Abstract
Fluoroquinolone (FLQ) drugs are a potent family of antibiotics used to treat infections including ocular infections. To determine if these antibiotics may be phototoxic to the eye, we exposed human lens epithelial cells to 0.125–1 mM FLQs [ciprofloxacin (Cipro), lomefloxacin (Lome), norfloxacin (Nor), and ofloxacin (Ofl)], the precursor quinolone nalidixic acid (Nalid), and UVA radiation (2.5 J/cm2). Based on fluorescence confocal microscopy, FLQs are diffused throughout the cytoplasm and preferentially located in the lysosomes of lens epithelial cells. Neither FLQ exposure alone nor UVA exposure alone reduced cell viability. However, with exposure to UVA radiation the FLQs studied (Cipro, Nor, Lome and Ofl) induced a phototoxic reaction that included necrosis, apoptosis, loss of cell viability as measured by MTS, and membrane damage as determined by the LDH assay. Both Nalid and all FLQs studied (Cipro, Nor, Lome and Ofl) photopolymerized the lens protein α-crystallin. Phototoxic damage to lens epithelial cells and/or α-crystallin will lead to a loss of transparency of the human lens. However, if precautions are taken to filter all UV radiation from the eye while taking these antibiotics, this eye damage may be prevented.
INTRODUCTION
Aside from the skin, the eye is the organ most affected by ambient radiation. Physiological barriers exist that prevent penetration of most substances into the eye. However, should a photoactive compound such as a drug or dye manage to pass the blood/retinal or lenticular barriers, it may result in the formation of a cataract and/or increased potential for macular degeneration, both of which may eventually lead to blindness.
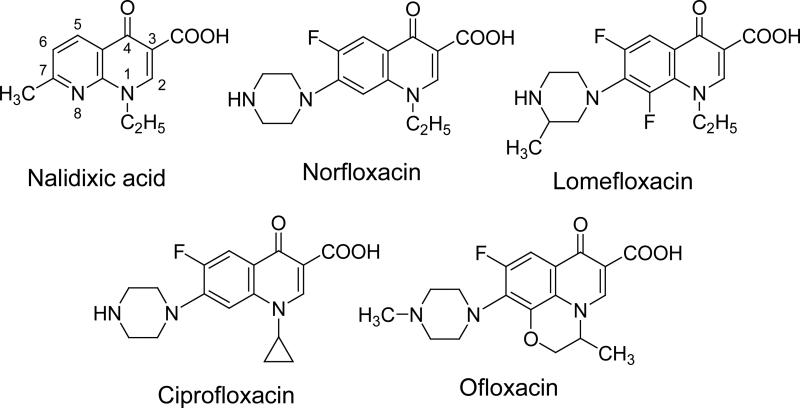
Fluoroquinolone (FLQ) drugs are a potent family of antibiotics used to treat various infections including ocular infections (1-6). The core structure of nalidixic acid (Nalid) is a quinolone, while the core structures of most FLQs consist of 4-oxo-1,4 dihydroquinoline or 4-quinolone (Figure 1). Fluorination at the 6-position enhances efficacy against Gram- negative pathogens and broadens the spectrum of activity to include Gram-positive pathogens. Substituents at the 7 position found in ciprofloxacin (Cipro) and ofloxacin (Ofl) further enhance activity against different microorganisms (7,8).
Figure 1.
Chemical structures of the quinolone Nalid and the four fluoroquinolones studied.
Drugs that absorb above 295 nm may induce a phototoxic reaction in the human lens (9). Quinolone (10) and FLQ drugs absorb UV radiation and induce long lived triplet states, which upon interaction with oxygen produce singlet oxygen, superoxide and other reactive species (11,12). Dawe et al (2003) have found that FLQ antibiotics are phototoxic to the skin (13). There have been further reports of in vitro dermal phototoxicity of Cipro at ambient levels of UV radiation (14). We have previously predicted ocular phototoxicity of antimalarial drugs, antipsychotic drugs, and herbal medications after irradiating these drugs with UV radiation and/or visible light in the presence of α-crystallin (a lens protein) and/or lens epithelial cells and examining the resultant damage (15-18). Our previous studies have shown that drugs that photochemically induce the production of singlet oxygen or superoxide will damage the lens, leading to loss of transparency and formation of a cataract in vivo (19). If a photosensitizing drug binds to the proteins in the lens (α, β, γ-crystallins) its retention time in the lens increases and the hazard is enhanced (20). Our previous studies on lens proteins have found that of the three lens crystallins (α, β, γ-crystallins), α-crystallin is most sensitive to photooxidation reactions (9). Maintenance of structural integrity of α-crystallin is particularly important for lens clarity because of its role as a molecular chaperone that prevents ultraviolet (A and B) induced protein aggregation (21,22).
Although FLQ antibiotics are taken orally for most infections, because of the strong blood-ocular barriers against exogenous substances, these antibiotics may be introduced into the eye by intraocular injection when used to overcome ocular infections (23). Although human toxicity studies are limited, a dose of Cipro of less than 25 μg for injections into the anterior portion of the eye (intracameral) is considered a safe amount in the anterior chamber volume and does not cause corneal decompensation. Intravitreal injections of 100 μg of ciprofloxacin into the posterior portion of the eye have been safely used without evidence of toxicity.
We present here a study to determine the potential ocular phototoxicity of FLQ antibiotics used for ocular infections. We have also included Nalid, the quinolone precursor to these antibiotics. We have specifically studied the UV phototoxicity of these drugs to human lens epithelial cells and their reaction with α-crystallin.
MATERIALS AND METHODS
Chemicals
Cipro, lomefloxacin (Lome), Nalid (free acid), norfloxacin (Nor), Ofl, were all purchased from Sigma Chemical Co. (St. Louis, MO) and have a purity of equal or higher than 98%. Bovine α-crystallin lens protein, Eagle's Minimum Essential Medium (MEM), gentamicin, L-glutamine, buffers, and other chemicals were also purchased from Sigma Chemical Co. (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Biofluids (Rockville, MD, USA). Trypsin-EDTA was purchased from GIBCO Invitrogen Corporation (Carlsbad, CA, USA).
