Fibrotic disorders and aging
Human fibrotic disorders affect a number of organ systems, including the cardiovascular system, the liver, kidneys and lungs. Fibrosis is a major cause for organ failure and death; it has been estimated to contribute to 45% of all cause mortality in the U.S. (Wynn, 2004). Fibrosis can be viewed as an “adaptive” physiological response to epithelial/endothelial injury when it is self-limited as in normal wound healing, a process that has been well studied in the skin (Martin, 1997). However, fibrosis is more commonly referred to in its pathological context, an irreversible, progressive, and ultimately fatal disease process. Fibrosis may occur at any age; however, its pathological occurrence appears to increase with advancing age. For example, the diagnosis of idiopathic pulmonary fibrosis (IPF) is rarely made in patients less than 40 years of age; both the incidence and prevalence of IPF markedly increase with age (Raghu et al., 2006).
The mechanisms that link aging with fibrosis are not well understood. Age-related diseases may be associated with telomere dysfunction (Armanios, 2009). Shortening of telomeres may result in reduced capacity for stem cell renewal and cellular senescence. Recent studies of familial and sporadic cases of IPF have been associated with telomere shortening (Tsakiri et al., 2007, Alder et al., 2008, Cronkhite et al., 2008), supporting the concept that IPF may represent an age-related degenerative disease process (Thannickal and Loyd, 2008). The cause(s) for shortened telomeres in the absence of telomerase mutations in IPF patients is currently unknown; however, oxidative stress represents at least one potential mechanism (Tchirkov and Lansdorp, 2003). Telomere shortening may lead to cellular senescence that influences the phenotypes and fates of epithelial and mesenchymal cells that reside in the adult (aging) lung. Animal studies suggest that the induction of a fibrotic response to injury requires telomerase activity, presumably through its effects on mesenchymal cells (Liu et al., 2007). The relationships between telomeres, telomerases, and cellular senescence and roles in determining cellular phenotypes/fates of tissue-resident progenitor cells require further study.
Asking the “why” question
Traditionally, we have addressed the problem of fibrosis by focusing on the what (definition – biochemical, morphological, and physiological) and the how (pathogenesis – genes/proteins and signaling pathways that mediate its formation). We have largely ignored the why question. Our current approaches, while providing an understanding of complex biological process of tissue repair and fibrosis in animal models of acute injury, have failed to identify a target that has translated into an effective drug therapy for human fibrotic disorders. A teleological understanding of why fibrosis develops or why it was evolutionarily conserved in mammals may be informative in defining optimal therapeutic targets that lead to successful drug discovery.
The basis of evolutionary theory is the concept that acquired and heritable variation of biological traits between individuals will select for individuals who are able to survive in a given environment. We speculate that “wound healing-fibrosis” represents a complex biological trait that provided a selective advantage for the survival of early vertebrates against various forms of tissue injury “in the wild”, including predation. Survival of these species likely necessitated the capacity to engender a primitive, conserved wound healing response that forms an initial barrier (coagulation-fibrosis) to prevent rapid blood loss and restrict pathogen invasion/spread. A beneficial role for a “fibrotic-type reaction” appears to be a key component of host defense in plants, the so-called “plant hypersensitivity response” which induces crosslinking of extracellular matrix proteins to form a barrier around pathogens at the site of contact (Levine et al., 1994). Similarly, the fibrotic “walling off” of bacterial lung abscesses, and of Mycocbacterium tuberculosis and fungal infections, may serve to restrict the spread of these pathogens in the host. These may also be considered examples of “evolutionary trade-offs” that would have allowed for host survival against invading infectious agents, albeit at the expense of loss of tissue structure/function.
