Summary of recent advances
Despite its importance for mammalian cell biology and human health, there are many basic aspects of cholesterol homeostasis that are not well understood. Even for the well-characterized delivery of cholesterol to cells via lipoproteins, a novel regulatory mechanism has been discovered recently, involving a serum protein called PCSK9, which profoundly affects lipoproteins and their receptors. Cells can export cholesterol by processes that require the activity of ABC transporters, but the molecular mechanisms for cholesterol transport remain unclear. Cholesterol levels in different organelles vary by 5–10 fold, and the mechanisms for maintaining these differences are now partially understood. Several proteins have been proposed to play a role in the inter-organelle movement of cholesterol, but many aspects of the mechanisms for regulating intracellular transport and distribution of cholesterol remain to be worked out. The endoplasmic reticulum is the main organelle responsible for regulation of cholesterol synthesis, and careful measurements have shown that the proteins responsible for sterol sensing respond over a very narrow range of cholesterol concentrations to provide very precise, switch-like control over cholesterol synthesis.
Introduction
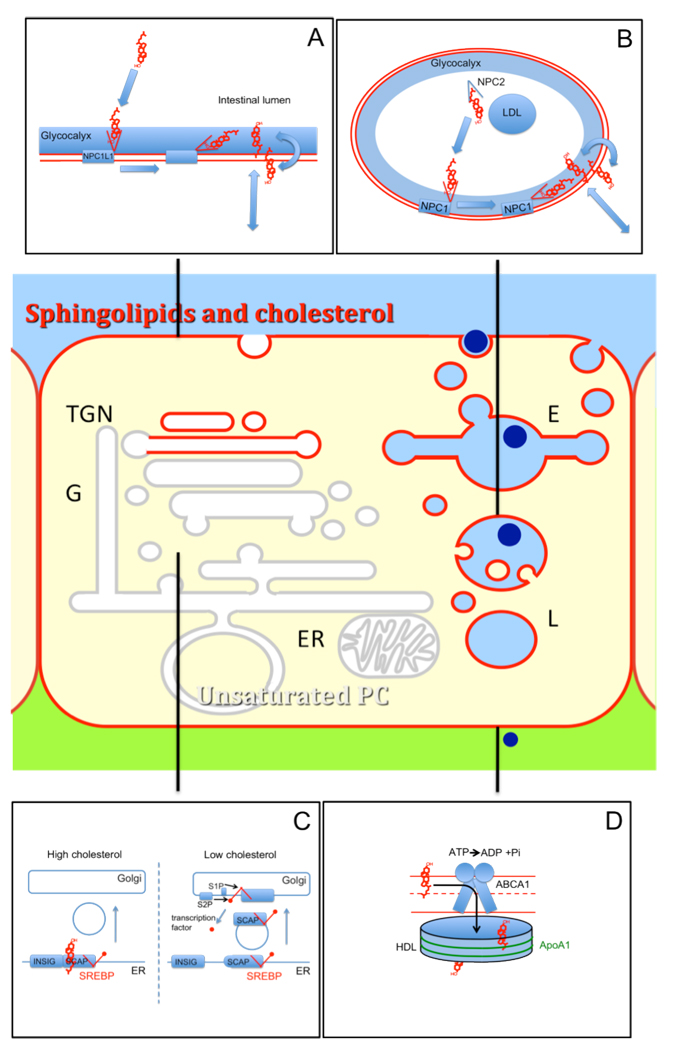
Cholesterol plays a unique role among the many lipids in mammalian cells. This is based in part on its biophysical properties, which allow it to be inserted into or extracted from membranes relatively easily. Additionally, it plays a special role in organizing the biophysical properties of other lipids in a bilayer. Because of its importance, cells have evolved complex mechanisms to tightly regulate the abundance and distribution of sterols within cells. In this brief review, we discuss new findings that are providing new insights into how the uptake and distribution of cholesterol are regulated and also how cholesterol moves among the organelles in a eukaryotic cell. We also point out significant gaps in our knowledge that will require further research. Some of the important cellular processes are illustrated in Figure 1.
Figure 1. Cholesterol homeostasis in mammalian cells.
