Abstract
Aging is associated with myocardial dysfunction although the underlying mechanism is unclear. AMPK, a key cellular fuel sensor for energy metabolism, is compromised with aging. This study examined the role of AMPK deficiency in aging-associated myocardial dysfunction. Young or old minwild-type (WT) and transgenic mice with overexpression of a mutant AMPK α2 subunit (kinase dead, KD) were used. AMPK α isoform activity, myocardial function and morphology were examined. DCF and JC-1 fluorescence probes were employed to quantify reactive oxygen species (ROS) and mitochondrial membrane potential (ΔΨm), respectively. KD mice displayed significantly reduced α2 but not α1 AMPK isoform activity at both ages with a greater effect at old age. Aging itself decreased α1 isoform activity. Cardiomyocyte contractile function, intracellular Ca2+ handling and SERCA2a levels were compromised with aging, the effects of which were exacerbated by AMPK deficiency. H&E staining revealed cardiomyocyte hypertrophy with aging, which was more pronounced in KD mice. TEM micrographs displayed severe disruption of mitochondrial ultrastructure characterized by swollen, irregular shape and disrupted cristae in aged KD compared with WT mice. Aging enhanced ROS production and reduced ΔΨm, the effects of which were accentuated by AMPK deficiency. Immunoblotting data depicted unchanged Akt phosphorylation and a significant decrease in mitochondrial biogenesis cofactor PGC-1α in aged groups. AMPK deficiency but not aging decreased the phosphorylation of ACC and eNOS. Expression of membrane Glut4 and HSP90 was decreased in aged KD mice. Moreover, treatment of the AMPK activator metformin attenuated aging-induced cardiomyocyte contractile defects. Collectively, our data suggest a role for AMPK deficiency in aging-induced cardiac dysfunction possibly through disrupted mitochondrial function and ROS production.
Keywords: AMPK, myocardial, cardiomyocytes, contractile function, morphology
INTRODUCTION
Myocardial contractile function, especially cardiac reserve during stress such as exercise, displays a dramatic decline with aging and contributes to the cardiac morbidity and mortality in the elderly (Lakatta, 1999;Yang et al., 2005). Although interactions among senescence, occult disease and physical inactivity are believed to predispose the senescence-associated changes in myocardial function and remodeling, the “aging process” per se is a rather unique determinant of ventricular performance and is considered an independent risk factor for cardiovascular diseases (Lakatta, 1999). Cardiac aging, an irreversible biological process, is characterized by a decrease in cardiac pump function and contractile reserve (Lakatta, 1999;Yang et al., 2005;Yang et al., 2006). A number of rationales have been put forward for the pathogenesis of cardiac aging including action potential prolongation, myosin heavy chain isozyme switch, mitochondrial defect and free radical accumulation, all of which may lead to the dysregulated intracellular Ca2+ homeostasis and excitation-contraction (E-C) coupling (Lakatta, 1999;Yang et al., 2005;Yang et al., 2006). Meanwhile, recent evidence favored an important and unique role for metabolic and energy metabolism as well as mitochondrial biogenesis in the onset and development of cardiac aging (Bhashyam et al., 2007;Yang et al., 2006). Nonetheless, the precise role of cardiac energy fuel and metabolism in cardiac aging has not been carefully defined.
AMP-activated protein kinase (AMPK) is a crucial cellular fuel sensor controlling fatty acid oxidation, mitochondrial biogenesis, myocardial morphology and contractile function (Arad et al., 2007;Young et al., 2005). AMPK is a heterotrimeric complex consisting of a catalytic subunit (α) and two regulatory subunits (β and γ). Two isoforms are identified for both α (α1 and α2) and β (β1 and β2) subunits while three isoforms have been reported for the γ subunit (γ1, γ2 and γ3) (Hardie et al., 1998). In response to a decrease in the ATP/AMP ratio, AMPK switches cells from an anabolic state where nutrients are taken up and stored, to a catabolic state where they are oxidized (Hardie and Carling, 1997). A plethora of evidence has demonstrated that AMPK serves as a therapeutic target for metformin and thiazolidinediones in the management of diabetes mellitus, ischemic heart disease and other metabolic diseases (Schimmack et al., 2006;Davis et al., 2006). More recent evidence has also revealed a possible role of AMPK signaling in the aging process (McCarty, 2004;Gonzalez et al., 2004;Reznick et al., 2007). Aging-associated loss in AMPK activity was reported in skeletal muscles which may contribute to the interrupted mitochondrial function and intracellular lipid metabolism in advanced age (Reznick et al., 2007). In addition, hypoxic insult was unable to turn on AMPK signaling in hepatic cells in aged rats, indicating a likely reduced stress tolerance with aging (Mulligan et al., 2005). However, the role of AMPK in cardiac aging has not been studied despite the high AMPK expression and demand in the heart (Stapleton et al., 1996). Hence, we took advantage of a transgenic model with overexpression of the dominant negative α2 subunit of AMPK. These adult AMPK kinase dead (KD) mice possess relatively smaller hearts, slightly reduced fractional wall thickening, normal myocyte diameter, normal left ventricular end-systolic and diastolic dimensions and fractional shortening, normal heart rate as well as mildly decreased ± dP/dt without overt morphological anomalies (Russell, III et al., 2004). Interestingly, the basal levels of ATP, glycogen and phosphocreatine appear to be normal with the transgenic overexpression of dominant-negative AMPK α2 subunit (D157A mutation) (Musi et al., 2005;Xing et al., 2003), suggesting a somewhat normal energy metabolism at least in young adults. To better understand the role of AMPK in cardiac aging, the state-of-the-art techniques were employed in our current research to evaluate myocardial morphology and contractile function, intracellular Ca2+ handling, generation of reactive oxygen species (ROS), mitochondrial function and the AMPK-associated signaling mechanisms in young or old wild-type (WT) and AMPK KD mice. Given the reported role of AMPK in aging-associated mitochondrial biogenesis in skeletal muscles (Reznick et al., 2007), expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), an essential mitochondrial biogenesis cofactor, its upstream regulator nitric oxide (Lopez-Lluch et al., 2008), the eNOS upstream signaling molecule Akt, and heat shock protein 90 (HSP90), a crucial ATP-dependent mitochondrial chaperone which may interact with eNOS to govern and reflect mitochondrial function (Sud et al., 2008), was also evaluated in young or old WT and AMPK KD mouse hearts.
RESULTS
General biometric and echocardiographic characteristics
The general biometric profiles of the young or old WT and KD mice are shown in Table 1. Transgenic overexpression of dominant negative AMPK α2 subunit did not elicit any notable effect on body, heart, liver and kidney weights or tibial length compared with the age-matched WT mice. Aged WT and KD mice displayed comparable heavier body and organ weights as well as enlarged heart but not liver and kidney size (normalized to body weight) when compared with their respective young counterparts. Cardiac hypertrophy persists in both aged groups when heart weight was normalized to tibial length, with a more pronounced effect in the aged KD mice compared with aged WT group. Neither aging nor AMPK deficiency significantly altered fasting blood glucose levels although the combination of the two overtly increased fasting glucose level. Both aging and AMPK deficiency independently elevated plasma insulin and HOMA-IR levels with a further increase of both indices in the aged AMPK KD mice. Aging but not AMPK deficiency significantly increased ventricular wall thickness, the effect of which was exaggerated in aged AMPK KD mice. To the contrary, AMPK deficiency but not aging increased ESD, the effect was further accentuated in aged AMPK KD mice. Neither aging nor AMPK deficiency, or both, significantly affected heart rate and EDD. Fractional shortening was significantly decreased in aged AMPK KD group but not in aging or young AMPK KD group.
Table 1.
