Abstract
Lymphoid chemokines, including CCL19, CCL21 and CXCL13, are critical in the development and organization of secondary lymphoid tissues and in the generation of adaptive immune responses. These molecules have also been implicated in the development of ectopic lymphoid structures in the setting of chronic inflammation. Here we review current knowledge on the production of lymphoid chemokines in the central nervous system during both homeostatic conditions and in disease states. Accumulating evidence suggests that constitutive expression of CCL19 plays a critical immunosurveillance role in healthy individuals. In contrast, aberrant induction of CCL19, CCL21 and CXCL13 may support the establishment of chronic autoimmunity and hematopoetic tumors within the CNS.
Keywords: Chemokines, experimental autoimmune encephalomyelitis, multiple sclerosis, Primary CNS lymphoma, lymphoid neogenesis, neuroborreliosis
1. Introduction
The lymphoid chemokines, CCL19, CCL21 and CXCL13, constitute a subclass of chemoattractant molecules originally identified based on their roles in the development and maintenance of secondary lymphoid tissues. These chemokines are constitutively expressed in lymph nodes, spleen and Peyer's patches. They regulate the homing of lymphocytes and myeloid cells to spatially segregated compartments within secondary lymphoid tissues, thereby facilitating cognate antigen-specific interactions and the generation of adaptive immune responses (Yoshie et al., 1997; Zlotnik et al., 1999; Mantovani, 1999).
CCL19 and CCL21 are produced by stromal cells in the T cell zones of lymph nodes and spleen. CCL21 is also expressed in high endothelial venules (HEVs) and lymphatic vessels (Cyster et al., 1999). Both of these chemokines bind to CCR7, which is expressed on the surface of naive T cells and mature dendritic cells (DC). CCL21 also binds CXCR3 with lower affinity. CXCL13 is produced by follicular DC within B cell follicles and binds to the receptor, CXCR5. It drives the migration of B cells and CD4+ helper T cells (follicular Th cells) to B cell rich areas (Förster et al., 1996; Legler et al., 1998; Ansel et al., 2000; Moser et al., 2002; Müller et al., 2003). Hence, CCL19 and CCL21 expedite antigen-specific CD4+ T cell immunity, while CXCL13 is critical for normal germinal center formation as well as for efficient immunoglobulin cross-switching and affinity maturation (Junt et al. 2005).
In addition to their role in the development and maintenance of secondary lymphoid tissues, CCL19, CCL21 and CXCL13 can be induced in non-lymphoid tissues during chronic inflammation. In this setting they have been implicated in the process of lymphoid neogenesis – the organization of infiltrating immune cells and stromal elements into structured aggregates that recapitulate many of the features of secondary lymphoid tissues (Hjelmstrom et al., 2001). When formed in response to persistent infection, these ectopic lymphoid structures have been shown to contain the spread of microbes (Halle et al., 2009; Moyron-Quiroz et al., 2004). However, lymphoid neogenesis also has the potential to aggravate autoimmune inflammation via the recruitment and activation of autoantigen-specific T and B lymphocytes within the target organ (Aloisi and Pujol-Borrell, 2006). Ectopic lymphoid follicles have been found in the salivary glands in Sjogren's syndrome (Salomonsson et al., 2002), the synovium in rheumatoid arthritis (Takemura et al., 2001) and the thyroid gland in Hashimoto's thyroiditis (Hjelmstrom et al., 2001).
Here, we will review the literature concerning the production of lymphoid chemokines in the central nervous system (CNS), both during homeostasis and in disease states. In certain circumstances, CNS lymphoid chemokines act to the benefit of the host. For example, there is growing evidence that constitutive expression of CCL19 and CCL21 in cerebrovascular endothelium and the choroid plexus plays a critical role in immune surveillance of the subarachnoid and perivascular spaces of healthy individuals. In addition, induction of lymphoid chemokines in CNS tissues might participate in the clearance of local infections, such as neuroborreliosis and Toxoplasmosis gondii. Conversely, aberrant production of CCL19, CCL21 and CXCL13 may support the establishment of hematopoetic tumors or chronic autoimmunity in the brain and spinal cord.