Identification of Nox4 as a pro-fibrotic gene
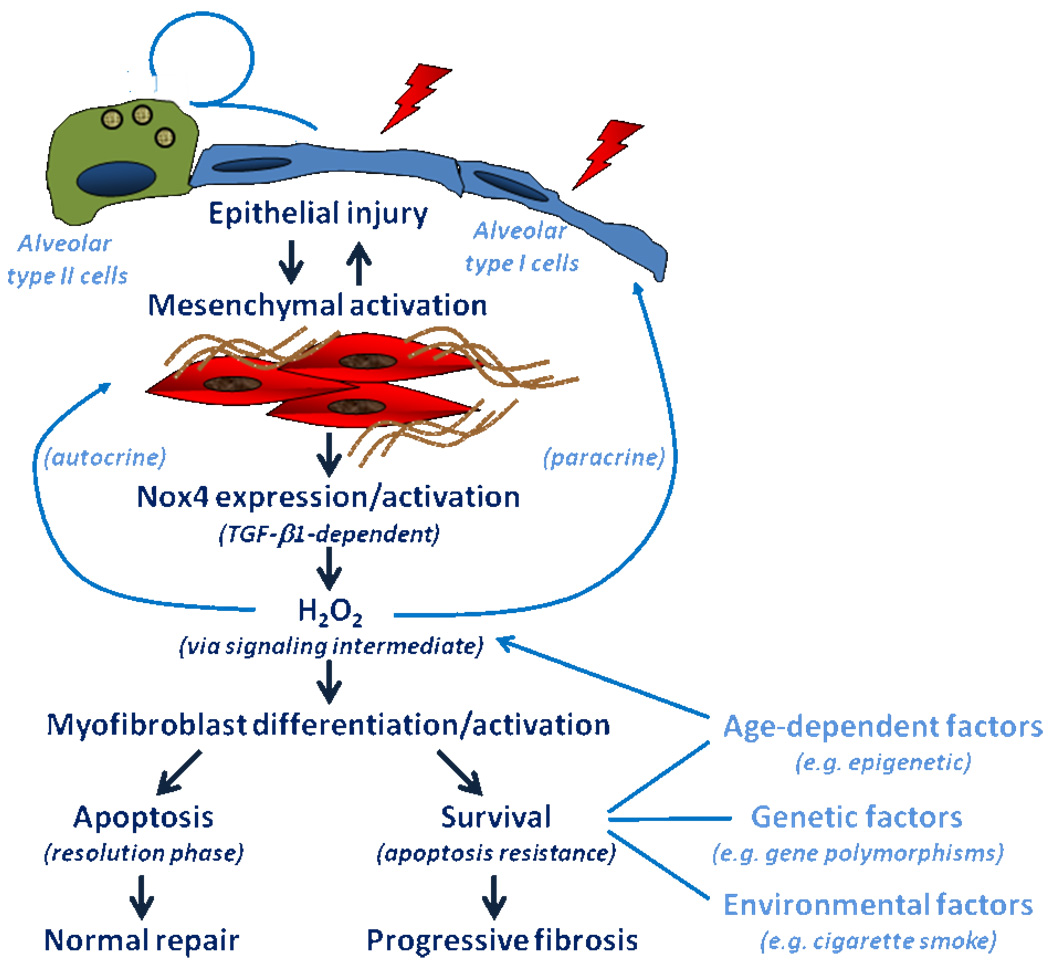
Our group has recently identified NADPH oxidase 4 (Nox4) as a critical regulator of the fibrotic response to acute lung injury in mammalian tissues. Nox4 was identified as one of the most highly induced genes in transforming growth factor-β1 (TGF-β1)-stimulated human lung mesenchymal cells by whole genome transcriptomal (Affymetrix) analysis (Hecker et al., 2009). Nox4, a member of reactive oxygen species (ROS)-generating Nox gene family, mediates myofibroblast differentiation, extracellular matrix (ECM) synthesis, and contractility of human lung fibroblasts isolated from human subjects with IPF (Fig. 1); it is highly expressed in myofibroblastic foci of IPF lung tissues (Hecker et al., 2009). Pharmacologic and genetic targeting of Nox4 protects against fibrogenesis in two different murine models of lung injury (Hecker et al., 2009).
