Abstract
Recent findings from our laboratory indicate that alterations in frontal cortex function, structural plasticity, and related social behaviors are persistent consequences of exposure to moderate levels of ethanol during prenatal brain development [24]. Fetal-ethanol-related reductions in the expression of the immediate early genes (IEGs) c-fos and Arc and alterations in dendritic spine density in ventrolateral and medial aspects of frontal cortex suggest a dissociation reminiscent of that described by Kolb et al. [38] in which these aspects of frontal cortex undergo reciprocal experience-dependent changes. In addition to providing a brief review of the available data on social behavior and frontal cortex function in fetal-ethanol-exposed rats, the present paper presents novel data on social-experience-related IEG expression in four regions of frontal cortex (Zilles LO, VLO, Fr1, Fr2) that are evaluated alongside our prior data from AID and Cg3. Social experience in normal rats was related to a distinct pattern of IEG expression in ventrolateral and medial aspects of frontal cortex, with generally greater expression observed in ventrolateral frontal cortex. In contrast, weaker expression was observed in all aspects of frontal cortex in ethanol-exposed rats, with the exception of an experience-related increase in the medial agranular cortex. Behaviors related to social investigation and wrestling/boxing were differentially correlated with patterns of activity-related IEG expression in the regions under investigation for saccharin- and ethanol-exposed rats. These observations suggest that recruitment and expression of IEGs in frontal cortex following social experience are potentially important for understanding the long-term consequences of moderate prenatal ethanol exposure on frontal cortex function, synaptic plasticity, and related behaviors.
Keywords: Immediate early gene expression, Social behavior, Ethanol teratogenesis
1. Introduction
The present paper has three major goals: (1) to provide a brief review of the literature on the persistent consequences of fetal ethanol exposure on social behavior in adulthood with an emphasis on recent data on structural plasticity and immediate early gene expression (IEG) in frontal cortex from our laboratory, (2) to evaluate previously published and novel data on social-experience-related IEG expression in the frontal cortex of fetal-ethanol-exposed rats to provide a more thorough characterization of the regional patterns of ethanol-related effects in frontal cortex, and (3) to evaluate the pattern of relationships between IEG expression and social behavior in normal and fetal-ethanol-exposed rats.
1.1. Fetal Alcohol Spectrum Disorders (FASDs)
Heavy consumption of alcohol during pregnancy can result in a set of profound morphological, neurological, and behavioral consequences in offspring referred to as Fetal Alcohol Syndrome (FAS; [2,14,30,31,32]). The incidence of FAS in the general population is relatively low (<0.5%) [1], however, 5–20% of women engage in moderate drinking during pregnancy (1–2 drinks/day; [17]) and it is estimated that 10–20 times more children are exposed to moderate levels of alcohol in utero that do not result in full-blown FAS [13]. At present, the long-term consequences of moderate fetal ethanol exposure are not well-characterized, however, such consequences may be of considerable importance because of their pervasiveness and persistence. A growing body of literature indicates that moderate exposure to alcohol during brain development can cause subtle behavioral and cognitive deficits in the absence of gross morphological effects and profound behavioral and cognitive deficits associated with heavy consumption. These deficits may not become apparent until the child is challenged during the educational years [15,36,59], may increase in severity as the child matures [58], and may persist throughout the life of the individual. Such considerations have recently led to a revised taxonomy of fetal ethanol effects, the Fetal Alcohol Spectrum Disorders (FASDs), which include several classifications for lesser-affected children including Partial FAS, Alcohol Related Neurodevelopmental Disorder (ARND) and Alcohol Related Birth Defects (ARBD) (see Ref. [27]).
1.2. Social behavior and fetal ethanol exposure
Among the numerous fetal-ethanol-related behavioral and cognitive impairments are a broad range of behavioral problems that can be classified as social in nature [24,33,49,51,62]. Animal models of fetal ethanol exposure have been central to the study of alcohol-related alterations in behavior and cognition and the multifaceted biological mechanisms that underlie these deficits [21]. Several studies have reported abnormal social behaviors in rats and mice exposed to high amounts of alcohol during brain development [19,34,35,39,40,41,44,53] including increased aggression [40,53], increased prepubertal play fighting [53], alterations in the normal pattern of sexually dimorphic play behavior [44], both decreases and increases in responsiveness to social stimuli [34,40,41], and deficits in socially acquired food preferences and social recognition memory [35]. Using a moderate prenatal ethanol exposure paradigm, work from our laboratory in non-social domains has consistently demonstrated that moderate prenatal ethanol exposure results in more subtle, yet persistent, behavioral and physiological alterations than are observed in heavy exposure treatments [60,61]. We recently demonstrated, however, that moderate prenatal ethanol exposure in the rat results in alterations in social interaction (e.g., investigation, wrestling) that persist into adulthood [24].
