Abstract
Chronic stress is a risk factor for many psychopathological conditions, including depression and anxiety disorders. Cognitive impairments associated with prefrontal cortical dysfunction are a major component of such illnesses. Using an attentional set shifting test (AST), we have previously shown that elevating noradrenergic activity in rat medial prefrontal cortex (mPFC) can facilitate cognitive set-shifting, and that chronic unpredictable stress (CUS) caused set-shifting deficits. It is not known, however, if noradrenergic modulatory function is compromised by chronic stress, perhaps contributing to the stress-induced cognitive deficit. Thus, the first study investigated whether acutely elevating noradrenergic activity in mPFC still enhances cognitive function after chronic stress. As previously demonstrated, CUS impaired cognitive set-shifting on the AST. This deficit was abolished by acute systemic administration of the α2-adrenergic autoreceptor antagonist, atipamezole. Microdialysis revealed no differences in extracellular norepinephrine (NE) levels in mPFC of CUS-exposed and unstressed control rats at baseline or during behavioral testing, and comparable increases after atipamezole. In the second experiment, rats were treated chronically with the selective NE reuptake blocker, desipramine, during the CUS treatment through behavioral testing. Again, CUS impaired cognitive set-shifting in vehicle-treated rats, and chronic desipramine treatment prevented such deficits. Acute blockade of post-synaptic α1-adrenergic receptors in mPFC prior to testing blocked the beneficial effect of desipramine on cognitive set-shifting. These results suggest that desipramine restores cognitive set-shifting capability that has been compromised by chronic stress by activating α1-adrenergic receptors in the mPFC. Thus, noradrenergic modulatory capability in mPFC remains intact after CUS, and this represents one possible substrate by which antidepressants may exert their beneficial effects in the treatment of depression.
Keywords: antidepressant, attentional set-shifting, cognitive flexibility, depression, norepinephrine, stress
Introduction
Cognitive impairments associated with prefrontal cortical dysfunction are major components of depression, anxiety disorders and other stress-related psychiatric illnesses (Anisman and Matheson, 2005; Beck, 1976; Beck et al., 1987; Fossati et al., 1999). The medial prefrontal cortex (mPFC) is particularly important in cognitive set-shifting and flexibility, i.e., using feedback from the environment to modify established behavioral patterns in an appropriate manner (Dalley et al., 2004; Ragozzino et al., 1999). Depressed patients exhibit cognitive flexibility deficits and mPFC hypoactivity (Austin et al., 2001; Beats et al., 1996; Davidson et al., 2002; Rogers et al., 2004). The attentional set-shifting test (AST) was developed to assess such prefrontal cortical executive capabilities in rats (Birrell and Brown, 2000). Lesions of rat mPFC specifically impaired cognitive set-shifting on the AST, confirming a function analogous to that of mPFC in humans and non-human primates (Dalley et al., 2004; Dias et al., 1996; Owen et al., 1991; Ragozzino et al., 1999).
The brain noradrenergic (NA) system modulates cognitive function in contexts such as arousal, attention, learning and memory (Berridge et al., 1993; Cole and Robbins, 1992; Devauges and Sara, 1990). We have previously shown that enhancing NA tone by acute systemic administration of the α2-adrenergic autoreceptor antagonist, atipamezole, can improve cognitive set-shifting on the AST, and that this effect was blocked by microinjections of the α1-adrenergic receptor antagonist, benoxathian, directly into mPFC (Lapiz and Morilak, 2006). Norepinephrine (NE) also plays an important role in the physiological and behavioral responses to stress (Aston-Jones et al., 1991; Morilak et al., 2005). Thus, dysregulation of NE has been implicated in the stress-related etiology of depression and anxiety disorders (Ressler and Nemeroff, 2000; Schatzberg and Schildkraut, 1995), and NE reuptake blockers are beneficial in their treatment (see Morilak and Frazer, 2004).
Unpredictable stress has greater negative impact than predictable stress (see Anisman and Matheson, 2005; Willner and Mitchell, 2002). Thus, to study possible mechanisms underlying stress-induced cognitive dysfunction in rats, we have employed a chronic unpredictable stress (CUS) model (Lu et al., 2006; Willner and Mitchell, 2002). CUS was shown to induce cognitive set-shifting deficits that were prevented by chronic treatment with the selective NE reuptake blocker, desipramine (DMI) (Bondi et al., 2008b). However, the mechanisms underlying the beneficial effects of DMI are not known, nor is it known if the CUS-induced cognitive deficit is associated with a loss of noradrenergic modulatory capability in mPFC. Thus, the purpose of experiment 1 was first to determine if CUS compromised noradrenergic modulatory function in mPFC, specifically whether the CUS-induced cognitive deficit was associated with a change in NE release evoked in mPFC, and whether acute pharmacological elevation of NE in mPFC is still capable of enhancing cognitive function after CUS. If noradrenergic facilitation of cognitive flexibility in mPFC remains intact after chronic stress, then this may serve a neuroadaptive function, helping to overcome the detrimental effects of chronic stress. Additionally, it may represent a substrate by which antidepressant drugs that block NE reuptake can exert their beneficial effects on cognition. Thus, to further examine potential mechanisms underlying antidepressant drug effects, experiment 2 tested the hypothesis that post-synaptic α1-adrenergic receptors in mPFC are responsible for the beneficial effects on cognition of chronic NE reuptake blockade during CUS. Portions of this work have been presented in abstract form (Bondi et al., 2008a).
Experimental Procedures
Animals
A total of 124 adult male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), weighing 200–250 g upon arrival, were used. They were initially housed in groups of three in 25 × 45 × 15 cm plastic cages, and maintained on a 12/12 h light/dark cycle (lights on at 0700 h), with food and water available ad libitum. Rats were acclimated to the housing facility for at least 4 days prior to any experimental procedures. For the social defeat procedure, male Long-Evans rats (Harlan, Indianapolis, IN, USA), weighing 400–450 g, were used as resident defeaters. They were each paired with an ovariectomized female and housed in a separate room in larger cages (80 × 55 × 40 cm), on the same light-dark cycle as the experimental animals. All experiments were carried out during the light portion of the cycle, between 0800–1700 h. All procedures were conducted according to NIH guidelines for the care and use of laboratory animals, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. All efforts were made to minimize animal pain, suffering or discomfort, and to minimize the number of rats used.
Chronic unpredictable stress (CUS)
The CUS procedure was conducted as described previously (Bondi et al., 2008b) with slight modifications for rats subjected to cranial implant surgery, as outlined below. Depending on the pretreatments required for each experiment, animals were individually housed 4–7 days prior to initiation of CUS. To maximize unpredictability, different stressors were applied daily, at different times of day (between 0800–1700 hr) for 14 consecutive days (see Table 1). All procedures were performed in an isolated room adjacent to the housing facility, requiring minimal transport and handling of the animals. After each stressor, rats were allowed to recover for 1 h in a separate room, following which they were transferred to clean cages with fresh bedding and returned to housing. Unstressed control animals were housed individually for the same period of time, and were handled daily for 30 s in the housing room.
Table 1.
Treatment Schedule for Chronic Unpredictable Stress
| Day 1 | 30-min restraint |
| Day 2 | 1-hr shaking/crowding |
| Day 3 | Social defeat |
| Day 4 | 10-min tail pinch in restrainer |
| Day 5 | 24-h wet bedding |
| Day 6 | Social defeat |
| Day 7 | 1-hr shaking/crowding |
| Day 8 | 15-min mild footshock |
| Day 9 | 30-min restraint |
| Day 10 | Social defeat (begin food restriction for AST) |
| Day 11 | 15-min mild footshock |
| Day 12 | 10-min tail pinch in restrainer |
| Day 13 | 24-h wet bedding |
| Day 14 | 15-min mild footshock |
| Day 15 | Habituation Day for AST |
| Day 16 | Training Day for AST |
| Day 17 | Testing Day for AST |
Treatment schedule for chronic unpredictable stress, including timing of the attentional set-shifting test. Stereotaxic surgery to implant microdialysis guide cannulae was performed 4–5 days prior to beginning CUS. For rats subjected to chronic antidepressant drug treatment and microinjections, stereotaxic surgery and osmotic minipump implantation were performed 6–8 days prior to beginning CUS. In experiment 1, for rats not subjected to stereotaxic surgery, swim stressors were administered in place of one each of the social defeat, tail pinch and restraint stressors, as in the previously published protocol (Bondi et al., 2008b).
