Abstract
Introduction
The influence of the autonomic nervous system on the pathogenesis of complex fractionated atrial electrograms (CFAE) during atrial fibrillation (AF) is incompletely understood. This study evaluated the impact of pharmacological autonomic blockade on CFAE characteristics.
Methods
Autonomic blockade was achieved with propanolol and atropine in 29 patients during AF. Three-dimensional maps of the fractionation degree were made before and after autonomic blockade using the Ensite Navx® system. In 2 patients, AF terminated following autonomic blockade. In the remaining 27 patients, 20113 electrogram samples of 5 seconds duration were collected randomly throughout the left atrium (10054 at baseline and 10059 after autonomic blockade). The impact of autonomic blockade on fractionation was assessed by blinded investigators and related to the type of AF and AF cycle length.
Results
Globally, CFAE as a proportion of all atrial electrogram samples were reduced after autonomic blockade: 61.6±20.3% vs. 57.9±23.7%, p=0.027. This was true/significant for paroxysmal AF (47±23% vs. 40±22%, p=0.003), but not for persistent AF (65±22% vs. 62±25% respectively, p=0.166). Left atrial AF cycle length prolonged with autonomic blockade from 170±33 ms to. 180±40 ms (p=0.001). Fractionation decreases only in the 14/27 patients with a significant (>6ms) prolongation of the AF cycle length (64±20% vs. 59±24%, p=0.027), while fractionation did not reduce when autonomic blockade did not affect the AF cycle length (58±21% vs. 56±25%, p=0.419).
Conclusions
Pharmacological autonomic blockade reduces CFAE in paroxysmal AF, but not persistent AF. This effect appears to be mediated by prolongation of the AF cycle length.
Keywords: Complex fractionated atrial electrograms, autonomic nervous system, ganglionated plexus, atrial fibrillation, catheter ablation, cycle length
INTRODUCTION
The autonomic nervous system (ANS) has been implicated in the pathogenesis of atrial fibrillation (AF) 1–3. Acetylcholine (Ach), which is released by ganglionic plexi (GPs), shortens action potential duration and the atrial effective refractory period2. Experimental animal studies have demonstrated that cholinergic AF is maintained by high-frequency sources predominantly distributed to the left atrium (LA)4, 5. Furthermore, radiofrequency ablation of the parasympathetic nervous system rendered AF non-inducible in a canine model6. In humans, directing ablation to sites of GPs distributed throughout both atria has also shown promise as a strategy for both paroxysmal and persistent AF6, 7.
On the other hand, sites harbouring complex fractionated atrial electrograms (CFAE) have been proposed as critical regions maintaining AF. Hypothesises concerning the underlying mechanisms about CFAE include local passive slow conduction8, 9, zones of colliding wavefronts or pivoting points between different wavelets participating in the AF process10 and relationship with dominant frequency areas11, 12. In addition, if the ANS does play an important role in the pathogenesis of AF, how it influences CFAE characteristics is less well understood. It has been hypothesized that areas harbouring CFAE are sites of rich innervation by GP, where local Ach release may directly and independently cause CFAE; however, this remains controversial6, 7, 13–15.
This study sought to evaluate the impact of pharmacological autonomic blockade on atrial electrograms characteristics.
METHODS
Study population
This study comprised 29 consecutive patients referred for catheter ablation for drug-refractory symptomatic AF. Antiarrhythmic drugs were withdrawn at least 5 half-lives before the study and all patients provided written informed consent.
AF was paroxysmal in 13 patients and persistent in 16. Paroxysmal AF was defined as AF that self terminated within 7 days while persistent AF was defined as AF which is sustained beyond seven days, or lasting less than seven days but necessitating pharmacologic or electrical cardioversion. Of note, in our study, all the patients with persistent AF had at least 7 days of continuous AF.
Study protocol
Electrophysiologic Study
Electrophysiological studies were performed as previously described16. Of note, a 20-polar mapping catheter (Pentaray, Biosense Webster Diamond Bar, CA, USA) was introduced in the LA for mapping and recording. This was stabilized using a long sheath (SL 0, St. Jude Medical, MN, USA) that was continuously perfused with heparinized saline.
Clinical variables
Heart rate, systolic and diastolic blood pressure were measured every minutes during 10 minutes before and after autonomic blockade. The AF cycle length was determined in the LA appendage immediately before and 5 minutes after the achievement of ANS blockade by averaging 30 consecutive cycles using automated monitoring software (Labsystem Pro, Bard Electrophysiology) as previously dscribed17, 18. A difference in the cycle length of more than 6 ms during two different analysis was considered as significant19.