Preparation of FLQ/quinolone solutions
The FLQ/quinolone solutions were freshly prepared by first dissolving in either 50 mM HCl (Cipro, Nor, and Ofl) or 100 mM NaOH (Nalid and Lome) to produce a 50 mM stock solution and then diluting according to the requirements of the experiments. For cell viability measurements, FLQ/quinolone stock solutions were diluted using Hanks’ Balanced Salt Solution (HBSS) and the final concentrations were 125, 250, 500 and 1000 μM.
Absorption spectra of FLQs and emission spectra of UVA lamp
All absorption spectra were recorded on an Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA). The UVA light source was composed of four parallel fluorescent UVA lamps (Houvalite F20T12BL-HO; National Biological Co. Twinsburg, OH). The emission spectrum of the UVA light source was measured with a spectroradiometer (LuzChem Research Inc., Ottawa, ON, Canada).
Photolysis of α-crystallin lens protein and gel electrophoresis analysis
Aliquots (0.5 mL) of α-crystallin lens protein samples [1 mg/ml in 100 mM phosphate buffered saline (PBS), pH 7.4] were placed in individual wells of a 24-well plate (Corning Incorporated, Corning, NY). After the addition of 500 μM FLQs, the samples were irradiated with UVA for 0, 10, and 20 minutes at irradiances of 0, 2.5, and 5.0 J/cm2 respectively, as measured with a YSI-Kettering Model 65A Radiometer (Yellow Springs Instrument Co., Yellow Springs, OH). Control samples containing either no HCl/NaOH or the same concentrations of HCl/NaOH were also irradiated under the same conditions.
After radiation, aliquots of lens protein samples were incubated with NuPAGE Sample Reducing Agent (Invitrogen, Carlsbad, CA) at 70° C for 10 minutes and then were electrophoresed under reducing conditions through NuPAGE 4–12% Bis-Tris gels (Invitrogen). Gels were stained with SimplyBlue SafeStain Reagent (Invitrogen) and scanned to ascertain the extent of photopolymerization of lens proteins. Gels were scanned on an HP Scanjet 7400C scanner (Torrance, CA) and analyzed using NIH Image software to quantitate protein concentrations. The gels’ backgrounds were comparatively normalized and the area below the peaks determined. The ratio of high-to-low molecular weight bands was calculated to determine the relative change between bands.
Cell Culture
An extended lifespan human lens epithelial cell line (HLE B-3) was used in these studies (24). Human lens epithelial cells were cultured by isolating epithelium fragments from infant human lenses and from patients who underwent treatment for retinopathy of prematurity and by allowing epithelial cells to grow from explants. Explants were processed as follows: a freshly dissected capsule-epithelial fraction from a single infant human lens was allowed to attach to a culture dish containing 20% fetal bovine serum in Eagles Minimum essential medium. Cell outgrowth was observed within a week. Cells were infected with an adenovirus, the 12-SV40 hybrid virus (Ad12-SV40), to increase their ability to propagate in culture.
Cells were grown in Eagle's MEM (Sigma) containing 2 mM L-glutamine, 50 μg/ml gentamicin, and 20% FBS in an atmosphere of 5% CO2 /95% air at 37°C. Cells were fed 3 times a week and after attaining confluence were passaged using trypsin (0.125%) – EDTA (0.5 mM).
Cell Viability
For photocytotoxicity tests, cells were exposed in the dark for 1 h at 37°C to different FLQs/quinolone in HBSS. Control cells were treated with either HBSS alone or the same concentration of HCl/NaOH in HBSS. After incubation the remaining medium was removed and replaced by 100 μL sterile HBSS. Cells were then irradiated with the aforementioned UVA light source for 10 minutes (2.5 J/cm2). After exposure, the HBSS solution was removed and replaced with cell culture medium and the cells were kept in the incubator overnight. Aliquots (10 μL) were removed and LDH release into the medium was assayed using the Cytotox 96 kit (Promega Corp., Madison, WI) according to the manufacturer's directions. The remaining medium was removed, the cells washed with HBSS, and the medium replaced with 120 μL/well of HBSS containing the MTS reagent (CellTiter 96 Aqueous Proliferation Assay; Promega Corp.). After incubation for 2 h at 37°C, the absorbance at 492 nm was recorded using a microplate reader (Spectrafluor Plus, Tecan US, RTP, NC).
Measurement of Apoptotic and Necrotic Cells
Apoptotic and necrotic cells were quantitatively evaluated by flow cytometry (25,26). After UVA treatment for 10 minutes (2.5 J/cm2), the cells were harvested by trypsinization and collected by centrifugation at 300 g for 5 min at room temperature. Cells were washed with cold PBS and stained with annexin V-FITC and propidium iodide (PI) using a TACS™ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD) according to the manufacturer's instructions. Cells positive for PI, for annexin V-FITC, or for both were quantified by flow cytometry using a Becton Dickinson FACSort (Becton Dickinson, Mountain View, CA).
Fluorescence confocal imaging
We employed fluorescence confocal microscopy to study cellular uptake and subcellular localization. Cells were seeded into 35 mm petri dishes containing a glass coverslip-covered 14 mm cutout (MatTek, Ashland, MA) for live cell microscopy measurement. Cells were incubated with 250 μM FLQs for 1 hour at 37°C. For measurement of subcellular localization, cells were stained with 100 nM MitoTracker Orange CMTMRos (Invitrogen/Molecular Probes, Eugene, OR), a specific fluorescent dye for mitochondria, or 60 nM LysoTracker Red DND-99 (Invitrogen/Molecular Probes, Eugene, OR), which is a specific fluorescence dye for lysosomes. Confocal fluorescence imaging was performed with a Zeiss LSM-510 META confocal microscope. For FLQ/quinolone fluorescence, excitation was carried out through a 364 nm laser source and emission was collected through a band pass filter for wavelengths between 385 and 545 nm. For MitoTracker or LysoTracker, excitation was carried out at 543 nm and emission collected for wavelengths between 560 and 615 nm.