For restraint stress, rats were placed for 30 min in a restraining tube made of Plexiglas and flexible nylon, secured by two Velcro strips, which restricted movement but allowed free respiration and air circulation. Shaking-crowding consisted of placing 6 rats in a plastic box for 1 hour atop a lab shaker set to generate 220 back-and-forth movements (approximately 2 inch lateral deflection) per min. The resident-intruder social defeat was carried out as described previously (Nikulina et al., 2005; Ruis et al., 1999). Prior to social defeat, the female was removed and the “intruder” Sprague-Dawley rat was introduced into the resident cage. Generally within approximately 2 min, the resident attacked and pinned the intruder. Once a full submissive posture occurred (motionless on the back for 4 sec), the intruder was placed under a small protective wire mesh cage (30 × 15 × 15 cm) in the resident cage for 45 min, which allowed for continuing olfactory, visual, and auditory contact between the two rats, but no further physical contact. At the end of the session, the experimental rat was placed back in its home cage and the ovariectomized female returned to the resident cage. Wet bedding consisted of 700–800 ml of water poured into the home cage, enough to saturate the bedding for 24 h. Footshock entailed 1.5 mA scrambled shock delivered through the grid floor of an isolated shock-chamber (5 cycles of: 5 s on/5 s off, repeated three times, then 155 s off, for a total of 15 min). Tail pinch involved placing the rat in the restraining device described above, and applying a clothespin 1 cm from the base of the tail for 10 min. Warm swim and cold swim were accomplished by placing the rat in a cylindrical tank (60 cm height × 30 cm diameter) filled with water to a 30 cm depth at 25 or 18° C, respectively. To avoid potential wound infection in rats subjected to stereotaxic surgery, the swim stressors were replaced with additional social defeat, tail pinch and restraint stimuli.
Attentional set-shifting test (AST)
Procedures for the AST were as described previously (Lapiz and Morilak, 2006), adapted from (Birrell and Brown, 2000). One week prior to behavioral testing (i.e., on day 10 of CUS), both CUS and unstressed control rats were restricted to 14 g of food per day, with water freely available. The testing apparatus was a custom-built rectangular wooden arena (width × length × height: 45 × 71 × 22 cm), painted white on all surfaces. A removable white divider separated one-third of the arena forming a start box, which was also used as a holding area between trials to allow the experimenter to clean the arena and change the pots. To begin each trial, the rat was given access to the arena by lifting the divider. A clear Plexiglas panel divided the opposite third of the arena into two sections, with one digging pot in each section. This separation enabled the experimenter to quickly remove the rat following a response. The terracotta digging pots (internal rim diameter 7 cm; depth 6 cm), were each defined by a pair of cues along two stimulus dimensions, the digging medium with which the pot was filled and an odor (see Table 2). To mark each pot with a distinct odor, two drops (10 μl/drop) of scented aromatic oil (Frontier Natural Brands, Boulder, CO, USA) were applied to the inner rim at least 5 days prior to use. Then, 1–2 μl were reapplied daily to maintain consistent odor intensity. A different pot was used for each combination of digging medium and odor, and only one odor was ever applied to a given pot. The bait, a 1/4 Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA), was buried 2 cm below the surface of the digging medium in the “positive” pot. A small quantity of powdered Cheerio dust was sprinkled over the digging media in both pots prior to beginning each task, to prevent the rat from locating the bait by smell rather than by learning the discrimination.
Table 2.
Attentional set-shifting test protocol
| Discrimination Stage | Dimensions | Example Combinations | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove/Sawdust | Nutmeg/Sawdust | |
| Compound (CD) | Odor | Medium |
Clove/Raffia Clove/Metallic Filler |
Nutmeg/Metallic Filler Nutmeg/Raffia |
| Reversal 1 (R1) | Odor | Medium |
Nutmeg/Raffia Nutmeg/Metallic Filler |
Clove/Metallic Filler Clove/Raffia |
| Intradimensional Shift (ID) | Odor | Medium |
Rosemary/Wood balls Rosemary/Plastic beads |
Cinnamon/Plastic beads Cinnamon/Wood balls |
| Reversal 2 (R2) | Odor | Medium |
Cinnamon/Plastic beads Cinnamon/Wood balls |
Rosemary/Wood balls Rosemary/Plastic beads |
| Extradimensional shift (ED) | Medium | Odor |
Velvet/Citronella Velvet/Thyme |
Crepe/Thyme Crepe/Citronella |
| Reversal 3 (R3) | Medium | Odor |
Crepe/Thyme Crepe/Citronella |
Velvet/Citronella Velvet/Thyme |
Representative examples of stimulus pairs, and the progression through the stages of the attentional set-shifting test. In this example, odor is the initial discriminative dimension, shifting to digging medium in the ED stage. For each task, the positive stimulus is in bold, and is paired randomly across trials with the two stimuli from the irrelevant dimension.
Digging was defined as a vigorous displacement of the medium to retrieve the reward buried within the pot. Simply investigating the rim of the pot or the surface of the digging medium with paws or snout without displacing material was not considered a dig, and the trial continued until a dig response was performed. Therefore, rats were able to access tactile, visual and olfactory characteristics of the pots to make their choices. Beginning on the day after the final stress session, the behavioral procedure entailed 3 days:
Day 1, Habituation
Rats were first trained to dig reliably in pots to obtain a food reward. Two unscented pots were placed in the home cage and re-baited every 5 min, with the Cheerio covered by increasing amounts of sawdust (3 times with no sawdust, 3 times with the pots one-third full, 3 times half-full and 3 times completely full). Once the rat was digging reliably, it was transferred to the testing arena and given three trials to retrieve reward from both sawdust-filled pots.
Day 2, Training
The following day, animals were trained on a series of simple discriminations (SD), to a criterion of six consecutive correct trials. First, animals learned to associate the food reward with an odor cue (lemon vs. rosewood, both pots filled with sawdust). After reaching criterion for the odor discrimination, new unscented-pots were introduced, and the rats had to learn to discriminate between digging media (felt strips vs. shredded-paper, no odor present). All rats were trained using the same pairs of stimuli, and in the same order. The positive and negative cues for each rat were randomly determined and equally represented. These training exemplars were not used again during testing. Any rat that failed to complete this training procedure was eliminated from subsequent testing.
Day 3, Testing
Rats were then tested on a series of increasingly difficult discrimination tasks (Table 2). To proceed to the next task, the rat had to reach a criterion of six consecutive correct trials. At each testing stage, the discriminative stimulus dimension and the positive cue within that dimension were varied according to the contingency schedule shown in Table 2. The first stage was a SD, again involving only one stimulus dimension (i.e. odor or medium). Half the animals in each group were required to discriminate between sawdust-filled pots differentiated by two odors, only one of which was associated with reward. For the other half, the first discrimination was between digging media in unscented pots (for clarity, the remainder of this description will consider only the example beginning with an odor discrimination). The second task was a compound discrimination (CD), in which the same discrimination was required (e.g., odor), and the second, irrelevant stimulus dimension (e.g., medium) was introduced. As in the SD task, only one odor was associated with reward, but two different digging media were paired randomly with the odors. The third stage was a reversal (R1) of the previous discrimination, in which the same odors and media were used. Odor remained the relevant dimension, however the negative odor from the previous stage became positive (i.e., was associated with reward), and the positive odor from the previous stage became negative (no reward). The fourth stage was an intra-dimensional (ID) shift, a new acquisition in which all new stimuli (odors and media) were introduced. Again, odor remained the relevant dimension and digging medium was still irrelevant. The fifth stage was a reversal of this discrimination (R2), in which the previously negative odor became positive, similar to R1. The sixth task required an extra-dimensional (ED) cognitive set-shift, in which all new stimuli were again introduced, but this time the dimension that had been repeatedly reinforced up to this point as the informative, relevant dimension (thus forming a “cognitive set”) was now irrelevant, and the previously distracting dimension, i.e., the digging medium, became the relevant dimension. Finally, the seventh stage was another reversal (R3), where the previously negative stimulus became positive, as in the previous reversals. The assignment of each exemplar in a pair as being positive or negative in a given stage, as well as the left-right positioning of the pots in each trial, were determined randomly in advance. The dependent measure was the number of trials required to reach criterion of six consecutive correct responses (Trials to Criterion, TTC) for each stage of the test.
Animals were allowed 10 minutes to make a choice on each trial. If a choice was not made within this interval, the trial was scored as an error and the rat was returned to the start box. Animals failing to make a choice on six consecutive trials, or failing to complete a stage within fifty trials were eliminated from further testing.