Considered as a whole, social behaviors engage and require a distributed set of neural circuitry, which, in addition to the amygdala and hippocampus, include several frontocortical regions. Damage to the orbital prefrontal cortex (OPFC) in primates, and corresponding regions in rodents (e.g., agranular insular cortex), has consistently been associated with alterations in social behavior including inappropriate aggression, withdrawal, agonistic behaviors [12,37] and altered play behavior [50]. In humans, damage to the prefrontal cortex (including orbital prefrontal cortex) is associated with a range of social behavior deficits [4] and functional neuroimaging studies have linked orbital prefrontal cortex to social cognition in humans [9,18]. The regional specificity of prefrontal circuitry involved in social behavior is indicated by the relative sparing of social behavior following lesions to dorsal frontal cortex in primates [12] and roughly corresponding medial prefrontal cortex of the rat [37]. Kolb [37] demonstrated persistent alterations in social behavior in male rats after lesions of lateral frontal cortex, including the agranular insular cortex (Zilles [66] area AID, see Fig. 1), but only transient deficits after medial frontal cortex lesions including prelimbic cortex (Zilles area Cg3). Recent evidence, however, suggests that deficits in juvenile and adult social behavior are persistent consequences of medial frontal cortex lesions in the rat [7,56]. Experience-dependent structural plasticity related to social experience in adult rats has also been demonstrated in AID and associated areas [8,24,25,57], with typically less robust experience-dependent changes observed in Cg3 [24,25] (but see Ref. [8]).
Fig. 1.
Coronal sections (adapted from Zilles, 1985) showing regions in which immediate early gene expression was quantified by Hamilton et al. [24] [agranular insular cortex (AID) and prelimbic cortex (Cg3)] and the additional regions of interest in the present study [lateral orbital area (LO), ventrolateral orbital area (VLO), and frontal cortex regions 1–2 (Fr1, Fr2)]. Distance anterior to bregma is provided next to each section. Abbreviations for other areas shown here but not included in the present analyses: aci = anterior commisure (intrabulbar), AO= anterior olfactory nucleus, Cl = claustrum, DPC = dorsal peduncular cortex, IL = infralimbic cortex, lo = lateral olfactory tract, Par1 = parietal cortex, Pir = piriform cortex, TT = taenia tecta, Tu = olfactory tubercle, MO=medial orbital area, VO= ventral orbital area.
Relatively little is known about the long-term consequences of heavy or moderate prenatal ethanol exposure on frontal cortex function and associated behaviors. Prenatal exposure to moderate levels of ethanol alters phospholipase C-β1 and A2 signaling in medial frontal cortex [3], and we have recently observed fetal-ethanol-related decreases in mGluR5 receptor density in Cg3, indicating a potential deficit in synaptic plasticity within this region [64]. Kelly and Dillingham [34] demonstrated a relationship between abnormal social behavior in female, but not male, rats exposed to alcohol during postnatal days 2–10 and altered DNA and dopamine metabolite (DOPAC) levels in the amygdala, which has prominent connections with AID [20,29,42,54]. Lawrence et al. [39] measured c-Fos immunoreactivity in adolescent rats exposed to alcohol during early perinatal brain development and found alcohol-related alterations in somatosensory cortex activity that corresponded to tactile stimulation associated with play behavior.
1.3. Frontocortical bases of alterations in social behavior following moderate fetal ethanol exposure
Work from our laboratory has been focused on better understanding the long-term consequences of moderate fetal ethanol exposure on learning, memory, and social behavior and the neurobiological bases of these effects. In our studies [24,55,63] we utilize a voluntary drinking protocol in which pregnant rat dams are provided with daily 4-h access to a 5% (v/v) ethanol solution in saccharin water during the dark cycle (from 1000 to 1400) throughout pregnancy (see Ref. [24] for details). This results in a mean blood ethanol concentration of 84 mg%, which approximates the legal limit for alcohol intoxication in most locations in the United States. Control animals consume saccharin water during the same 4-h period. Importantly, using this ethanol exposure protocol, ethanol consumption does not affect maternal weight gain during pregnancy, litter size or offspring birthweight, but has been shown to result in subtle, but persistent, effects on learning and synaptic plasticity in adult offspring [55,63]. For all conditions described here, adult offspring of rat dams that consumed ethanol or saccharin were pair-housed and no more than one offspring from a single breeding pair was assigned to any particular experimental condition.
1.3.1. Dendritic branching and spine density in AID and Cg3
Hamilton et al. [24] reported complementary alterations in dendritic spine density in AID and Cg3, with ethanol-related decreases observed in AID and modest increases observed in Cg3 (see also Ref. [26] and cf. Ref. [65] for effects of early postnatal binge ethanol exposure on dendritic spine density in medial prefrontal cortex). This pattern of results suggests somewhat different long-term consequences of ethanol on lateral and medial aspects of frontal cortex in the rat, and is reminiscent of reciprocal alterations involving these aspects of frontal cortex that have been observed for a broad range of pharmacologically and non-pharmacologically induced changes in dendritic complexity and spine density (reviewed in Ref. [38], see also Refs. [8,16]).