(A) Insertion of cholesterol from detergent micelles in the intestinal lumen into the apical membrane of enterocytes requires NPC1L1. The N-terminal extracellular loop of NPC1L1 may lift cholesterol across the glycocalyx as has been proposed for NPC1: (B) Soluble NPC2 extracts cholesterol from endocytosed LDL and hands it over to the cholesterol-binding lumenal loop 1 of NPC1. NPC1 may chaperone the hydrophobic cholesterol across the glycocalyx into the limiting membrane, which is followed by spontaneous transmembrane translocation and egress. (C) When the cholesterol concentration of the ER gets below 5 mol%, this results in release of the SCAP-SREBP complex from Insig into COPII vesicles and transport to the Golgi, where the subsequent action of the two endoproteases S1P and S2P releases the SREBP transcription factor that binds to sterol-responsive elements in the DNA and regulates transcription. (D) Extrusion of cholesterol by the ABC transporter ABCA1 from the cytoplasmic leaflet, across the plasma membrane onto lipid-poor apolipoprotein A1 releases cholesterol from peripheral tissues.
Cholesterol provides membranes with special physical properties
It has been long known that cholesterol condenses and rigidifies lipid bilayers containing phospholipids with unsaturated fatty acyl chains. In contrast, it fluidizes bilayers of di-saturated phospholipids and sphingolipids, which in its absence would be in a solid gel phase. In the past several years, studies of three component lipid mixtures containing cholesterol have shown that cholesterol can profoundly affect the phase separation behavior of lipids. In particular, cholesterol can promote the formation of nanoscopic phase separations in mixtures of unsaturated phospholipids with saturated phospholipids and/or sphingolipids, which would otherwise consist of one fluid phase or a well separated macroscopic fluid and a gel phase [1–3]. These small lipid domains in model membranes resemble the microdomains that can form in biological membranes, which are compositionally much more complex.
When eukaryotic cells obtained their internal membranes, they started to synthesize sterols [4] and sphingolipids [5]. This may have facilitated segregation of lipids into domains of different composition, which forms the basis for maintaining the 5–10-fold enrichment of sphingolipids and sterols in the plasma membrane as compared to the ER [6]. Evidence for preferential inclusion of sterols and sphingolipids into anterograde transport vesicles derived from the trans-Golgi network has been reported recently [7] as had their relative depletion from retrograde vesicles [8].
Lipid sorting in membrane trafficking processes
The role of other membrane components in determining the distribution of cholesterol among membranes raises the issue of how differences in these other membrane constituents are maintained. For proteins, the mechanisms of sorting based on molecular recognition are beginning to be understood in considerable detail. The same is not true for lipids. Maintenance of the distinct lipid compositions of organelles that are actively involved in membrane traffic suggests that there must be mechanisms for sorting lipids as transport vesicles and tubules are formed. This was proposed many years ago for the maintenance of the distinct lipid compositions of the apical and basolateral membranes of polarized epithelia [9]. Similarly, the membrane constituents of GPI-anchored proteins confer the ability to be sorted selectively in the biosynthetic and endocytic pathways [10]. In the endocytic pathway there is efficient sorting of fluorescent lipid analogs based on differences (length and saturation) in their hydrocarbon chains [11].
Three general mechanisms could account for sorting of lipids during vesicle budding. One possibility is that it is mediated mainly by binding of selected lipids to proteins in the forming vesicle buds. A second possibility is that curvature preferences help to select certain lipids for inclusion in the buds. A third possibility is that membrane microdomains form on the basis of order preferences, and these microdomains can be selected for inclusion or exclusion in forming buds. These mechanisms are not mutually exclusive, and some combination of these may be required.
In a study of narrow tubules pulled out from giant unilamellar vesicles, demixing of lipids was observed [12]. Two recent biophysical studies independently concluded that the free energy differences associated with curvature preferences could not account by themselves for efficient lipid sorting into highly curved membranes (e.g., tubules) [13,14]. These studies were based on low concentrations of the lipids exhibiting the curvature preference, and this indicated that lipid-lipid cooperativity must be invoked for lipid sorting based on curvature. A third biophysical study examined the effect of lipid composition on sorting into coexisting regions of different curvature [15]. Lipid sorting into regions of different curvature was found in lipid compositions that were close to a demixing point, suggesting lipid cooperativity may be required. In that study it was also observed that binding of small amounts of cholera toxin to GM1 could facilitate the curvature based sorting. It had been observed previously that binding of small amounts of cholera toxin to vesicles that were close to a demixing point could drive the formation of optically observable phase separations [16].