Experiment 1: Effect of elevating NE neurotransmission by acute systemic administration of the α2-autoreceptor antagonist, atipamezole, on the cognitive deficit seen after CUS
A total of 68 rats were randomly assigned to four groups (n=15–19/group), defined by chronic stress exposure (control or CUS), and acute drug treatment condition (vehicle or atipamezole). AST testing was conducted three days after the last stress session. On testing day, rats received an injection of either saline vehicle (2 ml/kg, i.p) or the α2-autoreceptor antagonist atipamezole HCl (1 mg/kg; Pfizer, Exton, PA, USA) to elevate tonic noradrenergic activity, 30 min prior to starting the test. Investigators conducting the behavioral tests were blind to the treatment condition of the animals.
In vivo microdialysis
A subset of 28 rats (n=6–8/group) from the groups described above were also subjected to in vivo microdialysis in mPFC, at baseline and during the AST procedure. These rats, weighing 230–290 g at the time of surgery, were anaesthetized with a cocktail of ketamine 43 mg/ml, acepromazine 1.4 mg/ml, xylazine 8.6 mg/ml, 1.0 ml/kg, i.m., with a 25% supplement given as needed. Once anesthetized, animals were placed in a stereotaxic apparatus with the incisor bar set at −3.3 mm, adjusted as needed to achieve a flat-skull position, indicated by equal DV coordinates for bregma and lambda. A microdialysis guide cannula (CMA/12; CMA Microdialysis, North Chelmsford, MA, USA), aimed at the mPFC, was implanted using a 10° lateral approach with the following coordinates relative to bregma: AP +2.6 mm, ML +1.4 mm, DV −1.7 mm. The guide cannula terminated 2 mm above the target at the boundary of areas IL and PrL in the mPFC, corresponding to plate 9 in the atlas of Paxinos and Watson (Paxinos and Watson, 1998). Approximately half the rats in each group were implanted on the left side, and half on the right. The guide cannula was anchored to the skull with jeweler s screws and dental acrylic, and an obdurator was inserted to maintain patency. After surgery, rats were treated prophylactically with antibiotic (penicillin G, 300,000 IU/ml, 2.0 ml/kg, s.c.), hydrated with 1 ml saline (s.c.), returned to a fresh cage and housed singly. Four to five days after surgery, the CUS procedure was initiated as described above.
On the test day, 3 days after the last CUS stress session, these rats were transported in their home cage to the testing room, and transferred to a plastic bucket (60 cm height × 30 cm diameter), to which the animals had been habituated for 10 min on each of the two previous training days. Immediately prior to placing the animal in the bucket, the obdurator was removed and a microdialysis probe (CMA/12) with molecular weight cutoff of 20 kDa (4 mm of active membrane) was inserted. The probe extended 4 mm beyond the tip of the guide cannula, centering the active membrane within the mPFC. Artificial cerebrospinal fluid (147 mM NaCl, 2.5 mM KCl, 1.3 mM CaCl2, 0.9 mM MgCl2, pH 7.4) was perfused through the probe at a flow rate of 1.0 μl/min. Rats were allowed a 2 h equilibration period before sample collection was initiated. Baseline sample collection time was 30 min, yielding a sample volume of 30 μl, collected into a tube containing 2.5 μl of stabilizing solution (0.2 μM EDTA, 0.2 μM ascorbic acid, 0.2 M perchloric acid). The timing of all procedures allowed for a 15 min transit time for the dialysate to travel from the active membrane at the probe tip to the outflow collection tube. Thus, 15 min prior to the end of the last baseline collection, the rat was injected with either saline (2 ml/kg, i.p.) or atipamezole (1 mg/kg, i.p.), and returned to the holding bucket for 30 min before behavioral testing. Previous studies demonstrated that some of the behavioral test stages require less time for completion than the 30-min microdialysis sample interval (Lapiz et al., 2007; Lapiz and Morilak, 2006). Thus, sampling during some of the behavioral tasks was combined as follows: a single sample was collected during the SD and CD test stages (SD/CD) and also during ID and R2 (ID/R2). The time required to complete the other test stages (R1, ED and R3) was sufficient to allow collection of a full 30-min dialysis sample without combining.
The amount of NE in the microdialysate samples was measured by high-performance liquid chromatography with coulometric detection (Coulochem2, ESA Inc., East Chelmsford, MA, USA). The mobile phase contained 60 mM sodium phosphate, 75 μM EDTA, 1.5 mM sodium 1-octanesulfonic acid, 6% methanol, pH 4.6, at a flow rate of 0.6 ml/min. Under these conditions, NE had a retention time of approximately 7.8 min. The amount of NE in each sample was quantified against a calibration curve run daily, ranging from 0.0 to 25 pg, with a detection limit of approximately 0.5 pg/sample. At the end of the experiment, rats were sacrificed by rapid decapitation, and the brains were removed for histological localization of the probe track. Any case in which the probe was located outside of mPFC was eliminated from analysis of microdialysis data a priori. Thirteen rats were eliminated from experiment 1 and were not included in the analyses. Five of these were eliminated because they did not complete the training procedure (all CUS-treated), and 8 did not complete the behavioral test procedure (4 CUS, 4 control). These animals were not included in the reported total number of rats used in this experiment. In addition, 2 rats implanted for microdialysis were eliminated from analysis of NE levels, one because of probe failure during sampling, and the other for probe misplacement. However, these two rats were included in the behavioral analysis of performance on the AST, and they were included in the reported total number of animals used.
Experiment 2: Role of post-synaptic α1-adrenergic receptors in mPFC in the beneficial effects of chronic DMI treatment in preventing the cognitive deficit induced by CUS
Following from the results of the previous study, the purpose of experiment 2 was to test the hypothesis that noradrenergic modulatory function in mPFC remained intact after CUS, and that blockade of α1-receptors in mPFC would thus attenuate the beneficial effects of chronic DMI treatment in improving cognitive set-shifting capability that had been compromised by CUS.
To test this hypothesis, 56 rats were randomly assigned to eight groups (n=5–8/group) defined by stress treatment (CUS or control), chronic drug condition (DMI or saline), and acute drug administration via local bilateral microinjection into mPFC on the testing day (benoxathian or vehicle). Rats weighing 275–310 g at the time of surgery were anesthetized as above. Osmotic minipumps (model 2ML4, Alzet Corp., Palo Alto, CA), preloaded with either vehicle (10% ethanol in saline) or DMI (Sigma, St Louis, MO) at a concentration calculated to deliver 7.5 mg/kg/day of free base, were implanted intraperitoneally via a ventral midline incision. In previous experiments, this treatment regimen resulted in mean plasma DMI concentrations of 183 ± 28 ng/ml (Bondi et al., 2008b), within the clinically relevant therapeutic range (Perry et al., 1994). Immediately following osmotic minipump surgery, rats were stereotaxically implanted with bilateral guide cannulae (22-gauge stainless steel tubing, Small Parts, Miramar, FL, USA) aimed at the mPFC using a 10° lateral approach. Cannulae terminated 1 mm above the target region based on the atlas of Paxinos and Watson (Paxinos and Watson, 1998) (coordinates relative to bregma: AP +2.6mm, ML ± 1.4 mm, DV −2.7 mm). The guide cannulae were anchored to the skull with jeweler s screws and dental acrylic, and fitted with 30-gauge stainless steel obdurators to maintain patency. Following surgery, rats were treated prophylactically with antibiotic (penicillin G, 300,000 IU/ml, 1.0 ml/kg, s.c.) and housed singly with food and water available ad libitum for 6–7 days prior to beginning CUS.
After the recovery period, CUS was administered as described above. For 7 days prior to behavioral testing, rats were restricted to 14 g of food per day, with water freely available. The AST procedure began the day after the final CUS stress session, thus testing occurred 3 days after the final stress session. On the test day, after completing the R2 task, obdurators were removed and 30-gauge stainless steel microinjectors were inserted into the guide cannulae, extending 1.0 mm beyond the ends of the guides, centering the tips in the mPFC. The microinjectors were connected via fluid-filled PE-10 tubing (Becton Dickinson, Sparks, MD, USA) to glass Hamilton syringes mounted on a syringe pump (KD Scientific, Holliston, MA, USA). Bilateral microinjections of vehicle (0.66% sterile saline) or the α1-receptor antagonist benoxathian (2.0 nmoles), were delivered into the mPFC in a volume of 0.5 μl/side, at a rate of 0.25 μl/min. This dose of benoxathian was determined from previous studies in our laboratory (Cecchi et al., 2002; Lapiz and Morilak, 2006) and others (Wellman and Davies, 1991). After microinfusions were completed, the injectors were left in place for 2 min to allow for diffusion. Behavioral testing on the ED set-shifting stage began 5 min after the injectors were removed.