Statistical analyses
For cell viability measurements, data were expressed as the mean of three independent experiments. Statistical significance was determined using an ANOVA, followed by Bonferroni's t-test using the StatView program (Abacus Concepts, Berkeley, CA). A two-sided P value of <0.05 was considered significant in all analyses.
RESULTS
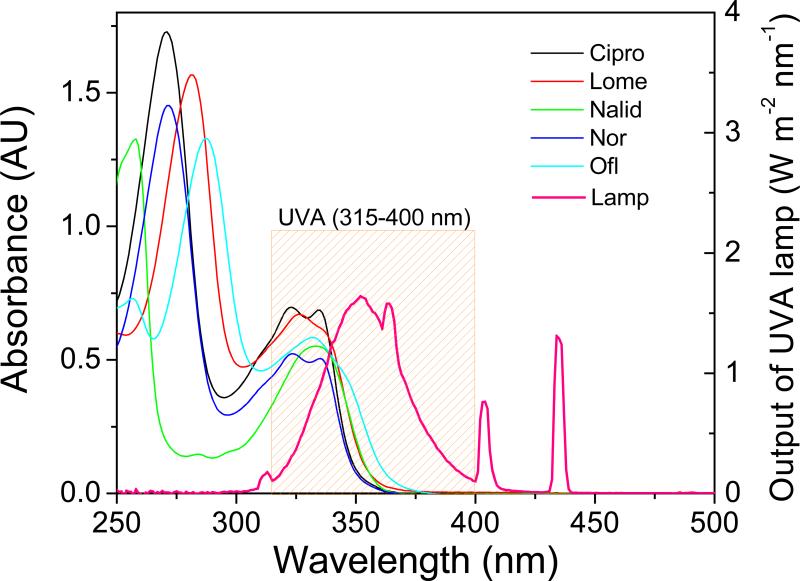
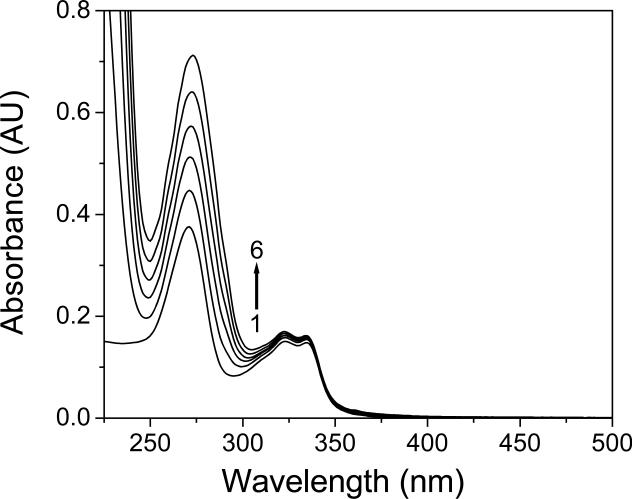
Figure 1 shows the chemical structures of the FLQs/quinolone studied in this paper. They all showed a strong absorption in the UVA (315 – 400 nm) region (Figure 2). We determined the potential binding of FLQ/quinolone to α-crystallin by monitoring the absorbance spectra. In our nascent publications on the effect of binding of photosensitizers to lens proteins (17,19,20,27), we found that binding of a photosensitizer (tetrasulfonatophenylporphyrin-TPPS) to lens proteins caused changes in the spectroscopic (red shift) and photophysical properties (triplet state lifetimes). The photosensitizers that did not have this shift and subsequent triplet state lifetime changes (Uroporphyrin) did not bind to lens proteins. Rose Bengal is known to phototoxidize lens proteins in vitro (28) and in vivo (29). Recently, Youssef et al (30) have determined that 5 μM Rose Bengal (RB) showed a 13 nm shift in absorbance in the presence of up to 0.6 mM α-crystallin, and the spectral shift and molar extinction coefficient changes were detectable at 0.05 mM α-crystallin. Cipro absorbs in the UVA region with a maximum absorbance of 324 nm (Figure 2). The maximum absorbance of l0 μM ciprofloxacin when titrated with α-crystallin lens protein was not modified. There was no broadening of this band nor shift in spectrum to the red which would indicate binding of Cipro to lens protein (Figure 3). Using similar procedures we found that none of the other examined FLQs/quinolone cause any changes in absorbance when titrated with α-crystallin (data not shown). Therefore we predict that non-photoinduced binding of these quinolones to lens proteins, if there is any, is less than 0.01 that of Rose Bengal or TPPS.
Figure 2.
UV-visible absorption spectra (AU, Absorbance Units) of FLQs/quinolone and emission spectra of UVA lamp (W m-2 nm-1) from four parallel fluorescent UVA lamps.
Figure 3.
UV-visible absorption spectra (AU, Absorbance Units) of ciprofloxacin (10 μM) in aqueous solutions (pH 7.4) containing 0.0 (1), 0.1 (2), 0.2 (3), 0.3 (4), 0.4 (5) and 0.5 mg/mL (6) α-crystallin lens protein.
There was a decrease in low molecular weight (LMW) bands and an increase in the high molecular weight (HMW) bands of α-crystallin treated with FLQs when irradiated with UVA for 10 and 20 minutes (Figure 4A-E). No obvious changes were observed when lens proteins were exposed to FLQs/quinolone (Figure 4A-E, 0 minutes) or to UVA alone (Figure 4F). Based on the ratio of high-to-low molecular weight bands (HMW/LMW), the quinolone Nalid and all FLQs studied (Cipro, Nor, Lome and Ofl) photopolymerized the lens protein α-crystallin (Figure 4G).
Figure 4.
Photolysis of α-crystallin lens protein by (A) Nalid; (B) Nor; (C) Ofl ; (D) Cipro; (E) Lome, and (F) control experiments after UVA radiation. From left to right of each figure, UVA duration 0, 10, 20 min. (G) Plot of quantification (ratio of high-to-low molecular weight) for each gel. *p < 0.05 compared with control experiments.