Several cytoplasmic proteins associated with vesicle or tubule formation have an inherent curvature on the side that would bind lipids. These include the BAR domain proteins, which have clusters of positively charged side chains that can associate with negatively charged phospholipids [17]. These are often flanked by more specific lipid binding domains such as PH or PX domains, which recognize lipids such as phosphoinositides that are present in specific organelles. It is unclear to what extent the curvature that is induced or stabilized by binding BAR domain proteins can lead to selective inclusion of lipids. From the model membrane studies, it seems possible that the curvature could promote lipid sorting if the donor membrane were close to a phase separation point.
A different type of role for protein-lipid interactions in lipid sorting has been proposed for the MAL protein, a small hydrophobic protein with four putative transmembrane helices. MAL is one of the proteins found in detergent-resistant low density membrane fractions. It sorts to the apical domains of polarized epithelial cells, and plays an undefined role in the transport of GPI-anchored proteins and some other molecules to the apical domain. A recent study [18] shows that clustering of MAL leads to co-clustering with lipids that have long, saturated chains and exclusion of lipid analogs with short or unsaturated acyl chains. The basis for this is proposed to be the degree of matching between the relatively long, hydrophobic transmembrane domains of MAL and the greater bilayer thickness of more highly ordered membranes. Consistent with this, a MAL homolog with shorter transmembrane helices does not induce similar lipid sorting. Evidence is presented that the MAL protein can self-associate, and this may promote cooperative formation of more ordered domains within the Golgi apparatus for transport to the apical plasma membrane.
Cholesterol, the dynamic lipid
As compared to other membrane lipids, cholesterol moves rapidly as a monomer across membranes (milliseconds-seconds). It can also move between membrane organelles on protein carriers (minutes). This does not imply, however, that cholesterol is distributed homogeneously among the cellular membranes. Cells maintain the sterol content of various membranes at very different levels even though there is a large amount of vesicular and non-vesicular transport among organelles. It is enriched in the late secretory pathway, the plasma membrane and some endocytotic membranes. As discussed in detail elsewhere [19,20], differences in the other components of the membranes (mainly the lipids) would significantly alter the chemical stabilization of cholesterol in the membranes, which would lead to different cholesterol contents even if cholesterol were equilibrated between the organelles. In model membranes it has been found that saturated or monounsaturated (sphingo)lipids have preferential interactions with cholesterol [21]. Unexpectedly, fluorescent sterols such as dehydroergosterol were found to be enriched in the cytosolic leaflet of some cellular membranes [22], whereas the sphingolipids are enriched in the exoplasmic leaflet. While the lipids in the plasma membrane, including those enriched in the cytoplasmic leaflet, have a somewhat higher degree of saturation than lipids in the total cell [23,24], this would not explain the higher level of sterols in the cytoplasmic leaflet. The basis for the preferential cytoplasmic localization of these fluorescent sterols remains to be determined, and it remains to be shown that cholesterol itself has the same transbilayer distribution. A cytoplasmic leaflet enrichment of sterols would facilitate exchange with cytoplasmic carrier proteins for non-vesicular transport between organelles.
Transport of cholesterol into and out of cells
Because cholesterol homeostasis is of extreme importance at the whole body level, cells have various dedicated pathways for the uptake of cholesterol from low density lipoproteins (LDL) and export to high density lipoprotein (HDL).
In most mammalian cells cholesterol can be synthesized endogenously, and it can also be delivered by lipoprotein carriers. The binding of LDL to its receptor and the uptake by receptor-mediated endocytosis have been summarized in a recent review by Brown and Goldstein, the discoverers of this pathway [25]. Although this system is very well characterized, a major new regulator of LDL-receptors, PCSK9 (proprotein convertase subtilisin/kexin type 9), has been identified from human genetic screens over the past few years [26,27]. PCSK9 is a protease that undergoes autocatalytic processing in the secretory pathway. The mature form is found in plasma, and it binds to the LDL-receptor’s EGF AB domains leading to lysosomal degradation of the LDL-receptor [28–30]. The loss of LDL receptors, particularly in hepatocytes, is associated with elevated circulating LDL levels. The physiological importance of this regulatory mechanism is highlighted by the identification of an adult woman with a compound heterozygous loss of PCSK9 function [31]. This person had no detectable circulating PCSK9 and LDL levels that were about 10% of typical values, with no reported problems associated with such low levels. In contrast, individuals with gain-of –function mutations in PCSK9 exhibit elevated circulating LDL.