1.3.2. Social-experience-related immediate early gene expression in frontal cortex
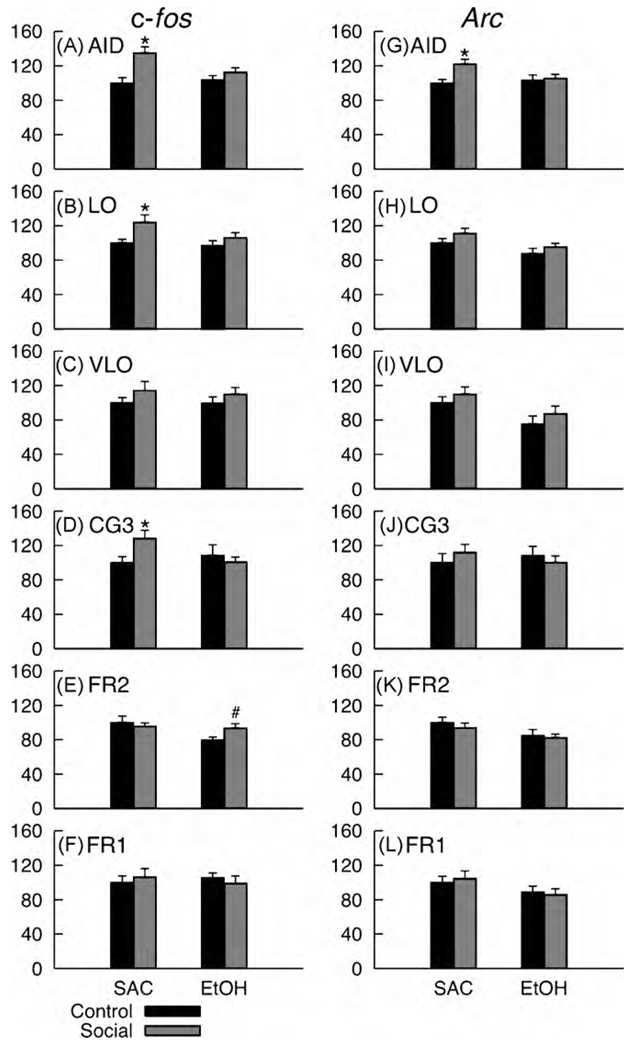
To further explore the dissociation between AID and Cg3 observed in moderately exposed rats, Hamilton et al. [24] quantified activity-related immediate early gene (IEG) expression in these regions following social interaction (see Fig. 2). A broad range of IEGs are expressed in neurons that have been recently active, and thus, quantification of their expression provides a measure of neuronal activity. Quantification of IEGs can also be performed with regional specificity allowing the relative experience-related engagement of brain regions to be compared. Such approaches, along with measuring proteins associated with IEGs, have proven useful in identifying brain regions implicated in alcohol-related behavioral deficits [28,39,48]. Hamilton et al. [24] quantified c-fos [5,46,47] and Arc [11,22,23] expression in AID and Cg3 following social interaction in adult rats that were either exposed to saccharin or moderate levels of ethanol during prenatal brain development. c-fos was selected for quantification because it is highly responsive to neural activity, and Arc was selected because of its additional linkage to synaptic plasticity [10,11,43,45,52].
Fig. 2.
Mean (S.E.M.) c-fos (A–F) and Arc (G–L) expression in each of the six regions of interest for each combination of Diet and Social condition. All values are expressed as the percentage of Saccharin-Control expression, which was calculated separately for each combination of immediate early gene and brain region. ‘*’ indicates a significant effect of Social experience within Diet condition at p < .05. ‘#’ indicates an effect that approached significance, at p = .059. Data from AID and Cg3 were previously published in Ref. [24].
Twenty-four rats served as caged controls and 40 rats were assigned to a social interaction condition. An equal number of rats for each sex and prenatal Diet condition were assigned to each of the Social experience conditions. Two brains were not analyzed (1 female ethanol rat in the Social condition, and 1 male saccharin rat from the caged Control condition), leaving 62 animals in the study. Social experience and brain extraction were performed when rats were 90–120 days old. Prior to social interaction, all rats, including those that served as caged controls, were placed in pairs with their cage-mate in the apparatus for 30 min on each of 3 successive days for habituation to the test environment. A chamber (95 cm × 47 cm × 43 cm) with a Plexiglas front was used for behavioral testing was placed in the center of a large room that was kept dark and quiet. Following the final habituation day, rats were housed in isolation for 24 h after which they were transported to the testing room in individual cages. Rats in the Social condition were placed in the test chamber with their cage-mate for 10 min. The apparatus was illuminated with infrared lights and behavior was video-taped with an infrared camera. After each session, the chamber was cleaned and fresh bedding was placed on the floor. Upon completion of the social interaction period, rats were returned to their individual cages and remained in the testing room for a 20-min holding period. Rats in the caged control condition were transferred individually to the testing room, but remained in their individual cages for a 20-min holding period. At the end of the holding period, rats were rapidly anesthetized with isoflurane and decapitated. The brains were quickly extracted and frozen until processed for fluorescence in situ hybridization (FISH).
Effects of fetal ethanol exposure on social behavior and expression of c-fos and Arc in AID (layer II/III) and Cg3 (layer III) were evaluated. Compared to control rats that did not engage in social interaction, rats that were exposed to saccharin prenatally demonstrated robust increases in c-fos expression in both regions, whereas increased Arc expression was only observed in AID (see Fig. 2(A), (D), (G), (J)). Because both signals indicate neural activity, and Arc is also strongly linked to synaptic plasticity, one interpretation of this pattern of results is that, although both AID and Cg3 are recruited during social interaction, the recruitment of intracellular processes involved in plasticity is more robust in AID. Rats exposed to ethanol prenatally showed less robust activity-related immediate early gene expression in AID and Cg3, and additionally showed no Arc expression in AID related to social interaction AID.
