bjorvell, Levander & Rossner, 1986; Nederkoorn, Braet, Van Eijs, Tanghe, & Jansen, 2006a). Impulsive responses on behavioral go/no-go and stop signal tasks also correlate positively with caloric intake (Guerrieri et al., 2007a) and BMI (Bonato & Boland, 1983a; Nederkoorn et al., 2006a; Nederkoorn, Jansen, Mulkens, & Jansen, 2006b; Nederkoorn, Smulders, Havermans, Roefs, & Jansen, 2006c).
Although self-report and behavioral data link impulsivity to obesity, functional neuroimaging studies have not previously tested whether BMI correlates with activation of brain regions involved in inhibitory control during response inhibition to palatable foods. Along with other widespread and diverse regions within parietal and subcortical areas, frontal lobe regions, including superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, medial prefrontal cortex (PFC), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and orbitofrontal cortex (OFC), are consistently implicated in response inhibition (Aron & Poldrack, 2005; Buchsbaum, Greer, Chang & Berman, 2005; Liddle et al., 2001; Mostofsky et al., 2003; Simmonds et al., 2008; Horn et al., 2003). Studies examining the neural correlates of impulsivity have demonstrated that individuals who show response inhibition deficits typically exhibit reduced activation in the response inhibition network, particularly within these frontal regions. For example, during inhibitory control task, self-reported impulsivity correlates negatively with activation in different areas within the response inhibition network, such as right inferior parietal lobule, superior medial frontal gyrus, bilateral ventral PFC, dorsal amygdala, and right dorsolateral PFC (Asahi et al., 2004; Brown et al., 2006; Horn et al., 2003). Similarly, individuals with versus without attention deficit hyperactivity disorder (ADHD) show hypoactivity in the response inhibition network, including right medial and inferior frontal cortex, right medial frontal cortex, caudate, and globus pallidus during response inhibition tasks (Booth et al., 2005; Rubia et al., 2001). More germane to the present study, a structural MRI study found lower gray matter density in the middle frontal gyrus of the PFC in obese versus lean individuals (Pannacciulli et al., 2006), a region involved in inhibition of inappropriate responses and the control of goal-directed behaviors. Similarly, higher BMI has been associated with lower baseline metabolism in PFC and cingulate gyrus, along with associated impairments in inhibitory control processes (Volkow et al., 2009). In addition, deficits in cognitive inhibition may lead to a failure to deactivate limbic food reward regions when required to do so, implicating another possible mechanism by which impairments in executive function may increase vulnerability to overeat (Wang et al., 2009).
In the present study, we used a food-specific go/no-go paradigm to assess whether BMI is related to the ability to inhibit prepotent responses to appetizing food items. The go/no-go paradigm is a measure of response inhibition that requires subjects to perform speeded responses on go trials and to inhibit responding on no-go trials. By having many more go than no-go trials, responding rather than inhibiting is made prepotent. We examined neural activations elicited during this task using functional magnetic resonance imaging (fMRI) to investigate potential BMI-related differences in neural recruitment during response inhibition. We also investigated whether individual differences in inhibitory control, at both the behavioral and the neural level, predict increases in BMI over a 1-year follow-up. We studied adolescent girls because PFC function is still developing in this age group (Zelazo, Carlson, & Kesek, 2008). Myelination of the PFC continues to progress through adolescence into early adulthood (Bartzokis et al., 2001), whereas gray matter volume shows gradual decreases from late childhood through adolescence (Paus, 2005). Because executive control has not yet fully matured in adolescents, response inhibition deficits should be more likely to emerge in this population. The present study is the first to employ a food specific go-no-go paradigm in an fMRI environment to test whether response inhibition deficits at the neural level to prepotent appetizing food stimuli correlate with BMI and future increases in BMI.
Subjects were instructed to respond rapidly when pictures of vegetables were displayed (go trials), but to inhibit their response to pictures of desserts (no-go trials). At the behavioral level, we hypothesized that BMI would correlate positively with number of commission errors (false positive responses), reflective of overweight subjects' higher levels of impulsivity and inability to inhibit prepotent responses to appetizing food. At the neural level, we hypothesized that BMI would be negatively correlated with activation in response inhibition regions, such as the right DLPFC and VLPFC, as well as middle, medial, inferior and superior frontal gyrus and the inferior parietal lobe. Reduced activation in the response inhibition network in overweight subjects would represent a neurofunctional correlate of impulsivity and poorer inhibitory control that may increase propensity towards overeating. We also hypothesized that BMI would correlate positively with activation in regions involved in encoding the reward value of food, such as the ventral striatum, insula and operculum, during no-go trials. Obese versus lean individuals report greater sensitivity to reward in general (Davis, Strachan, & Berkson, 2004) and show heightened response to appetizing food cues in regions of the brain that encode the sensory properties of food (Rothemund et al., 2007; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008; Stoeckel et al., 2008). The expected pattern of results would provide support for the thesis that overweight individuals are more influenced by the hedonic aspects of appetizing food and have a reduced ability to inhibit prepotent responses to these types of reward. Lastly, we expected that behavioral measures of impulsivity, reduced activation in response inhibition areas, and greater activation in food reward regions would predict weight gain over one-year follow-up.
Method
Participants
Participants were 39 adolescent girls (M age = 15.7; SD = 0.93); 2% Asian/Pacific Islanders, 2% African Americans, 86% European Americans, 5% Native Americans, and 5% mixed racial heritage. Participants from a larger study of female high school students who appeared to meet the inclusion criteria for the present imaging study were asked if they were interested in participating in a study on the neural response to presentation of food. Those who reported binge eating or compensatory behaviors in the past 3 months, any use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder were excluded. Data from other fMRI tasks completed by these participants have been reported elsewhere (Stice, Spoor, Bohon, & Small, 2008; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008; Stice, Yokum, Bohon, Marti, & Smolen, in press). Behavioral data from 4 of the 39 participants could not be used due to technical problems, resulting in a sample of 35 participants for behavioral analyses (M BMI = [kg/m2] = 24.5, range = 17.3 −38.9, ranging from the 15th to the 99th age-adjusted percentile). fMRI data from 10 participants were not analyzed because they showed excessive head movement during the scans, exceeding 2 mm (M = 3.4 mm, range 2.1 – 7.0 mm). This resulted in a final sample of 29 participants for fMRI analyses (BMI range = 17.3–38.9). The local Institutional Review Board approved this project. Participants and parents provided written consent.