Biometric and echocardiographic parameters of WT and KD mice at young and old age
| Parameter | WT-Young | WT-Old | KD-Young | KD-Old |
| Body Weight (g) | 24.0 ± 1.0 | 28.2 ± 1.0* | 24.4 ± 1.0 | 29.2 ± 0.6* |
| Heart Weight (mg) | 122 ± 5 | 160 ± 8* | 120 ± 7 | 181 ± 4* # |
| Tibial length (mm) | 16.7 ± 0.3 | 16.7 ± 0.2 | 17.3 ± 0.3 | 17.0 ± 0.2 |
| HW/TL (mg/mm) | 7.21 ± 0.25 | 9.12 ± 0.37* | 6.91 ± 0.38 | 10.70 ± 0.26* # |
| Heart/Body Weight (mg/g) | 5.13 ± 0.19 | 5.87 ± 0.25* | 4.82 ± 0.16 | 6.23 ± 0.17* |
| Liver Weight (g) | 1.22 ± 0.03 | 1.39± 0.05* | 1.36 ± 0.05* | 1.44 ± 0.02* |
| Liver/Body Weight (mg/g) | 52.7 ± 3.0 | 50.3 ± 1.6 | 52.0. ± 4.8 | 50.0 ± 1.2 |
| Kidney Weight (g) | 0.35 ± 0.01 | 0.42 ± 0.02* | 0.39 ± 0.02 | 0.43 ± 0.01* |
| Kidney/Body Weight (mg/g) | 14.1 ± 1.2 | 15.2 ± 0.4 | 13.8.± 1.3 | 14.9 ± 0.4 |
| Fasting Blood Glucose (mg/dl) | 114 ± 5 | 128 ± 5 | 125 ± 7 | 146 ± 5* # |
| Plasma Insulin (ng/ml) | 0.78 ± 0.21 | 2.51 ± 0.38* | 1.30 ± 0.56* | 4.77 ± 0.40* # |
| HOMA-IR (mmol/l*µU/ml) | 6.27 ± 2.06 | 18.59 ± 2.57* | 11.14 ± 4.86* | 39.55 ± 4.36* # |
| Heart Rate (bpm) | 501 ± 39 | 511 ± 22 | 508 ± 17 | 584 ± 36 |
| Wall Thickness (mm) | 0.71 ± 0.07 | 1.14 ± 0.10* | 0.65 ± 0.04 | 1.33 ± 0.07* # |
| EDD (mm) | 2.87 ± 0.26 | 2.93 ± 0.23 | 3.13 ± 0.10 | 3.27 ± 0.12 |
| ESD (mm) | 1.59 ± 0.11 | 1.63 ± 0.15 | 1.82 ± 0.27* | 2.11 ± 0.10* # |
| Fractional Shortening (%) | 43.2 ± 2.3 | 44.6 ± 1.7 | 41.9 ± 2.2 | 31.6 ± 3.9* # |
HW=heart weight; TL=tibial length; HOMA-IR=homeostatic model assessment of insulin resistance; EDD = end diastolic diameter; ESD = end systolic diameter; LV = left ventricular. Mean ± SEM, n = 12- 14 mice per group,
p < 0.05 vs. WT-young group,
p < 0.05 vs. WT-old group
α Isoform-specific AMPK activity
To delineate the impact of mutant AMPK α2 subunit overexpression on AMPK activity, a fluorescent polarization method was employed to assess α1 and α2 AMPK isoform activity. Our results revealed that AMPK α2 isoform activity was significantly reduced in young KD mice compared with the age-matched WT littermates, consistent with a previous report using the same model (Russell, III et al., 2004). Aging triggered a significant decrease in the AMPK α2 isoform activity in both WT and KD mice with a more pronounced reduction in AMPK KD mice. Overexpression of the mutant AMPK α2 subunit did not affect the AMPK α1 isoform activity although aging significantly dampened the AMPK α1 isoform activity in a comparable manner in both WT and KD mice (Fig. 1).
Fig. 1.
α-isoform-specific AMPK activity of left ventricular tissues from young or old wild-type (WT) and AMPK deficient KD mice. Mean ± SEM, n = 6–8 hearts per group, *p < 0.05 vs. WT young group, #p < 0.05 vs. WT- and KD-old groups.
Cardiomyocyte contractile and intracellular Ca2+ transient properties
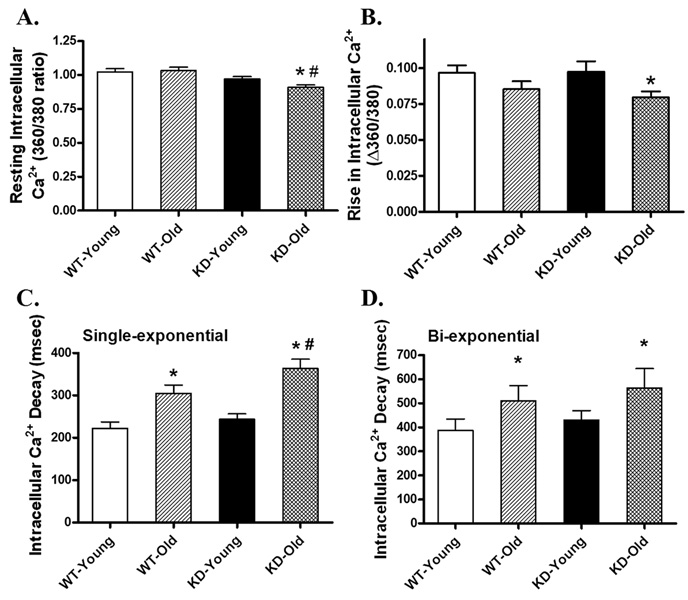
Neither aging nor AMPK deficiency significantly affected resting cell length. Aging itself significantly reduced PS and ± dL/dt as well as prolonged TR90 without affecting TPS. While AMPK deficiency itself did not elicit any significant effect on the mechanical parameters tested in young mice, it significantly accentuated the aging-induced alterations in ± dL/dt and TR90, as well as unveiled a prolonged TPS in aged KD mice. The aging-induced decline in PS was not affected by AMPK deficiency (Fig. 2). To explore the possible mechanism of action behind the interplay between aging and AMPK deficiency, intracellular Ca2+ handling was evaluated using Fura-2 fluorescence technique. Our data shown in Fig. 3 revealed that neither aging nor AMPK deficiency significantly affected resting intracellular Ca2+ levels and electrically-stimulated rise in intracellular Ca2+ although combination of the two overtly decreased both intracellular Ca2+ indices. Aging but not AMPK deficiency slowed down intracellular Ca2+ clearance rate (either single or bi-exponential). AMPK deficiency significantly exacerbated the aging-induced prolongation in single-exponential intracellular Ca2+ decay time without affecting the aging-induced prolongation of bi-exponential intracellular Ca2+ decay rate.
Fig. 2.
Cardiomyocyte mechanical function in left ventricular myocytes from young or old wild-type (WT) and AMPK deficient KD mice. (A) Resting cell length; (B) Peak shortening (PS, normalized to resting cell length); (C) Maximal velocity of shortening (+ dL/dt); (D) Maximal velocity of relengthening (−dL/dt); (E) Time-to-PS (TPS); and (F) Time-to-90% relengthening (TR90). Mean ± SEM, n= 95 – 96 cells from 5 mice per group, *p < 0.05 vs. respective young group, #p < 0.05 vs. WT-old group.
Fig. 3.
Intracellular Ca2+ property in cardiomyocytes from young or old wild-type (WT) and AMPK deficient KD mice. (A) Baseline intracellular Ca2+ levels (360/380 ratio); (B) Rise in intracellular Ca2+ levels in response to electrical stimulus (Δ360/380 ratio); (C) Single exponential intracellular Ca2+ decay rate; and (D) Bi-exponential intracellular Ca2+ decay rate. Mean ± SEM, n = 73 – 74 cells from 5 mice per group, *p < 0.05 vs. respective young group, #p < 0.05 vs. WT-old group.