Motivated by the more general observation that ventrolateral and medial aspects of rat frontal cortex undergo reciprocal or complementary experience-dependent changes, as described by Kolb et al. [38], and the more specific observation that social experience elicits differential patterns of IEG expression in AID and Cg3, we quantified c-fos and Arc expression in four additional frontal cortex regions in the same brains in which IEG expression was quantified by Hamilton et al. [24]. These novel data were primarily obtained to address the pattern of activity-related IEG expression in normal and ethanol-exposed rats, but also served an additional purpose. A failure to observe social-experience-related increases in c-fos expression in saccharin-exposed rats in other frontocortical regions would indicate that our previous observations were not reflective of a general experience-related effect in frontal cortex. Likewise, a demonstration of any experience-related effects in ethanol-exposed rats would rule out a generalized ethanol-related deficit. Using tissue archived from our previous work on activity-related IEG expression in AID and Cg3 in saccharin and ethanol-exposed rats, we extend our analyses to include the lateral orbital area (Zilles LO), ventrolateral orbital area (Zilles VLO), as well as frontal cortex regions Fr1 and Fr2 (lateral and medial agranular cortices; see Fig. 1), again focusing on populations of layer III neurons.
For the novel analyses presented here, cases were excluded on a region by region basis if valid measurements could not be obtained for a specific region. All results reported here include at least 6 samples per region for each combination of prenatal diet and social experience condition, and range from 6 to 11 samples for Control conditions and 11–20 samples for Social conditions. IEG expression was quantified as described in Ref. [24] and normalized to IEG expression in the saccharin-exposed control rats. Separate ANOVAs were conducted for each brain region and IEG with prenatal Diet condition and Social condition (Social interaction vs. caged Control) as factors. Preliminary analyses revealed no significant main effects or interactions involving Sex, so this factor was not included in the analyses reported here. Planned comparisons of Social and Control data were conducted within levels of the Diet condition to evaluate social-experience-related IEG expression separately for each Diet condition as was performed in our initial report [24]. Mean c-fos and Arc values, expressed as percentage of SAC-Control means, for combinations of Diet condition and Social condition are shown in Fig. 2 for the novel regions included in the present report (LO, VLO, Fr1, and Fr2) and the two regions from our previous study (AID and Cg3).
There was a significant Social main effect for LO [social > control; F(1,55) = 5.61], and a significant Diet main effect for Fr2 [saccharin > ethanol; F(1,55) = 4.62]. Planned comparisons revealed a significant activity-related increase in c-fos expression in LO for saccharin controls [F(1,26) = 4.55]. An activity-related increase in c-fos expression in Fr2 for ethanol-exposed rats approached significance [F(1,29) = 3.82, p = .059]. No significant main effects, interactions, or planned comparisons were observed for VLO or Fr1. Significant Diet main effects [saccharin > ethanol] in Arc expression were observed for LO [F(1,55) = 6.70], VLO [F(1,31) = 6.56], and Fr2 [F(1,55) = 5.84]. No other main effects or interactions were significant, and none of the planned comparisons revealed activity-related increases in Arc expression in LO, VLO, Fr1, or Fr2 for either of the Diet conditions. The overall pattern of significant planned comparisons, non-significant trends, and non-significant effects is summarized in Fig. 3.
Fig. 3.
Summary of social-experience-related increases in c-fos and Arc expression observed in Saccharin and EtOH rats (see Fig. 2 for means). Large, solid black arrows indicate significant activity-related increases within each Diet condition (p < .05). Dotted black arrows indicate non-significant activity-related increases that reached an arbitrary threshold of 10% (relative to the Control condition for each Diet condition).
1.3.3. Alterations in social behavior following moderate fetal ethanol exposure
For the study conducted by Hamilton et al. [24] the duration and frequency (over 10 min) of the following behaviors were quantified: anogenital sniffing, other sniffing of the partner’s body (body sniffing), sniffing/digging in the bedding, rearing, wrestling (including pinning), boxing, self-grooming, allogrooming (of the partner), and crawling over/under the partner [6]. These behaviors were selected to provide measurements of social behaviors directed toward the cage-mate (e.g., sniffing, wrestling), self-directed behaviors (e.g., grooming), and behaviors directed toward the environment (e.g., digging). Social behaviors included measures of investigation (e.g., anogenital sniffing), aggression (e.g., wrestling), and agonistic behaviors (e.g., crawling over/under). Behaviors classified as body sniffing were mainly directed toward the flank of the cage-mate. Instances of play behavior (chasing, attempting to contact the nape of the neck) were rare and not limited to one Diet condition.
Analyses of social behavior in this cohort of rats reported on here has been published elsewhere (Ref. [24], Table 2) and is not repeated in totality here. In summary, the only significant effect of moderate fetal ethanol exposure observed in the 10-min interaction following 24 h of isolation was a selective increase in the frequency and total time spent wrestling, and this was limited to male rats. There was a corresponding, non-significant ethanol-related increase in boxing among male rats. Hamilton et al. [24] also measured these social behaviors in a different cohort of rats where the cage-mate remained the same from day to day or was changed every 48 h. In this situation, ethanol-exposed males and females displayed similar increases in a variety of forms of social investigation (body sniffing, anogenital sniffing) in addition to increases in boxing and wrestling. Thus, the relative lack of ethanol-related effects with 24-h isolation may be related to differences in motivation as well as differences in the behavior of control rats. Although evaluation of the pattern of behavioral and gene expression data across the six frontocortical regions of interest can be informative, similar patterns among dependent measures may not actually reflect a relationship between alterations in IEG expression and behavior. Our previous report did not directly address correlations between IEG expression and social behavior, and more importantly, how these correlations are affected by fetal ethanol exposure. Although the differences in time and frequency of social behavior in ethanol-exposed rats observed in our previous study were modest, there may be important and informative correlational data for the variables of interest and how these correlations differ for the brain regions of interest.