Measures
Body Mass
The body mass index (kg/m2) was used to reflect adiposity (Dietz and Robinson, 1998). After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of height and weight were obtained and averaged at baseline and at 1-, 6- and 12-month follow-up assessments. The BMI correlates with direct measures of total body fat such as dual energy x-ray absorptiometry (r = .80 to .90) and with health measures including blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin levels, and diabetes mellitus in adolescent samples (Dietz & Robinson, 1998).
fMRI paradigm
Participants were asked to consume their regular meals, but to refrain from eating or drinking (including caffeinated beverages) for 4–6 hours immediately preceding their imaging session for standardization purposes. We selected this deprivation period to capture the hunger state that most individuals experience as they approach their next meal, which is a time when individual differences in response to food would logically impact caloric intake. Most participants completed the paradigm between 16:00 and 18:00, but a subset completed scans between 11:00 and 13:00 due to scheduling restraints. Before the imaging session, participants were familiarized with the fMRI paradigm through practice on a desktop computer.
The go/no-go paradigm was designed to examine inhibition of prepotent responses to appetizing food items. Two functional runs were carried out. Each run consisted of 48 trials. For each trial, a picture of a vegetable (go trial, 75% occurrence) or a picture of a dessert (no-go trial, 25% occurrence) was presented for 500 ms. Examples of go trials included pictures of broccoli, carrots, cabbage, and eggplants. Examples of no-go trials included pictures of chocolate cake, pie, ice cream, and cookies. Thus, there was substantial variation between trials within a given condition, reflecting real-life variation in food choices. Trials were separated by a fixation cross, which was presented for intervals ranging from 7 to 19 seconds in order to capture the full hemodynamic response. Subjects were instructed to respond with a button press to all vegetables, but to withhold their responses to desserts, and to respond as quickly and accurately as possible. Reaction times were measured from the beginning of trial onset and collected with a fiber-optic response box system. Trials were presented in pseudo-randomized order, designed so that desserts appeared with equal frequency after 1, 2, and 3 vegetable presentations. Stimuli were presented visually using the Presentation software package (Version 9, Neurobehavioral Systems, Davis, CA) and were displayed using a video projector that illuminated a rear projection screen located at the end of the magnet. Subjects viewed stimuli through an adjustable mirror attached to the head coil. MRI acquisition was synchronized with the paradigm.
Behavioral analyses
For each subject, median reaction times for go trials and for no-go trials (that were incorrectly responded to) were calculated. The mean rate of commission errors was calculated as the total number of failures of inhibition divided by the total number of no-go trials. The mean rate of omission errors was calculated as the total number of failures of response divided by the total number of go trials. Spearman's rho was used to calculate the correlation between reaction time, rate of commission errors, and BMI. For determining prospective change in body mass, BMI slope over 1-year was calculated based on BMI measurements taken at baseline, 1-, 6-, and 12-month follow-up visits.
Imaging and statistical analysis
Scanning was performed by a Siemens Allegra 3 Tesla head-only MRI scanner. A standard birdcage coil was used to acquire data from the entire brain. A thermo foam vacuum pillow and additional padding was used to restrict head motion. In total, 270 scans were collected during each of the functional runs. Functional scans used a T2* weighted gradient single-shot echo planar imaging (EPI) sequence (TE=30 ms, TR = 2000 ms, flip angle=80°) with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane as determined by the midsagittal section. Structural scans were collected using an inversion recovery T1 weighted sequence (MP-RAGE) in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans. High-resolution structural MRI sequences (FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈ 160) were acquired.
Data were pre-processed and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB (Mathworks, Inc., Sherborn, MA) (Worsley & Friston, 1995). The images were time-acquisition corrected to the slice obtained at 50% of the TR. For each participant, each functional image was then spatially realigned to the mean of all functional images for that participant, minimizing the effects of head movement. Functional and anatomical images were coregistered, and the images (both functional and anatomical) were normalized to the standard MNI template brain implemented in SPM5 (ICBM152, based on an average of 152 normal MRI scans). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel
Condition-specific effects at each voxel were estimated using general linear models. Vectors of the onsets for each event of interest were compiled and entered into the design matrix so that event-related responses could be modeled by the canonical hemodynamic response function (HRF), as implemented in SPM5, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 seconds and the subsequent undershoot. We also included temporal derivatives of the hemodynamic function to obtain a better model of the data (Henson, Price, Rugg, Turner, & Friston, 2002). A 128 second high-pass filter (per SPM5 convention) was used to remove low-frequency noise and slow drifts in the signal. Finally, the six movement parameters of the rigid body transformation applied by the realignment procedure were introduced as covariates in the first-level general linear model.
To explore main effects of this paradigm, we contrasted no-go (dessert) activations to baseline (fixation cross) activations, as well as no-go activations to go (vegetables) activations, across all subjects independently of BMI. In a separate analysis, to assess BMI-related differences in activation patterns, individual contrast maps of no-go trials versus fixation cross baseline and no-go trials versus go trials were entered into regression models using Spearman's rho with BMI scores as a covariate. We focused on no-go trials in the present report because we wanted to assess individual differences in response inhibition to appetizing foods. We contrasted no-go trials with a baseline fixation condition as our primary contrast condition to allow for the inclusion of all regions that may play a role in response inhibition, including those involved in response selection. Given that both go and no-go events involve active response selection, treating no-go and go trials as opposite contrasts in an fMRI design may lead to the incorrect conclusion that certain brain areas do not play a role in response inhibition because they are involved in response selection as well (Simmonds et al., 2008). Further, because go and no-go trials in our paradigm differed in both their instructional set (go versus not go) and stimulus type (vegetable versus dessert), contrasting no-go trials against a neutral baseline condition enabled us to avoid confounding instructional set and food type in our interpretation of the results. Nevertheless, we included a no-go > go contrast in secondary analyses to more thoroughly explore all effects. Lastly, we examined correlations between change in BMI over a 1-year period and brain activation, while controlling for baseline BMI.
For all analyses, we followed a regions-of-interest (ROI) approach. To test our hypothesis that BMI would be negatively correlated with activation in response inhibition regions, we selected ROIs that were previously identified in a meta-analysis of go/no-go tasks: superior frontal gyrus, middle frontal gyrus (MFG), inferior frontal gyrus, medial and lateral PFC, inferior parietal lobe, and orbitofrontal cortex (Nakata et al., 2008). To test our hypothesis that BMI would be positively related to activation in food reward regions, we selected the following ROIs: insula, operculum, striatum, and orbitofrontal cortex (Stice et al., 2008; Stoeckel et al., 2008). To examine main effects of our paradigm, we examined increased and decreased activation in both the response inhibition and food reward regions listed above. To examine effects of BMI change over a 1-year follow-up period, we included all ROIs listed above as well as peaks implicated in the original baseline BMI correlation analyses. ROIs were defined using the WFU Pickatlas and the AAL and Talairach Daemon Atlases (Lancaster et al, 2000; Maidjian et al., 2004; Tzourio-Mazoyer et al., 2002). T-maps are thresholded at p uncorrected < 0.001 with a cluster threshold of 3. Peaks were considered significant at pFDR corrected across the ROI. Reported pFDR values were corrected for the number of ROIs.