Three-dimensional electro-anatomical map
The 3-dimensional electro-anatomical maps were performed using the Ensite NavX® system (St. Jude Medical, MN, USA). A 3-dimensional anatomical geometry of the different chambers was created by using the 20 poles Pentaray catheter and the decapolar catheter for CS geometry. The surface interpolation (filling threshold) was set at 10 mm. The LA shell was divided into 8 segments on the NavX geometry: the right pulmonary veins (PV), the left PV’s, the anterior LA, the roof, the septum, the posterior LA, the inferior LA, and lateral LA. The respective PV region included ipsilateral PV’s and their ostia (defined as atrial tissue 0.5 to 1.0 cm from both ipsilateral PV’s). The posterior LA approximated a square, the corners of which were defined by the 4 PV orifices. The LA roof was defined as a band of 1 to 2 cm between the superior PV’s, which separates the posterior LA from the anterior LA. The anterior LA extended from the LA roof to the superior mitral annulus. The inferior LA extends from the lower aspect of the 2 inferior PV ostia to the inferior mitral annulus. The lateral LA was defined as atrial tissue between the posterolateral mitral annulus (4 o’clock, in left anterior oblique projection) and the lower lip of the LA appendage. The septal LA extended from the right veins to the anteroseptal mitral annulus.
Recordings were made as follows. In patients with paroxysmal AF who were in sinus rhythm at the start of the procedure, burst atrial pacing from the CS was performed to induce AF. Fractionation maps were taken using the Pentaray mapping catheter, which allowed 10 simultaneous atrial electrograms to be recorded at each Pentaray position.
Geometry surface modelling algorithm
A Ensite NavX® geometry model of a heart chamber begins as an icosahedron, or a polyhedron with 12 vertices and 20 triangular faces. These faces are further subdivided into a total of 1280 triangular faces. As the geometry model is collected, the vertices are pushed out from the centroid to match the chamber wall as the operator moves a catheter along it. When the model is complete, if any triangular face has one or more sides longer than 4 mm (unscaled Ensite NavX® distance), the face is subdivided into 2, 3 or 4 smaller triangles (depending on how many sides exceeded the length of 4 mm). Upon completion of this surface mesh refinement, it is common to have more than 5000 vertices and 10000 faces total for each patient. The density of vertices (size of the facets on the mesh) may vary regionally, depending upon the complexity of the geometry model and the local distance to the center of the geometry. Though the vertex distribution is not uniform, it is regionally constant when comparing maps before and after autonomic blockade.
Autonomic blockade
After obtaining the fractionation map at baseline, autonomic blockade was achieved with concomitant intravenous injection of propanolol 0.2 mg/kg at a rate of 1 mg/min, and atropine sulphate 0.04 mg/kg, as proposed by Jose20, 21 and modified by Jordan22. Heart rate, blood pressure, and left and right atrial cycle lengths were measured before and after autonomic blockade. A second fractionation map was performed immediately after the autonomic blockade using the same anatomical constructed shell.
In two patients with paroxysmal AF, AF terminated after having completed pharmacological autonomic blockade and was then non-inducible by burst pacing to atrial refractoriness despite multiple attempts. The second fractionation map could consequently not be performed and these two patients were excluded from the analysis about the impact of ANS blockade on CFAE. In the remaining 27 patients, AF was maintained following pharmacological autonomic blockade for the entire period of mapping and therefore the impact of pharmacological autonomic blockade could be analyzed.
Mapping was performed for both maps so as to ensure a similar distribution of electrogram samples across the LA. Furthermore, electrogram recordings were randomly collected in the LA after autonomic blockade.
Electrogram analysis
Signal analysis of the recordings obtained by the Ensite NavX® system was performed by investigators who were blinded to the baseline characteristics of the patient and to the degree of autonomic blockade. CFAE were defined as electrograms with a mean interval of less than 120 ms during a 5 seconds recording period. The peak to peak voltage threshold was set at 0.04 mV and was always above baseline noise. The duration threshold (slope) the refractory setting and the electrogram width were 20 ms, 40 ms and 0.1 ms, respectively. Electrograms were deleted when artefacts or poor contact were observed. Notably, all samples that projected more than 8 mm from the NavX endocardial geometry were deleted.
Statistical analysis
Continuous variables are expressed as mean±SD except for count and time variables which are expressed as median and interquartile (IQ) range. Statistical significance was assessed using the unpaired Student’s t test or Mann-Whitney test where necessary. Categorical variables, expressed as numbers or percentages, were analyzed with the Chi-square test or Fischer’s exact test. All tests were 2-tailed and a p value<0.05 was considered statistically significant.
RESULTS
Patient characteristics
There were 29 patients (19 men) with either paroxysmal (n=13) or persistent AF (n=16) of long duration (32 ± 41 months; range 3 to 120 months). Patients’ characteristics are summarized in table 1. Four patients with paroxysmal AF had spontaneous arrhythmia for 7 hours (range 3 to 13 hours) upon commencing the procedure; in the remaining 9 patients, it was induced by burst pacing and sustained for at least 10 minutes before mapping. In 2 patients with paroxysmal AF (one with spontaneous AF of 4 hours duration before the procedure and one with induced AF), AF terminated following complete autonomic blockade (after 2 and 15 minutes respectively) and was rendered non-inducible despite multiple attempts to re-induce with burst pacing. For these two patients, the implication of the autonomic nervous system on the AF process was strongly suspected. Nevertheless, the second fractionation map could not been performed and therefore, these two patients were withdrawn from subsequent analysis about the impact of autonomic blockade on CFAE characteristics.