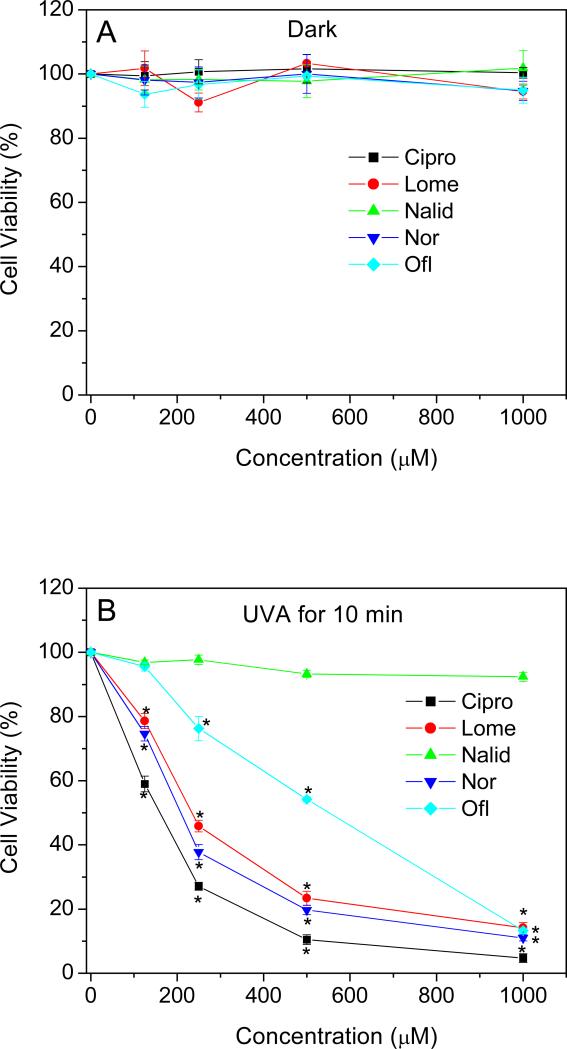
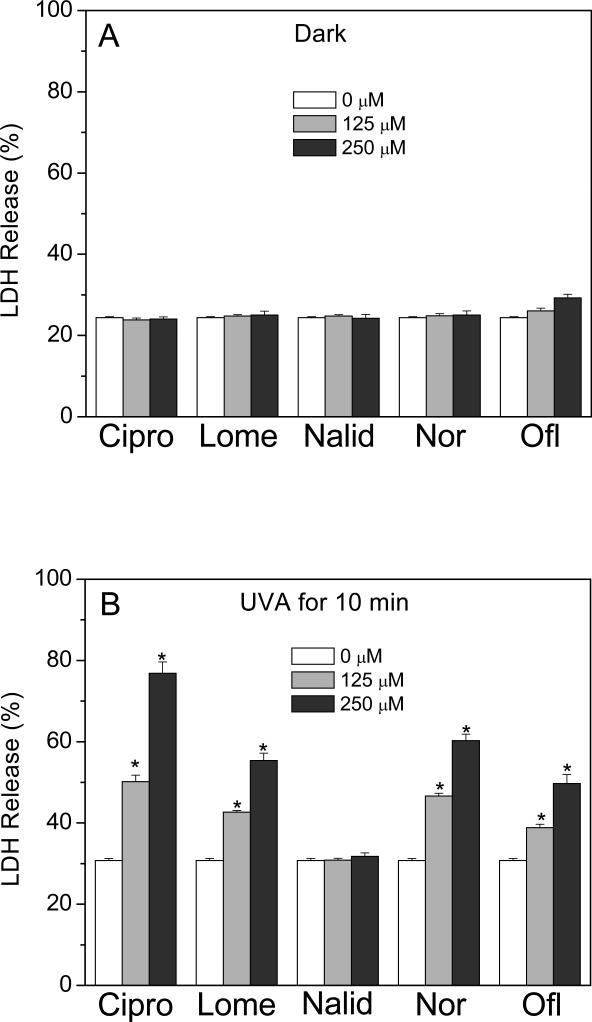
The phototoxicity of the four different FLQs and the quinolone, Nalid, towards HLE B-3 cells was determined using two different procedures. The MTS assay measures the activity of (mainly mitochondrial) dehydrogenases, while cell membrane damage is measured by the release of cytosolic LDH. As measured by the MTS assay, no decrease in viability was observed when HLE B-3 cells were exposed to compounds studied or to UVA alone (Figure 5A). However, in the presence of UVA radiation, the four FLQs (Cipro, Lome, Nor, and Ofl) caused concentration-dependent damage to HLE B-3 cells. The quinolone, Nalid, showed no phototoxicity towards HLE B-3 cells even at a high concentration of 1 mM (Figure 5B). A similar result was seen with the LDH release assay (Figure 6A and B).
Figure 5.
Effect of FLQs/quinolone exposure on the metabolic activity of HLE B-3 cells in the dark (A) or irradiated with UVA (B) as a function of FLQs/quinolone concentration, as measured by the MTS assay. Results were presented as the mean ± SEM from three independent experiments in duplicate. *p < 0.05 compared with cells without FLQ treatment.
Figure 6.
Effect of FLQs/quinolone exposure on the lactate dehydrogenase release of HLE B-3 cells in the dark (A) or irradiated with UVA (B) as a function of FLQs/quinolone concentration, as measured by the LDH assay. Results were presented as the mean ± SEM from three independent experiments in duplicate. * p < 0.05 compared with cells without FLQ treatment.