Results
Behavioral Data
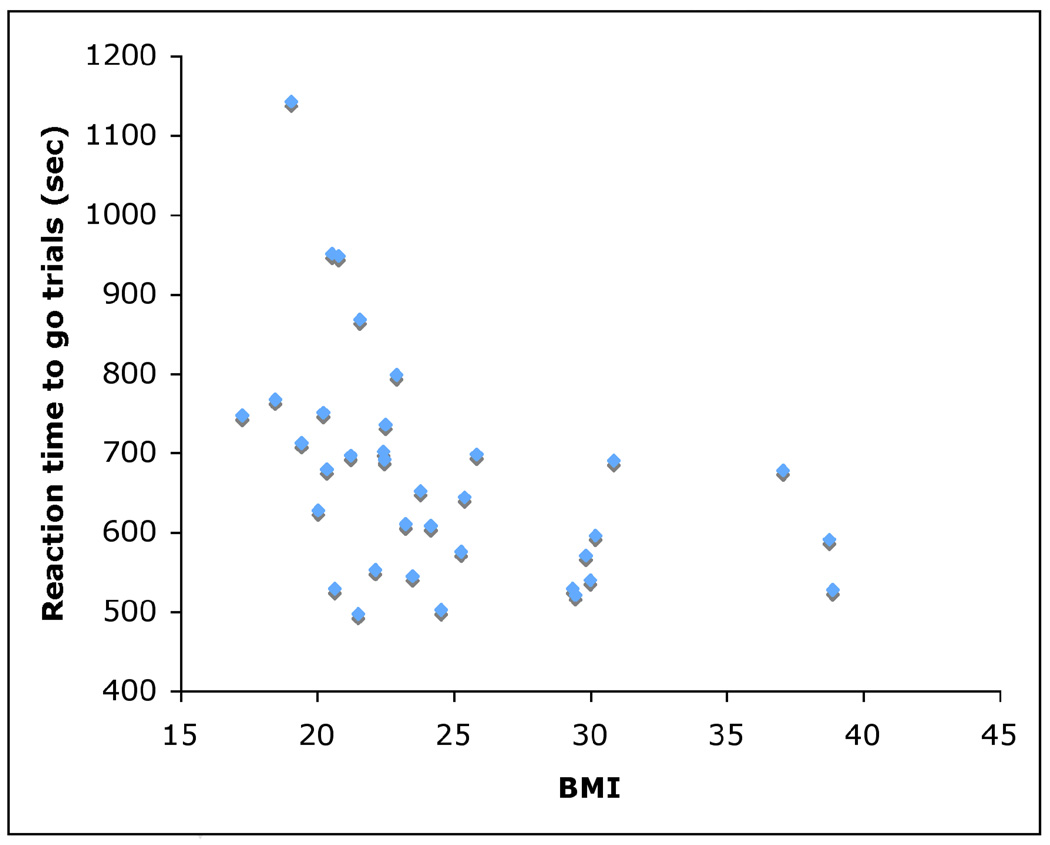
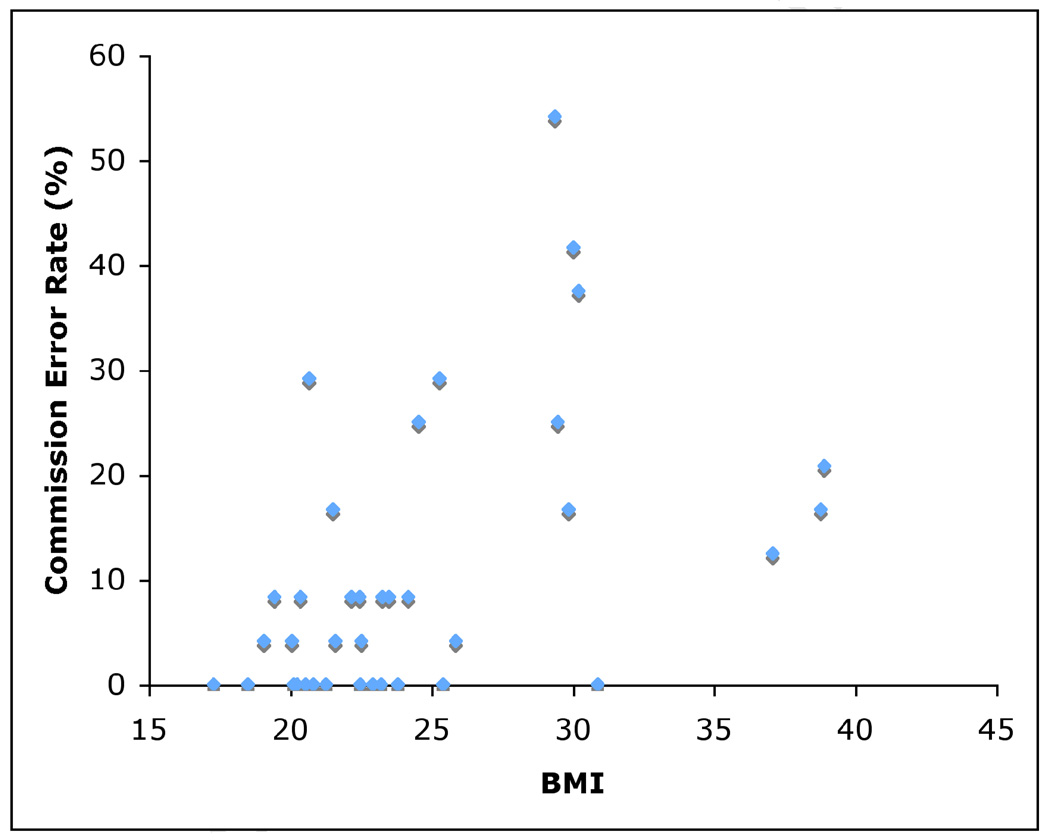
Median reaction time for go trials was 651 ms (SD 140 ms). Median reaction time for no-go trials that were incorrectly responded to was 588 ms (SD 261 ms). The mean rate of commission errors was 11.3% (SD 13.5) and the mean rate of omission errors was 2.5% (SD 4.5). Median reaction time to go trials was negatively correlated with baseline BMI (N = 35, rs = −0.54, p = 0.001), such that subjects with higher BMI scores showed significantly faster reaction times (Figure 1). Median reaction time to all food stimuli (both go trials and incorrectly responded-to no-go trials) was also negatively correlated with BMI (N = 35, rs = −0.54, p = 0.001). Rate of commission errors was positively correlated with baseline BMI (N = 35, rs = 0.50, p = 0.002), such that subjects with higher BMI scores showed significantly more false positive responses (Figure 2). Change in body mass over a 1-year period was not significantly correlated with any behavioral measures of response inhibition deficits (N = 35, range r = 0.382 to −0.322, n.s.).