1.3.4. Correlations between social behavior and IEG expression in frontal cortex: effects of fetal ethanol exposure
In the present section we report correlations between IEG expression in the six regions of frontal cortex under investigation and social behaviors for saccharin- and fetal-ethanol-exposed rats. One consideration of some importance is the rather large number of potential correlations between behavior and IEG expression that could be computed, which led us to restrict evaluation and discussion of behavioral and IEG expression correlations to those that were most clearly relevant to the manipulation of prenatal diet. To restrict our treatment in an objective manner, we focused on IEG/behavior correlations within each brain area for which the regression slopes significantly differed for the two Diet treatment groups for at least one of the behavior/IEG expression correlation. Separate Analyses of Covariance (ANCOVAs) were performed to evaluate heterogeneity of regression slopes with individual behavioral measures as a dependent measure, Diet as a between-subject fixed factor, and IEG expression in each of the six brain regions from the present study and the earlier report of Hamilton et al. [24] as covariates. IEG × Diet interactions at p < .05 indicate significant differences in IEG/behavior correlations for the two Diet conditions. Preliminary analyses failed to detect any significant Sex effects for these analyses, and due to the small number of observations for some relationships male and female data were combined for all tests. Significant differences in regression slopes for the ethanol- and saccharin-exposed rats were observed for the relationship between c-fos expression and body sniffing (time), anogenital sniffing (time), and the combined frequency of boxing and wrestling.1 These differences, including scatterplots and regression lines for ethanol- and saccharin-exposed rats for these behavioral measures and c-fos expression in the six brain regions of interest are illustrated in Fig. 4.
Fig. 4.
Scatterplots and regression lines for c-fos expression in each of six frontal cortex regions and three behavioral measures: body sniffing (total time), anogenital (total time), and the combined frequency of boxing and wrestling during the 10 min session. Data for each brain region are presented in separate rows and data for each behavior are presented in separate columns. Data are presented separately for saccharin-exposed and ethanol-exposed rats within each individual graph. The x-axes represent c-fos expression (% of Saccharin-Control rats). ‘*’ in the top left corner of each graph indicate significant differences in regression slopes for saccharin-exposed and ethanol-exposed rats.
Significantly different regression slopes for the two Diet conditions were observed for body(flank) sniffing and for c-fos expression in AID [F(1,35) = 3.89; rSAC(18) = .608, p = .004; rEtOH(17) = .153, p = .52] and Cg3 [F(1,35) = 4.81; rSAC(18) = .365, p = .11; rEtOH(17) = −.345, p = .15]. For anogenital sniffing, there were significant differences in regression slopes for the two Diet conditions for c-fos expression in VLO [F(1,18) = 9.68; rSAC(9) = .646, p = .03; rEtOH(9) = −.481, p = .13], Cg3 [F(1,35) = 5.00; rSAC(18) = .36, p = .11; rEtOH(17) = −.34, p = .16], and Fr1 [F(1,18) = 5.58; rSAC(9) = .56, p = .07; rEtOH(9) = −.38, p = .25]. For boxing and wrestling, there were significantly different regression slopes among the Diet conditions in LO [F(1,32) = 8.23; rSAC(15) = −.20, p = .43; rEtOH(17) = .52, p = .02] and Fr2 [F(1,32) = 8.23; rSAC(15) = −.04, p = .88; rEtOH(17) = .60, p = .007]. There were no significant differences in regression slopes for correlations between individual behavioral measures and Arc expression in any of the frontal cortex regions. There were no significant gene expression behavior correlations for these behaviors when the data were collapsed across Diet conditions.
Several features of the pattern of gene expression–behavior relationships are apparent. Perhaps most important, although there were numerous behavioral measures that were quantified, the only measures for which there were significantly different regression slopes for IEG expression and behavior correlations were social behavioral measures related to investigation (sniffing) or other forms of interaction (wrestling/boxing); Measures of environment-directed behaviors (e.g., rearing) were not correlated with IEG expression in frontal cortex and did not differentiate saccharin- and ethanol-exposed rats with respect to these correlations. With respect to AID, increased c-fos expression in saccharin-exposed rats was associated with increased body sniffing, but decreased anogenital sniffing and wrestling/boxing. In contrast, for ethanol-exposed rats there was little relationship between c-fos expression in AID for body sniffing. A similar pattern for saccharin- and ethanol-exposed rats is apparent in the graphs for LO. With the exception of the correlations for body sniffing and expression in VLO, for regions VLO, Cg3, Fr1, and Fr2 there were positive correlations for saccharin-exposed rats for body sniffing and anogenital sniffing, whereas the correlations were either negative or weak for ethanol-exposed rats. This is captured particularly well by the significant differences in regression slopes for anogenital sniffing and c-fos expression in VLO, Cg3, and Fr1. A general characteristic of the relationship between c-fos expression and wrestling/boxing was that the relationships were positive for most areas for ethanol-exposed rats, whereas the relationships were negative for saccharin-exposed rats. This is most obvious in LO and Fr2. Overall, a broad interpretation of the pattern of correlations reported here suggests that AID (and to a lesser degree LO) and Cg3 are important for distinguishing Diet conditions with respect to one form of social investigation (body sniffing), whereas more medial regions are more important for distinguishing Diet conditions with respect to anogenital sniffing. With respect to wrestling/boxing, Diet conditions are distinguished by different directions of correlations compared to measures of investigation, with the strongest distinctions made in areas LO and Fr2.