The mechanism by which PCSK9 redirects the receptor from its normal recycling route to the late endosomes and lysosomes is not fully characterized. The catalytic activity of PCSK9 is required for autocatalytic processing but not for down regulation of LDL-receptors [32,33]. Studies of natural mutations in PCSK9 that increase the efficiency of LDL-receptor degradation may provide clues about its mechanism. PCSK9 that is secreted by cultured cells has a tendency to aggregate either with itself or with other proteins, and at least one of the gain-of-function mutations causes an increase in self-association [34]. The binding of PCSK9 to LDL-receptors and its self-association are both enhanced at the low pH that is encountered in endosomes [30,34]. For many recycling receptors, occupancy by multi-valent ligands causes retention in the cell or delivery to late endosomes [35], so it is likely that oligomerization of PCSK9 that is bound to LDL-receptors would be sufficient to block efficient recycling. Whatever the mechanism, PCSK9 regulation of LDL-receptors is now a major target of therapeutic strategies for reducing circulating LDL.
Macrophages, which convert to foam cells in atherosclerotic lesions, have been shown to have a novel mechanism for the uptake of cholesterol derived from extracellular lipoprotein deposits. In the wall of blood vessels, macrophages encounter aggregates of lipoproteins that are tightly linked to the extracellular matrix, chemically modified (e.g., oxidation), and acted upon by lipases including lipoprotein lipase and sphingomyelinase [36]. In a cell culture model of macrophages interacting with aggregated lipoproteins, it was found that the macrophages create an acidified extracellular compartment (a lysosomal synapse) into which lysosomal contents are secreted [37,38]. This leads to extracellular hydrolysis of the cholesterol esters in the core of the lipoproteins by lysosomal acid lipase. The released cholesterol is transferred to the macrophage. This process may play an important role in the early stages of formation of atherosclerotic plaques.
Intestinal cells take up cholesterol from the gut in monomeric form. NPC1L1 is the essential and limiting protein in intestinal cholesterol absorption [39]. NPC1L1 is expressed mainly in the apical membranes of enterocytes and hepatocytes. Cholesterol can also be taken up from the blood as cholesterol or cholesteryl ester monomers from lipoproteins or as part of lipoproteins via scavenger receptors like SR-A, SR-BI and CD36 [40], which are now known to fulfill broader (signaling) functions in lipid physiology. On the other hand, sterols are secreted from cells by a variety of dedicated mechanisms. Whereas intestinal and liver cells assemble and secrete lipoproteins, these cells prevent inclusion of especially plant sterols by selectively extruding them via the apical ABC transporter, ABCG5/8 heterodimers [41]. Other cells utilize ABCA1 and ABCG1 for releasing cholesterol onto apoA1 and the apoA1 containing HDL [42]. The molecular mechanism and the relative importance of each of these processes remain to be elucidated [43].
Transport through and out of lysosomes
Many aspects of the intracellular transport of cholesterol are still poorly understood. As with LDL uptake, studies of inherited human disorders have provided important clues about these processes. Niemann-Pick disease type C (NPC) is a recessive inherited lysosomal storage disease in which cholesterol and other lipids accumulate in lysosome-like storage organelles (LSOs) [44]. Defects in two genes, NPC1 and NPC2, are responsible for NPC disease. NPC2 is a small, soluble cholesterol binding protein that is targeted to lysosomes by a mannose 6-phosphate post-translational modification. NPC2 can exchange sterol with lipid bilayers, especially if they contain acidic phospholipids such as bis(monoacylglycero)phosphate (BMP), which is abundant in lysosomal membranes [45]. The role of NPC1, which is a polytopic membrane protein that is found primarily in late endosomes, remains less clear. The N-terminal domain of NPC1, which projects into the lumen, contains a cholesterol-binding domain [46,47]. Exchange of sterols on and off the NPC1 N-terminal domain was very slow, but it was accelerated significantly by interaction with NPC2 [48]. Recent structural studies showed that the sterol is bound to NPC1 with the hydroxyl group pointing inward, in contrast to NPC2, which has the hydroxyl pointing outward, toward the opening of the binding pocket [49,50]. This provides a potential path for the sterol to slide easily from one binding pocket to the other without significant exposure to the aqueous environment. This finding led to a proposal that cholesterol that is produced by hydrolysis of cholesterol esters associated with lipoproteins may first associate with NPC2 and then be transferred to the N-terminal domain of NPC1, which would then insert the sterol into the limiting membrane of the lysosome [49], analogous to the uptake of long-chain fatty acids into and across the E. coli outer membrane [51].