Effect of increasing stimulation frequency on myocyte shortening
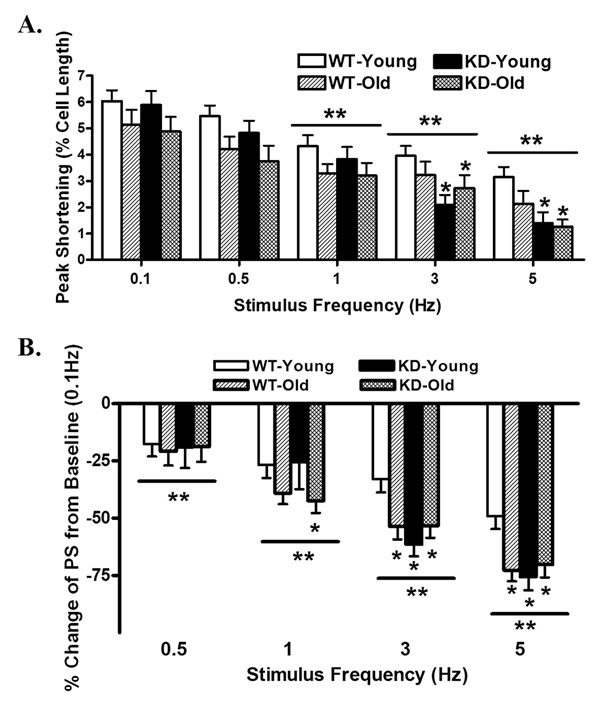
Rodent hearts normally contract at high frequencies, whereas our mechanical recording was performed at 0.5 Hz. To evaluate the impact of aging and/or AMPK deficiency on cardiac contractile function under higher frequencies, we increased stimulus frequency up to 5.0 Hz (300 beats/min) and recorded the steady-state peak shortening. Cardiomyocytes were initially stimulated to contract at 0.5 Hz for 5 min to ensure a steady-state before commencing the frequency response. Fig. 4 displays a negative staircase of PS with increased stimulus frequency in all 4 groups with a steeper decline in both absolute and normalized PS value (to PS value at 0.1 Hz of the same myocyte) in mice from aging, AMPK KD or old AMPK KD groups. These data favor a possible role of reduced intracellular Ca2+ cycling or stress tolerance capacity under aging and AMPK deficiency.
Fig. 4.
Peak shortening (PS) amplitude of cardiomyocytes from young or old wild-type (WT) and AMPK deficient KD mice at different stimulus frequencies (0.1 – 5.0 Hz). (A) Absolute peak shortening (normalized to resting cell length) amplitude at various stimulus frequencies; (B) Normalized PS value at various stimulus frequencies (PS was shown as % change from PS value obtained at 0.1 Hz from the same cell). Mean ± SEM, n = 17 – 24 cells from 5 mice per group, *p < 0.05 vs. WT-young group, **p < 0.05 vs. respective baseline value (0.1 Hz).
Effect of aging and AMPK deficiency on intracellular Ca2+ regulatory proteins
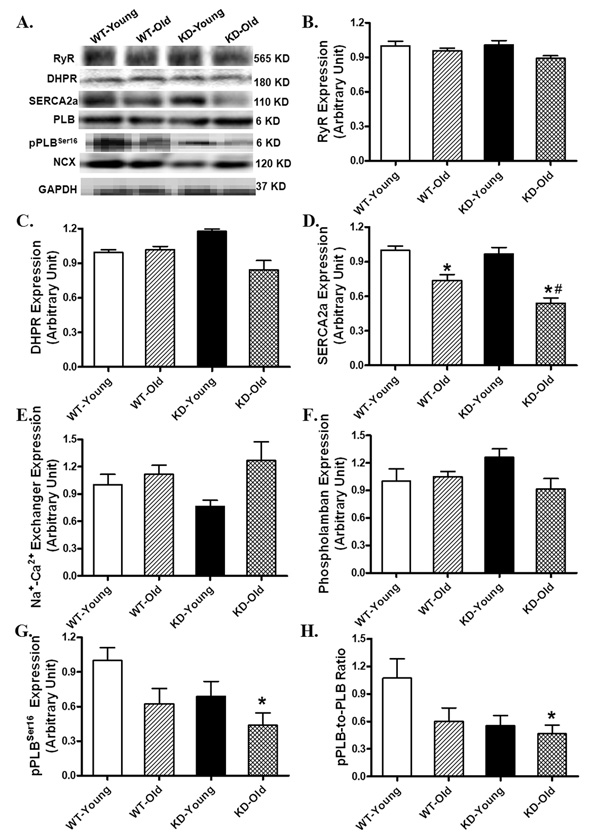
To further elucidate the possible mechanism(s) behind aging and/or AMPK deficiency-induced intracellular Ca2+ mishandling, a cascade of intracellular Ca2+ transporting/regulatory proteins was examined in myocardium from aging, AMPK deficiency, or both, groups. Our data depicted that aging but not AMPK deficiency significantly downregulated SERCA2a expression, the effect of which was accentuated by AMPK deficiency. Neither aging nor AMPK deficiency, or both, significantly affected the expression of ryanodine receptor (RyR), L-type Ca2+ channel dihydropyridine receptor (DHPR), Na+/Ca2+ exchanger and phospholamban. Although aging or AMPK deficiency did not affect the phosphorylation of phospholamban (either absolute value or pPLB-to-PLB ratio), the combination of the two significantly dampened the phosphorylation of phospholamban (either absolute value or normalized ratio), an inhibitor of SERCA2a with its phosphorylation removes the blockade on SERCA2a (Fig. 5).
Fig. 5.
Western blot analysis depicting protein expression of ryanodine receptor (RyR), L-type Ca2+ channel dihydropyridine receptor (DHPR), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), Na+/Ca2+ exchanger (NCX), phospholamban (PLB) and phosphorylated PLB (pPLB, Ser16) in myocardium from young or old wild-type (WT) and AMPK deficient KD mice. (A) Representative gel blots of RyR, DHPR, SERCA2a, NCX, PLB, pPLB and GAPDH (loading control) using specific antibodies; (B) RyR expression; (C) DHPR expression; (D) SERCA2a expression; (E) NCX expression; (F) PLB expression; (G) pPLB (Ser16) expression and (H) pPLB-to-PLB ratio. Mean ± SEM, n = 3 – 6 mice per group, *p < 0.05 vs. respective young group, #p < 0.05 vs. WT-old group.
Histological and TEM examination
To evaluate the impact of aging and/or AMPK deficiency on cardiac histology and ultrastructure, myocardial sections from young or old WT and AMPK KD mice were stained with H&E. Light microscopic observation did not reveal any distinct histological difference between WT and KD mouse hearts at the young age. However, myocardial sections from old AMPK KD mice exhibited greater cardiomyocyte hypertrophy and more pronounced increase in interstitial space and myofiber disorganization compared with aged WT hearts. Similarly, TEM sections from old KD mouse hearts exhibited marked mitochondrial alterations including swelling, irregular shape and disrupted cristae compared with the aged-matched WT mice (Fig. 6).
Fig. 6.
Histological examination of hearts from (A) WT-young; (B) KD-young; (C) WT-old; and (D) KD-old mice stained with hematoxylin and eosin (H&E). Panel E/F displays representative transmission electron micrographs of cardiomyocytes from WT-old (E) and KD-old (F) mouse hearts. Scale bars = 50 µm (A–D) and 1 µm (E and F); G: Quantitative analysis of cardiomyocyte cross-sectional area in young or old WT and AMPK KD mice. Data were obtained by averaging areas of at least 200 nucleated myocytes per section from each mouse (3–5 mice per group).