2. CCR7-CCL19 interactions in immune surveillance of the CNS
Low numbers of T cells are consistently found in normal human and rat brains and cerebrospinal fluid (CSF)( Booss et al., 1983; Wekerle et al, 1987), indicating that the CNS is continuously patrolled by activated T cells. The importance of such immune surveillance is suggested by the emergence of opportunistic infections and tumors in the brains and spinal cords of immunosuppressed individuals (Snider et al., 1983; Schneck and Penn, 1971). Disruption of leukocyte trafficking to the CNS by adhesion molecule blockade in patients with multiple sclerosis (MS) increases the risk of progressive multifocal leukoencephalopathy, a demyelinating disease mediated by the JC virus (Langer-Gould et al., 2005). Furthermore, primary CNS lymphoma occurs in the context of AIDS as well as global immunosuppressive treatments to prevent transplant rejection (Snider et al., 1983; Schneck and Penn, 1971). Still, relatively little is known about the chemokines that regulate leukocyte migration to the uninflamed CNS.
Lymphocyte trafficking through the intact blood brain barrier (BBB) is tightly controlled by specialized cerebrovascular endothelial cells and astrocytic processes. The presence of the BBB and the absence of lymphatic vessels has led some to view the CNS as an immunologically privileged site. However, adoptive transfer studies have shown that activated T cells access the CNS as readily as other tissues (Hickey et al., 1991; Wekerle et al., 1987). In addition to traversing the cerebrovascular endothelium, leukocytes could bypass the BBB by entering the subarachnoid space via the meningeal veins or the choroid plexus (Krumbholz et al., 2007; Kivisakk et al., 2004). Accumulating evidence indicates that these are the primary routes by which lymphocytes survey the CNS during homeostasis and that CCL19/ CCR7 interactions regulate this process (Axtell et al., 2009; Kivisaak et al., 2003).
More than 80% of cells in the CSF of healthy individuals are central memory T (TCM) cells that express high levels of CCR7, CD27, CD45RO and L-selectin (Giunti et al., 2003; Kivisakk et al., 2003; Kivisakk et al., 2004). The presence of L-selectin and CCR7 suggests these cells are not terminally committed effector T cells (TEFF) but instead retain the ability to recirculate to secondary lymphoid organs. CCL19 has been detected in the CSF of healthy humans (Krumbholz et al., 2007; Pashenkov et al., 2003). Furthermore, CCL21 is constitutively expressed in the choroid plexus (Kivisakk et al., 2004). Taken together, these data support the hypothesis that TCM enter the subarachnoid space in response to CCL19/ CCL21 gradients to carry out routine surveillance. If a patrolling TCM encounters its cognate antigen presented by a resident antigen presenting cell, it will downregulate CCR7 and CD27 and upregulate CCR5 and CXCR3, evolving to an effector phenotype (TEFF) with the ability to traverse the glia limitans and infiltrate the parenchyma (Giunti et al., 2003; Kivisakk et al., 2003). In support of this model, TEFF accumulate in active demyelinating lesions but are sparse in the CSF of healthy individuals and MS patients (Giunti et al., 2003; Kivisakk et al., 2004; Hintzen et al., 1995).
3. Lymphoid chemokines in non-inflammatory neurological disease
A. CCL21/CXCR3 interactions in cerebrovascular ischemia and glutamate mediated excitotoxicity
As will be discussed below, induction of lymphoid chemokines in the CNS is most prominently associated with inflammatory processes, such as autoimmune demyelinating disease and Lyme neuroborreliosis. However, non-inflammatory insults can also trigger their expression by CNS resident cells. Hence, CCL21 mRNA is rapidly and persistently upregulated in coritcal neurons in response to ischemic injury (Biber et al., 2001). Consistent with these findings, CCL21 is expressed in cultured neurons, but not in astrocytes or microglia, that have been subjected to various treatments known to induce cell death. Moreover, the isoform of CCL21 expressed by neurons in the ischemic CNS or in culture is identical to that expressed by stromal cells in the T cell rich areas of the spleen and lymph nodes (Vassileva et al., 1999; Rappert et al., 2002). The related chemokine, CCL19, which shows a similar expression pattern to CCL21 in peripheral lymphoid organs, is not induced in response to ischemic insult (Biber et al., 2001).
The main target of the neuronal derived CCL21 appears to be CXCR3 expressing glial cells. CCR7, the principal receptor for CCL19 and CCL21 (Yoshida et al., 1997; Yoshida et al., 1998), is not present on cultured glia or neurons, or in ischemic brain tissue (Biber et al., 2001). Given the lack of CCR7 on infiltrating leukocytes, it is unlikely that CCL21 upregulation mediates their recruitment from the circulation to the ischemic brain. In contrast, CXCR3, which also binds CCL21 (Soto et al., 1998), is expressed on both cultured microglia as well as in ischemic brain tissue (Biber et al., 2001). CCL21 exerts direct biological effects on microglia via a CXCR3 dependent pathway. Hence, Kettenmann and colleagues reported that CCL21 triggers a chloride current and chemotaxis in cultured murine microglia (Rappert et al., 2002). Microglia from CXCR3-deficient, but not CCR7-deficient, mice were unresponsive to CCL21. Furthermore, CCL19, which binds CCR7 but not CXCR3, had no effect on the microglia.