Figure 1.
Scatterplot showing reaction times to go trials plotted as a function of BMI (N = 35). Subjects with higher BMIs showed significantly faster reaction times.
Figure 2.
Scatterplot showing rate of commission errors (%), calculated as the number of commission errors divided by the total number of no-go trials, plotted as a function of BMI (N = 35). Subjects with higher BMIs showed a significantly higher rate of commission errors.
Functional Imaging Data
Activation During Go/No-Go Task
A table of main effects, examining increased and decreased activation to no-go trials relative to both baseline and go trials, is presented in Table 1. A number of peaks within superior frontal gyrus and inferior frontal gyrus, regions that are commonly implicated in response inhibition (Nakata et al., 2008), were found to be activated in the no-go > go contrast. A smaller number of peaks within regions that have been previously implicated in food reward (Stice et al., 2008; Stoeckel et al., 2008), namely insula and frontal operculum, also showed greater activation in one or both of these contrasts. Significant deactivations to no-go trials were not observed at our statistical threshold, but a number of deactivations that were observed at a more lenient threshold of pFDR ≤ 0.05 are reported as a footnote for interested readers.
Table 1.
Main Effects: Regions Showing Activation During no-go Trials Across Subjects, Independent of BMI (N=29)1
| x | y | z | Number of voxels in cluster |
Activation cluster Z |
pFDR corrected |
|
|---|---|---|---|---|---|---|
|
No-go>Baseline: increased activation Insula |
45 | 12 | −9 | 80 | 3.48 | 0.003 |
| 39 | 9 | 3 | 80 | 4.03 | 0.003 | |
|
No-go > Go: increased activation Superior frontal gyrus |
−6 | 30 | 57 | 98 | 4.88 | 0.001 |
| −3 | 39 | 51 | 98 | 4.68 | 0.002 | |
| 21 | 48 | 39 | 5 | 3.75 | 0.005 | |
| Inferior frontal gyrus | −33 | 24 | −12 | 103 | 4.17 | 0.003 |
| −36 | 21 | −3 | 103 | 3.99 | 0.003 | |
| −45 | 24 | 15 | 103 | 4.33 | 0.003 | |
| 27 | 24 | −6 | 73 | 4.63 | 0.002 | |
| 33 | 18 | −15 | 73 | 4.35 | 0.003 | |
| Anterior insula/ | −30 | 24 | 3 | 67 | 4.02 | 0.005 |
| frontal operculum | −36 | 21 | −3 | 67 | 3.99 | 0.005 |
T-maps are thresholded at p uncorrected < 0.001 with a cluster threshold of 3. Peaks were considered significant at P≤0.005, false-discovery rate (FDR) corrected across the ROI.
Peaks within the following additional regions were significant at a more lenient threshold of pFDR ≤ 0.05:
No-go > baseline contrast: increased activation: frontal/temporal operculum
No-go > baseline contrast: decreased activation: medial frontal gyrus, VLPFC, medial PFC, OFC
No-go > Go: increased activation: medial frontal gyrus, inferior parietal lobe
No-go > Go: decreased activation: postcentral gyrus, putamen
Correlation of Activation During Go/No-Go Task and BMI
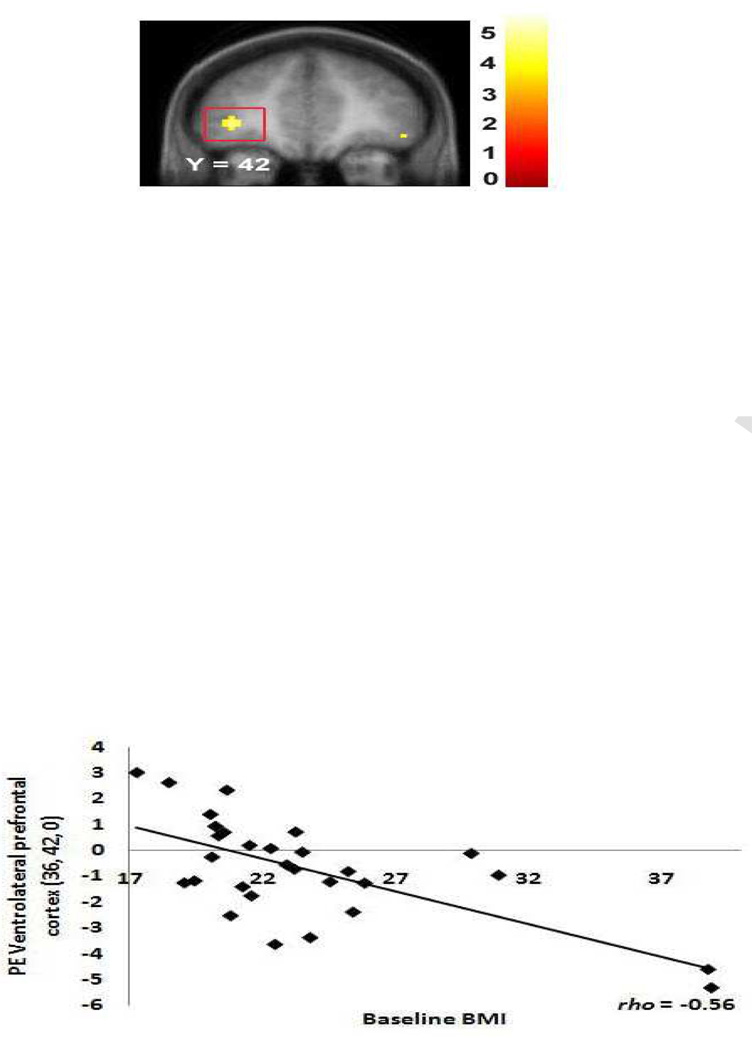
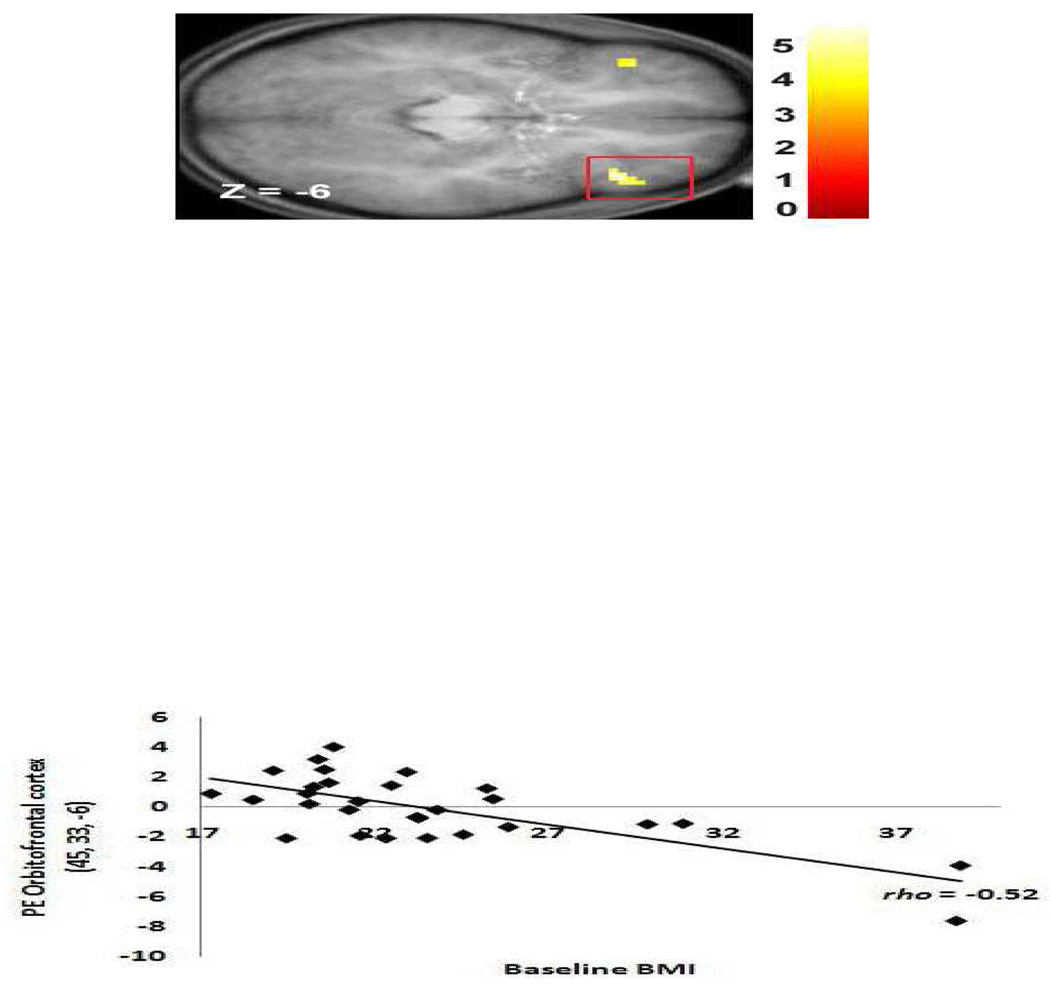
Regression models investigated whether BMI scores (N = 29) showed significant negative correlations with activation in response to no-go trials versus a baseline fixation in regions that have been previously implicated in response inhibition (Nakata et al., 2008). As hypothesized, BMI was negatively related to activation in frontal regions (Table 2), including peaks in superior frontal gyrus (Figure 3), middle frontal gyrus, VLPFC (Figure 4), medial PFC , and OFC (Figure 5). The effect sizes from these analyses were all medium to large per Cohen's (1998) criteria (range rs = −0.39 to −0.79, with a mean rs = −0.57 ).