Effect of AMPK deficiency on ROS levels and mitochondrial membrane potential
Given that aging is known to elicit myocardial damage through accumulation of ROS (Yang et al., 2006), it is plausible to speculate a role of ROS production in aging and/or AMPK deficiency-induced myocardial contractile dysfunction. Using the intracellular fluoroprobe CM-H2DCFDA, our data shown in Fig. 7A depicted significantly elevated ROS generation in aged WT cardiomyocytes, the effect of which was accentuated by AMPK deficiency. To examine the potential role of mitochondria in aging and/or AMPK KD-induced effect on ROS production, mechanical and intracellular Ca2+ properties, mitochondrial membrane potential was measured using the JC-1 fluorescence technique. Results displayed in Fig. 7B revealed significantly reduced mitochondrial membrane potential in old WT cardiomyocytes. Consistent with its effect on ROS production, AMPK deficiency significantly exacerbated the aging-elicited decrease in mitochondrial membrane potential. Last but not the least, AMPK deficiency did not exhibit any significant effect on ROS generation or mitochondrial membrane potential at the young age.
Fig. 7.
Measurement of ROS production (A) and mitochondrial membrane potential (Ψm, B) in cardiomyocytes from young or old wild-type (WT) and AMPK deficient KD mice. Cells were loaded with the intracellular fluoroprobe CM-H2DCFDA (1 µM) at 37°C for 30 min prior to ROS detection. Quantification of Ψm was expressed as ratio between monomer and J-aggregate fluorescence (Red/Green). Mean ± SEM, n = 4 – 6 isolations per group, *p – 0.05 vs. respective young group, # p – 0.05 vs. WT-old group.
Effect of aging and/or AMPK deficiency on expression of Akt, pAkt, AMPK and pACC
To further elucidate the potential signaling mechanisms involved in aging and/or AMPK deficiency-induced myocardial dysfunction, western blot analysis was performed on a cascade of signaling molecules involved in the AMPK cascade. Immunoblotting study revealed a significantly downregulated Akt expression in old AMPK KD myocardium despite absence of notable change in either aging or AMPK KD group. Neither aging nor AMPK deficiency affected the level of Akt phosphorylation (pAkt). Expression of AMPK α2 subunit was significantly elevated by ~40% in the young KD mice due to overexpression of the dominant negative mutant AMPK α2 subunit, consistent with our previous report using the same model (Li et al., 2005). Aging itself did not affect the expression of AMPK α subunit in either WT or AMPK KD mice. Coinciding with the α-isoform AMPK activity assay, ACC phosphorylation at Ser79, a down-stream target of AMPK, was significantly and comparably reduced in the AMPK KD mice at both ages (Fig. 8). Total ACC expression was unaffected by either aging or AMPK deficiency, or both (data not shown).
Fig. 8.
Expression of Akt, pAkt, AMPK and pACC in myocardium from young or old wild-type (WT) and AMPK deficient KD mice. A: Akt; B: pAkt; C: AMPK; and D: pACC (ser79). Inset: Representative gel blots of Akt, pAkt, AMPK and pACC using specific antibodies. GAPDH was used as the loading control. Mean ± SEM, n = 3 – 6 hearts per group, *p – 0.05 vs. respective young group, #p – 0.05 vs. WT-old group.
Effects of metformin treatment on aging-induced cardiomyocyte contractile dysfunction
To further evaluate the role of AMPK and AMPK activation in the aging-induced cardiac contractile defect, both young and aged adult male WT mice were treated with intraperitoneal injection of metformin, a well-established activator of AMPK (300 mg/kg/b.w., for 5 days)(Davis et al., 2006) before cardiomyocyte mechanical function was evaluated. While metformin failed to elicit any significant mechanical response in the young mice, it significantly alleviated or abrogated the aging-induced cardiomyocyte contractile dysfunctions including reduced PS and ± dL/dt without affecting the aging-induced response on TPS and TR90 (Fig. 9). These data strongly support a beneficial role of AMPK activation against aging-induced cardiac contractile dysfunction.
Fig. 9.
Cardiomyocyte contractile function in left ventricular myocytes from young or old wild-type (WT) mice with or without treatment of the AMPK agonist metformin (300 mg/kg b.w., for 5 days). (A) Resting cell length; (B) Peak shortening (PS, normalized to resting cell length); (C) Maximal velocity of shortening (+ dL/dt); (D) Maximal velocity of relengthening (− dL/dt); (E) Time-to-PS (TPS); and (F) Time-to-90% relengthening (TR90). Mean ± SEM, n = 63 – 69 cells from 3 – 4 mice per group, *p < 0.05 vs. WT young group, #p < 0.05 vs. WT-old group.
Effect of aging and/or AMPK deficiency on expression of peNOS, PGC-1α, HSP90 and membrane Glut4
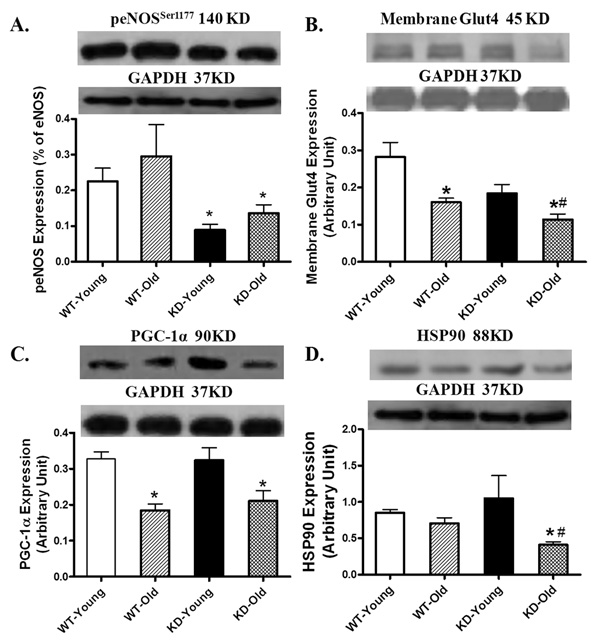
To better understand possible cellular mechanisms behind AMPK deficiency-exacerbated myocardial dysfunction in aging, western blot analysis was performed on mitochondrial function and glucose transport proteins. Immunoblotting study revealed that eNOS phosphorylation was significantly reduced in young and old AMPK KD mice with little difference between the two age groups. Aging itself did not affect phosphorylation of eNOS. Aging reduced Glut4 translocation shown as the membrane fraction of Glut4. Although AMPK deficiency itself failed to significantly affect the membrane Glut4 expression, it accentuated aging-induced decline in Glut4 membrane translocation. Our data further depicted that aging but not AMPK KD significantly downregulated the expression of the mitochondrial biogenesis cofactor PGC-1α, the effect was unaffected by AMPK deficiency. The mitochondrial chaperone protein HSP90 was not altered by either aging or AMPK deficiency although the combination of the two significantly reduced HSP90 expression (Fig. 10).
Fig. 10.
Expression of peNOS (Ser1177, A), membrane fraction of Glut4 (B), PGC1α (C) and HSP90 (D) in myocardium from young or old wild-type (WT) and AMPK deficient KD mice. Inset: Representative gel blots of peNOS, Glut4, PGC1α and HSP90 using specific antibodies. GAPDH was used as the loading control. Mean ± SEM, n = 4 – 6 mice per group, * p < 0.05 vs. respective young group, # p < 0.05 vs. WT-old group.
DISCUSSION
The major findings of our present study revealed that AMPK deficiency accentuated the aging-associated cardiomyocyte hypertrophy, cardiomyocyte dysfunction, intracellular Ca2+ mishandling and morphological alteration. The observation that AMPK deficiency significantly enhanced the aging-associated ROS generation and mitochondrial membrane potential drop in conjunction with histological, ultrastructural, functional and protein expression changes favors that a possible role of mitochondrial function in AMPK deficiency-induced myocardial alteration with aging. Involvement of impaired cardiac mitochondrial function was further substantiated by reduced mitochondrial chaperone HSP90 and membrane Glut4 levels in aged KD mice. Our observation that short-term in vivo treatment of the AMPK activator metformin alleviated aging-induced cardiomyocyte dysfunction also supported a likely role of AMPK in the maintenance of normal cardiac mechanical function during aging. These results have collectively prompted for a possible role of AMPK in the maintenance of myocardial morphology and function in the aging process and more importantly, the therapeutic potential of AMPK in cardiac aging.