Table 1.
Baseline Characteristics of Overall Study Population (n=29)
| Age (years) | 58±10 |
| Male | 19/29 (66%) |
| Structural heart disease | 5/29 (17%) |
| Hypertension | 9/29 (31%) |
| Failed antiarrhythmic drugs (n) | 2±1 |
| AF history before ablation (months) | 86±73 |
| LA diameter (mm) | 46±6 |
| LVEF (%) | 62±11 |
| Left end diastolic ventricular diameter (mm) | 53±7 |
| Procedural duration (min) | 244±94 |
| Fluoroscopy duration (min) | 97±40 |
| Duration of radiofrequency delivery (min) | 58±25 |
Effect of autonomic blockade on patients’ clinical parameters
Systolic and diastolic blood pressure and heart rate did not differ significantly before and after autonomic blockade: 128±17 mm Hg vs. 130±18 mm Hg (p=0.39), 83±10 mm Hg vs. 84±11 mm Hg (p=0.32) and 70±13 bpm vs. 71±13 bpm (p=0.63), respectively. Notably, AF cycle length in the LA appendage was significantly longer for the entire group following autonomic blockade (180±40 vs. 170±33 ms at baseline, p=0.001). However, only 17/27 patients (63%, 10 persistent and 7 paroxysmal) had a significant lengthening of the LAA cycle length (of more than 6 ms).
In total, 9/13 patients with paroxysmal AF (including the 2 patients no longer inducible) and 10/16 patients with persistent AF had a significant impact of the autonomic blockade on the AF cycle length (p=0.86).
Electrograms recordings and distribution of the vertices
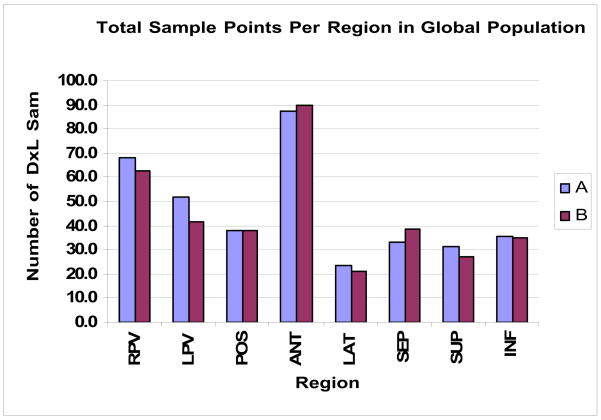
There were 20113 electrogram samples of 5 seconds collected randomly in the left atrium and which were subsequently analyzed (10054 at baseline and 10059 after autonomic blockade) for paroxysmal (7404 samples) and persistent AF (12709 samples). This represents a mean of 372 electrogram samples analyzed per map and per patient. The distribution of electrogram samples for each region is represented in Figure 1.
Figure 1. Distribution of electrogram samples for each region before and after pharmacological autonomic blockade.
RVs: Right pulmonary veins; LVs: Left pulmonary veins; Post: Posterior left atrium; Ant: Anterior left atrium; Lat: Lateral left atrium; Sept: left interatrial septum; Inf: Inferior left atrium
After the subdivision of the LA shell into vertices, as described in the method section, a fractionation value could be assigned to 68% of the vertices in patients with persistent AF and to 66% of the vertices in patients with paroxysmal AF. For the other vertices, the distance between the vertex and adjacent recorded electrogram samples was too great (>8 mm) to allow accurate interpretation.
Validation of Vertex Analysis
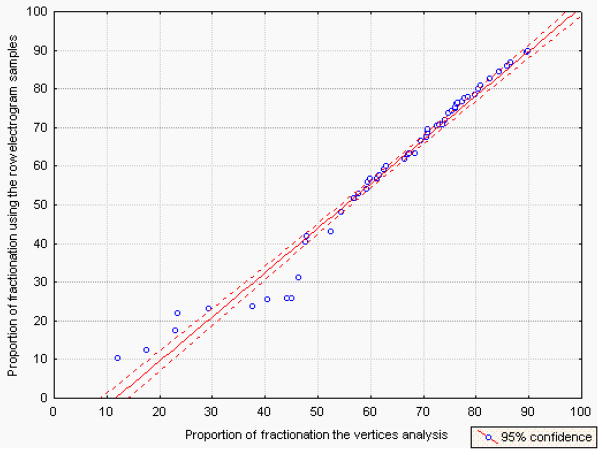
The values derived from analysis of the vertices and those derived from the raw electrogram samples were highly correlated: the global proportion of CFAE (before and after autonomic blockade) was 63.8±18.9% using the vertices analysis and 59.7±21.9% using the raw values (p<0.001, r=0.985) (Figure 2).