Glutamate mediated excitotoxicity is an important mechanism that underlies neuronal death in stroke (Castillo et al., 1996). It was recently demonstrated that cultured cortical neurons and hippocampal neurons in organotypic slices release CCL21 following exposure to high concentrations of glutamate (de Jong et al., 2005). These investigators found that CCL21 is stored in secretory granules that are transported along neuronal processes to presynaptic structures. Furthermore, microglia migrate towards supernatants from glutamate-treated neuronal cultures. Interestingly, microglia cultured from CXCR3-deficient mice can still migrate towards these supernatants, albeit less effectively, indicating the presence of as yet unidentified factors released by the neurons that act as chemoattractants for microglia (de Jong et al., 2005). Collectively, these data indicate that production and release of CCL21 by neurons is a stereotypic response to excitotoxic insults and provides a bridge between neuronal stress and microglial activation and chemotaxis. Furthermore, axonal transport of CCL21 in secretory granules suggests a mechanism for the activation of microglia remote from the primary lesion, a phenomenon that has been observed in both stroke and traumatic brain and spinal cord injury (Williams, et al. 2007).
B. Lymphoid chemokines in hematopoetic tumors of the CNS
A growing body of data suggests that local production of lymphoid chemokines plays an important role in the establishment and/or maintenance of hematopoietic tumors within the CNS. The specific chemokine(s) involved depend on the cell of origin of the neoplasm. Hence, CCL19 was recently implicated in CNS infiltration by T cell acute lymphoblastic leukemia (T-ALL), while CXCL13 has been associated with the development of diffuse large B cell lymphomas in the CNS (Buonamici et al., 2009; Smith et al., 2003; Tun et al., 2008).
It has long been observed that T-ALL tends to relapse in the CNS. However, the factors controlling T-ALL cell migration across the BBB remain poorly understood. Recently, leptomeningeal invasion by leukemic cells was shown to be CCR7-dependent in two animal models of T-ALL (Buonamici et al., 2009). Here, CCR7 expression is driven by the T-ALL oncogene, Notch-1. Similarly, CCR7 is expressed in the 80% or more of the human T-ALL tumors that carry Notch 1 activating mutations. CCL19 was detected in endothelial cells of brain venules in the vicinity of the infiltrating cancer cells. Deficiency of either CCR7 or CCL19 prevented the recruitment of cancer cells to the CNS. Notably, CCR7 expression did not correlate with neoplastic infiltration of non-CNS tissues, and CCR7 expression was not required for CNS involvement in mouse models of B cell ALL (Buonamici et al., 2009).
Primary CNS lymphoma (PCNSL) is a diffuse large B cell lymphoma that is confined to the CNS. In several studies, development of this rare tumor has been associated with ectopic expression of CXCL13 in the brain. Smith and colleagues found that CXCL13 was expressed in all 24 PCNSL specimens, but none of the healthy brain biopsy specimens they examined (Smith et al., 2003). Immunohistochemical staining localized the chemokine to the leukemic cells themselves as well as to the cerebrovascular endothelium. However, in situ hybridization pinpointed the leukemic cells as the primary source. Tumor cells stained positively for CXCR5, the primary receptor for CXCL13. In a complementary study, CXCL13 was found to be elevated in the CSF of patients with PCNSL compared to patients with other CNS malignancies or with systemic lymphomas without CNS involvement (Fischer et al., 2009). CSF CXCL13 levels fell in a small number of patients who responded to chemotherapy. Finally, microarray analyses identified CXCL13 and regulator of G-protein signaling 13 (RGS13), a modulator of CXCR5 signaling, as molecules upregulated in PCNSL in comparison to non-CNS diffuse B cell lymphoma (Tun et al., 2008). The question of whether CXCL13 alters the survival or spread of the neoplastic B cells, or represents an epiphenomenon, remains unanswered.