Myocardial morphological and contractile changes especially hypertrophied ventricles, compromised contractility and prolonged diastolic duration are commonly seen in aged hearts (Yang et al., 2005;Yang et al., 2006;Fang et al., 2006;Lakatta, 1999). Reduced intrinsic contractile function and structural changes (mainly cardiomyocyte hypertrophy and interstitial fibrosis) within left ventricular myocardium has been demonstrated in senescence, which results in reduced early diastolic filling in left ventricle (Lakatta, 1999;Wei, 1992;Yang et al., 2005). Nevertheless, ventricular ejection fraction, the essential measure of left ventricular systolic performance, is often preserved during the aging process, probably due to enlarged ventricular end diastolic volume (Wei, 1992;Yang et al., 2005). This is consistent with the unchanged fractional shortening from our echocardiographic measurement in aging hearts. However, this apparent compensation against aging was gone with AMPK deficiency. Our echocardiographic finding also revealed significantly increased ventricular wall thickness associated with aging but not AMPK deficiency. This observation, in conjunction with the more pronounced changes in heart mass, heart weight-to-tibial length, cardiomyocyte cross-sectional area, ventricular wall thickness, ESD and fractional shortening in aged AMPK KD mice, favors a more prominent role of AMPK deficiency in aging hearts as opposed to the young age. ESD, which is better preserved in aging than AMPK deficiency, is significantly enhanced with concurrent aging and AMPK deficiency, and is mainly responsible for the reduced fractional shortening in aged AMPK KD mice. Ventricular hypertrophy and fibrosis are common manifestations of aging heart and may lead to heart failure (Wei, 1992). Our data revealed that old AMPK KD hearts possess greater heart mass, heart weight-to–tibial length ratio and cardiomyocyte size compared to their WT littermates. Although it is beyond the scope of our study with regards to the precise mechanism responsible for cardiac hypertrophy in the old KD mice, deficiency in AMPK, which is known to inhibit protein synthesis associated with cardiac hypertrophy (Chan et al., 2004), may be responsible for the exacerbated cardiac hypertrophy in old KD mice. Our study of in vitro cardiomyocyte function revealed depressed peak shortening and maximal velocity of shortening/relengthening associated with prolonged duration of relaxation in old WT mice, consistent with the previous findings (Li et al., 2008;Li et al., 2007). Although AMPK deficiency itself did not affect cardiomyocyte mechanical function, it significantly augmented aging-elicited cardiomyocyte dysfunction (with the exception of peak shortening) and unveiled a prolongation in contraction duration (TPS).
Our study further noted unchanged resting intracellular Ca2+ levels and intracellular Ca2+ release in response to excitation as well as delayed intracellular Ca2+ clearance in old WT cardiomyocytes, somewhat in agreement with our previous findings (Li et al., 2007;Li and Ren, 2007;Yang et al., 2006). Although AMPK deficiency did not affect intracellular Ca2+ homeostasis in young mice, it unveiled a reduced resting as well as electrically-stimulated rise in intracellular Ca2+ levels with aging while further exacerbating the aging-induced decrease in intracellular Ca2+ clearance (single exponential). These findings depict that AMPK deficiency overtly disrupted intracellular Ca2+ homeostasis especially Ca2+ re-sequestration with aging. The fact that AMPK deficiency itself did not significantly affect myocardial contractile and intracellular Ca2+ properties in young mouse hearts indicates that deficiency of this kinase early on in life may not be innately harmful to cardiac function despite the increased insulin and HOMA-IR levels. Previous report using slightly older (4–6 month-old) mice indicated normal myocyte diameter, normal echocardiographic properties (including left ventricular end-systolic and diastolic dimensions, fractional shortening, and heart rate), as well as slightly reduced fractional wall thickening and ± dP/dt in these AMPK KD mice (Russell, III et al., 2004). The subtle difference between this previous finding and our current study may be related to factors such as cardiac function assessment technique (whole heart versus isolated cardiomyocytes) and age of mice. It is worth mentioning the discrepant findings between the unchanged fractional shortening and the reduced cardiomyocyte contractile capacity in aging WT group may be associated with the absence of nonmyocyte factors such as fibroblasts and connective tissues as well as the non-loading isotonic nature in vitro.
Existence of intracellular Ca2+ mishandling in aging and/or AMPK deficiency is further supported by the reduced stress tolerance manifested as the steeper negative staircase in the shortening-frequency response. This also coincides with changes in intracellular Ca2+ handling proteins especially SERCA2a and phospholamban phosphorylation. Our present data revealed downregulated SERCA2a expression with unchanged Na+-Ca2+ exchanger, phospholamban, RyR and DHP receptor, somewhat consistent with our previous finding (Li et al., 2007). While AMPK deficiency failed to affect these machineries for intracellular Ca2+ cycling, it accentuated or unveiled, respectively, the aging-induced depression on SERCA2a expression and phosphorylation of phospholamban, an endogenous SERCA inhibitor. Dampened SERCA2a expression and phospholamban phosphorylation may account for, at least in part, intracellular Ca2+ mishandling, prolonged intracellular Ca2+ clearance and cardiomyocyte relaxation in senescent AMPK KD myocytes.
Several mechanisms may be proposed for the AMPK KD-elicited exaggeration of aging-induced mechanical and intracellular Ca2+ dysregulation. First, our data revealed that AMPK deficiency is capable of exaggerating aging-induced ROS generation and mitochondrial damage (evaluated by mitochondrial membrane potential). Enhanced ROS accumulation, oxidative damage and mitochondrial damage directly interrupt cardiac contractile function and intracellular Ca2+ homeostasis (Chien, 1999;Goldhaber and Qayyum, 2000). The mitochondrial variant of the free radical theory of aging suggests that ROS accumulation attacks mitochondrial constituents, causing mitochondrial DNA damage, leading to further ROS production, oxidative damage to lipids and proteins, and a decline in overall cardiac function (Dai et al., 2009). Overexpression of antioxidants such as catalase targeted to mitochondria is known to attenuate cardiac aging (Dai et al., 2009). This is consistent with the notion that resveratrol, a known antioxidant and AMPK activator, exerted a cardioprotective effect against ROS-induced cell death (Hwang et al., 2008). Secondly, our observation revealed that AMPK deficiency significantly accentuated the aging-induced decline in Glut4 membrane translocation, indicating possible involvement of glucose metabolism and fatty acid uptake in AMPK KD-elicited effects. An aging-associated decline in Glut4 expression has been demonstrated (Qiang et al., 2007). The more dramatic reduction in the expression of membrane Glut4 is consistent with the observation of the dramatic increases in blood glucose, plasma insulin and HOMA-IR index in aged AMPK KD mice. Moreover, our data indicated that metformin treatment significantly improved aging-induced cardiomyocyte dysfunction. This is consistent with a recent report where metformin significantly alleviated ventricular dysfunction in a murine model of heart failure through activation of AMPK and subsequently preservation of PGC-1α expression and eNOS phosphorylation (Gundewar et al., 2009). Our data suggest that metformin effectively alleviated all aging-associated cardiomyocyte mechanical dysfunctions with the exception of prolonged relaxation duration. It is possible that the dose and duration of metformin treatment as well as possible existence of certain AMPK-independent machineries may contribute to lack of drug response in the relengthening duration associated with aging in our current experimental setting.