Table 2.
Regions Showing Activation During no-go Trials Correlated with Body Mass Index (N=29)
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Number of voxels in cluster |
Activation cluster Z |
pFDR corrected |
Effect size rs |
|
|
No-go > Fixation: Negative Correlations Superior frontal gyrus |
−21 | 12 | 57 | 73 | 3.96 | 0.0031 | −0.72**** |
| 21 | 15 | 63 | 3 | 3.84 | 0.003 | −0.53** | |
| 24 | 12 | 54 | 22 | 3.73 | 0.003 | −0.79*** | |
| 9 | 33 | 48 | 3 | 3.39 | 0.005 | −0.61*** | |
| Middle frontal gyrus | 21 | 15 | 60 | 4 | 3.90 | 0.0021 | −0.66*** |
| VLPFC | −36 | 42 | 0 | 16 | 3.93 | 0.0081 | −0.56** |
| Medial PFC | −9 | 54 | −3 | 12 | 4.41 | 0.0021 | −0.53** |
| 6 | 54 | −6 | 13 | 3.81 | 0.006 | −0.52** | |
| OFC | −39 | 33 | −9 | 10 | 3.56 | 0.008 | −0.45* |
| 45 | 33 | −6 | 19 | 4.30 | 0.002 | −0.52** | |
| 45 | 42 | −9 | 19 | 3.32 | 0.008 | −0.39* | |
|
No-go>Fixation: Positive Correlation |
|||||||
| Temporal & frontal operculum/Insula |
51 | 9 | −6 | 81 | 3.99 | 0.003 | 0.60*** |
T-maps are thresholded at p uncorrected < 0.001 with a cluster threshold of 3. Peaks were considered significant at P ≤ 0.008, false-discovery rate (FDR) corrected across the ROI.
p < 0.05 whole brain corrected
Correlation is significant at the 0.001 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
Figure 3.
Coronal section showing less activation in the bilateral superior frontal gyrus (−21, 12, 57, Z = 3.96, pFDR = 0.003) extending into middle frontal gyrus in response to picture of dessert versus baseline fixation as a function of BMI. Although the correlation appears to be driven by 2 outliers, the effect remains significant at the p = 0.05 level when outliers are excluded.
Figure 4.
Coronal section showing less activation in the right VLPFC (36, 42, 0, Z = 3.93, pFDR = 0.008) in response to picture of dessert versus baseline fixation as a function of BMI. Again, exclusion of the 2 apparent outliers still results in a significant effect at the p = 0.05 level.
Figure 5.
Axial section of less activation in a region of the OFC (45, 33, −6, Z = 4.30, pFDR = 0.002) in response to picture of dessert versus baseline fixation as a function of BMI. Again, the effect remains significant at the p = 0.05 level when the two apparent outliers are excluded.
Regression models also tested whether baseline BMI scores (N = 29) showed positive relations to regions that have been previously found to encode the reward value of food in response to no-go trials versus baseline (Stice et al., 2008; Stoeckel et al., 2008). BMI was positively correlated with bilateral temporal operculum activation, which extended to frontal operculum and insula (Table 2). The activation was greater in the right hemisphere, and did not reach statistical significance at our threshold values on the left. This effect size was also in the medium to large range (rs = 0.60). Although there were two apparent outliers that may have driven the baseline BMI-BOLD correlations, the effects remained significant at the p = 0.05 level when the outliers were excluded. BMI was not significantly related to activation, either positive or negative, in the no-go > go contrast.