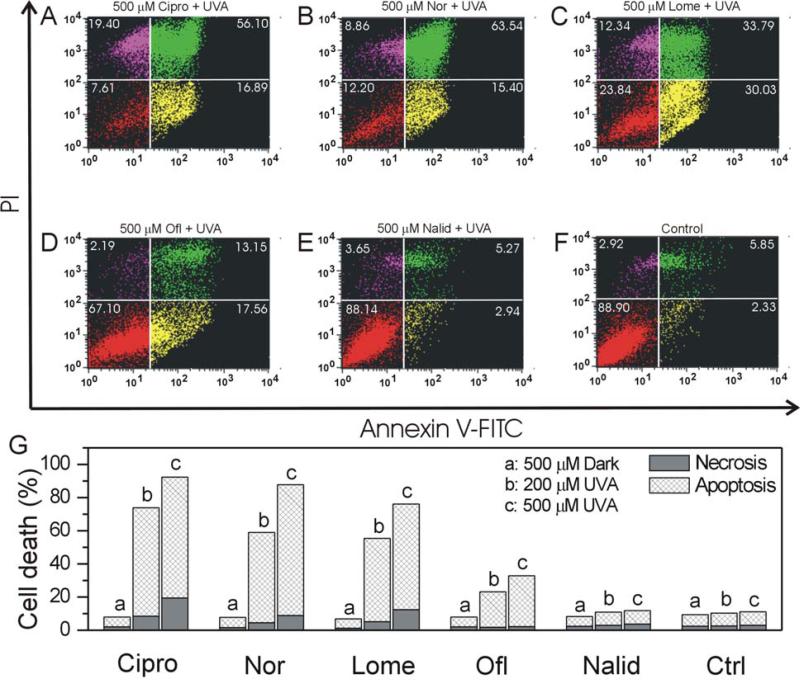
We used flow cytometry to quantify necrotic and apoptotic HLE B-3 cells induced by FLQs/quinolone and UVA radiation. Early apoptosis was detected by positive staining for Annexin V-FITC, while later stage apoptosis was detected through positive staining for both Annexin V and PI. We measured necrosis by determining the percentage of cells which were positive only for PI. In the fluorescence dot plot histogram of Annexin V/PI stained cells (Figure 7A-F), the lower left quadrant shows normal viable cells which are negative for both annexin V and PI; the lower right quadrant shows early-apoptotic cells which are positive for annexin V; the upper left quadrant shows necrotic cells which are positive for PI, while the upper right quadrant shows late-apoptotic cells which are positive for both annexin V and PI. In the absence of UVA radiation, all compounds studied had no effect on apoptosis or necrosis (Figure 7G). However, cells exposed to 500 μM FLQs (Cipro, Nor, Lome, and Ofl) plus UVA exhibited a significant increase in apoptosis (Figure 7A-E, G). Exposure of HLE B-3 cells to 500 μM of the quinolone Nalid plus UVA or UVA alone had no detectible effect on apoptosis or necrosis (Figure 7F and G). Also from the ratio between necrosis and apoptosis, the photo-induced necrosis by Cipro and Lome was higher than that of Nor and Ofl.
Figure 7.
FLQ/quinolone-induced apoptotic and necrotic death in HLE B-3 cells with UVA radiation (A: Cipro, B: Nor, C: Lome, D: Ofl, E: Nalid, and F: Control). Cells were seeded in plastic petri dishes (60 cm2) and pretreated with different FLQs at 500 μM concentration in HBSS for 1 hour and then exposed to UVA (10 min). After radiation, the cells were incubated overnight in cell culture medium and then stained with annexin V-FITC and propidium iodide. Apoptotic and necrotic cell death were determined with flow cytometry. The lower left quadrant shows normal viable cells, the lower right and upper right quadrants show apoptotic cells, while the upper left quadrant shows necrotic cells. (G) Graphs illustrating apoptosis and necrosis induced by UVA with different FLQs/quinolone.
After incubation with FLQs/quinolone for 1 h and upon excitation at 364 nm, the HLE B-3 cells showed a strong intracellular fluorescence, indicating substantial uptake of the FLQs/quinolone (Figure 8). After treatment with FLQs/quinolone, we further stained the cells with either MitoTracker Orange CMTMRos or LysoTracker Red DND-99, which are specific fluorescent dyes for mitochondria and lysosomes, respectively. It can be seen that the fluorescence caused by the FLQs/quinolone (excitation: 364 nm, emission: 385-545 nm) is diffused throughout the cytoplasm and is partially taken up by the mitochondria as indicated by the fluorescence caused by MitoTracker (excitation: 543 nm, emission: 560-615 nm). In addition, FLQs/quinolone were partially localized in lysosomes as visualized by co-staining cells with LysoTracker. When the overlays of FLQs/quinolone with Mitotracker and Lysotracker are compared, it appears that more FLQs/quinolone are co-localized with LysoTracker staining.
Figure 8.
Visualization of intracellular fluorescence of HLE B-3 cells using filter sets specific for FLQs/quinolone (in green; excitation: 364 nm, emission: 385-545 nm), the MitoTracker or LysoTracker (in red; excitation: 543 nm, emission: 560-615 nm), and the corresponding superimposed images.
DISCUSSION
The orderly arrangement of protein fibers in the lens causes the lens to be highly transparent (31). Chronic exposure to sunlight damages the lens epithelial cells and lens proteins and when this damage becomes extensive the lens becomes sufficiently cloudy to obstruct vision, and the individual is said to have a cataract. Exposure to sunlight while taking photosensitizing medication dramatically accelerates this process (9).
In order for a photochemical reaction to occur in the eye, the UV radiation or visible light must be absorbed in a particular ocular tissue. The human cornea filters all radiation shorter than 295 nm, while the adult human lens absorbs the remaining UV-B and all UV-A (295–400 nm), so that only visible light reaches the retina (32). Therefore drugs that absorb above 295 nm may induce a phototoxic reaction in the human lens (9). The quinolone and FLQ drugs studied here absorb UV radiation and are therefore potential lenticular photosensitizers. As the FLQ drugs studied here have been designed to either cross blood-ocular barriers or to be directly injected into the eye, they have a particular risk of inducing phototoxic reactions in the eye.
The lens of the eye contains three crystallin proteins, the α-, β- and γ-crystallin lens proteins. Our previous work with endogenous photoreactive compounds has shown that α-crystallin lens protein is the most susceptible to photooxidative reactive substances (9,15-19). Alpha crystallin is the main chaperone protein of the lens, and damage to this protein has been shown to lead to the induction of cataracts (33). Therefore we measured the effect of UVA radiation with FLQs/quinolone on photopolymerization of α-crystallin. Both the quinolone Nalid and all FLQs studied (Cipro, Nor, Lome and Ofl) photopolymerized the lens protein α-crystallin. The extent of photopolymerization is consistent with the ability of each FLQ to produce singlet oxygen in aqueous solutions at pH 7, when rates were corrected for the number of photons absorbed by each compound over the radiation wavelengths (11).