Figure 2.
Correlation between the values derived from analysis of the vertices and those derived from the raw electrogram samples.
Baseline characteristics of the fractionation
The proportion of atrial electrograms defined as being CFAE at the baseline (prior to autonomic blockade) was significantly higher in patients with persistent AF as compared to patients with paroxysmal AF (65.0±21.8% vs. 47.1±22.9 % respectively, p=0.040). However, although there was a trend, the proportion of atrial electrograms displaying complex fractionation was not significantly different between patients with persistent and paroxysmal AF in each of the 8 LA regions: anterior LA (68.8±28.0% vs. 56.6±25.4% respectively, p=0.272), roof (82.3±30.9% vs. 80.4±12.3% respectively, p=0.859), posterior LA (77.8±31.5% vs. 76.9±17.7% respectively, p=0.933), right PV’s (46.1±28.5% vs. 34.1±24.8% respectively, p=0.298), left PV’s (38.7±25.0% vs. 36.2±12.7% respectively, p=0.780), inferior LA (80.3±28.1% vs. 72.3±30.8% respectively, p=0.681), septum (81.2±28.1% vs. 71.3±14.6% respectively, p=0.311) and lateral LA (74.7±33.0% vs. 54.2±25.2% respectively, p=0.103). The roof region displayed the highest proportion of atrial electrograms that were fractionated, in patients with either persistent or paroxysmal AF (82.3±30.9% and 80.4±12.3% respectively).
Impact of autonomic blockade on global CFAE proportion
The global proportion of atrial electrograms that were defined as being CFAE was significantly lower following autonomic blockade: 61.6±20.3% vs. 57.9±23.7%, p=0.027. This reduction in the proportion of CFAE after autonomic blockade was significant for paroxysmal AF (47.1±22.9% vs. 40.1±22.1%, p=0.003), but not for persistent AF (65.0±21.8% at baseline vs. 62.1±25.2% after ANS blockade, p=0.166); see examples in figure 3.
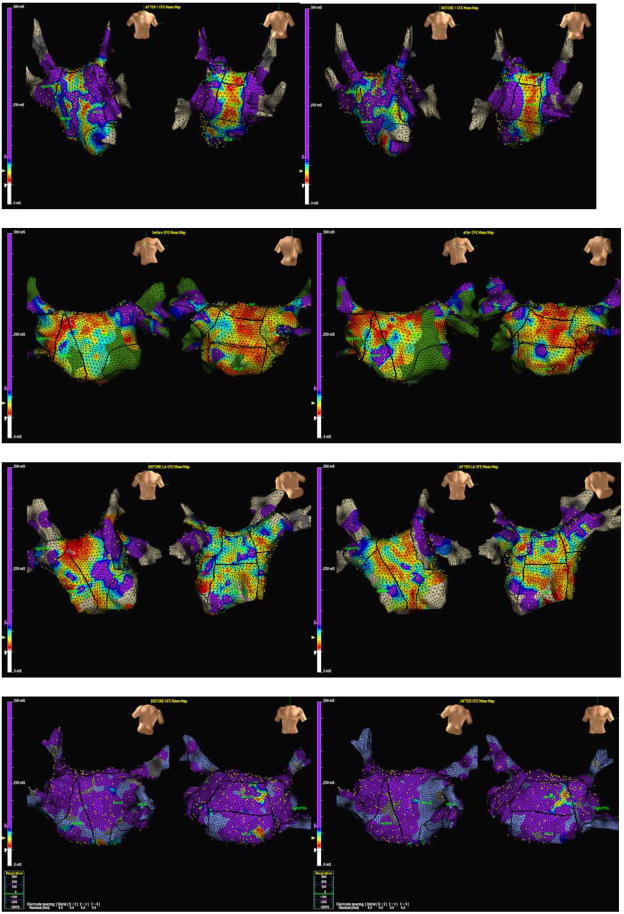
Figure 3. Fractionation maps before and after autonomic blockade.
Two typical examples demonstrating the difference in fractionation before and after pharmacological autonomic blockade. Panels A (before autonomic blockade) and B (after autonomic blockade) are from a patient with paroxysmal atrial fibrillation and a decrease in fractionation can be seen in panel B on the inferior left atrium (arrow).
Panels C (before autonomic blockade) and D (after autonomic blockade) are from a patient with persistent atrial fibrillation and a small decrease in fractionation can be seen in the anterior and posterior wall.