4. Lymphoid chemokines in infections of the CNS
Lyme neuroborreliosis (NB) is a CNS infection caused by the spirochete Borrelia burgdoferi (B. burgdoferi). It can manifest as a basilar meningitis with cranial neuropathies, radiculitis, transverse myelitis, or encephalitis causing focal white matter lesions (Ruppercht et al., 2008). Myeloid cells are a major source of CNS CXCL13 in animal models of NB. CXCL13 has been localized to microglia and infiltrating macrophages/ DC in brain and spinal cord sections of rhesus macaques with acute NB (Ramesh et al., 2009; Narayan et al., 2005). Interestingly, sonicates of B. burgdoferi stimulate human myeloid and plasmacytoid DC to produce CXCL13 in vitro (Narayan et al., 2005).
Multiple laboratories have found that CXCL13 is significantly elevated in the CSF of patients with NB compared to other neurological diseases, including Guillane Barre syndrome and Bell's palsy (Ruppercht et al., 2009; Senel et al., 2009). There is no correlation between CSF CXCL13 and serum CXCL13 or measures of BBB disruption, indicating that the chemokine is produced intrathecally. In a recent study, CSF CXCL13 revealed a higher combined sensitivity and specificity for the diagnosis of NB than any other parameter investigated (Senel et al., 2009). Its level fell in response to antibiotic treatment faster than the other parameters. In a separate study, B cell chemotaxis towards CSF from patients with acute NB was reduced over 50% when the samples were preincubated with an anti-CXCL13 neutralizing antibody (Ruppercht et al., 2009). This suggests that CXCL13 may be involved in the recruitment of B cells to the subarachnoid space of B. burgdoferi infected patients.
CCL19 and CCL21 have clearly been demonstrated to play a protective role in an animal model of another CNS infection. Hence, transcripts encoding both chemokines rise significantly in the CNS following infection of mice with the protozoan parasite, Toxoplasmosis gondii (Noor et al., 2010). CCR7-deficient mice succumb to disease early in association with increased parasite burden in the brain. Their increased susceptibility may be due to impaired priming of effector T cells in the periphery as well as to a reduction in leukocyte trafficking to the brain, since serum IFNγ levels are reduced in the infected knock-out mice.
5. CCL19/CCL21 expression in neuroinflammation associated with autoimmune demyelination
CCL19 and CCL21 have been detected in inflamed CNS venules and in perivascular cuffs in EAE lesions, both by in situ hybridization and immunohistochemistry (Alt et al., 2002; Columba-Cabezas et al., 2003). Several groups have reported that CCL19, CCL21 and CCR7 levels rise in the spinal cord in association with clinical relapses and chronic progression of EAE (Bagaeva et al., 2006; Columba-Cabezas et al., 2003; Dijkstra et al., 2006). In parallel, CCR7+ cells accumulate within inflammatory cuffs and meningeal infiltrates (Alt et al., 2002; Columba-Cabezas et al., 2003). Encephalitogenic lymphoblasts express CCR7 and CXCR3 proteins on the cell surface, migrate towards CCL19 and CCL21 in vitro, and specifically bind to exposed cerebral vessels in frozen sections of EAE brains in a CCR7/ CXCR3 dependent manner. CCR7 is also upregulated on activated microglia in white matter adjacent to active EAE lesions (Dijkstra et al., 2006), and cultured microglia upregulate CCR7 and migrate towards CCL21 following treatment with the TLR4 agonist, LPS (Dijkstra et al., 2004). By extension, proinflammatory factors might induce CCR7 expression by microglia in vivo.
Similar observations have been made in MS specimens. Hence, CCL19 transcripts are elevated in both active and inactive lesions (Krumbholz et al. 2007), and CCL19 concentrations are elevated in the CSF from MS patients compared to controls (Pashenkov et al., 2003 Krumbholz et al., 2007; Giunti et al., 2003). CCL21 expression has also been described in the choroid plexus of MS specimens (Kivisakk et al., 2004; Krumbholz et al., 2007). CSF CCL19 levels correlate with intrathecal IgG production, suggesting that CCL19 may play a role in the maintenance or expansion of B cells in the MS brain (Krumbholz et al., 2007; Pashenkov et al., 2003). Interestingly, lymphocytes infiltrating MS lesions do not express CCR7, although microglia and infiltrating DC do (Kivisakk et al., 2004; Serafini et al., 2006). This may be secondary to downregulation of the receptor following the migration of lymphocytes across the BBB. In support of this theory, CCR7 is consistently found on CD4+ memory T cells in the CSF of healthy individuals and of patients with MS, prior to their conversion to effector cells. CCR7 is also expressed by a subset of DC in the CSF of MS patients (Krumbholz et al., 2007; Kivisakk et al., 2004).