AMPK has been considered as a critical regulator involved in initiating mitochondrial biogenesis (Zong et al., 2002). AMPK stimulates PGC-1α phosphorylation, the latter triggers other transcription factors involved in mitochondrial biogenesis (Jager et al., 2007). This is consistent with the observation that the AMPK activator β-guanidinopropionic acid failed to increase PGC-1α expression or mitochondrial content in skeletal muscles from AMPK KD mice (Zong et al., 2002). Somewhat to our surprise, our current finding that AMPK deficiency failed to affect aging-induced downregulation in PGC-1α does not favor a major role of AMPK in aging-associated cardiac mitochondrial biogenesis. A number of reports have indicated that AMPK deficiency and aging may independently decrease mitochondrial biogenesis (Winder et al., 2000;Jager et al., 2007;Bhashyam et al., 2007;Reznick et al., 2007), possibly through eNOS phosphorylation (Chen et al., 1999). Nonetheless, our data failed to associate any patterns of responsiveness in PGC-1α expression and eNOS phosphorylation. Our Western blot analysis depicted a significant reduction of the mitochondrial chaperon HSP90 in aging AMPK KD mice, an event deemed secondary to uncoupling of eNOS en route to mitochondrial and endothelial dysfunction associated with cardiovascular diseases (Sud et al., 2008). Indeed, metformin-induced interaction of eNOS with HSP90 was markedly depressed following transfection with the dominant-negative mutant of AMPK α2 subunit (Davis et al., 2006). We demonstrated earlier that AMPK-mediated glucose uptake and translocation of Glut4 are partly mediated though NO in the hearts (Li et al., 2004). Activation of eNOS by AMPK offers protection against ischemia-reperfusion injury (Calvert et al., 2008), the effect of which may be blunted with AMPK or eNOS deficiency (Gundewar et al., 2009). Nevertheless, further study is warranted to elucidate the precise role of AMPK in aging-associated change in cardiac mitochondrial biogenesis and mitochondrial function.
Our data revealed reduced ACC phosphorylation in KD mice from both age groups. To our surprise, the degrees of decrease in ACC phosphorylation were similar between the two KD groups (Fig. 8D), inconsistent with the finding from isoform-specific AMPK activity assay (Fig. 1). It was demonstrated that AMPK regulates intracellular fatty acid oxidation via inactivating ACC in a phosphorylation manner (Kudo et al., 1995). ACC serves as a downstream signaling molecule for AMPK. Change of pACC levels in aging not only provides evidence that AMPKα plays a role in aging-associated disturbance in intracellular fat oxidation and insulin signaling, but also suggests involvement of ACC-mediated pathway in AMPK deficiency-exacerbated cardiac aging responses. Although the nature of the ACC pathway is beyond the scope of our current study, its important role in cardiac aging should not be underestimated. Our observation of accentuated myocardial function and morphology with AMPK deficiency associated with normal Akt phosphorylation depicts a rather minor role of Akt in AMPK deficiency-associated exaggeration of cardiac aging response. However, it should be pointed out that other signals such as leptin (Minokoshi et al., 2002) and β-adrenergic receptors (Hattori et al., 2010), may also be involved in the regulation of ACC. Additional study is in demand to explore and validate the role of these AMPK-dependent and -independent signaling molecules in ACC phosphorylation during cardiac aging process.
Cardiac aging is an irreversible biological process characterized by cardiac hypertrophy and the progressive decline of myocardial contractile function (Lakatta, 1999;Yang et al., 2005). Accumulation of free radicals and oxidative stress are perhaps the main players leading to aging-related cardiovascular diseases (Lakatta, 1999;Yang et al., 2005). Our finding suggests that AMPK may play an essential role in the preservation of cardiac function in the elderly. While our data did not favor an aging-associated decline in the cardiac AMPK expression, a decrease in AMPK activity or blunted responsiveness of AMPK activity with advanced age may well exist as shown here as well as previously (Qiang et al., 2007;Mulligan et al., 2005), suggesting a potential therapeutic target for AMPK in cardiac aging. Although our study sheds some light on the interaction of oxidative stress, mitochondrial function and cardiac aging-associated mechanical and intracellular Ca2+ defects, the pathogenesis of cardiac dysfunction under senescence still deserves further in-depth investigation. As life expectancy throughout the world continues to rise, cardiac implications of the normal aging process are expected to remain the focus of much attention for physicians and scientists.
METHODS AND MATERIALS
Mice overexpressing dominant-negative α2 subunit of AMPK and metformin treatment
Adult male mice overexpressing the dominant negative AMPK α2 subunit (kinase dead, KD, obtained from Dr. Morris Birnbaum, University of Pennsylvania, Philadelphia, PA) and their littermates expressing wild-type (WT) of α2 subunit of AMPK were used in this study. These transgenic mice overexpress a kinase dead rat α2 isoform (K45R mutation) which is driven by a muscle-specific creatine kinase promoter to the skeletal muscle and heart. Due to the replacement of functional α1, α2 and α3 isoforms by the KD α2 isoform, KD mice display very low AMPK activity in skeletal and cardiac muscles (Mu et al., 2001). Founder transgenic mice were genotyped by the polymerase chain reaction (PCR) (Mu et al., 2001). Either 4–6 month-old (denoted as "young") or 24–28 month-old (denotes as "old") male WT and KD mice were maintained with a 12/12-light/dark cycle with free access to tap water until experimentation. At the time of sacrifice, blood glucose and plasma insulin levels were measured using a glucose monitor and an ELISA commercial kit, respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) was used to estimate insulin resistance based on the following equation: fasting insulin (µU/ml) x fasting blood glucose (mmol/l)/22.5 (Matthews et al., 1985). In addition, left tibia of each mouse was isolated and the tibial length was measured with a micrometer. To evaluate the effect of AMPK activation in cardiac aging, young and old WT mice were treated with the AMPK activator metformin (300 mg/kg/b.w., i.p.) for 5 days (Davis et al., 2006)prior to examination of the cardiomyocyte mechanical property. All animal procedures were approved by the Animal Care and Use Committee at the University of Wyoming (Laramie, WY).
α isoform-specific AMPK activity
An AMPK KinEASE™ FP Fluorescein Green Assay kit (Millipore, Billerica, MA) was used to determine the α-isoform-specific AMPK kinase activity from left ventricular protein lysates using the anti-AMPKα1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-AMPKα2 antibodies (Cell Signaling Technology Inc., Beverly, MA) according to the protocol supplied by the vendor (Kandadi et al., 2010).
Echocardiographic assessment
Cardiac geometry and function were evaluated in anesthetized (Avertin 2.5%, 10 µl/g, b.w., i.p.) mice using the 2-D guided M-mode echocardiography (Sonos 5500) equipped with a 15-6 MHz linear transducer. Left ventricular (LV) anterior and posterior wall dimensions during diastole and systole were recorded from three consecutive cycles in M-mode using method adopted by the American Society of Echocardiography (Doser et al., 2009). Fractional shortening was calculated from LV end-diastolic (EDD) and end-systolic (ESD) diameters using the equation (EDD-ESD)/EDD. Heart rates were averaged over 10 cardiac cycles.
Isolation of mouse cardiomyocytes
Hearts were rapidly removed from anesthetized mice and mounted onto a temperature-controlled (37°C) Langendorff system. After perfusing with a modified Tyrode's solution (Ca2+ free) for 2 min, the heart was digested with a Ca2+-free KHB buffer containing liberase blendzyme 4 (Hoffmann-La Roche Inc., Indianapolis, IN) for 20 min. The modified Tyrode solution (pH 7.4) contained the following (in mM): NaCl 135, KCl 4.0, MgCl2 1.0, HEPES 10, NaH2PO4 0.33, glucose 10, butanedione monoxime 10, and the solution was gassed with 5% CO2–95% O2. The digested heart was then removed from the cannula and left ventricle was cut into small pieces in the modified Tyrode's solution. Tissue pieces were gently agitated and pellet of cells was resuspended. Extracellular Ca2+ was added incrementally back to 1.20 mM over 30 min. A yield of at least 50–60% viable rod-shaped cardiomyocytes with clear sacromere striations was achieved. Cardiomyocytes with obvious sarcolemmal blebs or spontaneous contraction were not chosen for mechanical study. Only rod-shaped myocytes with clear edges were selected for contractile and intracellular Ca2+ studies (Doser et al., 2009).