At the end of the experiment, rats were killed by rapid decapitation, brains were removed, and cannulae placements were determined histologically. Cases in which one or both microinjector tips were found to be located outside of mPFC were eliminated a priori from any further analyses. A total of 6 animals were eliminated from analyses in this experiment: one for misplacement of an injection cannula, and five for failure to successfully complete the training process prior to behavioral testing (3 CUS-DMI, 2 CUS-Vehicle). These animals were not included in the total number reported as being used.
Data analysis
In the microdialysis experiment, to first test for any differences in tonic baseline NE levels in mPFC of CUS-treated rats compared to unstressed controls, the mean of the four baseline samples was calculated for each animal, and then compared by Student s t-test. Subsequently, the effect of acute atipamezole administration on NE levels in mPFC, expressed as pg/sample, was analyzed using a three-way Multivariate ANOVA (MANOVA) with repeated measures, defined by two grouping factors (CUS and Drug) and one within-subjects factor (Time). Where significant main treatment effects or interactions were indicated, data were further analyzed by post hoc comparisons using the Newman-Keuls test.
For analysis of behavior on the AST, the number of trials to criterion was recorded for each test stage. In experiment 1, these data were analyzed by three-way MANOVA (Chronic Stress × Acute Drug Treatment × Task), with repeated measures over Task. Where significant main effects or interactions were indicated, post hoc comparisons were performed using the Newman-Keuls test. In experiment 2, data for all tasks preceding the microinjections were analyzed by three-way MANOVA (Chronic Stress × Chronic Drug Treatment × Task), with repeated measures over Task. The effects of local microinjections in mPFC specifically on cognitive performance on the ED set-shifting task were then analyzed separately by three-way ANOVA (Chronic Stress × Chronic Drug Treatment × Microinjected Drug). The a priori hypotheses dictating the design of this experiment predicted an isolated single-cell effect to which the ANOVA interaction term can be insensitive when embedded in a complex multi-factorial design in which several of the groups are expected to show no effect (e.g., see Anderson, 1961). Therefore, these a priori hypotheses were tested specifically by planned contrast analyses, first comparing CUS-treated rats receiving chronic DMI to those receiving chronic vehicle treatment (both with vehicle microinjections in mPFC), and then comparing CUS-DMI treated rats microinjected with vehicle to those microinjected with benoxathian into mPFC prior to ED testing. Significance for all statistical analyses was set at p<0.05. Thus, with the Bonferroni correction applied, the α in the contrast analyses was adjusted to p<0.025.
Results
Experiment 1: Effect of elevating NE neurotransmission by acute systemic administration of the α2-autoreceptor antagonist, atipamezole, on the cognitive deficit seen after CUS
The first experiment assessed whether acutely elevating NE release by systemic administration of the α2-autoreceptor antagonist, atipamezole, was able to reverse the detrimental effects of CUS on attentional set-shifting performance. A comparison between rats implanted with microdialysis cannulae versus those that were not revealed no significant difference in behavior on the AST (F1,66 = 0.30, p=0.58). Thus, data from these subsets of rats were included in the behavioral analyses of the otherwise identical atipamezole and CUS- treated groups. Further, there were no significant differences between treatment groups (CUS vs. control) in trials to criterion for the SD task on the training day (F1,66 = 0.02, p=0.89).
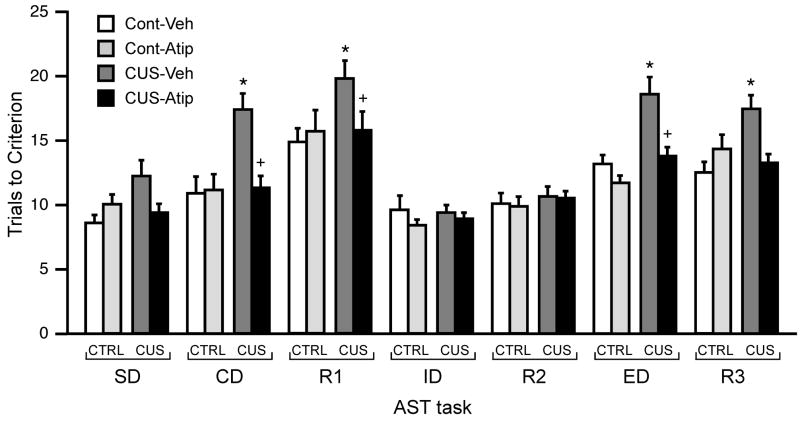
Figure 1 shows the effects of CUS exposure (14 days) and acute systemic atipamezole administration (1 mg/kg, i.p.) on the trials to criterion (TTC) for each task of the AST. Repeated measures MANOVA revealed significant effects of Stress (F1,64 = 14.52, p < 0.001), Drug (F1,64 = 9.31, p < 0.005) and Task (F6,384 = 36.61, p < 0.001). Moreover, this analysis showed significant interactions of Stress × Drug (F 1,64 = 11.97, p < 0.001) and Drug × Task (F6,384 = 2.10, p < 0.05), as well as a Stress × Drug × Task interaction (F6,384 = 2.30, p = 0.05). The Stress × Task interaction approached significance (F6,384 = 1.81, p = 0.096). Post hoc analyses of the main effect of Task across all groups showed the R1, ED, and R3 tasks to be significantly more difficult than all other task stages. Further, post hoc comparisons showed that in vehicle-treated rats, CUS induced significant performance deficits relative to unstressed controls (p<0.005, Figure 1) on CD, R1, ED and R3 tasks. Acute systemic administration of the α2-autoreceptor antagonist, atipamezole, reversed the CUS-induced deficits on CD, R1 and ED, (p<0.05, compared to vehicle-treated CUS rats; for stage R3, p = 0.10), decreasing the number of TTC to a level equivalent to that of unstressed controls (see Figure 1).
Figure 1.
Effects of acute atipamezole injection (1 mg/kg, i.p.) on cognitive performance after CUS exposure. In vehicle-treated rats, CUS induced significant performance deficits relative to unstressed controls, seen as increases in trials to criterion on CD, R1, ED and R3 tasks (*p < 0.005 compared to vehicle-treated unstressed controls on the same stage). Acute systemic administration of atipamezole reversed the CUS-induced deficits on three of the four tasks impacted by CUS, namely CD, R1 and ED. (+p < 0.05 compared to vehicle-treated stressed rats on the same stage; mean ± S.E.M., n = 15–19/group).
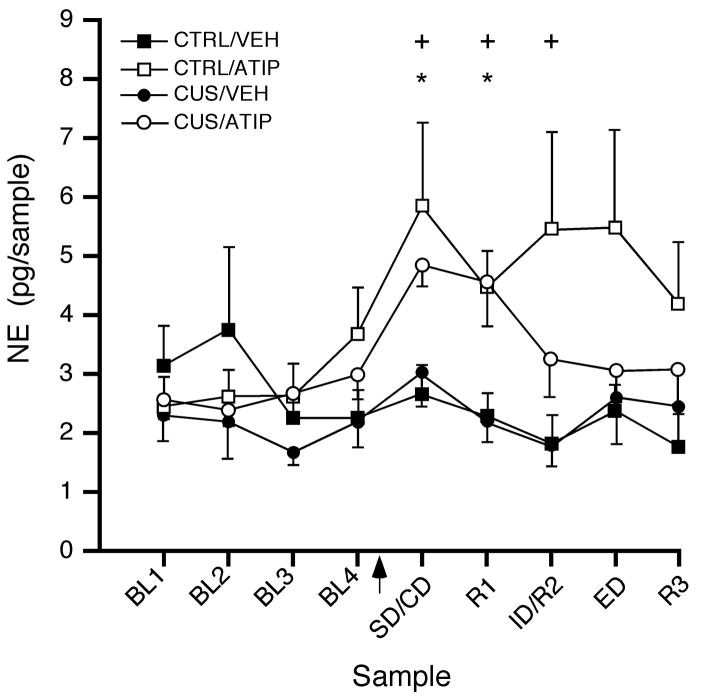
Figure 2 shows a representative example of a microdialysis guide cannula track implanted unilaterally in the medial prefrontal cortex. There were no differences in NE collected from right and left mPFC, either comparing mean baseline NE levels (t12 = 1.174, p = 0.26 and t12 = 0.309, p = 0.76 for control and CUS-treated rats, respectively), or in the full factorial analysis (main effect of hemisphere: F1,20 = 0.16, p = 0.69, with no interactions of hemisphere with any other factor or factors). Figure 3 shows extracellular NE levels collected in microdialysate samples from mPFC at baseline and during performance on the attentional set-shifting test following acute atipamezole or vehicle administration. There were no baseline differences in NE levels between CUS rats and unstressed controls before acute systemic injection of atipamezole or vehicle (t26 = 1.05, p = 0.30, n=14/group). Repeated measures MANOVA revealed a significant main effect of Drug (F1,24 = 6.44, p < 0.02) and Sample (F8,192 = 3.16, p<0.005), but not Stress (F1,24 = 0.94, p = 0.34). There was also a Drug × Sample interaction in this analysis (F 8,192 = 3.50, p<0.001). Collapsing across stress condition, post hoc comparisons showed that NE levels in mPFC were significantly higher after drug injection in atipamezole-treated rats compared to vehicle controls, in samples corresponding to behavioral tasks SD/CD, R1 and ID/R2 (p<0.01). Within the atipamezole-treated group, NE levels were significantly elevated in the first two samples after drug injection (p<0.05), representing SD/CD and R1, relative to baseline. The NE levels remained moderately elevated for the duration of the testing period, but this was not significant at the later time points (p<0.06 and p<0.08 for ID/R2 and ED, Figure 3). Consistent with our previous studies (Lapiz et al., 2007), there were no significant differences in NE levels collected in mPFC across behavioral tasks during testing in either of the vehicle-treated groups.