While the above findings support a role for CCL19 and/ or CCL21 in lymphocyte recruitment and microglial activation/ chemotaxis during autoimmune demyelinating disease, a cause-and-effect relationship has not been established. In fact, transgenic expression of CCL21 or CCL19 in oligodendrocytes did not result in lymphocyte recruitment or lymphoid neogenesis in white matter (Chen et al., 2002). However, transgenic expression of CCL21 did cause a severe neuropathological condition, characterized by neutrophil and eosinophil infiltration, hypomyelination, spongiform myelinopathy, myelin breakdown and reactive astrogliosis. These mice died within the first four weeks of life (Chen et al., 2002). One explanation for this unexpected phenotype is that CCL21 constitutively produced by oligodendrocytes does not simulate the biological functions of the chemokine induced in endothelial cells during neuroinflammation. Pathological changes were not observed in the CNS of CCL19 transgenic mice, suggesting a role for CXCR3, rather than CCR7, in mediating the effects of oligodendrocyte derived CCL21.
6. CXCL13 in autoimmune demyelination
CXCL13 is not expressed in the healthy CNS. However, we and others have found that it is upregulated in the spinal cord of mice with EAE (Bagaeva et al., 2006; Magliozzi et al., 2004). This observation was consistent across several different mouse strains, autoantigens and methods of disease induction. CXCL13 is first detectable in the CNS at the onset of the initial clinical episode. Its levels rise during subsequent relapses and during chronic progression. We showed that both CXCL13 deficiency and CXCL13 neutralization reduce the severity of the early clinical course (Bagaeva et al., 2006). The role of CXCL13 at this stage of disease does not seem to be recruitment of leukocytes to the CNS, since the frequency of infiltrating CXCR5+ leukocytes did not differ between myelin immunized CXCL13 deficient and wildtype mice. Our studies indicated that infiltrating myeloid DC are at least one source of CXCL13 in the inflamed CNS. This observation lead us to postulate that CXCL13 induces the migration of encephalitogenic T cells towards DC in the CNS, thereby increasing the frequency of their cognate interaction within the target organ. Myeloid DC have been described as highly efficient antigen presenting cells in the CNS during EAE (McMahon et al., 2005).
Later in the disease course, CXCL13 might assume the alternative role of initiating lymphoid neogenesis. CXCL13-expressing lymphoid follices were detected in the meninges of Biozzi mice during the chronic progressive stage of EAE (Magliozzi et al., 2004). These follicles contained B cells and a network of CXCL13+ and FDC-M1+ follicular DC. Blockade of LTα1β2 (LTβ, which is expressed on B cells and subsets of encephalitogenic T cells) suppressed clinical EAE and prevented the induction of CXCL13 as well as the formation of organized ectopic lymphoid follicles in the CNS of myelin-immunized mice (Columba-Cabezas et al., 2006). These observations suggest that, analogous to the regulation of CXCL13 in secondary lymphoid tissues, engagement of LTβ receptor on stromal cells by LTβ on lymphocytes, induces CXCL13 expression in the CNS.
Similar observations have been made in MS. Hence, LT and CXCL13 are upregulated in the CSF of MS patients compared to healthy controls or patients with non-inflammatory neurological diseases (Corcione et al., 2004; Krumbholz et al., 2006; Sellebjerg et al., 2009). CSF CXCL13 expression correlated with other indices of inflammation, including the presence of B cells, plasmablasts, and T cells and intrathecal immunoglobulin synthesis (Krumbholz et al., 2006; Sellebjerg et al., 2009). The CXCL13 receptor, CXCR5, was expressed on all CSF B cells and approximately 20% of CSF T cells (Krumbholz et al., 2006). Furthermore, serum CXCL13 fluctuated in association with radiological disease activity (Festa et al., 2009). Conversely, CXCL13 levels and CSF B cell counts fell after treatment of MS patients with natalizumab or methylprednislone (Sellebjerg et al., 2009; Stüve et al., 2006; Krumbholz et al., 2008). If the above studies are reproduced in additional cohorts of patients, CSF and/ or serum CXCL13 might become useful as a biomarker of MS disease activity.
In complementary studies, CXCL13 was detected in active, but not inactive, MS lesions (Corcione et al., 2004; Krumbholz et al., 2006; Sellebjerg et al., 2009). CXCL13 expression localized to infiltrating immune cells, particularly macrophages, both in the perivascular cuffs and within the parenchyma. Similar to the findings in Biozzi mice with chronic EAE, lymphoid follicles were discovered in the meninges of 40% of brain autopsy specimens from individuals with secondary progressive MS (Serafini et al., 2004; Magliozzi et al., 2007). These follicles contained a well developed network of proliferating B cells, plasma cells, T cells and CXCL13-expressing follicular DC in close association with inflamed meningeal blood vessels. Future studies will be required to determine whether the immunoglobulins produced in the meningeal follicles (or less structured B cell aggregates during earlier phases of MS) are the main source of oligoclonal bands in the CSF, a frequent finding in MS.