Cell shortening/relengthening
Mechanical properties of cardiomyocytes were assessed using a SoftEdge MyoCam® system (IonOptix Corporation, Milton, MA) (Doser et al., 2009). In brief, cells were placed in a Warner chamber mounted on the stage of an inverted microscope (Olympus, IX-70) and superfused (~1 ml/min at 25 °C) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, at pH 7.4. The cells were field stimulated with supra-threshold voltage at a frequency of 0.5 Hz (unless otherwise stated), 3 msec duration, using a pair of platinum wires placed on opposite sides of the chamber connected to a FHC stimulator (Brunswick, NE). The myocyte being studied was displayed on the computer monitor using an IonOptix MyoCam camera. An IonOptix SoftEdge software was used to capture changes in cell length during shortening and relengthening. Cell shortening and relengthening were assessed using the following indices: peak shortening (PS) - indicative of peak ventricular contractility, time-to-PS (TPS) - indicative of contraction duration, and time-to-90% relengthening (TR90) - represents cardiomyocyte relaxation duration, maximal velocities of shortening (+dL/dt) and relengthening (−dL/dt) - indicatives of maximal velocities of ventricular pressure rise/fall. In the case of altering stimulus frequency from 0.1 to 5.0 Hz, the steady state contraction of myocyte was achieved (usually after the first 5–6 beats) before PS was recorded.
Intracellular Ca2+ transient measurement
Myocytes were loaded with fura-2/AM (0.5 µM) for 10 min and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). Cardiomyocytes were placed on an Olympus IX-70 inverted microscope and imaged through a Fluor × 40 oil objective. Cells were exposed to light emitted by a 75W lamp and passed through either a 360 or a 380 nm filter, while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 and 520 nm by a photomultiplier tube after first illuminating the cells at 360 nm for 0.5 sec then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration were inferred from the ratio of fura-2 fluorescence intensity (FFI) at two wavelengths (360/380). Fluorescence decay time was measured as an indication of the intracellular Ca2+ clearing rate. Both single and bi-exponential curve fit programs were applied to calculate the intracellular Ca2+ decay constant (Doser et al., 2009).
Intracellular reactive oxygen species (ROS)
Production of ROS was evaluated by fluorescence intensity changes resulting from oxidation of the intracellular fluoroprobe 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA). In brief, isolated cardiomyocytes were loaded with the non-fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, 1 µM, Molecular Probes, Eugene, OR) at 37 °C for 30 min. The myocytes were rinsed and the fluorescence intensity was then measured using a fluorescent micro-plate reader at an excitation wavelength of 480 nm and an emission wavelength of 530 nm (Molecular Devices, Sunnyvale, CA). Untreated cardiomyocytes showed no fluorescence and were used to determine background fluorescence, which was subtracted from the treated samples. The final fluorescent intensity was normalized to the protein content in each group (Privratsky et al., 2003).
Mitochondrial membrane potential
Isolated cardiomyocytes were suspended in HEPES-saline buffer and mitochondrial membrane potential (ΔΨm) was detected as described (Di et al., 1995). Briefly, cells were incubated with 5 µM JC-1 fluorochrome (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethyl-benzimidazolocarbocyanineiodide) (Molecular Probes) for 10 min at 37°C. Cells were then washed twice using PBS buffer before examination using a spectrofluorimeter (Spectra MaxGeminiXS) at 530 nm (monomer form of JC-1, green) and at 590 nm (aggregate form of JC-1, red). The mitochondrial uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP, 10 µM) was used as a positive control for mitochondrial membrane potential (Reers et al., 1991).
Histological examination
Following anesthesia, hearts were excised and immediately placed in 10% neutral-buffered formalin at room temperature for 24 hrs after a brief rinse with PBS. The specimen were embedded in paraffin, cut in 5 µm sections and stained with hematoxylin and eosin (H&E). Cardiomyocyte cross-sectional areas were calculated on a digital microscope (x400) using the Image J (version1.34S) software (Doser et al., 2009).
Electron microscopy
Following perfusion fixation, left ventricular and interventricular septal tissues were minced to blocks of 1 mm3 followed by fixation and postfixation. Tissue blocks were embedded in Epon/Araldite and cured 48 hr at 60°C. Thin sections were collected on naked copper (300-mesh) grids, stained with lead citrate and uranyl acetate, and imaged with a Hitachi 7500 transmission electron microscope (Ceylan-Isik et al., 2009).
Western blot analysis
Murine heart tissues were homogenized and sonicated in a lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 0.1% sodium dodecyl sulfate (SDS), and a protease inhibitor cocktail. Protein levels of Akt, phosphorylated Akt (pAkt), AMPK, phosphorylated acetyl CoA Carboxylase (pACC), eNOS, phosphorylated eNOS (peNOS), PGC-1α, Glut4, HSP90, sarco(endo)plasmic reticulum Ca2+− ATPase (SERCA2a), phospholamban (PLB), phosphorylated PLB (pPLB), Na+/Ca2+ exchanger, dihydropyridine Ca2+ receptor (DHPR) and ryanodine receptor (RyR) were examined by standard western immunoblotting. Membranes were probed with anti-Akt (1:1,000), anti-pAkt (Thr473, 1:1000), anti-AMPK (1:1,000), anti-pACC (Ser79, 1:1,000), anti-HSP90 (1:1,000 ), anti-peNOS (Ser1177, 1:1,000), anti-Glut4 (1:1,000, all from Cell Signaling), anti-PGC-1α (1:500) anti-DHPR (1:200), anti-RyR (1:100, all from Santa Cruz Biotechnology, Santa Cruz, CA), anti-SERCA2a (1:1,000, Bethyl Laboratories Inc., Montgomery, TX), anti-PLB (1:1,000), anti-pPLB (Ser16, 1:1000 dilution; both from Upstate, Lake Placid, NY), anti- Na+/Ca2+ exchanger (Abcam Inc., Cambridge, MA) and anti-GAPDH (1:1,000, internal loading control, Cell Signaling) antibodies. The membranes were then incubated with horseradish peroxidase (HRP)-coupled anti-rabbit, anti-mouse (Cell Signaling) or anti-goat (Santa Cruz) secondary antibodies. After immunoblotting, the film was scanned and detected with a Bio-Rad Calibrated Densitometer and the intensity of immunoblot bands was normalized to that of GAPDH (Ceylan-Isik et al., 2009).
Myocardial membrane protein extraction
Myocardial membrane protein was extracted using a membrane protein extraction kit (Biovision Inc., Mountain View, CA). The membrane protein was subsequently used for Western blot analysis of Glut-4 (Dong et al., 2008).
Data analysis
Data were Mean ± SEM. Statistical significance (p < 0.05) was estimated by one-way analysis of variation (ANOVA) followed by a Tukey’s test for post hoc analysis.
ACKNOWLEDGMENTS
This work was supported in part by NIH/NCRR P20 RR016474.