Figure 2.
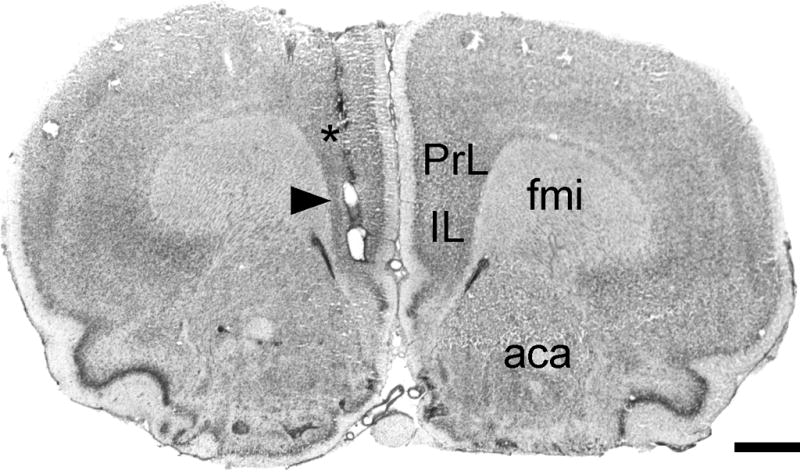
Representative photomicrograph of a Cresyl Violet-stained coronal section through mPFC, corresponding to plate 9 in the atlas of Paxinos and Watson (1998), showing the track of a microdialysis guide cannula implanted unilaterally in mPFC. The microdialysis probe extended 4 mm past the tip of the guide cannula (*), centering the active membrane in mPFC (arrowhead). Abbreviations: aca, anterior commissure, anterior limb; fmi, forceps minor, internal capsule; IL, infralimbic cortex; PrL, prelimbic cortex. Scale bar = 1 mm.
Figure 3.
Effects of acute atipamezole injection (1 mg/kg, i.p., arrowhead) on extracellular NE levels in microdialysate samples collected from mPFC after CUS exposure. Data expressed as absolute NE levels, measured as pg/sample (mean ± S.E.M.; n = 6–8/group). There were no baseline differences between CUS and control rats before the drug injection, and NE levels were significantly elevated in mPFC following acute atipamezole injection, independent of stress condition (Main effect of Drug, p < 0.02; post hoc comparisons: *p < 0.05 compared to baseline in the same drug-treatment group; +p < 0.01 compared to corresponding time point in the vehicle-injected group).
Experiment 2: Role of post-synaptic α1-adrenergic receptors in mPFC in the beneficial effects of chronic DMI treatment in preventing the cognitive deficit induced by CUS
In the second experiment, there were no significant differences in trials to reach criterion for the SD task between treatment groups on the training day, indicating no pre-existing difference between treatment groups in the ability to learn the contingency and perform the reward location and retrieval tasks required on the AST (all p>0.05). Figure 4 shows the effects of CUS and chronic DMI treatment on the test stages preceding drug microinjection into the mPFC, in replication of our previously published results (Bondi et al., 2008b). Three-way repeated-measures MANOVA (Stress × Drug × Task) for the tasks preceding microinjections indicated significant main effects of Stress (F1,52 = 5.96, p<0.02), Drug (F1,52 = 13.88, p<0.001) and Task (F4,208 = 51.41, p<0.001), and significant interactions for Stress × Drug (F1,52 = 8.11, p < 0.01), Stress × Task (F 4,208 = 4.88, p<0.001) and Drug × Task (F4,208 = 3.39, p<0.02), but not a significant Stress × Drug × Task interaction (F4,208 = 0.61, p = 0.66).
Figure 4.
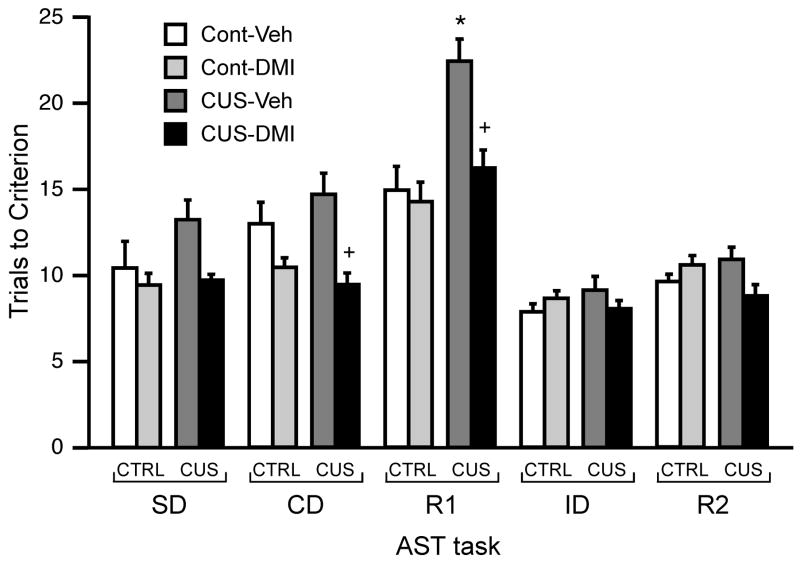
Effects of chronic DMI treatment (7.5 mg/kg/day) during CUS on AST performance in the tasks tested prior to drug microinjections into mPFC. In vehicle-treated rats, CUS produced a significant performance deficit on the first reversal task (R1), manifest as significantly higher number of trials required to reach criterion (*p < 0.001 compared to vehicle-treated unstressed control rats on the same task). This effect was prevented by chronic treatment with DMI (+p<0.001 compared to vehicle-treated rats subjected to CUS on the same task; data expressed as mean ± S.E.M.; n = 11–16/group).
As in experiment 1, post hoc analysis of the Task effect across groups showed the number of trials required to reach criterion on R1 to be significantly higher than on the other tasks (Figure 4). Post hoc comparisons also showed that CUS induced a significant performance deficit relative to unstressed controls (p<0.001, Figure 4) on the first reversal task (R1), and that this effect was prevented by chronic DMI treatment (p<0.001, Figure 4).
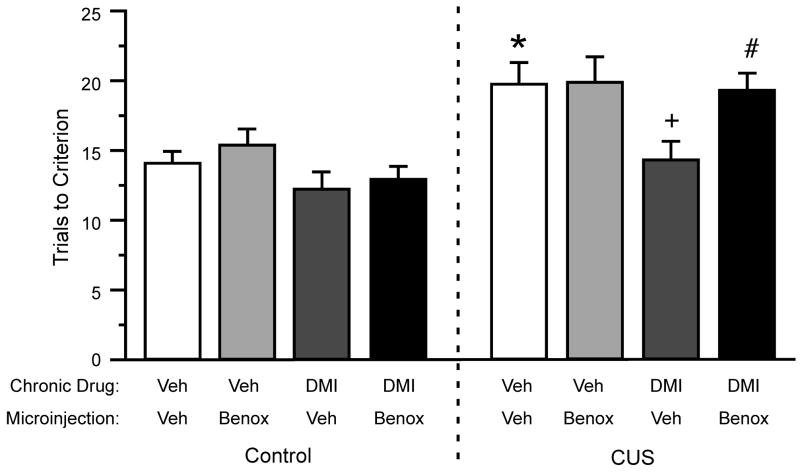
Figure 5 shows a representative example of localization of bilateral microinjection cannulae to the mPFC. Three-way ANOVA for performance on the ED set-shifting task after local microinjections into mPFC (Figure 6) found significant main effects of Stress (F1,48 = 23.59, p<0.001) and Chronic Drug treatment (F1,48 = 7.34, p<0.01). The main effect of Microinjected Drug approached significance (F1,48 = 3.48, p = 0.068). No significant interactions were revealed for the ED stage (stress × drug × microinjection, p = 0.16). However, as expected, planned contrasts showed that in rats receiving vehicle microinjections, chronic DMI treatment prevented the CUS-induced cognitive performance deficit on the ED task (p<0.01). Moreover, bilateral administration of the α1-receptor antagonist, benoxathian (2.0 nmol), into the mPFC of DMI-treated CUS animals significantly attenuated the protection conferred by DMI against the CUS-induced deficit (p<0.024), increasing the number of TTC in the ED set shifting task to a level comparable to that in CUS-exposed rats that were vehicle-treated and vehicle-microinjected (Figure 6).