In every instance, ectopic follicles were located deep within the sulci, adjacent to underlying cortical plaques. Extensive subpial demyelination, pronounced microglial activation and neurite loss and an increased number of active lesions were present within the cortical areas adjacent to ectopic B-cell follicles (Magliozzi et al., 2007). This finding supports the view that ectopic follicles have a direct role in cortical injury, possibly as a source of diffusible factors such as autoantibodies, proinflammatory cytokines and/or proteolytic enzymes that cause neuronal injury either directly or via microglial activation (Magliozzi et al., 2007).
8. Conclusions
Evidence is accumulating that lymphoid chemokines play pleiotrophic roles when expressed in the CNS. During homeostasis, CCL19 and CCL21 may facilitate immune surveillance of the subarachnoid space via recruitment of central memory T cells. Conversely, ectopic expression of these chemokines by CNS resident cells may trigger microglial activation and/ or the recruitment and organization of infiltrating leukocytes in response to specific danger signals. These chemokines are induced de novo (CXCL13) or above baseline levels (CCL19 and CCL21) in response to certain CNS infections and neoplasms, as well as in the setting of autoimmune inflammation. In addition to regulating leukocyte entry across the BBB, they have been associated with the development of ectopic lymphoid structures in the meninges during chronic EAE and secondary progressive MS. Data from animal models suggest that CNS lymphoid chemokines play a beneficial role in homeostatic immune surveillance and clearance of immune infections. Conversely, aberrant expression of these molecules appears to be detrimental with regard to the development of hematopoetic tumors and autoimmune demyelination within the CNS. A causal relationship between CNS lymphoid chemokines and pathological and clinical outcomes remains to be established in human disease. Nonetheless, the current data suggests that CXCL13, CCL19 and CCL21 will ultimately be useful as biomarkers and/ or therapeutic targets.
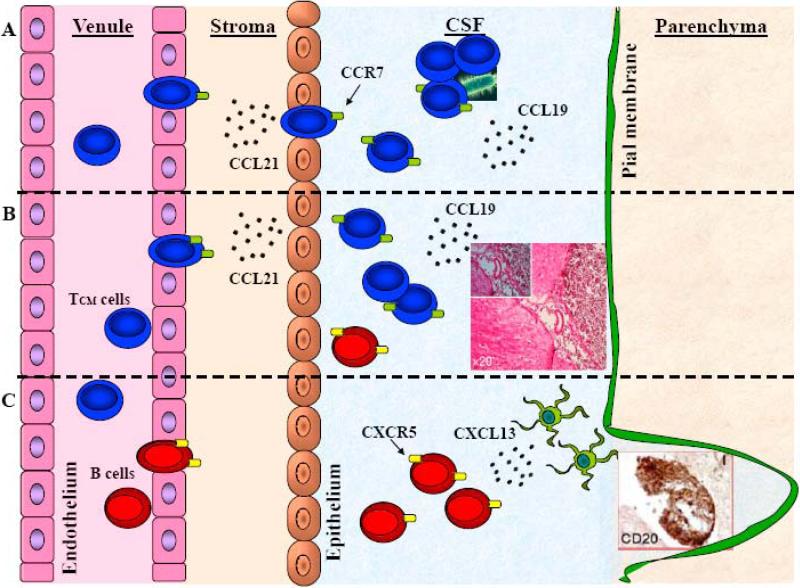
Figure 1. The role of lymphoid chemokines in CNS homeostatic and pathogenic states.
CCL19 and CCL21 are constitutively expressed in cerebrovascular endothelium and the choroid plexus. The majority of cells in the CSF are TCM cells that express CCR7 and may play a role in immunosurveillance of the subarachnoid and perivascular spaces, including in the clearance of local infections, A. Conversely, aberrant production of CCL19, CCL21 and CXCL13 may support CNS infiltration by leukemic T and B cells and the establishment of hematopoetic tumors (inset) in the brain and spinal cord, B. CXCL13 is not expressed in the healthy CNS but is upregulated during autoimmune inflammation where it may have a role in the cognate interaction of encephalitogenic T cells and DC, while later in disease CXCL13 has been associated with the development of ectopic lymphoid structures (inset) in the meninges, C.