NPC1 also contains a canonical sterol sensing domain in its transmembrane segments [52], which may have regulatory function based on the presence of similar sequences in the SCAP and Insig proteins [53]. However, at present the function of the sterol sensing domains remains unclear.
Recent studies have shown that the cholesterol-chelating β-cyclodextrins can bypass the need for either NPC1 or NPC2. NPC1-deficient mice that are injected repeatedly with hydroxypropyl-β-cyclodextrin have a remarkable extension in their lifespan, delay in the onset of pathology in various tissues, and reduction in accumulation of cholesterol and glycosphingolipids [54,55]. Studies in cultured cells defective in NPC1 or NPC2 have shown that after cyclodextrin treatment, cholesterol is removed from the LSOs and distributed to organelles such as the ER, where it can be re-esterified [56]. The effective cyclodextrin is delivered to the LSOs by endocytic uptake, and it remains effective for several days after removal of cyclodextrin from the culture medium [57]. The effectiveness of cyclodextrin in the absence of functional NPC1 suggests that the essential role of NPC1 and NPC2 may be just to get cholesterol into the limiting membrane of late endosomes and lysosomes.
Soluble sterol binding proteins as sterol carriers and sensors
In addition to being carried by inter-organelle membrane transport as part of the bilayer, lipids including cholesterol are transported between organelles by lipid transport proteins. There is good evidence that sterols move rapidly between organelles by non-vesicular transport, which requires association with a carrier because of the low solubility of cholesterol in water [58]. Several candidate carrier proteins have been shown to bind cholesterol and/or to facilitate transport between membranes in vitro. These include some of the proteins containing lipid-binding START domains. The founding member of this family, StARD1, has been shown to transport cholesterol from the mitochondrial outer membrane to the inner membrane in vivo, where the sterol is converted to steroid hormones [59]. Other family members, including MLN64 [60] and StARD4 [61] have also been shown to bind cholesterol. Other START family members bind and transport other lipids, including ceramide, which is transported by CERT [62]. Overexpression of StARD4 leads to an increase in esterification of cholesterol [61], which might be attributed to increased transport to the ER, the site of the esterifying enzyme, ACAT. However, except for StARD1, direct evidence for in vivo lipid transport by START family members is still lacking.
Another family of sterol binding proteins are related to oxysterol binding protein (OSBP), which was identified based on its binding of 25-hydroxycholesterol [63]. The family of OSBP related proteins binds sterols through their OSBP homology (Osh) domains [64]. In vitro, several of the yeast Osh proteins can transport sterols between membranes [65]. One issue in considering whether sterol transport is a major function of Osh proteins has been the relative abundance of these proteins compared to estimates of sterol transport rates in cells (reviewed by Mesmin & Maxfield [19]). It has been noted, however, that many Osh proteins have membrane-binding domains, and transport between closely apposed membranes should be more rapid than diffusion between well separated organelles.