Figure 5.
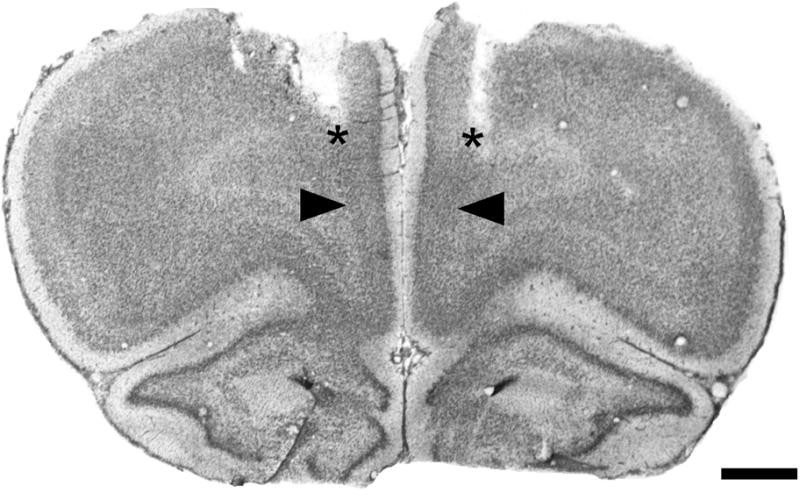
Representative photomicrograph of a Cresyl Violet-stained coronal section through mPFC, corresponding to plate 9 in the atlas of Paxinos and Watson (1998), showing the tracks of implanted guide cannulae (asterisks), and the microinjection sites, located 1 mm below the guide cannulae (arrowheads). See fig. 2 for region labels. Scale bar = 1 mm.
Figure 6.
Effects of local bilateral microinjections of the α1-adrenergic receptor antagonist benoxathian into mPFC, given immediately prior to testing on the ED task, on the protective effect of DMI against CUS-induced cognitive alterations. CUS induced a significant ED set-shifting deficit (main effect of CUS: *p < 0.001), that was prevented by chronic DMI treatment ( +p < 0.01 compared to CUS-exposed rats that were vehicle-treated and vehicle-microinjected). Microinjections of the α1-antagonist, benoxathian (2.0 nmoles per side) into mPFC prior to ED testing significantly blocked the protective effect of chronic DMI treatment against the detrimental effects of CUS (#p < 0.024 compared to CUS-exposed rats that were DMI-treated and vehicle-microinjected; all data expressed as mean ± S.E.M., n=5–8/group).
In this experiment, CUS failed to significantly alter performance in the third reversal (R3) task in vehicle-treated rats (F1,48 = 1.59, p = 0.21, data not shown). We have noted in previous studies that the CUS effect on R3 is less robust and less consistent than the effect on ED (Bondi et al., 2008b). Thus, due to the extended and sometimes variable time between drug microinjections in mPFC and testing on the third reversal stage, and because reversal learning is thought to depend more on orbitofrontal cortex than on medial prefrontal cortex, the effects of mPFC drug microinjections on the R3 reversal task were not further analyzed.
Discussion
The purpose of this study was to investigate whether chronic stress may compromise noradrenergic modulation of cognitive processes in prefrontal cortex, and to elucidate potential mechanisms by which chronic antidepressant drug treatment might exert beneficial effects against stress-induced cognitive dysfunction. We provide evidence that the capacity for noradrenergic facilitation of cognitive flexibility in medial prefrontal cortex is preserved after chronic stress, thus potentially functioning as an adaptive mechanism to counteract the detrimental effects of chronic stress. Replicating previous results (Bondi et al., 2008b), 2 weeks of CUS exposure induced a cognitive deficit on the AST tasks reflecting behavioral flexibility (i.e., reversals and cognitive set-shifting). In vivo microdialysis conducted during behavioral testing revealed no differences in extracellular NE levels in mPFC, either at baseline or during behavioral testing in vehicle-treated CUS rats compared to controls. Enhanced cognitive performance after acute administration of the α2-adrenergic autoreceptor antagonist, atipamezole, was accompanied by increased extracellular NE levels in mPFC, but again there were no differences in the elevated NE levels induced by atipamezole in CUS-treated and unstressed control rats. Thus, it does not appear that the chronic stress-induced cognitive deficit was caused by a pre-synaptic dysregulation of NE release. Rather, if the noradrenergic system was in fact compromised by CUS, it would seem more likely to have involved changes in post-synaptic receptor function. However, elevating NE transmission by atipamezole was still capable of enhancing cognitive function that had been compromised by CUS, and this was further demonstrated in experiment 2 with chronic DMI treatment. Moreover, the beneficial effect of DMI was blocked by α1-receptor antagonism specifically in mPFC. Therefore, these findings, taken together, would instead support the hypothesis that noradrenergic facilitatory capability in mPFC remains intact after CUS, thus providing a likely substrate by which the beneficial effects of chronic antidepressant drug treatment may be mediated.
Effects of elevating NE pharmacologically in animals compromised by CUS
We have shown previously that elevating noradrenergic activity in mPFC by acute atipamezole administration can improve cognitive set-shifting (Lapiz and Morilak, 2006). In the present study, atipamezole administration enhanced cognitive function, specifically ED set-shifting, that had been compromised by CUS treatment. Blockade of α2-adrenergic autoreceptors enhances NE release in brain regions such as neocortex (Devauges and Sara, 1990; Garcia et al., 2004; Haapalinna et al., 1998), and improves performance in various learning and memory tests (Bunsey and Strupp, 1995; Devauges and Sara, 1990; Sara et al., 1994). Atipamezole has been shown to enhance acquisition in a linear-arm maze test, improve choice accuracy of poorly performing rats in a delayed three-choice test (Haapalinna et al., 1998), and facilitate passive avoidance retention in aged rats (Riekkinen et al., 1992). However, it is important to acknowledge that α2-adrenoreceptors are also located both pre- and post-synaptically on cells other than noradrenergic neurons, and that these receptors can also modulate the release of other neurotransmitters, such as histamine, serotonin (5-HT) and dopamine (Gulat-Marnay et al., 1989; Raiteri et al., 1990; Trendelenburg et al., 1994). Therefore, effects seen after systemic administration of α2-adrenoceptor antagonists such as atipamezole may not be attributable exclusively to increased NE release. However, the fact that elevating extracellular NE levels by chronic treatment with the selective NE reuptake blocker, DMI, exerted effects on cognitive set-shifting that were similar to those induced by acute atipamezole administration would suggest that the effects of atipamezole were unlikely to be attributable to post-synaptic α2-adrenergic receptor blockade. Moreover, the enhancement of cognitive set-shifting induced in previous studies by systemic atipamezole treatment was blocked by selective post-synaptic α1-adrenergic receptor blockade in mPFC (Lapiz and Morilak, 2006).
Acute administration of atipamezole and chronic treatment with DMI were similarly effective in improving cognitive performance after CUS. It is possible that the requirement for chronic treatment with reuptake blockers could be related to regulatory changes that may be necessary to allow sufficient elevation of neurotransmitter levels to be effective, resulting in the so-called “therapeutic lag” in antidepressant efficacy (Blier and de Montigny, 1994). By contrast, acute autoreceptor blockade can rapidly and robustly elevate extracellular neurotransmitter levels. However, even in depressed patients, it is being increasingly recognized that various parameters and symptoms respond at different rates after treatment begins, and are presumably sensitive to different types of alteration in neurotransmitter function (Katz et al., 1996/1997). For instance, an acute dose of the NE reuptake inhibitor, reboxetine, significantly improved measures of emotional processing in depressed patients, e.g., recognition of positive facial expression and recall for positive personality descriptors, but had no effect on subjective ratings of mood or anxiety (Harmer et al., 2009). Such findings suggest that some aspects of depression may be improved by acute changes in neurotransmitter function, but that others may require chronic treatment before effects become evident. Thus, different measures and circuits may respond differently to acute versus tonic elevations of NE, or more specifically, to rapid and transient elevations compared to more gradual and stable elevations.