Acknowledgements
This work was supported by the National Multiple Sclerosis Society (Grant RG3866-A3) and the National Institutes of Health (Grant NS047687-01A1). We thank David Irani for critical review of our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32:2133–2144. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ansel K, McHeyzer-Williams L, Ngo V, McHeyzer-Williams M, Cyster J. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell R, Steinman L. Gaining entry to an uninflamed brain. Nat Immunol. 2009;10:453–455. doi: 10.1038/ni0509-453. [DOI] [PubMed] [Google Scholar]
- Bagaeva L, Rao P, Powers J, Segal B. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- Biber K, Sauter A, Brouwer N, Copray S, Boddeke H. Ischemia-induced neuronal expression of the microglia attracting chemokine Secondary Lymphoid-tissue Chemokine (SLC). Glia. 2001;34:121–133. doi: 10.1002/glia.1047. [DOI] [PubMed] [Google Scholar]
- Booss J, Esiri MM, Tourtellotte WW, Mason DY. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. Journal of the Neurological Sciences. 1983;62:219–232. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- Buonamici S, Trimarchi T, Ruocco M, Reavie L, Cathelin S, Mar B, Klinakis A, Lukyanov Y, Tseng J, Sen F, Gehrie E, Li M, Newcomb E, Zavadil J, Meruelo D, Lipp M, Ibrahim S, Efstratiadis A, Zagzag D, Bromberg J, Dustin M, Aifantis I. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459:1000–1004. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Dávalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke. 1996;27:1060–1065. doi: 10.1161/01.str.27.6.1060. [DOI] [PubMed] [Google Scholar]
- Chen S, Leach M, Chen Y, Cai X, Sullivan L, Wiekowski M, Dovey-Hartman B, Zlotnik A, Lira S. Central nervous system inflammation and neurological disease in transgenic mice expressing the CC chemokine CCL21 in oligodendrocytes. J Immunol. 2002;168:1009–1017. doi: 10.4049/jimmunol.168.3.1009. [DOI] [PubMed] [Google Scholar]
- Columba-Cabezas S, Griguoli M, Rosicarelli B, Magliozzi R, Ria F, Serafini B, Aloisi F. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. J Neuroimmunol. 2006;179:76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Columba-Cabezas S, Serafini B, Ambrosini E, Aloisi F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003;13:38–51. doi: 10.1111/j.1750-3639.2003.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi G, Uccelli A, Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E, Dijkstra I, Hensens M, Brouwer N, van Amerongen M, Liem R, Boddeke H, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra I, de Haas A, Brouwer N, Boddeke H, Biber K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia. 2006;54:861–872. doi: 10.1002/glia.20426. [DOI] [PubMed] [Google Scholar]
- Dijkstra I, Hulshof S, van der Valk P, Boddeke H, Biber K. Cutting edge: activity of human adult microglia in response to CC chemokine ligand 21. J Immunol. 2004;172:2744–2747. doi: 10.4049/jimmunol.172.5.2744. [DOI] [PubMed] [Google Scholar]
- Festa E, Hankiewicz K, Kim S, Skurnick J, Wolansky L, Cook S, Cadavid D. Serum levels of CXCL13 are elevated in active multiple sclerosis. Mult Scler. 2009;15:1271–1279. doi: 10.1177/1352458509107017. [DOI] [PubMed] [Google Scholar]
- Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk H, Cakiroglu H, Widmann T, Thiel E. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15:5968–5973. doi: 10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- Förster R, Mattis A, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle M, Pedemonte E, Noonan D, Albini A, Bernardi G, Mancardi G, Battistini L, Uccelli A. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol. 2003;73:584–590. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- Halle S, Dujardin H, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hämmerling G, Garbi N, Sutter G, Worbs T, Förster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W, Hsu B, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hintzen R, Fiszer U, Fredrikson S, Rep M, Polman C, van Lier R, Link H. Analysis of CD27 surface expression on T cell subsets in MS patients and control individuals. J Neuroimmunol. 1995;56:99–105. doi: 10.1016/0165-5728(94)00137-d. [DOI] [PubMed] [Google Scholar]
- Hjelmström P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- Junt T, Fink K, Förster R, Senn B, Lipp M, Muramatsu M, Zinkernagel R, Ludewig B, Hengartner H. CXCR5-dependent seeding of follicular niches by B and Th cells augments antiviral B cell responses. J Immunol. 2005;175:7109–7116. doi: 10.4049/jimmunol.175.11.7109. [DOI] [PubMed] [Google Scholar]