There was a significant correlation between alterations in AF cycle length and the proportion of global CFAE after autonomic blockade (p=0.044, r=0.39, figure 4). Importantly, in the 17/27 (10 persistent and 7 paroxysmal) patients with a significant prolongation of the AF cycle length after autonomic blockade (≥6 ms), there was a significant reduction in the CFAE proportion (64.2±20.4% vs. 59.2±23.6%, p=0.027), while this was not the case when autonomic blockade did not affect the AF cycle length (58.3±20.6% at baseline vs. 56.2±24.8% after ANS blockade, p=0.419).
Figure 4.
Correlation between change in atrial fibrillation cycle length (AFCL) and the proportion of global complex fractionated atrial electrograms (CFAE) after autonomic blockade
Impact of autonomic blockade on regional CFAE proportion
Following autonomic blockade, the proportion of atrial electrograms displaying complex fractionated activity was significantly lower compared to baseline at the lateral LA (67.1±31.5% at baseline vs. 58.8±38.5% after ANS blockade, p=0.049) and at the roof (81.6±25.3% vs. 71.6±34.2%, respectively, p=0.004). However, although there was a trend towards lowering fractionated electrograms in some other regions, autonomic blockade did not significantly affect the proportion of CFAE: anterior LA (64.3±27.3% vs. 59.9±30.2% respectively, p=0.09), posterior LA (77.4±26.8% vs. 71.1±29.5% respectively, p=0.426), right PV’s. (41.9±27.4% vs. 40.5±28.2%, respectively, p=0.585), left PV’s (37.8±21.3% vs. 35.6±26.3% respectively, p=0.584), inferior LA (77.3±28.8% vs. 73.9±29.1% respectively, p=0.194), and septum (77.5±24.1% vs. 77.1±27.7% respectively, p=0.882).
Impact of autonomic blockade on the individual vertices CFAE values
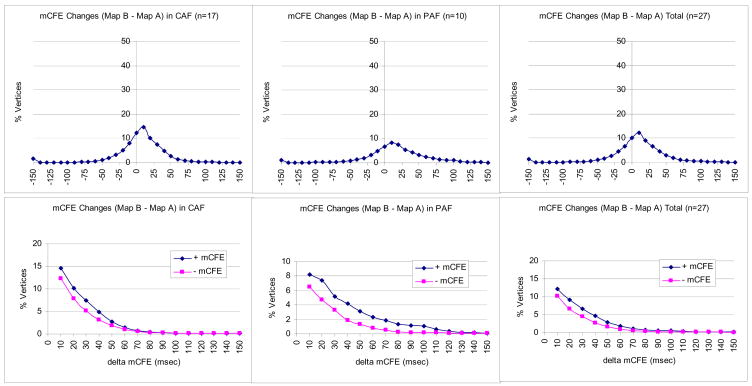
Student’s t test for dependent samples demonstrated a statistically significant (although small) reduction of individual CFAE values after autonomic blockade (109.6±40.0 ms vs. 113.2±40.8 ms, p<0.001). Interestingly, the comparison of individual CFAE values showed a significant correlation before and after autonomic blockade in 64.7% of the cases (p<0.001, r=0.647). Furthermore, 31% of the vertices had differences in CFAE values of less than 10 ms and 94% of the vertices had differences in the CFAE values of less than 50 ms (Figure 5).
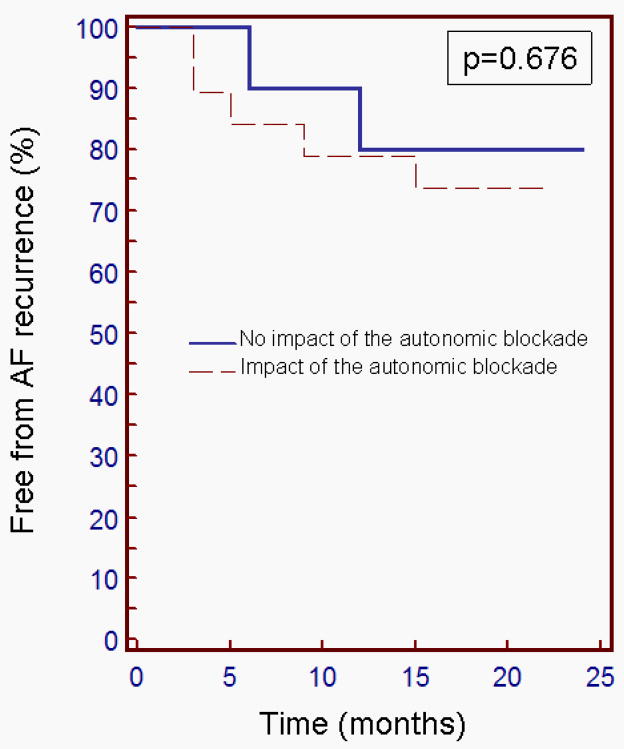
Figure 5. Kaplan-Meier analysis of freedom from atrial fibrillation (AF) recurrence depending on the impact of the autonomic blockade on the AF process.