Beneficial effects of NE on set-shifting after CUS are mediated by α1-receptors in mPFC
We then investigated the potential involvement of post-synaptic α1-adrenergic receptors in mPFC in mediating the beneficial effects of chronic DMI treatment. DMI treatment initiated prior to, and continuing throughout CUS exposure, prevented the stress-induced deficits in cognitive set-shifting on the AST, and bilateral blockade of post-synaptic α1-adrenergic receptors in mPFC immediately prior to the ED set-shifting task attenuated the beneficial effects of DMI. Thus, these results suggest that postsynaptic α1-adrenergic receptors in mPFC mediate the beneficial effects of enhanced noradrenergic activity induced by chronic DMI treatment in animals whose cognitive function has been compromised by chronic stress. At high concentrations, DMI can non-selectively block α1-adrenergic receptors (Hall and Ogren, 1981). However, it is unlikely that the effects of DMI in the present study were attributable to blockade of α1-adrenergic receptors, as the dose of DMI was relatively low (7.5 mg/kg/day), although sufficient to generate clinically relevant plasma drug levels (Bondi et al., 2008b; Perry et al., 1994). More importantly, the cognitive effects of local α1-antagonist administration in mPFC were opposite to those of DMI, further suggesting that the beneficial effects of DMI treatment against CUS-induced detrimental effects on cognition are not mediated via α1-adrenergic receptor blockade.
As in our previous studies, α1-antagonist administration into mPFC had no apparent effect on cognitive performance of control rats, suggesting that NE neurotransmission contributes little to performance on the cognitive set-shifting task under basal conditions, perhaps due to the relatively low level of tonic basal NA activity in a non-aroused waking state (Aston-Jones et al., 1991; Garcia et al., 2004; Jacobs et al., 1991). Further, in experiment 1, there were no detectable differences in NE levels in dialysate samples collected during behavioral testing in mPFC of vehicle-treated CUS rats compared to controls. Thus, if CUS-induced deficits in cognitive capability are related in any way to changes in noradrenergic modulatory function in mPFC, it appears that such deficits may be associated with changes in post-synaptic adrenergic receptor expression or sensitivity, rather than to changes in NE release itself.
This conclusion must be tempered, however, by recognition of the relatively poor temporal resolution of the microdialysis technique, with a sampling interval in this study of 30 min. Thus, any potential differences in transient changes in NE release that may occur at critical moments in the cognitive process, for instance at the moment an error is detected, or when a contingency is learned, would not be evident. Fast-scan cyclic voltammetry has been used to detect phasic, sub-second changes in anticipatory dopamine (DA) release seconds before bar-pressing for cocaine self-administration (Phillips et al., 2003). Thus, although detection of NE by in vivo voltammetry is challenging, it would be informative if microdialysis and voltammetry could be used in combination, to monitor changes in tonic extracellular NE levels in conjunction with more dynamic, phasic changes in NE release at critical points in the behavioral task. Such an approach could allow an examination of more subtle changes in NE release that may have been impacted by CUS, but were not detectable by microdialysis alone. This may also account for the variable and modest elevation in NE seen after atipamezole during the ED set-shifting stage, when clear cognitive improvement was nonetheless evident.
Potential mechanisms of stress-related pathology and antidepressant efficacy
One potential mechanism underlying the cognitive set-shifting deficit seen after CUS treatment might involve stress-induced structural and functional changes that have been described in PFC after chronic stress, and which have been implicated as potentially important factors in the pathophysiology of depression and in symptoms reflecting cognitive dysfunction (Davidson et al., 2002; Rogers et al., 2004). Atrophy of apical dendrites and dendritic spine retraction have been described in pyramidal neurons in PFC after chronic restraint stress (Liston et al., 2006; Radley et al., 2006; Radley et al., 2004). In a more recent report, apical dendritic atrophy was also observed in PFC pyramidal neurons after 6 weeks of chronic mild stress (Bessa et al., 2009), which was attenuated by concomitant treatment with imipramine, a dual 5-HT and NE uptake inhibitor, but not by fluoxetine, an SSRI, although both classes of drugs reversed stress-induced morphological changes in the CA3 region of the hippocampus. Therefore, in the present experiments, chronic DMI treatment of CUS rats may have preserved the morphology and function of dendrites in the mPFC that are likely targets of noradrenergic modulatory input. Alternatively, it is also possible that the effects of chronic NE reuptake blockade were mediated in part by an increase in DA transmission in PFC, which has also been linked to improvements in ED set-shifting (Crofts et al., 2001; Ragozzino, 2002; Tunbridge et al., 2004). The noradrenergic innervation of PFC is relatively dense compared to that of DA (Slopsema et al., 1982), and the NE transporter has a high affinity for DA (Raiteri et al., 1977). Thus, the NE transporter can play a significant role in DA clearance in mPFC (Bymaster et al., 2002; Gresch et al., 1995; reviewed in Morilak and Frazer, 2004), and it is possible that blockade of the NE transporter by DMI may have also increased DA influence on the neuronal circuitry engaged in PFC by cognitive set-shifting. Still, the effects of DMI were completely blocked by microinjection of an α1-adrenergic receptor antagonist into mPFC, suggesting that any potential contribution of elevated DA levels to the enhancement of cognitive set-shifting by DMI was relatively minor.
Exposure to CUS consistently produced deficits in cognitive set-shifting in both the present study and in our previous studies (Bondi et al., 2008b). Deficits were also seen on the reversal tasks, R1 and R3, although less consistently than on ED. Reversal learning has been shown to be dependent more on the function of orbitofrontal cortex (OFC) than on medial prefrontal cortex (Clarke et al., 2007; McAlonan and Brown, 2003), and to be preferentially modulated by cortical serotonergic activity (Clarke et al., 2005; Clarke et al., 2007). Reversal of spatial learning in the Morris water maze was also reported to be impaired by CUS (Hill et al., 2005), whereas repeated restraint stress produced effects on both cognition (i.e., set-shifting) and cellular morphology in mPFC, but not OFC (Liston et al., 2006). In the present study, then, it may be that CUS produced modest or inconsistent effects in orbitofrontal cortex. Likewise, CUS may have only modestly or inconsistently altered serotonergic activity, although we have shown that enhancing serotonergic function by chronic SSRI treatment can also attenuate the effects of CUS on cognitive set-shifting (Bondi et al., 2008b). This is perhaps further evidence of the need to investigate interactions and convergence of serotonergic and noradrenergic influence, as either chronic SSRI or selective NRI administration can modulate the activity of both systems (Huang et al., 2006; Koch et al., 2001; Szabo and Blier, 2001; Szabo et al., 2000).
An important difference noted between the current results and those of our previous studies is that facilitation of cognitive set-shifting was previously observed in unstressed rats treated acutely with atipamezole, and also in control rats treated chronically with DMI (Lapiz et al., 2007; Lapiz and Morilak, 2006). Such facilitation was not observed in unstressed animals in the present study. We suspect this may be a consequence of the moderately better baseline performance seen on the ED task in this study compared to the earlier ones. Although the test procedure was the same in both sets of experiments, the test apparatus had been relocated to a larger room since the earlier studies, and it is possible that some environmental factor may have influenced baseline performance. For instance, a reduction in background noise, or a difference in air flow that minimized the commingling of odors, may have rendered the discrimination tasks slightly less challenging, and thus less sensitive to noradrenergic facilitation after atipamezole. However, any conclusive definition of the environmental factors that can influence baseline performance, and as a consequence may also influence the degree of pharmacological facilitation that is possible, would require an extensive parametric analysis and systematic manipulation of many such potential factors that is well beyond the scope of the present study. Nonetheless, when facilitation of cognitive set-shifting was observed in the present study after atipamezole administration in CUS-treated animals, that facilitation was attributable to the same α1-adrenergic receptor mechanism as in the previous studies, suggesting that noradrenergic facilitatory mechanisms in mPFC remained intact in animals in which cognitive set-shifting had been compromised by chronic stress exposure.
Once they reached the testing stage, CUS-treated rats did not have any more difficulty completing the behavioral test protocol than control rats, as equal numbers of CUS and control rats were eliminated from analysis for this reason. However, CUS-treated rats did show greater difficulty in completing the training session prior to behavioral testing, as all rats eliminated for this reason (5 in each experiment) were CUS-treated. This may have created a selection bias that, if anything, would have led to an underestimation of the behavioral impact of CUS. Anecdotally, in an earlier pilot study we attempted to test rats subjected to 5-weeks of CUS, and >50% of these animals refused to participate in any behavioral training or testing procedures. Thus, there is obviously a point at which the severity of chronic stress treatment precludes informative behavioral assessment on the AST. Although we did begin to see some drop-out at the training stage after 2-weeks CUS treatment in this study (approximately 11% and 16% in experiments 1 and 2), those rats that did complete the training showed no greater failure to complete testing than did controls, yet they still showed a clear deficit in ED set-shifting.