For a quick screening of binding of phototoxic drugs, we have found that a red shift and decrease in the absorption spectrum of the drug in the presence of a-crystallin is a good indication that the drugs bind to lens proteins (9,16). As none of the drugs tested in this work had their absorbance spectra modified in this assay, we did not examine exact binding sites (18) of FLQ's to α-crystallin with other techniques for this paper. The screening of FLQ's for binding to the lens protein α-crystallin suggests that these drugs should be transported away from the eye and not retained if photo-crosslinking is not induced by ocular UV radiation exposure. This result is similar to what had previously been seen with the photosensitizer 8-methoxy psoralen (8-MOP) used in the treatment of psoriasis (34).
We found that lysosomes are a preferential site of FLQ/quinolone photosensitization in human lens epithelial cells, as determined by the fluorescence overlay of the FLQs/quinolone and lysosomal probe LysoTracker (Figure 8). This is in agreement with previous work, where lysosomes were found to be the primary site of phototoxicity of FLQs for skin fibroblasts (35). Lysosomes were also recently found to be a preferential site of photosensitization for FLQs in keratinocyte cells (unpublished data). It appears that the phototoxicity induced by the FLQ studies (Cipro, Nor, Lome and Ofl) generally follows the ability of each FLQ to dehalogenate and form superoxide and/or carbene free radicals (11,12,36). However, as the exact concentration of these drugs in each compartment of the lens cells was not measured using radioactive labeling these data are only suggestive. The quinolone Nalid, which is not fluorinated, induced little phototoxic damage to lens epithelial cells. Early apoptosis is characterized by plasma membrane reorganization (25,26,37) and is detected by positive staining for Annexin V-FITC, while later stage apoptosis indicating DNA damage shows positive staining for both Annexin V and PI. We measured necrosis by determining the percentage of cells which were positive only for PI. All the FLQs (Cipro, Nor, Lome and Ofl) upon UVA radiation induced a significant increase in apoptosis in human lens epithelial cells, and apoptosis has also been shown to lead to the development of cataracts (38).
The FLQ drugs involved in our present studies are of particular significance as they are used against ocular infections because of their known ability to cross blood/ocular barriers (2-8,39) and to be absorbed into various ocular structures such as the cornea (40,41), aqueous humor (42-45), vitreous humor and retina (40,46-52). Therefore, further studies are necessary to ascertain the short and long term ocular phototoxicity of these drugs in vivo.
Intraocular reactions resulting from light exposure may be prevented by appropriate use of sunglasses designed to filter light of wavelengths less than 400 nm (including UVB and UVA radiation). As has been seen with 8-MOP (32), filtration may prevent initial photo-oxidation reactions from occurring, subsequently protecting the eye from any resulting phototoxicity.
Although the FLQs/quinolone induced only moderate damage to lens proteins, damage to the lens is not repaired and is cumulative, so FLQ antibiotics that we have tested must be considered to be potential exogenous phototoxic agents in the lens.
CONCLUSION
We found that all FLQs (Cipro, Nor, Lome and Ofl) and quinolone (Nalid) studied had little or no toxicity without UV radiation. However, all of the FLQ drugs (Cipro, Nor, Lome and Ofl) absorb UV radiation that reaches the human lens and caused phototoxic damage to human lens epithelial cells and lens proteins. Phototoxic damage to either lens epithelial cells and/or α-crystallin will lead to a loss of transparency of the human lens (33). Thus, Cipro and other FLQ antibiotics taken systemically or injected intravitreally are potentially phototoxic to the eye and could contribute to early cataractogenesis. However, if proper precautions are taken to prevent UV radiation from reaching the eye by using appropriate wrap-around UV-B and -A blocking sunglasses while taking these antibiotics, this eye damage may be prevented.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and by the Dreyfus Senior Scientist Mentor Initiative Awarded to Mary G Hamilton. The authors are indebted to Dr. Ann Motten, NIEHS, for preparation and critical reading of the manuscript, Dr. Carl Bortner and Maria Sifre for their assistance with flow cytometry, and Dr. C. J. Tucker for his assistance with confocal microscopy.
Footnotes
This invited paper is part of the Symposium-in-Print: “Phototoxicity of the Skin and Eye”, in honor of Dr Colin Chignell.