Reference List
- Arad M, Seidman CE, Seidman JG. AMP-Activated Protein Kinase in the Heart: Role During Health and Disease. Circ Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- Bhashyam S, Parikh P, Bolukoglu H, Shannon AH, Porter JH, Shen YT, Shannon RP. Aging Is Associated With Myocardial Insulin Resistance and Mitochondrial Dysfunction. Am J Physiol Heart Circ Physiol. 2007;293:H3063–H3071. doi: 10.1152/ajpheart.00163.2007. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute Metformin Therapy Confers Cardioprotection Against Myocardial Infarction Via AMPK-ENOS-Mediated Signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Guo KK, Carlson EC, Privratsky JR, Liao SJ, Cai L, Chen AF, Ren J. Metallothionein Abrogates GTP Cyclohydrolase I Inhibition-Induced Cardiac Contractile and Morphological Defects: Role of Mitochondrial Biogenesis. Hypertension. 2009;53:1023–1031. doi: 10.1161/HYPERTENSIONAHA.108.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-Activated Protein Kinase Inhibits Protein Synthesis Associated With Hypertrophy in the Cardiac Myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-Activated Protein Kinase Phosphorylation of Endothelial NO Synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Chien KR. Stress Pathways and Heart Failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-Activated Kinase by Antidiabetes Drug Metformin Stimulates Nitric Oxide Synthesis in Vivo by Promoting the Association of Heat Shock Protein 90 and Endothelial Nitric Oxide Synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Di LF, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial Membrane Potential in Single Living Adult Rat Cardiac Myocytes Exposed to Anoxia or Metabolic Inhibition. J Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Kandadi MR, Ren J, Sreejayan N. Chromium (D-Phenylalanine)3 Supplementation Alters Glucose Disposal, Insulin Signaling, and Glucose Transporter-4 Membrane Translocation in Insulin-Resistant Mice. J Nutr. 2008;138:1846–1851. doi: 10.1093/jn/138.10.1846. [DOI] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic Overexpression of Aldehyde Dehydrogenase-2 Rescues Chronic Alcohol Intake-Induced Myocardial Hypertrophy and Contractile Dysfunction. Circulation. 2009;119:1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein Antagonizes Aging-Induced Cardiac Contractile Dysfunction: Role of PTP1B, Insulin Receptor Tyrosine Phosphorylation and Akt. Aging Cell. 2006;5:177–185. doi: 10.1111/j.1474-9726.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Qayyum MS. Oxygen Free Radicals and Excitation-Contraction Coupling. Antioxid Redox Signal. 2000;2:55–64. doi: 10.1089/ars.2000.2.1-55. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Saupe KW. Effects of Aging on Cardiac and Skeletal Muscle AMPK Activity: Basal Activity, Allosteric Activation, and Response to in Vivo Hypoxemia in Mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1270–R1275. doi: 10.1152/ajpregu.00409.2004. [DOI] [PubMed] [Google Scholar]
- Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, naya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-Activated Protein Kinase by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-Activated Protein Kinase--Fuel Gauge of the Mammalian Cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-Activated/SNF1 Protein Kinase Subfamily: Metabolic Sensors of the Eukaryotic Cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hattori A, Mawatari K, Tsuzuki S, Yoshioka E, Toda S, Yoshida M, Yasui S, Furukawa H, Morishima M, Ono K, Ohnishi T, Nakano M, Harada N, Takahashi A, Nakaya Y. Beta-Adrenergic-AMPK Pathway Phosphorylates Acetyl-CoA Carboxylase in a High-Epinephrine Rat Model, SPORTS. Obesity (Silver Spring) 2010;18:48–54. doi: 10.1038/oby.2009.145. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol Protects ROS-Induced Cell Death by Activating AMPK in H9c2 Cardiac Muscle Cells. Genes Nutr. 2008;2:323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle Via Direct Phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandadi MR, Rajanna PK, Unnikrishnan MK, Boddu SP, Hua Y, Li J, Du M, Ren J, Sreejayan N. 2-(3,4-Dihydro-2H-Pyrrolium-1-Yl)-3oxoindan-1-Olate (DHPO), a Novel, Synthetic Small Molecule That Alleviates Insulin Resistance and Lipid Abnormalities. Biochem Pharmacol. 2010;79:623–631. doi: 10.1016/j.bcp.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High Rates of Fatty Acid Oxidation During Reperfusion of Ischemic Hearts Are Associated With a Decrease in Malonyl-CoA Levels Due to an Increase in 5'-AMP-Activated Protein Kinase Inhibition of Acetyl-CoA Carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular Aging Research: the Next Horizons. J Am Geriatr Soc. 1999;47:613–625. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Li J, Hu X, Selvakumar P, Russell RR, III, Cushman SW, Holman GD, Young LH. Role of the Nitric Oxide Pathway in AMPK-Mediated Glucose Uptake and GLUT4 Translocation in Heart Muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, III, Young LH. AMP-Activated Protein Kinase Activates P38 Mitogen-Activated Protein Kinase by Increasing Recruitment of P38 MAPK to TAB1 in the Ischemic Heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of Insulin-Like Growth Factor 1 Reduces Sensitivity to Aging-Associated Cardiomyocyte Dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ren J. Influence of Cardiac-Specific Overexpression of Insulin-Like Growth Factor 1 on Lifespan and Aging-Associated Changes in Cardiac Intracellular Ca2+ Homeostasis, Protein Damage and Apoptotic Protein Expression. Aging Cell. 2007;6:799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-Specific Overexpression of Insulin-Like Growth Factor 1 Attenuates Aging-Associated Cardiac Diastolic Contractile Dysfunction and Protein Damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de C R. Mitochondrial Biogenesis and Healthy Aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Chronic Activation of AMP-Activated Kinase As a Strategy for Slowing Aging. Med Hypotheses. 2004;63:334–339. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A Role for AMP-Activated Protein Kinase in Contraction- and Hypoxia-Regulated Glucose Transport in Skeletal Muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging Elevates Basal Adenosine Monophosphate-Activated Protein Kinase (AMPK) Activity and Eliminates Hypoxic Activation of AMPK in Mouse Liver. J Gerontol A Biol Sci Med Sci. 2005;60:21–27. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, Ahmad F, Berul CI, Seidman JG, Tian R, Goodyear LJ. Functional Role of AMP-Activated Protein Kinase in the Heart During Exercise. FEBS Lett. 2005;579:2045–2050. doi: 10.1016/j.febslet.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, Bagchi M, Bagchi D, Maulik N. Niacin Bound Chromium Treatment Induces Myocardial Glut-4 Translocation and Caveolar Interaction Via Akt, AMPK and ENOS Phosphorylation in Streptozotocin Induced Diabetic Rats After Ischemia-Reperfusion Injury. Biochim Biophys Acta. 2009;1792:39–48. doi: 10.1016/j.bbadis.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 Blockade Prevents Glucose-Induced Cardiac Dysfunction in Ventricular Myocytes: Role of the AT1 Receptor and NADPH Oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging Impairs Insulin-Stimulated Glucose Uptake in Rat Skeletal Muscle Via Suppressing AMPKalpha1. Exp Mol Med. 2007;39:535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- Reers M, Smith TW, Chen LB. J-Aggregate Formation of a Carbocyanine As a Quantitative Fluorescent Indicator of Membrane Potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-Associated Reductions in AMP-Activated Protein Kinase Activity and Mitochondrial Biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-Activated Protein Kinase Mediates Ischemic Glucose Uptake and Prevents Postischemic Cardiac Dysfunction, Apoptosis, and Injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack G, Defronzo RA, Musi N. AMP-Activated Protein Kinase: Role in Metabolism and Therapeutic Implications. Diabetes Obes Metab. 2006;8:591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-Activated Protein Kinase Subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric Dimethylarginine Inhibits HSP90 Activity in Pulmonary Arterial Endothelial Cells: Role of Mitochondrial Dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JY. Age and the Cardiovascular System. N Engl J Med. 1992;327:1735–1739. doi: 10.1056/NEJM199212103272408. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-Activated Protein Kinase Increases Mitochondrial Enzymes in Skeletal Muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose Metabolism and Energy Homeostasis in Mouse Hearts Overexpressing Dominant Negative Alpha2 Subunit of AMP-Activated Protein Kinase 2. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J. Metallothionein Prolongs Survival and Antagonizes Senescence-Associated Cardiomyocyte Diastolic Dysfunction: Role of Oxidative Stress. FASEB J. 2006;20:1024–1026. doi: 10.1096/fj.05-5288fje. [DOI] [PubMed] [Google Scholar]
- Yang X, Sreejayan N, Ren J. Views From Within and Beyond: Narratives of Cardiac Contractile Dysfunction Under Senescence. Endocrine. 2005;26:127–137. doi: 10.1385/ENDO:26:2:127. [DOI] [PubMed] [Google Scholar]
- Young LH, Li J, Baron SJ, Russell RR. AMP-Activated Protein Kinase: a Key Stress Signaling Pathway in the Heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP Kinase Is Required for Mitochondrial Biogenesis in Skeletal Muscle in Response to Chronic Energy Deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]