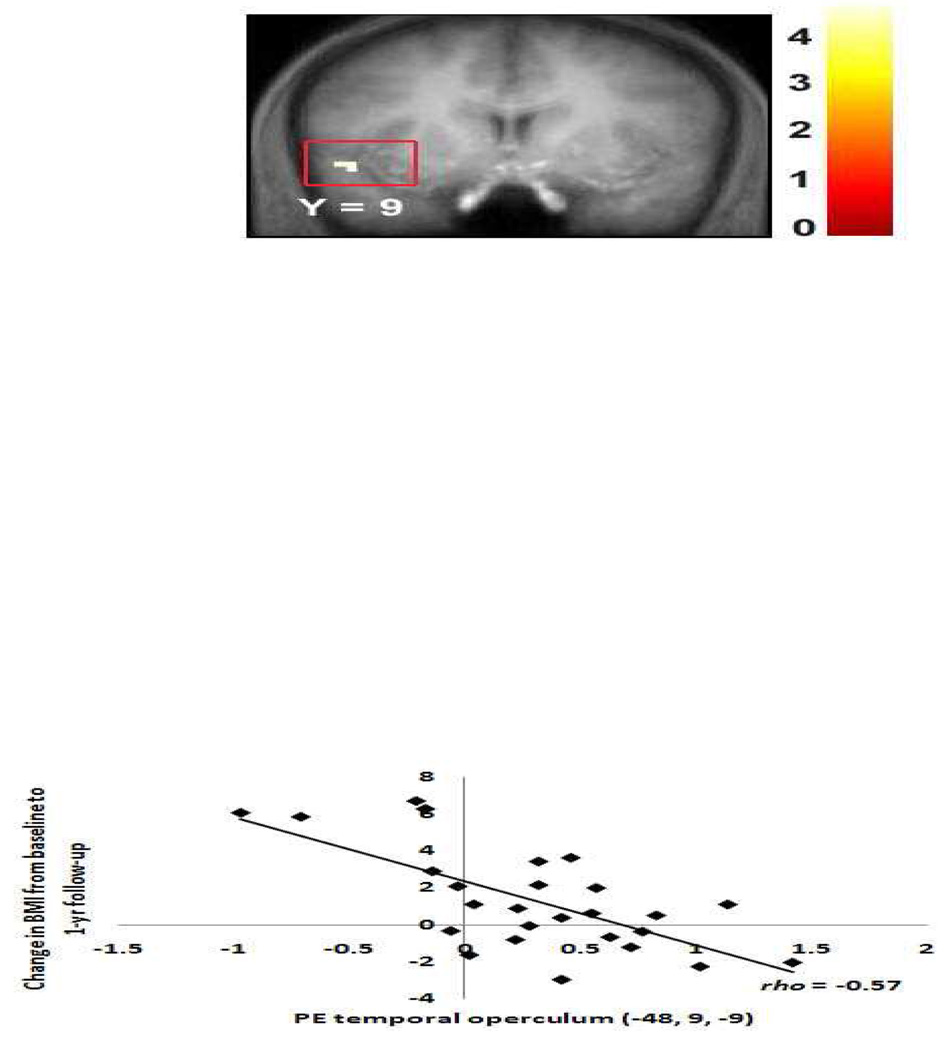
Change in body mass over a 1-year period after controlling for baseline BMI was negatively correlated with activation in the temporal operculum (−48, 9, −9, Z = 4.46, pFDR = .01, rs = −0.57; Figure 6) in the no-go > baseline condition, such that subjects who gained the most weight showed weaker activation in this area. There were no significant positive relations between brain activation and change in BMI at 1-year follow-up in the no-go > baseline condition. There were also no significant relations between brain activation during the no-go > go contrast and future weight change (neither negative nor positive).
Figure 6.
Activation in a region of the temporal operculum (−48, 9, −9, Z = 4.46, pFDR =0.01) in response to dessert versus baseline fixation was related to risk for future weight gain.
Discussion
In the current study, we used a food-specific go/no-go task to investigate behavioral and neural correlates of response inhibition to appetizing food in adolescent girls who ranged from lean to obese. Behaviorally, participants with higher BMI scores responded significantly more quickly to go food stimuli and made a significantly greater number of commission errors, failing to inhibit their responses to the images of desserts. Results suggests that participants with higher BMIs are more willing to sacrifice accuracy for speed compared to their leaner counterparts and consequently have greater difficulty inhibiting prepotent responses to go trials. This general pattern of behavioral results is indicative of lower control of behavioral and greater impulsivity. These findings are consistent with previous studies that have used more general, non-food-specific go/no-go tasks, which have also found that obese children and adults have more difficulty with response inhibition and are more impulsive than lean individuals (Nederkoorn et al., 2006a, 2006b, 2006c). Collectively, these results provide evidence that impulsivity is positively related to overeating and adiposity as measured by BMI.
Results from our fMRI analyses indicate that the correlation between BMI and impulsivity is observable at the neural level as well, with more overweight individuals showing different patterns of brain activation when asked to inhibit prepotent responses to images of appetizing foods compared to their leaner counterparts. Specifically, subjects with higher BMIs appear to less effectively recruit regions involved in response inhibition, particularly within the frontal lobes, including loci within superior frontal gyrus, middle frontal gyrus, VLPFC, medial PFC, and OFC. This pattern of hypoactivation in these regions may contribute to these individuals' greater difficulties with response inhibition. At the same time, individuals with higher BMIs show increased activation in right temporal operculum extending to frontal operculum and insula, which are food reward regions.
Activation During Go/No-Go Task
Across all subjects, no-go trials elicited greater activation relative to go trials in a number of peaks within superior frontal gyrus and inferior frontal gyrus, regions which have previously been shown to play key roles in response inhibition (Nakata et al., 2008). This finding indicates that our food-specific go/no-go task successfully engaged the response inhibition system, recruiting neural areas implicated in inhibitory control as intended. Peaks within two regions that have been implicated in food reward processing, the insula and frontal operculum, were also activated more in the no-go (dessert) condition relative to the go (vegetable) condition and the baseline fixation condition. The operculum and insula play a role in encoding the reward value of food (Small et al., 2001, 2008; Stice et al., 2008; Schur et al., 2009; Rothemund et al., 2007; Stoeckel et al., 2008). Thus our finding of greater activation in these food reward regions to no-go (dessert) trials relative to go (vegetable) trials suggests that, across all subjects, the images of desserts were indeed viewed as more appetizing and rewarding than the images of vegetables, recruiting neural circuitry associated with reward processing as intended. This conclusion is supported by participants' average pre-scan palatability ratings of a subportion of the vegetable and dessert images used in the paradigm. Desserts were rated as appetizing significantly more often than vegetables (t = 3.41, p = 0.002), while vegetables were rated as unappetizing significantly more often than desserts (t = 6.33, p < 0.001). In sum, these results give support for the validity of this new paradigm.