A recent paper [65] has proposed that yeast Osh proteins can simultaneously associate with two membranes, and this might provide a mechanism for rapid sterol exchange between membranes. However, other studies have suggested that membrane binding by Osh proteins is related to lipid sensing and regulation. For example, in mammalian cells OSBP can act as a sterol sensor that regulates activity of phosphoprotein phosphatases in the ERK signaling pathway [66] and also regulates CERT-dependent synthesis of sphingomyelin [67]. Another family member, OSBP-related protein 2, binds to lipid droplets, but is released upon sterol binding. Reduction in the level of OSBP-related protein 2 led to slowed triacylglyceride hydrolysis and an increase in cholesteryl ester formation [68], indicating that this protein may regulate lipid metabolism in a sterol-dependent manner. OSBP-related protein 9 is a cholesterol-binding protein that interacts with the trans-Golgi/trans-Golgi network and ER, and is necessary to maintain Golgi structure and ER–Golgi protein trafficking [69]. A truncated version of the protein missing a PH domain inhibits both ER–Golgi protein transport and cell growth. As yet another example, cholesterol in late endosomes can be sensed by OSBP-related protein 1L in a process that regulates interaction with microtubule-associated motors and influences the positioning of late endosomes in the cell. This may be related to the clustering of late endosomes near the center of the cell in Niemann-Pick type C disease and other lysosomal storage diseases [70].
Studies that demonstrate a cholesterol/lipid sensing role for Osh proteins raise an interesting conundrum: are the Osh proteins primarily cholesterol and lipid sensors with a minor role in sterol transport? Or are they multi-function proteins that play an important role in sterol transport in addition to their regulatory activities? Further work will be required to resolve this issue.
Cellular cholesterol homeostasis
Cells maintain the sterol content of various membranes at very different levels, even though there is extensive vesicular and non-vesicular transport among organelles. As discussed in detail elsewhere [6,19], even if cholesterol were equilibrated between the organelles, differences in the other components of the membranes (i.e., lipids and proteins) would significantly alter the chemical stabilization of cholesterol, which would lead to differences in cholesterol content.
The ER is the site of much of the regulation of cholesterol levels. It contains ACAT, the enzyme responsible for esterifying excess cholesterol for storage in lipid droplets. It is also the site of cholesterol synthesis, including the key regulated step catalyzed by HMG-CoA-reductase. The Insig-SCAP-SREBP complexes are one of the most important sensors of sterol levels. At high cholesterol levels the complex is retained in the ER, but at lower levels the SCAP-SREBP enters transport vesicles. In the Golgi, SREBP undergoes two steps of proteolysis, releasing a soluble transcription factor that regulates many genes associated with cholesterol and lipid metabolism. This leads to increased synthesis of cholesterol and increased levels of LDL receptors. In a recent study, it was found that there is a sharp, cooperative response to cholesterol levels in the ER, with a mid-point for cholesterol content around 5% of total ER lipids [71]. This switch-like response can help to keep cellular cholesterol in a narrow range. It is unclear at present whether the sharp transition is due to cooperative protein-protein interactions between SCAP molecules, or if it is instead due to an abrupt change in the chemical activity coefficient of cholesterol in the ER membrane when it crosses a threshold value [71]. The level of expression of Insig-1 protein can influence the cholesterol-dependent transition point, and reduction of cholesterol levels leads to proteasomal degradation of Insig-1 [72], which would sensitize cells to cholesterol depletion.
Conclusion
Its high spontaneous mobility and its dramatic effects on the phase behavior of biological lipid mixtures, make cholesterol particularly suited as a modulator of the dynamic lipid organization in cells. The presence of so many dedicated cholesterol binding, transporting and sensing proteins shows that cells use cholesterol as a central lipid for regulating the cellular lipid homeostasis. An important challenge for the next years is to elucidate how the cholesterol-centered regulatory mechanisms are integrated into the overall control of cellular physiology.
Acknowledgements
FRM is supported in part by grants from the National Institutes of Health (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim Biophys Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Feigenson GW. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim Biophys Acta. 2009;1788:47–52. doi: 10.1016/j.bbamem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4. Desmond E, Gribaldo S. Phylogenomics of Sterol Synthesis: Insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol Evol. 2009:364–381. doi: 10.1093/gbe/evp036.. * An interesting perspective on the evolution of sterols
- 5.Freilich S, Goldovsky L, Ouzounis CA, Thornton JM. Metabolic innovations towards the human lineage. BMC Evol Biol. 2008;8:247. doi: 10.1186/1471-2148-8-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145.. ** This paper shows that the TGN has the capacity to sort membrane lipids. Furthermore, the observation that the immuno-isolated vesicles exhibited a higher membrane order than the late Golgi membrane strongly suggests that lipid rafts play a role in the TGN-sorting machinery.