REFERENCES
- 1.Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein EJ, Citron DM, Bendon L, Vagvolgyi AE, Trousdale MD, Appleman MD. Potential of topical norfloxacin therapy. Comparative in vitro activity against clinical ocular bacterial isolates. Arch. Ophthalmol. 1987;105:991–994. doi: 10.1001/archopht.1987.01060070135043. [DOI] [PubMed] [Google Scholar]
- 3.Hobden JA, Reidy JJ, O'Callaghan RJ, Insler MS, Hill JM. Quinolones in collagen shields to treat aminoglycoside-resistant pseudomonal keratitis. Invest. Ophthalmol. Vis. Sci. 1990;31:2241–2243. [PubMed] [Google Scholar]
- 4.Malet F, Colin J, Jauch A, Abalain ML. Bacterial keratitis therapy in guinea pigs with lomefloxacin by initially high-followed by low-dosage regimen. Ophthalmic. Res. 1995;27:322–329. doi: 10.1159/000267743. [DOI] [PubMed] [Google Scholar]
- 5.Osato MS, Jensen HG, Trousdale MD, Bosso JA, Borrmann LR, Frank J, Akers P. The comparative in vitro activity of ofloxacin and selected ophthalmic antimicrobial agents against ocular bacterial isolates. Am. J. Ophthalmol. 1989;108:380–386. doi: 10.1016/s0002-9394(14)73305-7. [DOI] [PubMed] [Google Scholar]
- 6.Sugar A, Cohen MA, Bien PA, Griffin TJ, Heifetz CL, Mehta S. Treatment of experimental Pseudomonas corneal ulcers with enoxacin, a quinolone antibiotic. Arch. Ophthalmol. 1986;104:1230–1232. doi: 10.1001/archopht.1986.01050200136068. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Agents. 2000;16:5–15. doi: 10.1016/s0924-8579(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 8.Bryskier A, Chantot JF. Classification and structure-activity relationships of fluoroquinolones. Drugs. 1995;49(Suppl 2):16–28. doi: 10.2165/00003495-199500492-00005. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JE. Screening for ocular phototoxicity. Int. J. Toxicol. 2002;21:491–500. doi: 10.1080/10915810290169918. [DOI] [PubMed] [Google Scholar]
- 10.Dayhaw-Barker P, Truscott TG. Direct detection of singlet oxygen sensitized by nalidixic acid: the effect of pH and melanin. Photochem. Photobiol. 1988;47:765–767. doi: 10.1111/j.1751-1097.1988.tb02777.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez LJ, Sik RH, Chignell CF. Fluoroquinolone antimicrobials: singlet oxygen, superoxide and phototoxicity. Photochem. Photobiol. 1998;67:399–403. [PubMed] [Google Scholar]
- 12.Sauvaigo S, Douki T, Odin F, Caillat S, Ravanat JL, Cadet J. Analysis of fluoroquinolone-mediated photosensitization of 2'-deoxyguanosine, calf thymus and cellular DNA: determination of type-I, type-II and triplet-triplet energy transfer mechanism contribution. Photochem. Photobiol. 2001;73:230–237. doi: 10.1562/0031-8655(2001)073<0230:AOFMPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Dawe RS, Ibbotson SH, Sanderson JB, Thomson EM, Ferguson J. A randomized controlled trial (volunteer study) of sitafloxacin, enoxacin, levofloxacin and sparfloxacin phototoxicity. Br. J. Dermatol. 2003;149:1232–1241. doi: 10.1111/j.1365-2133.2003.05582.x. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal N, Ray RS, Farooq M, Pant AB, Hans RK. Photosensitizing potential of ciprofloxacin at ambient level of UV radiation. Photochem. Photobiol. 2007;83:1226–1236. doi: 10.1562/2006-10-12-RA-1059. [DOI] [PubMed] [Google Scholar]
- 15.He YY, Chignell CF, Miller DS, Andley UP, Roberts JE. Phototoxicity in human lens epithelial cells promoted by St. John's Wort. Photochem. Photobiol. 2004;80:583–586. doi: 10.1562/0031-8655(2004)080<0583:PIHLEC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen S, Wang RH, Tonnesen HH, Dillon J, Roberts JE. Photoreactivity of biologically active compounds. VIII. Photosensitized polymerization of lens proteins by antimalarial drugs in vitro. Photochem. Photobiol. 1995;61:124–130. [PubMed] [Google Scholar]
- 17.Roberts JE. The photodynamic effect of chlorpromazine, promazine, and hematoporphyrin on lens protein. Invest. Ophthalmol. Vis. Sci. 1984;25:746–750. [PubMed] [Google Scholar]
- 18.Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE. Photooxidation of lens alpha-crystallin by hypericin (active ingredient in St. John's Wort). Photochem. Photobiol. 2000;72:200–203. doi: 10.1562/0031-8655(2000)072<0200:polcbh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JE, Kinley JS, Young AR, Jenkins G, Atherton SJ, Dillon J. In vivo and photophysical studies on photooxidative damage to lens proteins and their protection by radioprotectors. Photochem. Photobiol. 1991;53:33–38. doi: 10.1111/j.1751-1097.1991.tb08464.x. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JE, Atherton SJ, Dillon J. Detection of porphyrin excited states in the intact bovine lens. Photochem. Photobiol. 1991;54:855–857. doi: 10.1111/j.1751-1097.1991.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 21.Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J. Biol. Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- 22.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J. Biol. Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 23.van Wirdum E, van Best J, Bruining GJ, de Beaufort C, Oosterhuis J. Blood-retinal and blood-aqueous barrier permeability, lens autofluorescence and transmission in insulin-dependent diabetic youngsters. Graefes Arch. Clin. Exp. Ophthalmol. 1989;227:26–29. doi: 10.1007/BF02169820. [DOI] [PubMed] [Google Scholar]
- 24.Andley UP, Rhim JS, Chylack LT, Jr., Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- 25.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reno F, Burattini S, Rossi S, Luchetti F, Columbaro M, Santi S, Papa S, Falcieri E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem. Cell Biol. 1998;110:467–476. doi: 10.1007/s004180050308. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JE, Atherton SJ, Dillon J. Photophysical studies on the binding of tetrasulfonatophenylporphyrin to lens proteins. Photochem Photobiol. 1990;52:845–848. doi: 10.1111/j.1751-1097.1990.tb08691.x. [DOI] [PubMed] [Google Scholar]