Activation of Inhibitory Control Regions Correlate Negatively with BMI
We observed bilateral activation in superior frontal gyrus, middle frontal gyrus, VLPFC, medial PFC, and OFC that correlated negatively with BMI. As noted, these different regions are part of a distributed frontal lobe inhibitory control network and have been commonly implicated in response inhibition during go/no-go tasks (Buchsbaum et al., 2005; Liddle, Kiehl & Smith, 2001; Mostofsky et al., 2003; Simmonds et al., 2008; Aron & Poldrack, 2005; Horn et al., 2003; Casey et al., 1997). Different regions within this network have been proposed to play different roles in top-down inhibitory control. For example, predominantly left hemispheric regions of a bilateral network of middle and inferior frontal regions are especially important in response selection, while predominantly right areas have been proposed to mediate the withholding of a prepared motor response (Rubia et al., 2001; Chevrier et al., 2007). Similarly, the superior medial frontal cortex has been proposed to directly mediate response inhibition independent of signal monitoring and post-response processing (Li et al., 2006). The VLPFC is involved in the maintenance of information in working memory and low-level control (Wolf, Vasic, & Walter, 2006; Rahm et al., 2006). The OFC has also been implicated in behavioral inhibition through lesion studies (Fuster, 1997; Paradiso, Chemerinsku, Yazici, Tartaro, & Robinson, 1999), and plays a key role in decision-making involving reward and response cost (Bechara, Tranel, & Damasio, 2000).
Activation in many of these regions has been previously shown to correlate negatively with different measures of impulsivity. Self-reported impulsivity has correlated negatively with activation in the right middle frontal gyrus, medial superior frontal gyrus, and bilateral bentral PFC. (Asahi et al., 2004; Brown et al., 2006; Horn et al., 2003). Li et al. (2006a) found that greater activation of left superior medial and precentral frontal cortices correlated with more efficient response inhibition, as measured by shorter stop-signal processing times. Casey et al. (1997) showed a significant negative correlation between number of commission errors and volume of activation in OFC, implying that hypoactivity in this area predisposes individuals to poor response inhibition.
In sum, findings from the present study support the idea that hypoactivity in these frontal regions is related to poorer inhibitory control, and establish direct links between hypoactivation in the response inhibition network, behavioral inhibitory deficits, and predisposition to overweight. Results suggest that, relative to more overweight individuals, leaner participants may more readily recruit neural regions involved in response inhibition, allowing them to more effectively withhold prepotent responses when required to do so.
Activation of Food Reward Regions Correlate Positively with BMI
Although most of the significant activations during no-go trials were found to correlate negatively with BMI, activation in right temporal operculum, extending to frontal operculum and insula, was positively correlated with BMI. The paradigm used in this preliminary study confounded stimulus type (vegetable or dessert) with instruction set (go or no-go) and thus neural activity observed during no-go trials may have been associated with either the perception of images of appetizing food or with response inhibition. Previous evidence, however, suggests that this activation more likely reflects the processing of images depicting appetizing food. For example, Schur et al. (2009) found that viewing photographs of fattening foods compared to nonfood objects resulted in greater activation in the right insular cortex, among other regions, in healthy non-obese women. Rothemund et al. (2007) found greater activation in right anterior insula, among other areas, in response to pictures of high-calorie foods versus a neutral utensil condition in obese relative to lean individuals and also found that BMI correlated positively with activation in this region. Stoeckel et al. (2008) reported greater activity in left insula, among other areas, in response to pictures of high-calories versus low-calorie foods for obese relative to lean individuals. Thus, reward processing associated with viewing images of appetizing food appears to be the most likely explanation for our finding of positive BMI-correlated activation in temporal and frontal operculum and insula. Overweight individuals may be captured by the appetizing characteristics of the food images and engage to a greater extent in reward processing associated with the stimuli. As a result, perhaps they may have fewer neural resources to invest in the experimental task, consequently showing poorer response inhibition.
An alternative explanation to consider, however, is that the insula activation observed is associated with response inhibition rather than food reward processing, as the insula has been implicated previously in go/no-go tasks (Nakata et al., 2008; Mostofsky, 2003, Simmonds, 2008). Some evidence suggests that enhanced activation in the insula during response inhibition tasks is characteristic of subjects with greater difficulties in inhibitory control, consistent with our finding. For example, Horn et al. (2003) reported that right anterior insula activation during response inhibition was positively correlated with self-reported impulsivity. Similarly, Pliszka et al. (2006) found that children and adolescents with ADHD activated the right insula during response inhibition, while control subjects did not show activation in this area. Thus, another possibility is that our observed insula activation, positively correlated with BMI, represents a neural correlate of inhibitory control deficits in overweight relative to leaner individuals during response inhibition.
Prospective BMI Results
We found no evidence that either behavioral or neural measures of response inhibition predict weight gain at one-year follow-up. Change in body mass over a 1-year period was not significantly correlated with any behavioral measures of response inhibition, nor was it correlated with any peaks that correlated negatively with baseline BMI. The only finding to reach significance was a negative correlation between weight change after one year and activation in left temporal operculum. This area plays a role in reward processing associated with appetitive food stimuli. The finding that subjects who show the most weight gain over one-year follow-up show weaker activation in this area accords with the reward deficit model of obesity, which posits that people who experience weaker activation of dopamine-based reward circuitry overeat to compensate for this reward deficit (Blum et al., 1996). It is possible that we did not observe more effects because we had limited power to detect prospective effects in this preliminary study. The present study was sufficient to detect only large effects (e.g., power = 0.81 for r = 0.50), and was not adequate to detect medium effects (power = 0.36 for r = 0.30) or small effects (power = 0.08 for r = 0.10). Thus, given this limited power, effect sizes for behavioral and neural predictors of changes in BMI were likely not large enough to be detected. Future studies with a larger number of participants and a longer follow-up period should be carried out to examine whether inhibitory control differences at either the behavioral or neural level predict subsequent weight gain.