Effect of autonomic blockade on complex fractionated electrograms (CFAE). The upper panels demonstrate the absolute difference in CFAE value after autonomic blockade using the vertices analysis. The lower panels show the positive and negative delta in individual vertices after autonomic blockade using the vertices analysis. PsAF = persistent atrial fibrillation; PAF = paroxysmal atrial fibrillation.
DISCUSSION
Main findings
This study demonstrates that pharmacological autonomic blockade results in a small but significant reduction in the proportion of atrial electrograms that display complex fractionation in patients with paroxysmal but not persistent AF. Furthermore the study suggests that the effect of the autonomic system on complex fractionation may be mediated via a slowing of the AF cycle length.
Electrophysiological mechanisms of complex fractionated atrial electrograms
Multiple electrophysiological mechanisms have been suggested to try and explain CFAE, including local slow conduction9-possibly in the vicinity of areas displaying rapid activity12, areas of colliding wavefronts, and pivot points of re-entrant circuits10. Other authors demonstrated that fractionated electrograms were increased when the atrial cycle length was shortened8. Another emerging hypothesis is that fractionation is a result of the autonomic nervous system6, 7, 15. Indeed, acetylcholine infusion results in appearance of fractionated potentials at the vicinity of GPs at remote sites. Furthermore, some authors suggest a correlation between the localization of GPs and the most fractionated areas7.
The present study confirms that there is a small but significant action of the autonomic nervous system on the overall proportion of atrial electrograms displaying fractionated activity. However, our results suggest that this is secondary to the impact on AF cycle length, as patients with no AF cycle length change also had no alteration in the proportion of electrograms exhibiting CFAE. Furthermore, areas displaying CFAE were stable in 65% of the cases, similar to previous studies describing spontaneous CFAE stability23, 24, again suggesting that the impact of the autonomic nervous system on CFAE is limited.
Role of the autonomic system in AF maintenance and implication for AF mechanisms
This study confirms that there is an effect of the ANS on the pathophysiology of AF in patients with either paroxysmal or persistent AF. This effect is not uniform throughout the patients but shows considerably interpatient variability. Indeed, following pharmacological autonomic blockade, 2 patients cardioverted to sinus rhythm and were then non-inducible, while 17 patients had a significant lengthening of AF cycle length.
Several authors have suggested that increased vagal balance is involved in the mechanisms for AF1–3. Parasympathetic stimulation shortens the atrial effective refractory period and decreases the wavelength of atrial re-entrant circuits25. Furthermore, vagal stimulation increases spatial atrial heterogeneity, thereby favouring AF26.
The differential effect of the autonomic nervous system on paroxysmal and persistent AF is controversial. The initial models of vagal AF were derived from patients with paroxysmal AF1, 2. Different data suggested a similar role for persistent AF. Indeed, in atrial biopsies obtained from patients with chronic AF, acetylcholinesterase activity was significantly reduced compared to patients with sinus rhythm27. Infusion of adenosine (which uses the same receptor as acetylcholine) accelerates DFmax, suggesting re-entry as a mechanism of AF maintenance25, although this effect was restricted to patients with paroxysmal AF.
In our study, the proportion of patients with a significant impact of the ANS blockade was similar in paroxysmal and persistent AF; however, both patients with AF termination and subsequent non inducibility after ANS blockade had paroxysmal AF. This could still suggest a higher contribution of the ANS in paroxysmal AF although this requires confirmation in larger prospective studies.
Pharmacological autonomic blockade
Jose et al. studied the effect of combined sympathetic and parasympathetic blockade in man on heart rate and function by infusing atropine and propanolol20. They found that after a dose of 0.04 mg/kg atropine and 0.2 mg/kg propanolol, there was no evidence of an chronotropic response to isoproterenol for 20 minutes in all of the patients. They assumed that total or near total isolation of the autonomic nervous system was ascertained during this period of time. Notably, our mapping was completed within this 20 minute period of time.
In our study, two lines of reasoning support an effect of the humoral autonomic nervous system on AF. First, AF stopped after the protocol in 2 patients with paroxysmal AF, and was rendered non inducible despite consistent induction prior to autonomic blockade. Secondly, there was a significant impact on the AF cycle length measured in the LA appendage. Recent studies suggest that the impact of neural and humoral stimulation of the autonomic nervous system have several similarities28. However, it remains unclear from our results whether the effects of elimination of nervous efferents (such as, via ablation of GP’s) would differ.