We did not detect any significant correlations or differences in regression slopes for the two Diet conditions with respect to Arc expression and behavior correlations. One possibility is that the lack of significant correlations, in the context of increased social-induced Arc expression in AID for saccharin-exposed rats, may be related to multiple, combined experiential factors that were not analyzed here. Future studies with greater statistical power should employ multiple regression analyses to address the combined behavioral/experiential factors as they relate to gene expression in brain regions involved in social behavior.
2. Discussion
Several observations from the combined findings of our prior experiments [24] along with the present findings are relevant to the goals of the present paper. The major findings from our previous study [24] and the present analyses regarding social-experience-related c-fos and Arc in saccharin-exposed and ethanol-exposed rats are summarized in Fig. 3. Rats exposed to saccharin prenatally displayed robust social-experience-related increases in c-fos expression in agranular insular cortex (AID)[24], prelimbic cortex (Cg3)[24], and lateral orbital cortex (LO), with a similar trend toward increased c-fos expression in the ventrolateral orbital area (VLO). Robust increases in Arc expression were observed in AID [24], however, non-significant increases in Arc expression were observed in LO, VLO, and Cg3. No experience-related alterations in c-fos or Arc expression were observed in frontal cortex regions Fr1–Fr2 for saccharin controls. In contrast, fetal-ethanol-exposed rats failed to display significant increases in c-fos or Arc in any of the regions under investigation. There were, however, non-significant trends toward increases in both c-fos and Arc expression in VLO and a trend toward increased c-fos expression in AID. Further, an increase in c-fos expression in Fr2 that approached significance also differentiated fetal-ethanol-exposed rats from saccharin controls.
These observations indicate that, in normal rats, ventrolateral aspects of frontal cortex, regions along the dorsal bank of the rhinal sulcus, and regions in the medial bank of frontal cortex display social-experience-related increases c-fos expression. In contrast, dorsomedial and dorsolateral aspects of rodent frontal cortex do not appear to display social-experience-related increases in c-fos expression. This pattern is somewhat different than that observed for Arc in that the most robust experience-related increases in expression were observed in the most ventrolateral aspects of frontal cortex (AID), with less robust increases in expression in the more medial regions along the dorsal bank of the rhinal sulcus, and no apparent increases in expression in medial frontal cortex or the dorsomedial/dorsolateral aspects of frontal cortex. One broad conclusion from this observation, based on the role of Arc expression in synaptic plasticity, is that the ventrolateral aspects of frontal cortex are more critically involved in social-experience-related plasticity. It is interesting to note that social experience has been associated with more robust structural plasticity in AID compared to medial aspects of frontal cortex [24,57], therefore, one possibility is that there is a correspondence between the pattern of social-experience-related Arc expression and structural plasticity in frontal cortex. The precise linkages between these two observations have not been systematically investigated, however, both suggest that, in normal rats, there is an important dissociation between ventrolateral and medial aspects of frontal cortex that is consistent with other demonstrations of either independent or reciprocal effects in medial frontal and orbitofrontal cortices [8,16,38]. Although there were no significant effects of sex observed in the present analyses or our previous findings, it is important to note that the pattern of numerical differences between males and females observed in the frontal cortex regions under investigation here (data not shown) fit well with the major theme of this paper. Saccharin-exposed males displayed more robust experience-related IEG expression in the ventrolateral aspects of frontal cortex, whereas saccharin-exposed females displayed more robust experience-dependent IEG expression in medial aspects of frontal cortex, particularly in Cg3.
The basic pattern of experience-related IEG expression in frontal cortex observed in normal rats did not hold for rats exposed to moderate levels of ethanol during prenatal brain development. Social-experience-related IEG expression was either weak (AID, VLO) or not observed (LO, Cg3, Fr1) throughout frontal cortex, with the exception of a near significant increase in c-fos expression in medial agranular cortex (Fr2). Collectively, these observations indicate that various regions of frontal cortex are not recruited normally in ethanol-exposed rats, with increased activation observed in some regions not normally recruited during social interaction. Importantly, the findings in Fr2 indicate that experience-related increases in IEG can be observed in ethanol-exposed rats, thus, ethanol-related deficits in IEG expression observed in other regions are not related to a general decrease in capacity for experience-related IEG expression.