Limitations and Future Directions
There are several limitations to this study that should be noted. First, data from several of our subjects could not be analyzed due to excessive head movements in the scanner, resulting in a smaller sample than originally anticipated. By reducing the number of subjects in the study, statistical power was also reduced, making it more likely that certain a priori expected results, such as BMI-correlated activations in the inferior frontal gyrus and inferior parietal lobe (Nakata et al., 2008), were not found to be significant. Although our sample size was larger than most previously published neuroimaging studies that compared obese to lean individuals and a number of significant relations were observed, future studies with larger samples should attempt to replicate these relations. Second, our sample is limited to adolescent girls and thus results may not be generalizable to other demographic groups, particularly given the evidence that there may be gender and age-related differences in activation patterns (e.g., Casey et al., 1997; Li, Huang, Constable, & Sinha, 2006). Third, as noted, in this preliminary food-specific go/no-go paradigm, go trials always consisted of the presentation of a vegetable, while no-go trials always consisted of the presentation of a dessert. Thus, instruction type (go or no-go) and stimulus type (vegetable or dessert) were confounded. Activation during no-go trials may have been caused either by the processing of appetizing food images or by response inhibition. Although this design was adopted to maximize the number of trials requiring inhibition of prepotent responses to appetizing food, representing our main condition of interest, future research might incorporate a balanced design to answer other related research questions. By disentangling instruction type and stimulus type, subsequent research will be able to clarify whether similar results are observed when subjects are instructed to inhibit their responses to less palatable, low-calorie foods such as vegetables. Such future studies could also parse which activations are caused by the processing of appetizing food images and which are solely accounted for by response inhibition.
Conclusions
In sum, our neuroimaging findings indicate that BMI is negatively correlated with activation throughout diverse loci in the frontal lobes, including superior frontal gyrus, middle frontal gyrus, VLPFC, medial PFC, and OFC, when subjects are required to inhibit prepotent responses to appetizing foods. As these areas have been shown to play a key role in response inhibition, one explanation for this observation is that recruitment of the neural circuitry underlying inhibitory control occurs to a lesser extent in more overweight individuals. Thus, the lower activation can be interpreted as a neurofunctional correlate of impulsivity and poor inhibitory control, traits which have been previously shown to contribute to unhealthy eating patterns and weight gain. At the same time, more overweight individuals show greater activation in certain food reward areas, suggesting that they may be "captured" by the hedonic reward aspect of appetizing food presentations, recruiting more neural resources to process the appetitive characteristics of these stimuli at the expense of completing the experimental task, response inhibition. These two elements, increased sensitivity to appetitive stimuli and deficits in inhibitory control, would be expected to predict poorer performance in our experimental task as well as to increase the risk of problems in real-life situations that require inhibitory control, such as resisting the temptation of appetizing, high-calorie food.
These findings contribute to our understanding of the link between impulsivity and obesity. Individuals whose response inhibition neural networks are recruited to a lesser extent during response inhibition, or who preferentially engage in the processing of food reward stimuli, may be at risk for impulsive behavior, including overeating and making unhealthy food choices. By elucidating the neural basis of risk factors underlying obesity, we may contribute towards reaching a better understanding of overweight and obesity and to designing and implementing effective interventions and treatments to combat this prevalent problem. For instance, it is possible that cognitive training to improve recruitment of regions involved in response inhibition may reduce the frequency of intake of high-fat foods.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura Batterink, University of Oregon.
Sonja Yokum, Oregon Research Institute.
Eric Stice, Oregon Research Institute.
References
- Aron AR, Poldrack RA. The Cognitive Neuroscience of Response Inhibition: Relevance for Genetic Research in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related Changes in Frontal and Temporal Lobe Volumes in Men: A Magnetic Resonance Imaging Study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of foods. Journal of Neuroscience. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara D, Tranel H, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato DP, Boland FJ. Delay of gratification in obese children. Addictive Behaviors. 1983;8:71–74. doi: 10.1016/0306-4603(83)90059-x. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Braet C, Claus L, Verbeken S, van Vlierberghe L. Impulsivity in overweight children. European Child & Adolescence Psychiatry. 2007;16(8):473–483. doi: 10.1007/s00787-007-0623-2. [DOI] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-Analysis of Neuroimaging Studies of the Wisconsin Card-Sorting Task and Component Processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schacharr R. Dissociation of Response Inhibition and Performance Monitoring in the Stop Signal Task Using Event-Related fMRI. Human Brain Mapping. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and obesity. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dietz WH, Robinson TN. Use of body mass index (BMI) as a measure of overweight in children and adolescents. The Journal of Pediatrics. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. New York: Raven Press; 1997. [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007a;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007b;48:119–122. doi: 10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Bjorvell H, Levander S, Rossner S. Personality traits predicting weight loss outcome in obese patients. Acta Psychiatrica Scandinavica. 1986;74:384–387. doi: 10.1111/j.1600-0447.1986.tb06258.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R. Imaging Response Inhibition in a Stop-Signal Task: Neural Correlates Independent of Signal Monitoring and Post-Response Processing. Journal of Neuroscience. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006b;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach Atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cognitive Brain Research. 2003;15:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, Ferretti A, Perrucci MG, Del Gratta C, Kakigi R, Romani GL. Somato-motor inhibitory processing in humans: An event-related functional MRI study. NeuroImage. 2008;39:1858–1866. doi: 10.1016/j.neuroimage.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: The role of impulsivity. Eating Behaviors. 2006a;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy. 2006b;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders F, Havermans R, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006c;47:253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Pannacciulli M, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: A voxel-based morphometric study. NeuroImage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Chemerinski E, Yazici KM, Tartaro A, Robinson RG. Frontal lobe syndrome reassessed: Comparison of patients with lateral or medial frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1999;67:664–667. doi: 10.1136/jnnp.67.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, Xiong J, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. American Journal of Psychiatry. 2006;163:957–960. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Rahm B, Opwis K, Kaller CP, Spreer J, Schwarzwald R, Seifritz E, et al. Tracking the subprocesses of decision-based action in the human frontal lobes. NeuroImage. 2006;30:656–667. doi: 10.1016/j.neuroimage.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H, Klingeblel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rydén A, Sullivan M, Torgerson JS, Karlsson J, Lindroos A-K, Taft C. Severe obesity and personality: A comparative controlled study of personality traits. International Journal of Obesity. 2003;27:1534–1540. doi: 10.1038/sj.ijo.0802460. [DOI] [PubMed] [Google Scholar]
- Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. International Journal of Obesity advance online publication 14 April 2009. 2009 doi: 10.1038/ijo.2009.56. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation Between Obesity and Blunted Striatal Response to Food is Moderated by TaqIA A1 Allele. Science. 2008;332:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small DM. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008;117:924–934. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward Circuitry Responsivity to Food Predicts Future Increases in Body Mass: Moderating Effects of DRD2 and DRD4. Neuroimage. doi: 10.1016/j.neuroimage.2010.01.081. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JF. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crvello F, Etard O, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, Pradhan K. Inverse Association Between BMI and Prefrontal Metabolic Activity in Healthy Adults. Obesity. 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Walter H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia. 2006;44:2558–2563. doi: 10.1016/j.neuropsychologia.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. Development of executive function in childhood. In: Nelson C, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2008. [Google Scholar]