Clinical implications
In addition to PV isolation29 and LA linear lesions30, 31, ablation based on electrogram analysis including CFAE 32, 33 and ablation of GP’s6, 7 are the main techniques currently available for AF ablation. Some authors have suggested that CFAE ablation could be indirectly beneficial secondary to ablation of the autonomic nervous system, in particular the GPs7. However, the present study suggests that the impact of the autonomic nervous system on the CFAE is small and present only for patients with paroxysmal AF. Furthermore, the variability of the total CFAE as a proportion of all atrial electrograms is similar to the intrinsic temporal variability of CFAE23 and the reduction in CFAE proportion is present only for patients with a significant prolongation of the AF cycle length. Therefore, our study suggests that, while the ANS has a clear role in the AF process, it may play a minor role in CFAE. Therefore, CFAE ablation should not be used as a surrogate for GP ablation and conversely GP ablation may not result in the elimination of all CFAE.
Limitations
The patient population in this study was not pre-selected as having AF during clinical vagal circumstances. It may be that the small effect that was seen in the patients with paroxysmal AF was due to taking an unselected population. Additionally, the patients with persistent AF were unselected, and it may be that the effect of the ANS is not lost in a cohort of patients with vagally mediated AF. Although autonomic blockade typically lasts for only 20 minutes, recording the second map always fell within this timeframe. Moreover, we sampled our second map in random sequence to reduce the possibility that specific regions of the atrium would consistently be sampled earlier or later after autonomic blockade. Although our protocol was robust for humoral autonomic blockade, we accept that this may not fully eliminate neural contributions from local efferent discharge; our conclusions must be interpreted with that caveat. The effect of a antiarrhythmic drugs on CFAE has not been investigated although this could have added some useful information concerning the relationship between CFAE and AF cycle length. Finally, the reproducibility of AF cycle length as measured in the LA appendage has not been assessed in this study; however, this has already been investigated and established previously by our group17–19.
Conclusions
Pharmacological autonomic blockade results in a small reduction of the CFAE proportion but only for paroxysmal AF. This effect seems to act via a lengthening of the AF cycle length.
Acknowledgments
Sébastien Knecht is supported by the Belgian “Funds for cardiac surgery”.
Mark O’Neill is supported by the British Heart Foundation.
References
- 1.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15 (Suppl A):9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 2.Schuessler R, Grayson T, Bromberg B, Cox J, Boineau J. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992 Nov;71(5):1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 3.Sharifov OF, Zaitsev AV, Rosenshtraukh LV, Kaliadin AY, Beloshapko GG, Yushmanova AV, Schuessler RB, Boineau JP. Spatial Distribution and Frequency Dependence of Arrhythmogenic Vagal Effects in Canine Atria. J Cardiovasc Electrophysiol. 2000;11(9):1029–1042. doi: 10.1111/j.1540-8167.2000.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 4.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal Periodicity During Atrial Fibrillation in the Isolated Sheep Heart. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 5.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001 May 29;103(21):2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 6.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000 Nov 28;102(22):2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 7.Lemery R, Birnie D, Tang ASL, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3(4):387–396. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Rostock T, Rotter M, Sanders P, Takahashi Y, Jais P, Hocini M, Hsu LF, Sacher F, Clementy J, Haissaguerre M. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006;3(1):27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Pak H-N, Kim SK, Jang JK, Choi JI, Lim HE, Hwang C, Kim Y-H. Electrophysiologic Characteristics of Complex Fractionated Atrial Electrograms in Patients with Atrial Fibrillation. J Cardiovasc Electrophysiol. 2009;20(3):266–272. doi: 10.1111/j.1540-8167.2008.01321.x. [DOI] [PubMed] [Google Scholar]
- 10.Konings KTS, Smeets JLRM, Penn OC, Wellens HJJ, Allessie MA. Configuration of Unipolar Atrial Electrograms During Electrically Induced Atrial Fibrillation in Humans. Circulation. 1997;95(5):1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 11.Stiles MK, Brooks AG, Kuklik P, John B, Dimitri H, Lau DH, Wilson L, Dhar S, Roberts-Thomson RL, Mackenzie L, Young GD, Sanders P. High-Density Mapping of Atrial Fibrillation in Humans: Relationship Between High-Frequency Activation and Electrogram Fractionation. J Cardiovasc Electrophysiol. 2008;19(12):1245–1253. doi: 10.1111/j.1540-8167.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of Wave Fractionation at Boundaries of High-Frequency Excitation in the Posterior Left Atrium of the Isolated Sheep Heart During Atrial Fibrillation. Circulation. 2006 February 7;113(5):626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 13.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically Induced Conversion of Pulmonary Vein Focal Firing Into Atrial Fibrillation. Journal of the American College of Cardiology. 2005;45(11):1878–1886. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic Mechanism to Explain Complex Fractionated Atrial Electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18(11):1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Scherlag BJ, Lin J, Niu G, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Autonomic Mechanism for Complex Fractionated Atrial Electrograms: Evidence by Fast Fourier Transform Analysis. J Cardiovasc Electrophysiol. 2008;19(8):835–842. doi: 10.1111/j.1540-8167.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 16.Knecht S, Hocini M, Wright M, Lellouche N, O’Neill MD, Matsuo S, Nault I, Chauhan VS, Makati KJ, Bevilacqua M, Lim K-T, Sacher F, Deplagne A, Derval N, Bordachar P, Jais P, Clementy J, Haissaguerre M. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008 July 8; doi: 10.1093/eurheartj/ehn302. In press. [DOI] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Lim K-T, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P, Virag N. Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace. 2007 November 1;9(suppl_6):vi64–70. doi: 10.1093/europace/eum208. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Lellouche N, Wright M, Bevilacqua M, Knecht S, Nault I, Lim K, Arantes L, O’Neill M, Platonov P, Carlson J, Sacher F, Hocini M, Jaïs P, Haïssaguerre M. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J Am Coll Cardiol. 2009;54(9):788–795. doi: 10.1016/j.jacc.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, O’Neill MD, Hocini M, Dubois R, Matsuo S, Knecht S, Mahapatra S, Lim K-T, Jais P, Jonsson A, Sacher F, Sanders P, Rostock T, Bordachar P, Clementy J, Klein GJ, Haissaguerre M. Characterization of Electrograms Associated With Termination of Chronic Atrial Fibrillation by Catheter Ablation. Journal of the American College of Cardiology. 2008;51(10):1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Jose A. Effect of combined sympathetic and parasympathetic blockade on heart rate and cardiac function in man. Am J Cardiol. 1966;18:476–478. doi: 10.1016/0002-9149(66)90073-7. [DOI] [PubMed] [Google Scholar]
- 21.Jose ADC. The normal range and determinants of the intrinsic heart rate in man. Circ Res. 1970;4(2):160–167. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- 22.Jordan J, Yamaguchi M, Mandel W. Studies on the mechanism of sinus node dysfunction in the sick sinus syndrome. Circulation. 1978;57(2):217–223. doi: 10.1161/01.cir.57.2.217. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Wulffhart Z, Beardsall M, Whaley B, Hill C, Khaykin Y. Spatial and Temporal Stability of Complex Fractionated Electrograms in Patients with Persistent Atrial Fibrillation over Longer Time Periods: Relationship to Local Electrogram Cycle Length. Heart rhythm: the official journal of the Heart Rhythm Society. 2008;5(8):1127–1133. doi: 10.1016/j.hrthm.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Roux J-F, Gojraty S, Bala R, Liu CF, Hutchinson MD, Dixit S, Callans DJ, Marchlinski F, Gerstenfeld EP. Complex Fractionated Electrogram Distribution and Temporal Stability in Patients Undergoing Atrial Fibrillation Ablation. J Cardiovasc Electrophysiol. 2008;19(8):815–820. doi: 10.1111/j.1540-8167.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 25.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of Inward Rectifier Potassium Channels Accelerates Atrial Fibrillation in Humans: Evidence for a Reentrant Mechanism. Circulation. 2006 December 5;114(23):2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 26.Arora R, Ng J, Ulphani J, Mylonas I, Subacius H, Shade G, Gordon D, Morris A, He X, Lu Y, Belin R, Goldberger JJ, Kadish AH. Unique Autonomic Profile of the Pulmonary Veins and Posterior Left Atrium. Journal of the American College of Cardiology. 2007;49(12):1340–1348. doi: 10.1016/j.jacc.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez R, Campos E, Karmelic C, Moran S, Inestrosa N. Acetylcholinesterase changes in hearts with sinus rhythm and atrial fibrillation. Gen Pharmacol. 1993 Jan;24(1):111–114. doi: 10.1016/0306-3623(93)90019-t. [DOI] [PubMed] [Google Scholar]
- 28.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li Y-H, Scherlag BJ, Po SS. Sodium-Calcium Exchange Initiated by the Ca2+ Transient: An Arrhythmia Trigger Within Pulmonary Veins. J Am Coll Cardiol. 2006;47(6):1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Haissaguerre M, Shah DC, Jais P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clementy J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102(20):2463–2465. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 30.Jais P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clementy J, Haissaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110(19):2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 31.Gaita F, Riccardi R, Caponi D, Shah D, Garberoglio L, Vivalda L, Dulio A, Chiecchio A, Manasse E, Gallotti R. Linear Cryoablation of the Left Atrium Versus Pulmonary Vein Cryoisolation in Patients With Permanent Atrial Fibrillation and Valvular Heart Disease: Correlation of Electroanatomic Mapping and Long-Term Clinical Results. Circulation. 2005 January 18;111(2):136–142. doi: 10.1161/01.CIR.0000151310.00337.FA. [DOI] [PubMed] [Google Scholar]
- 32.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43(11):2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 33.Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi F, Jr, Bogun F, Morady F. Radiofrequency Catheter Ablation of Chronic Atrial Fibrillation Guided by Complex Electrograms. Circulation. 2007 May 22;115(20):2606–2612. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]