- 28.Goosey JD, Zigler JS, Jr., Kinoshita JH. Cross-linking of lens crystallins in a photodynamic system: a process mediated by singlet oxygen. Science. 1980;208:1278–1280. doi: 10.1126/science.7375939. [DOI] [PubMed] [Google Scholar]
- 29.Jernigan HM, Jr., Fukui HN, Goosey JD, Kinoshita JH. Photodynamic effects of rose bengal or riboflavin on carrier-mediated transport systems in rat lens. Exp. Eye Res. 1981;32:461–466. doi: 10.1016/s0014-4835(81)80025-5. [DOI] [PubMed] [Google Scholar]
- 30.Youssef T, Kassem M, Abdella T, Harith MA, Lenci F. Photosensitized effects of Rose Bengal on structure and function of lens protein “alpha-crystallin”. Photochem. Photobiol. 2009;85:1306–1313. doi: 10.1111/j.1751-1097.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 31.Benedek GB. Theory of Transparency of Eye. Appl. Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 32.Bachem A. Ophthalmic ultraviolet action spectra. Am. J. Ophthalmol. 1956;41:969–975. doi: 10.1016/0002-9394(56)91044-3. [DOI] [PubMed] [Google Scholar]
- 33.Datiles MB, 3rd, Ansari RR, Suh KI, Vitale S, Reed GF, Zigler JS, Jr., Ferris FL., 3rd Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch. Ophthalmol. 2008;126:1687–1693. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerman S, Jocoy M, Borkman RF. Photosensitization of the lens by 8-methoxypsoralen. Invest. Ophthalmol. Vis. Sci. 1977;16:1065–1068. [PubMed] [Google Scholar]
- 35.Ouedraogo G, Morliere P, Bazin M, Santus R, Kratzer B, Miranda MA, Castell JV. Lysosomes are sites of fluoroquinolone photosensitization in human skin fibroblasts: a microspectrofluorometric approach. Photochem. Photobiol. 1999;70:123–129. [PubMed] [Google Scholar]
- 36.Fasani E, Profumo A, Albini A. Structure and medium-dependent photodecomposition of fluoroquinolone antibiotics. Photochem. Photobiol. 1998;68:666–674. [PubMed] [Google Scholar]
- 37.Singh N. A simple method for accurate estimation of apoptotic cells. Exp. Cell Res. 2000;256:328–337. doi: 10.1006/excr.2000.4810. [DOI] [PubMed] [Google Scholar]
- 38.Okamura N, Ito Y, Shibata MA, Ikeda T, Otsuki Y. Fas-mediated apoptosis in human lens epithelial cells of cataracts associated with diabetic retinopathy. Med. Electron. Microsc. 2002;35:234–241. doi: 10.1007/s007950200027. [DOI] [PubMed] [Google Scholar]
- 39.Thompson AM. Ocular toxicity of fluoroquinolones. Clin. Experiment. Ophthalmol. 2007;35:566–577. doi: 10.1111/j.1442-9071.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 40.Koch HR, Kulus SC, Roessler M, Ropo A, Geldsetzer K. Corneal penetration of fluoroquinolones: aqueous humor concentrations after topical application of levofloxacin 0.5% and ofloxacin 0.3% eyedrops. J. Cataract. Refract. Surg. 2005;31:1377–1385. doi: 10.1016/j.jcrs.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 41.McDermott ML, Tran TD, Cowden JW, Bugge CJ. Corneal stromal penetration of topical ciprofloxacin in humans. Ophthalmology. 1993;100:197–200. doi: 10.1016/s0161-6420(13)31672-8. [DOI] [PubMed] [Google Scholar]
- 42.Celebi S, Ay S, Aykan U, Bulut V, Alagoz G, Celiker UO. Penetration of oral and topical ciprofloxacin into human aqueous humor. Acta. Ophthalmol. Scand. 1998;76:683–685. doi: 10.1034/j.1600-0420.1998.760610.x. [DOI] [PubMed] [Google Scholar]
- 43.Donnenfeld ED, Schrier A, Perry HD, Aulicino T, Gombert ME, Snyder R. Penetration of topically applied ciprofloxacin, norfloxacin, and ofloxacin into the aqueous humor. Ophthalmology. 1994;101:902–905. doi: 10.1016/s0161-6420(13)31248-2. [DOI] [PubMed] [Google Scholar]
- 44.Huber-Spitzy VN, Czejka M, Georgiew L, Arocker-Mettinger E, Grabner G. Penetration of norfloxacin into the aqueous humor of the human eye. Invest. Ophthalmol. Vis. Sci. 1992;33:1723–1726. [PubMed] [Google Scholar]
- 45.von Gunten S, Lew D, Paccolat F, Vaudaux P, Brazitikos PD, Leuenberger PM. Aqueous humor penetration of ofloxacin given by various routes. Am. J. Ophthalmol. 1994;117:87–89. doi: 10.1016/s0002-9394(14)73019-3. [DOI] [PubMed] [Google Scholar]
- 46.Cekic O, Batman C, Yasar U, Basci NE, Bozkurt A, Kayaalp SO. Human aqueous and vitreous humour levels of ciprofloxacin following oral and topical administration. Eye. 1999;13(Pt 4):555–558. doi: 10.1038/eye.1999.137. [DOI] [PubMed] [Google Scholar]
- 47.Cekic O, Batman C, Yasar U, Basci NE, Zilelioglu O, Bozkurt A. Subretinal fluid levels of topical, oral, and combined administered ciprofloxacin in humans. Br. J. Ophthalmol. 2000;84:1061–1063. doi: 10.1136/bjo.84.9.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cekic O, Batman C, Yasar U, Totan Y, Basci NE, Bozkurt A, Zilelioglu O, Kayaalp SO. Penetration of topical, oral, and combined administered ofloxacin into the subretinal fluid. Br. J. Ophthalmol. 1999;83:1183–1185. doi: 10.1136/bjo.83.10.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donnenfeld ED, Perry HD, Snyder RW, Moadel R, Elsky M, Jones H. Intracorneal, aqueous humor, and vitreous humor penetration of topical and oral ofloxacin. Arch. Ophthalmol. 1997;115:173–176. doi: 10.1001/archopht.1997.01100150175004. [DOI] [PubMed] [Google Scholar]
- 50.Lesk MR, Ammann H, Marcil G, Vinet B, Lamer L, Sebag M. The penetration of oral ciprofloxacin into the aqueous humor, vitreous, and subretinal fluid of humans. Am. J. Ophthalmol. 1993;115:623–628. doi: 10.1016/s0002-9394(14)71460-6. [DOI] [PubMed] [Google Scholar]
- 51.Ozturk F, Kurt E, Inan UU, Kortunay MC, Ilker SS, Basci NE, Bozkurt A. Penetration of topical and oral ofloxacin into the aqueous and vitreous humor of inflamed rabbit eyes. Int. J. Pharm. 2000;204:91–95. doi: 10.1016/s0378-5173(00)00482-8. [DOI] [PubMed] [Google Scholar]
- 52.Vrabec TR, Sergott RC, Jaeger EA, Savino PJ, Bosley TM. Reversible visual loss in a patient receiving high-dose ciprofloxacin hydrochloride (Cipro). Ophthalmology. 1990;97:707–710. doi: 10.1016/s0161-6420(90)32518-6. [DOI] [PubMed] [Google Scholar]