- 8.Brügger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–518. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Meer G, Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988;36:51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]
- 10.Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamal MM, Mills D, Grzybek M, Howard J. Measurement of the membrane curvature preference of phospholipids reveals only weak coupling between lipid shape and leaflet curvature. Proc Natl Acad Sci U S A. 2009;106:22245–22250. doi: 10.1073/pnas.0907354106.. * See comment after [15].
- 14. Tian A, Baumgart T. Sorting of lipids and proteins in membrane curvature gradients. Biophys J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067.. * See comment after [15].
- 15. Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, Bassereau P. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci U S A. 2009;106:5622–5626. doi: 10.1073/pnas.0811243106.. * These papers examine the role of curvature in lipid sorting in model membrane systems.
- 16.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci U S A. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magal LG, Yaffe Y, Shepshelovich J, Aranda JF, de Marco Mdel C, Gaus K, Alonso MA, Hirschberg K. Clustering and lateral concentration of raft lipids by the MAL protein. Mol Biol Cell. 2009;20:3751–3762. doi: 10.1091/mbc.E09-02-0142.. * MAL-mediated association with raft lipids is driven at least in part by positive hydrophobic mismatch between the lengths of the transmembrane helices of MAL and membrane lipids. These data place MAL as a key component in the organization of membrane domains.
- 19.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radhakrishnan A, McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc Natl Acad Sci U S A. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol Biol Cell. 2009;20:581–588. doi: 10.1091/mbc.E08-07-0785.. * This paper presents evidence that sterols are preferentially on the cytosolic leaflet even though sphingolipids are enriched in the exofacial/lumenal leaflet.
- 23.Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 24.Kalvodova L, Sampaio JL, Cordo S, Ejsing CS, Shevchenko A, Simons K. The lipidomes of Vesicular Stomatitis Virus, Semliki Forest Virus, and the host plasma membrane analyzed by quantitative shotgun mass spectrometry. J. Virol. 2009;83:7996–8003. doi: 10.1128/JVI.00635-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50 Suppl:S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mousavi SA, Berge KE, Leren TP. The unique role of proprotein convertase subtilisin/kexin 9 in cholesterol homeostasis. J Intern Med. 2009;266:507–519. doi: 10.1111/j.1365-2796.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 28. Bottomley MJ, Cirillo A, Orsatti L, Ruggeri L, Fisher TS, Santoro JC, Cummings RT, Cubbon RM, Lo Surdo P, Calzetta A, et al. Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants. J Biol Chem. 2009;284:1313–1323. doi: 10.1074/jbc.M808363200.. * See comment after [34]
- 29. Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 2008;105:13045–13050. doi: 10.1073/pnas.0806312105.. * See comment after [34].
- 30. Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200.. * See comment after [34].
- 31. Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, Cohen JC, Hobbs HH. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–523. doi: 10.1086/507488.. * See comment after [34].
- 32. Li J, Tumanut C, Gavigan JA, Huang WJ, Hampton EN, Tumanut R, Suen KF, Trauger JW, Spraggon G, Lesley SA, et al. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem J. 2007;406:203–207. doi: 10.1042/BJ20070664.. * See comment after [34].
- 33. McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282:20799–20803. doi: 10.1074/jbc.C700095200.. * See comment after [34].
- 34. Fan D, Yancey PG, Qiu S, Ding L, Weeber EJ, Linton MF, Fazio S. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 2008;47:1631–1639. doi: 10.1021/bi7016359.. * This group of papers examines the mechanisms by which PCSK9 can down-regulate surface expression of LDL receptors.