It is tempting to speculate that the ethanol-related behavioral effects described by Hamilton et al. [24] may be direct consequences of the alterations in recruitment of the frontal cortex regions under investigation here. As described above, the primary social behavioral alteration observed in the cohort of rats in this study was an increase in wrestling among male ethanol-exposed rats [24]. Based on extant lesion data [7,37,50] it seems reasonable to expect that reduced recruitment of ventrolateral frontal cortex may contribute to increases in this and similar behaviors. It is also important, however, to recognize that alterations in behavior and experience during social interaction likely contribute to the differential patterns of IEG expression observed in the frontal cortices of ethanol-exposed rats. Social experience and interaction provide a rich source of sensory, cognitive, and behavioral stimulation, and the precise components of experience and behavior that may have led to the observed results is not known. The results of the correlational analyses described in Section 1.3.4 suggest that different patterns of c-fos expression are related to different patterns of social behaviors in saccharin- and ethanol-exposed rats. Of particular interest are the observations that the overall patterns of correlations between behaviors and c-fos expression in frontal cortex were different for saccharin- and ethanol-exposed rats. The aforementioned increase in wrestling amongst ethanol-exposed rats is related to increasing levels of c-fos expression in several regions of frontal cortex, whereas decreased c-fos in the same regions were associated with increased wrestling in saccharin-exposed rats. Body sniffing was most strongly associated with increased c-fos expression in the AID of saccharin-exposed rats, whereas negative relationships in the same region were observed for anogenital sniffing and wrestling/boxing. Although the correlational data presented here provide initial evidence that the pattern of relationships between IEG expression and behavior differ for ethanol- and saccharin-exposed rats, the results are not as compelling as the planned comparisons conducted for social-experience-related IEG expression with respect to the medial vs. ventrolateral dissociation that motivated the present paper. Future efforts should include multiple regression approaches that allow non-unique contributions of the variables of interest to be accounted for. This approach was not possible in the present analyses due to the comparatively low numbers of observations for some of the brain regions. Further, the present approach was focused exclusively on relationships between a small number of behavioral measurements and IEG expression. It is also possible, if not probable, that the ethanol-related effects observed in the present study are also due to other factors such as stress, locomotor activity during social interaction, and potentially many other aspects of experience that were not quantified. Additional research is needed to clarify the precise factors that contributed to the observed ethanol-related alterations in IEG expression. Regardless of the precise experiential factors that underlie the observed effects on IEG expression in frontal cortex, the present findings are nonetheless consistent with a functional dissociation along a medial vs. ventrolateral axis, and demonstrate that this pattern is not represented normally in ethanol-exposed rats.
We wish to emphasize the importance of the observations described here for understanding functional dissociations between lateral and medial aspects of frontal cortex in normal animals [38], as well as understanding the long-term consequences of moderate prenatal ethanol exposure. Moderate prenatal ethanol exposure results is a broad range of subtle deficits, many of which can reasonably be linked to alterations in frontal cortex function (discussed in Ref. [24]). Considerable emphasis has been placed on the effects of prenatal ethanol exposure on the hippocampus and cerebellum and related behavioral and cognitive deficits. The results presented here as well as the results from other recent studies [24,26,65] indicate that alterations in frontal cortex are also important consequences of ethanol-exposure during brain development. The various aspects of frontal cortex investigated here contribute to a diverse set of behavioral, sensory, and cognitive functions. Persistent ethanol-related alterations in frontal cortex structure and function may, therefore, result in a variety of long-lasting behavioral and cognitive deficits, with social interaction being just one of many related behavior and cognitive deficits to which ethanol-related alterations in frontal cortex function contribute. Given the diversity of potential behavioral and cognitive effects that could result from alterations in frontal cortex function and plasticity, the development of rational treatment strategies that target frontal cortex may hold considerable importance for attenuating a broad spectrum of behavioral and cognitive consequences associated with moderate fetal ethanol exposure. The dissociation between lateral and medial aspects of frontal cortex with respect to experience-related IEG expression suggested here may further provide a novel dimension along which the effectiveness of rational treatment strategies for alleviating effects of prenatal ethanol exposure can be developed and evaluated.
Acknowledgements
Funding: NIAAA/NIH AA015356 to DAH and AA017068 to DDS. The authors wish to thank Alexandra Lusk for assistance with data analysis.
Footnotes
The use of combined boxing and wrestling data was based on preliminary analyses that revealed the combination to be a better predictor of IEG expression than either of the individual measures.