Figure 1. The proposed role of Nox4 in myofibroblast differentiation/activation and factors that influence alveolar epithelial cell injury-repair.
Injury to the alveolar epithelium is typically accompanied by a regenerative response involving the differentiation of “cuboidal” alveolar type II cells to “squamous” alveolar type I cells that constitute >95% of the alveolar surface lining. This regenerative response is facilitated by a provisional extracellular matrix elaborated by tissue-resident mesenchymal cells. Myofibroblasts are a distinct subpopulation of mesenchymal cells with contractile properties and that differentiate in response to transforming growth factor-β1 (TGF-β1)-induced activation of NADPH oxidase isoform 4 (Nox4). Apoptosis and clearance of myofibroblasts facilitate the resolution of the repair process, leading to restitution of normal alveolar structure/function. The persistence of activated myofibroblasts (due to the acquisition of apoptosis-resistant and/or proliferative phenotypes) leads to progressive fibrosis that characterizes a number of human fibrotic diseases, including idiopathic pulmonary fibrosis. The inability to “deactivate” myofibroblasts may be related to a number of host (age, epigenetic and genetic factors) and environmental factors. Age may also influence the susceptibility of the alveolar epithelium to injury/apoptosis, thus impairing normal alveolar regeneration.
Is Nox4 an example of antagonistic pleiotropy?
The theory of antagonistic pleiotropy, originally proposed by George Williams to explain senescence (Williams, 1957), proposes that some genes are beneficial at earlier ages but harmful at later ages. Accordingly, the theory of antagonistic pleiotropy is based on two assumptions: (1) a particular gene has more than one “trait” in an organism (pleiotropy); and (2) the pleiotropic effects of the gene affect organismal fitness in an antagonistic manner (beneficial in early life and detrimental in later life). Such genes will be maintained in the population and “conserved” through evolution due to their positive effects on fitness/survival at young age, despite their negative effects at post-reproductive, older age. We speculate that Nox4 may function in an antagonistically pleiotropic manner; providing a beneficial role by mediating myofibroblast differentiation to promote wound “closure” and normal healing in early age, while playing a detrimental role by mediating myofibroblast persistence, epithelial injury and progressive fibrosis in later life (Thannickal, 2009, Hecker et al., 2009). The precise mechanisms for its putative role in progressive fibrotic reactions in association with aging require further study. Such studies might include not only the autocrine effects of Nox4-mediated ROS generation on mesenchymal cells, but its potential paracrine effects on adjacent reparative epithelial cells susceptible to senescence-related phenotypes and/or apoptosis (Waghray et al., 2005). Increasing complexity of multicellular eukaryotes has been associated with expanding diversification of the Nox gene family (Kawahara et al., 2007, Thannickal, 2009). Although many physiological functions have been attributed to Nox enzymes, the best established role for this gene family is in host defense against invading pathogens in almost all species studied. The prototypical and best characterized member of this family, Nox2 (also known as gp91phox), is critical for host defense against infectious microbes. Interestingly, Nox4 emerged later during multicellular evolution in chordates (Kawahara et al., 2007, Sumimoto, 2008). We propose a related, parallel “host defense” function for Nox4 that appears to be fundamentally involved in wound repair functions, and that also mediates age-associated fibrotic disorders. Therapeutic targeting of this “antagonistically pleiotropic” gene may prove be an effective strategy for these recalcitrant human disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, Choi YY, Ishikawa F, Phan SH. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Tchirkov A, Lansdorp PM. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum Mol Genet. 2003;12:227–232. doi: 10.1093/hmg/ddg023. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol. 2009;40:507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Loyd JE. Idiopathic pulmonary fibrosis: a disorder of lung regeneration? Am J Respir Crit Care Med. 2008;178:663–665. doi: 10.1164/rccm.200807-1127ED. [DOI] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]