Upon superficial consideration, the present results may seem in apparent disagreement with those of Arnsten and colleagues regarding the role of α1-adrenergic receptors in modulating cognitive capability in mPFC (see Arnsten and Li, 2005). Specifically, they reported that activation of prefrontal cortical α1-receptors impaired spatial working memory in both rats and primates (Arnsten et al., 1999; Birnbaum et al., 2000; Mao et al., 1999). However, the natures of the cognitive tasks assessed in our respective studies are quite different. The delayed alternation T-maze test used by Arnsten requires animals to retain a recently acquired spatial memory and to recall a previously-established response-reward contingency. This task demands focused attention and the ability to maintain and reinforce prior learning. By contrast, the AST requires behavioral flexibility, meaning the ability to suppress previously-learned contingencies that are no longer applicable in the face of changing environmental cues, in order to acquire a new rule. This entails a state of scanning attention, to which the facilitatory influence of noradrenergic transmission in mPFC may contribute (Aston-Jones et al., 1999; Lapiz and Morilak, 2006; McGaughy et al., 2008; Tait et al., 2007). Thus, activation of the brain NE system may play an important role in cognitive processes that specifically promote behavioral flexibility and adaptation.
Conclusion
Understanding the mechanisms by which chronic stress alters activity of the mPFC, or the noradrenergic modulation of that activity, may provide important clues to the mechanisms underlying stress-related psychopathology and its treatment. The fact that the noradrenergic system is still capable of exerting a facilitating influence in mPFC after cognitive capabilities have been compromised by chronic stress provides a viable substrate by which antidepressant drugs that block NE reuptake may exert their beneficial effects. Neither the manner in which dysregulation of noradrenergic function can lead to pathology, nor the mechanisms by which drug-induced changes in noradrenergic function can normalize mood are well understood. The data presented herein suggest a complex modulatory role for NE in the mPFC related to behavioral flexibility and adaptation in a changing environment, a role that may be an important component in the effective treatment of depression and related psychiatric disorders.
Acknowledgments
We thank Ms. Ashley Furr for outstanding technical assistance. We also thank Dr. Jim Mintz, Departments of Psychiatry and Epidemiology & Biostatistics, UTHSCSA for his insight and advice with the statistical analyses. This work was supported by research grants from the National Institute of Mental Health (MH053851 and MH072672) and by the San Antonio Neuroscience Alliance. The authors have no conflicts of interest to report, nor any involvement to disclose, financial or otherwise, that may bias the conduct, interpretation or presentation of this work.
Abbreviations
- AST
attentional set-shifting test
- CD
compound discrimination
- CUS
chronic unpredictable stress
- DMI
desipramine
- ED
extra-dimensional
- EDTA
ethylenediaminetetraacetic acid
- ID
intra-dimensional
- IL
infralimbic subregion of medial prefrontal cortex
- MANOVA
multivariate analysis of variance
- mPFC
medial prefrontal cortex
- NA
noradrenergic
- NE
norepinephrine
- PFC
prefrontal cortex
- PrL
prelimbic subregion of medial prefrontal cortex
- R1
first reversal task
- R2
second reversal task
- R3
third reversal task
- SD
simple discrimination
- TTC
trials to criterion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson NH. Scales and statistics: Parametric and nonparametric. Psychological Bulletin. 1961;58:305–316. doi: 10.1037/h0042576. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Revs. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Li B-M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Matthew R, Ubriani R, Taylor JR, Li B-M. α-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Austin M-P, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. Brit J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26:591–604. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. Int. Univ. Press; New York: 1976. [Google Scholar]
- Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: A test of the cognitive content-specificity hypothesis. J Abnormal Psychol. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OFX, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:767–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AFT. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharm Biochem Behav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Effects of acute atipamezole treatment on performance in an attentional set-shifting test after chronic unpredictable stress. Soc Neurosci Abstr. 2008a;34 Online Program no. 195.9. [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008b;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bunsey MD, Strupp BJ. Specific effects of idazoxan in a distraction task: Evidence that endogenous norepinephrine plays a role in selective attention in rats. Behav Neurosci. 1995;109:903–911. doi: 10.1037//0735-7044.109.5.903. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Forebrain norepinephrine: Role in controlled information processing in the rat. Neuropsychopharmacology. 1992;7:129–142. [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cerebral Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal cognitive and executive functions in rodents: Neural and neurochemical substrates. Neurosci Biobehav Revs. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagallli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Ann Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res. 1990;39:19–28. doi: 10.1016/0166-4328(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex of the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Garcia AS, Barrera G, Burke TF, Ma S, Hensler JG, Morilak DA. Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. J Neurochem. 2004;91:683–693. doi: 10.1111/j.1471-4159.2004.02748.x. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–116. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- Gulat-Marnay C, Lafitte A, Arrang J-M, Schwartz J-C. Modulation of histamine release and synthesis in the brain mediated by α2-adrenoceptors. J Neurochem. 1989;53:513–518. doi: 10.1111/j.1471-4159.1989.tb07364.x. [DOI] [PubMed] [Google Scholar]
- Haapalinna A, Sirviö J, Lammintausta R. Facilitation of cognitive functions by a specific [alpha]2-adrenoceptor antagonist, atipamezole. Eur J Pharmacol. 1998;347:29–40. doi: 10.1016/s0014-2999(98)00077-6. [DOI] [PubMed] [Google Scholar]
- Hall H, Ogren SO. Effects of antidepressant drugs on different receptors in the brain. Eur J Pharmacol. 1981;70:393–407. doi: 10.1016/0014-2999(81)90172-2. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Huang M, Ichiwaka J, Li Z, Dai J, Meltzer HY. Augmentation by citalopram of risperidone-induced monoamine release in rat prefrontal cortex. Psychopharmacology. 2006;185:274–281. doi: 10.1007/s00213-005-0206-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog Brain Res. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FPN. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: An in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2001;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-Y, Kim CS, Frazer A, Zhang W. Leptin: A potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z-M, Arnsten AFT, Li B-M. Local infusion of an α-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatr. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioral effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP. Prolonged effects of repeated social defeat stress on mRNA expression and function of μ-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacology. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalohippocampectomy. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Perry PJ, Zeilmann C, Arndt S. Tricyclic antidepressant concentrations in plasma: An estimate of their sensitivity and specificity as a predictor of response. J Clin Psychopharmacol. 1994;14:230–240. [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, RMC Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learning & Memory. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Maura G, Folghera S, Cavazzani P, Andrioli GC, Schlicker E, Schalnus R, Göthert M. Modulation of 5-hydroxytryptamine release by presynaptic inhibitory α2-adrenoceptors in the human cerebral cortex. Naunyn-Schmiedeberg’s Arch Pharmacol. 1990;342:508–512. doi: 10.1007/BF00169037. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 (Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Riekkinen PJ, Sirviö J, Riekkinen M, Lammintausta R, Riekkinen P. Atipamezole, an alpha 2 antagonist, stabilizes age-related high-voltage spindle and passive avoidance defects. Pharm Biochem Behav. 1992;41:611–614. doi: 10.1016/0091-3057(92)90381-o. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ruis MAW, te Brake JHA, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: A clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Schildkraut JJ. Recent studies on norepinephrine systems in mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press, Ltd; New York: 1995. pp. 911–920. [Google Scholar]
- Slopsema JS, Van der Gugten J, De Bruin JPC. Regional concentrations of noradrenaline and dopamine in the frontal cortex of the rat: Dopaminergic innervation of the prefrontal subareas and lateralization of prefrontal dopamine. Brain Res. 1982;250:197–200. doi: 10.1016/0006-8993(82)90970-2. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci. 2001;13:2077–2087. doi: 10.1046/j.0953-816x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P. Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2000;3:1–11. doi: 10.1017/S1461145700001772. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Starke K, Limberger N. Presynaptic alpha-2A-adrenoceptors inhibit the release of endogenous dopamine in rabbit caudate nucleus slices. Naunyn-Schmiedeberg’s Arch Pharmacol. 1994;350:473–481. doi: 10.1007/BF00173016. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-Methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Davies BT. Reversal of phenylpropanolamine anorexia in rats by the alpha-1 receptor antagonist benoxathian. Pharmacol Biochem Behav. 1991;38:905–908. doi: 10.1016/0091-3057(91)90261-y. [DOI] [PubMed] [Google Scholar]
- Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]