References
- 1.Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- 2.Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 1994;16:467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Allan AM, Weeber EJ, Savage DD, Caldwell KK. Effects of prenatal ethanol exposure on phospholipase C-beta 1 and phospholipase A2 in hippocampus and medial frontal cortex of adult rat offspring. Alcohol Clin Exp Res. 1997;21:1534–1541. [PubMed] [Google Scholar]
- 4.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 5.Asanuma M, Ogawa N. Pitfalls in assessment of c-fos mRNA expression in the brain: effects of animal handling. Rev Neurosci. 1994;5:171–178. doi: 10.1515/revneuro.1994.5.2.171. [DOI] [PubMed] [Google Scholar]
- 6.Barnett SA. A study in behaviour: principles of ethology and behavioural physiology displayed mainly in the rat. London: Camelot Press; 1963. [Google Scholar]
- 7.Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behav Neurosci. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- 8.Bell HC, Pellis SM, Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2010;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- 10.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp. 1972;32:525–565. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Report. Frequent alcohol consumption among women of childbearing age – behavioral risk factor surveillance system. J Am Med Assoc. 1994;271:1820–1821. [PubMed] [Google Scholar]
- 14.Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- 15.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol Clin Exp Res. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 16.Crombag H, Gorny G, Li Y, Kolb B, Robinson T. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 17.Day NL, Cottreau CM, Richardson GA. The epidemiology of alcohol, marijuana, and cocaine use among women of childbearing age and pregnant women. Clin Obstet Gynecol. 1993;36:232–245. doi: 10.1097/00003081-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 19.Elis J, Krsiak M. Proceedings:. Effect of alcohol administration during pregnancy on social behaviour of offsprings in mice. Act Nerv Super (Praha) 1975;17:281–282. [PubMed] [Google Scholar]
- 20.Gerfen CR, Clavier RM. Neural inputs to the prefrontal agranular insular cortex in the rat: horseradish-peroxidase study. Brain Res Bull. 1979;4:347–353. doi: 10.1016/s0361-9230(79)80012-x. [DOI] [PubMed] [Google Scholar]
- 21.Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–184. [PMC free article] [PubMed] [Google Scholar]
- 22.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 23.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton DA, Akers KG, Rice JP, Johnson T, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton DA, Silasi G, Carroll CE, Pellis SM, Kolb BE. Experience differetially affects the orbital and medial frontal cortex of the rat. Soc Neurosci Abstr. 2004;771:16. [Google Scholar]
- 26.Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyme H, May P, Kalberg W, Kodituwakku P, Gossage J, Trujillo P, et al. A practical clinical approach to diagnosis of Fetal Alcohol Spectrum Disorders: clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang MH, Jung SB, Lee MH, Kim H, Lee SJ, Sim YJ, et al. Influence of maternal alcohol administration on c-Fos expression in the hippocampus of infant rats. Neurosci Lett. 2005;378:44–48. doi: 10.1016/j.neulet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- 30.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 31.Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12:1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- 32.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- 33.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 35.Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 36.Kodituwakku PW. Defining the behavioral phenotype in children with Fetal Alcohol Spectrum Disorders: a review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Kolb B. Social behavior of rats with chronic prefrontal lesions. J Comp Physiol Psychol. 1974;87:466–474. doi: 10.1037/h0036969. [DOI] [PubMed] [Google Scholar]
- 38.Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence RC, Bonner HC, Newsom RJ, Kelly SJ. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behav Brain Res. 2008;188:209–218. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lugo JN, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 41.Lugo JN, Marino MD, Gass JT, Wilson MA, Kelly SJ. Ethanol exposure during development reduces resident aggression and testosterone in rats. Physiol Behav. 2006;87:330–337. doi: 10.1016/j.physbeh.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald A, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 43.Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- 45.Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 47.Morgan PF, Linnoila M. Regional induction of c-fos mRNA by NMDA: a quantitative in-situ hybridization study. Neuroreport. 1991;2:251–254. doi: 10.1097/00001756-199105000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Nagahara AH, Handa RJ. Fetal alcohol exposure alters the induction of immediate early gene mRNA in the rat prefrontal cortex after an alternation task. Alcohol Clin Exp Res. 1995;19:1389–1397. doi: 10.1111/j.1530-0277.1995.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 49.National Institute on Alcohol Abuse and Alcoholism. 10th special report to the U.S. congress on alcohol and health. Washington, DC: National Institutes of Health; 2000
- 50.Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, et al. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behav Neurosci. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- 51.Riley EP, McGee CL. Fetal Alcohol Spectrum Disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez JJ, Davies HA, Silva AT, De Souza IE, Peddie CJ, Colyer FM, et al. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur J Neurosci. 2005;21:2384–2396. doi: 10.1111/j.1460-9568.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- 53.Royalty J. Effects of prenatal ethanol exposure on juvenile play-fighting and postpubertal aggression in rats. Psychol Rep. 1990;66:551–560. doi: 10.2466/pr0.1990.66.2.551. [DOI] [PubMed] [Google Scholar]
- 54.Sarter M, Markowitsch HJ. Convergence of basolateral amygdaloid and mediodorsal thalamic projections in different areas of the frontal cortex in the rat. Brain Res Bull. 1983;10:607–622. doi: 10.1016/0361-9230(83)90029-1. [DOI] [PubMed] [Google Scholar]
- 55.Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, et al. Effects of a novel cognition-enhancing agent on fetal-ethanol-induced learning deficits. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2010.01266.x. in press, doi:10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacol. 2005;30:944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- 57.Silasi G, Hamilton DA, Kolb B. Social instability blocks functional restitution following motor cortex stroke in rats. Behav Brain Res. 2008;188:219–226. doi: 10.1016/j.bbr.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 58.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. J Am Med Assoc. 1991;265:1961–1967. [PubMed] [Google Scholar]
- 59.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 61.Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 62.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–533. [PubMed] [Google Scholar]
- 63.Varaschin RK, Rosenberg MJ, Akers KG, Hamilton DA, Savage DD. Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. J Pharmacol Exp Ther. doi: 10.1124/jpet.109.165027. in press, doi:10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varaschin RK, Savage DD. Unpublished observations. [Google Scholar]
- 65.Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zilles K. The cortex of the rat: a stereotaxic atlas. Berlin: Springer; 1985. [Google Scholar]