- 35.Marsh EW, Leopold PL, Jones NL, Maxfield FR. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol. 1995;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol. 2008;28:1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosheva I, Haka AS, Qin C, Pierini LM, Maxfield FR. Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler Thromb Vasc Biol. 2009;29:1615–1621. doi: 10.1161/ATVBAHA.109.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haka AS, Grosheva I, Chiang E, Buxbaum AR, Baird BA, Pierini LM, Maxfield FR. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol Biol Cell. 2009;20:4932–4940. doi: 10.1091/mbc.E09-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis HR, Jr., Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim Biophys Acta. 2009;1791:679–683. doi: 10.1016/j.bbalip.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J Lipid Res. 2009;50:2235–2244. doi: 10.1194/jlr.M900036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 42.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Xie C, Turley SD, Dietschy JM. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J Lipid Res. 2009;50:1316–1329. doi: 10.1194/jlr.M900024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sturley SL, Patterson MC, Balch W, Liscum L. The pathophysiology and mechanisms of NPC disease. Biochim Biophys Acta. 2004;1685:83–87. doi: 10.1016/j.bbalip.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Xu Z, Farver W, Kodukula S, Storch J. Regulation of sterol transport between membranes and NPC2. Biochemistry. 2008;47:11134–11143. doi: 10.1021/bi801328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 47.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 48.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049.. * See Comment after [50].
- 50. Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200.. * These papers show the structural basis for cholesterol binding to NPC1 and NPC2.
- 51.Hearn EM, Patel DR, Lepore BW, Indic M, van den Berg B. Transmembrane passage of hydrophobic compounds through a protein channel wall. Nature. 2009;458:367–370. doi: 10.1038/nature07678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci U S A. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15:259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 54. Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of the newborn or mature NPC1 mouse leading to excretion of the sequestered cholesterol as bile acid. J. Lipid Res. 2009 doi: 10.1194/jlr.M000257. jlr.M000257.. * See annotation to [56].
- 55. Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951.. * See annotation to [56].
- 56. Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci U S A. 2009;106:19316–19321. doi: 10.1073/pnas.0910916106.. * The NPC1 or NPC2 block in cholesterol export from lysosomes can be overcome by beta-cyclodextrins, which leads to a marked increase in ACAT-mediated cholesterol esterification. The buildup of cholesteryl esters in the cytosol is expected to be much less toxic than the buildup of free cholesterol in the lysosomes of patients with mutations in NPC1 or NPC2.
- 57. Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0914309107.. * See annotation to [56].
- 58.Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 59.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 60.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol. 2000;7:408–414. doi: 10.1038/75192. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez-Agudo D, Ren S, Wong E, Marques D, Redford K, Gil G, Hylemon P, Pandak WM. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J Lipid Res. 2008;49:1409–1419. doi: 10.1194/jlr.M700537-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 63.Lehto M, Olkkonen VM. The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism, and cell signalling. Biochim Biophys Acta. 2003;1631:1–11. doi: 10.1016/s1388-1981(02)00364-5. [DOI] [PubMed] [Google Scholar]
- 64.Olkkonen VM, Johansson M, Suchanek M, Yan D, Hynynen R, Ehnholm C, Jauhiainen M, Thiele C, Lehto M. The OSBP-related proteins (ORPs): global sterol sensors for coordination of cellular lipid metabolism, membrane trafficking and signalling processes? Biochem Soc Trans. 2006;34:389–391. doi: 10.1042/BST0340389. [DOI] [PubMed] [Google Scholar]
- 65.Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang PY, Weng J, Anderson RG. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005;307:1472–1476. doi: 10.1126/science.1107710. [DOI] [PubMed] [Google Scholar]
- 67.Perry RJ, Ridgway ND. Oxysterol-binding protein and VAMP-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hynynen R, Suchanek M, Spandl J, Back N, Thiele C, Olkkonen VM. OSBP-related protein 2 is a sterol receptor on lipid droplets that regulates the metabolism of neutral lipids. J Lipid Res. 2009;50:1305–1315. doi: 10.1194/jlr.M800661-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ngo M, Ridgway ND. Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol Biol Cell. 2009;20:1388–1399. doi: 10.1091/mbc.E08-09-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008.. * By careful measurements of ER sterol concentrations, this paper shows that a SREBP-2 responds to small changes in cholesterol levels, providing a mechanism for tight control of cellular cholesterol content.
- 72.Ikeda Y, Demartino GN, Brown MS, Lee JN, Goldstein JL, Ye J. Regulated endoplasmic reticulum-associated degradation of a polytopic protein: p97 recruits proteasomes to Insig-1 before extraction from membranes. J Biol Chem. 2009;284:34889–34900. doi: 10.1074/jbc.M109.044875. [DOI] [PMC free article] [PubMed] [Google Scholar]