- Kivisäkk P, Mahad D, Callahan M, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis S, Lassmann H, Ransohoff R. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Kivisäkk P, Mahad D, Callahan M, Trebst C, Tucky B, Wei T, Wu L, Baekkevold E, Lassmann H, Staugaitis S, Campbell J, Ransohoff R. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Meinl I, Kümpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology. 2008;71:1350–1354. doi: 10.1212/01.wnl.0000327671.91357.96. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisäkk P, Ransohoff R, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Steinmeyer F, Cepok S, Hemmer B, Hofbauer M, Farina C, Derfuss T, Junker A, Arzberger T, Sinicina I, Hartle C, Newcombe J, Hohlfeld R, Meinl E. CCL19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J Neuroimmunol. 2007;190:72–79. doi: 10.1016/j.jneuroim.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas S, Green A, Bollen A, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Legler D, Loetscher M, Roos R, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- McMahon E, Bailey S, Castenada C, Waldner H, Miller S. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- Moser B, Schaerli P, Loetscher P. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002;23:250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz J, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland D, Lund F, Randall T. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Müller G, Lipp M. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation. 2003;10:325–334. doi: 10.1038/sj.mn.7800197. [DOI] [PubMed] [Google Scholar]
- Narayan K, Dail D, Li L, Cadavid D, Amrute S, Fitzgerald-Bocarsly P, Pachner A. The nervous system as ectopic germinal center: CXCL13 and IgG in lyme neuroborreliosis. Ann Neurol. 2005;57:813–823. doi: 10.1002/ana.20486. [DOI] [PubMed] [Google Scholar]
- Noor S, Habashy A, Nance J, Clark R, Nemati K, Carson M, Wilson E. CCR7 dependent immunity during acute Toxoplasma gondii infection. Infect Immun. 2010 doi: 10.1128/IAI.01314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashenkov M, Söderström M, Link H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J Neuroimmunol. 2003;135:154–160. doi: 10.1016/s0165-5728(02)00441-1. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Borda J, Gill A, Ribka E, Morici L, Mottram P, Martin D, Jacobs M, Didier P, Philipp M. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard N, Gerard C, Boddeke H, Kettenmann H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J Immunol. 2002;168:3221–3226. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- Rupprecht T, Elstner M, Weil S, Pfister H. Autoimmune-mediated polyneuropathy triggered by borrelial infection? Muscle Nerve. 2008;37:781–785. doi: 10.1002/mus.20929. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelmström P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Schneck S, Penn I. De-novo brain tumours in renal-transplant recipients. Lancet. 1971;1:983–986. doi: 10.1016/s0140-6736(71)91384-5. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Börnsen L, Khademi M, Krakauer M, Olsson T, Frederiksen J, Sørensen P. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73:2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- Senel M, Rupprecht T, Tumani H, Pfister H, Ludolph A, Brettschneider J. The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2009.195438. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi G, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Smith J, Braziel R, Paoletti S, Lipp M, Uguccioni M, Rosenbaum J. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- Snider W, Simpson D, Aronyk K, Nielsen S. Primary lymphoma of the nervous system associated with acquired immune-deficiency syndrome. N Engl J Med. 1983;308:45. doi: 10.1056/NEJM198301063080112. [DOI] [PubMed] [Google Scholar]
- Soto H, Wang W, Strieter R, Copeland N, Gilbert D, Jenkins N, Hedrick J, Zlotnik A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci U S A. 1998;95:8205–8210. doi: 10.1073/pnas.95.14.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüve O, Marra C, Jerome K, Cook L, Cravens P, Cepok S, Frohman E, Phillips J, Arendt G, Hemmer B, Monson N, Racke M. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- Takemura S, Braun A, Crowson C, Kurtin P, Cofield R, O'Fallon W, Goronzy J, Weyand C. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- Tun H, Personett D, Baskerville K, Menke D, Jaeckle K, Kreinest P, Edenfield B, Zubair A, O'Neill B, Lai W, Park P, McKinney M. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200–3210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick J, Lira S. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]
- Williams A, Wei H, Dave J, Tortella F. Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J Neuroinflammation. 2007;4:17. doi: 10.1186/1742-2094-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nagira M, Kitaura M, Imagawa N, Imai T, Yoshie O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998;273:7118–7122. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol. 1997;62:634–644. doi: 10.1002/jlb.62.5.634. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Morales J, Hedrick J. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]