Abstract
The chemokine CXCL12 and its receptor, CXCR4, regulate neuronal migration, differentiation, and survival. Alterations of CXCL12/CXCR4 signaling are implicated in different neuropathologies, including the neurological complications of HIV infection. Opiates are important co-factors for progression to neuroAIDS and can disrupt the CXCL12/CXCR4 axis in vitro and in vivo. This paper will review recently identified mechanisms of opiate-induced CXCR4 impairment in neurons and introduce results from pilot studies in human brain tissue, which highlight the role of the protein ferritin heavy chain in HIV neuropathology in patients with history of drug abuse.
Keywords: CXCR4, CXCL12, opiates, ferritin heavy chain, neuroAIDS, NR2B
1: Introduction
Human immunodeficiency virus (HIV) continues to profoundly impact public health within the United States and around the world. With over 50,000 new cases of HIV-1 infection reported annually in the United States alone, the prevalence of individuals diagnosed with HIV/AIDS is increasing steadily according to the most recent reports from the Centers for Disease Control and Prevention (CDC, 2009). The social impact of HIV/AIDS disproportionally affects males and overwhelmingly affects minorities, underscoring disparities in risk factors and public health access across the socioeconomic spectrum (CDC, 2009). One such risk factor, illicit opiate abuse through intravenous drug use (IVDU), increases the risk of HIV exposure but also accelerates disease progression and exacerbates neurological involvement (Hu et al., 2005, Khurdayan et al., 2004, Hauser et al., 2006). Given the success of combined antiretroviral therapy (cART) and efforts to broaden treatment to include medically underserved areas, the average life expectancy of HIV positive individuals has been significantly extended (Harrison et al., 2010), revealing the neurological impact of chronic HIV infection on an aging population (Valcour et al., 2004) and highlighting the importance of identifying the molecular mechanisms essential to the ability of opiates to promote neurological dysfunction.
2: HIV neuropathology and Drug abuse
The natural history of HIV infection, coupled with the typically poor central nervous system (CNS) penetration of cART, predispose individuals for CNS complications over time. Following an acute, systemic viremia HIV seeds numerous tissues in the body, including the CNS, allowing it to harbor itself in an immunologically privileged environment and replicate chronically (Ghafouri et al., 2006). The resulting infection within the CNS can remain asymptomatic for years; ultimately leading to neuroAIDS or HIV associated neurological disease (HAND). HAND can be subcategorized into three groups based on the nature and severity of neurological symptoms: asymptomatic neurocognitive impairment (ANI); minor neurocognitive disorder (MND); HIV-associated dementia (HAD) (Antinori et al., 2007).
Approximately 30% of HIV positive individuals, within developed countries, are intravenous (IV) drug users (Donahoe and Vlahov, 1998, Hauser et al., 2005). The molecular mechanisms underlying the neuropathogenesis of HAND are complex and multifaceted, particularly in the context of opiate use (Ghafouri et al., 2006, Hauser et al., 2006). While a substantial body of literature demonstrates the role of glia in promoting neurotoxicity through the release of exitotoxic mediators and secretion of the toxic viral proteins tat (Sabatier et al. 1991; Haughey et al. 1999), gp120 (Bansal et al, 2000; Lipton et al, 1991; Meucci and Miller, 1996) and vpr (Rom et al., 2009, Jones et al., 2007), numerous mechanisms of direct neuronal toxicity and dysregulation have emerged as a significant contributor to neurological impairment. These include alteration of neuronal/glial signaling via chemokine receptors, such as CXCR4 and CCR5, which are not only the primary HIV co-receptors in vivo (Moore et al., 2004) but also regulate fundamental physiological processes in the CNS (Rostene et al., 2007, Miller and Meucci, 1999, Gonzalez-Scarano and Martin-Garcia, 2005, Miller et al., 2008).
The neurological impact of HIV infection can be detected, through functional magnetic resonance imaging, even before neurocognitive symptoms appear (Ernst et al., 2002) and changes that are independent of cell death, such as synaptodendritic injury, can be seen in neurons of HIV patients (Ellis et al., 2007). This suggests that the neuropathology is potentially reversible in its earliest stages. Though HIV neuropathology shares many of the hallmarks of other neuroinflammatory diseases, some important differences appear to exist when the HIV patient also abuses drugs. When compared to non-drug abusing HIV positive individuals, drug abusing HIV-positive subjects show a greater frequency of HIV encephalitis (brain inflammation with leuckocyte infliltration and glial activation) (Bell et al., 2006, Davies et al., 1998), enhanced microglia activation (Arango et al., 2004), giant cell formation (Martinez et al., 1995), and blood brain barrier disruption (Bell et al., 2006). These neuropathological findings suggest that drug abuse, including opiates, target the CNS creating an additive (if not synergistic) effect with HIV. However, due to the inherently heterogeneous nature of drug abusing patient populations, the specific impact of opiate abuse on neuronal function and survival has been difficult to ascertain in vivo. Developing methodologies to untangle the complex network of interactions between host and viral factors, within neurons in vivo, remains an active area of research.
3: Regulation of neuronal CXCR4 by opiates
The chemokines are a family of small soluble proteins, between 8–10 KDa, which signal through G protein coupled receptors (GPCRs) (Ransohoff, 2009). The alpha chemokine CXCL12 and its specific receptor, CXCR4, play essential roles in cellular migration and cell signaling during organogenesis as well as adulthood (Tran and Miller, 2003). CXCL12 is constitutively expressed in the CNS and, during embryogenesis, secreted by mesenchymal cells to guide the migration of CXCR4 expressing cells (Stumm et al., 2007, Tiveron and Cremer, 2008, Tran and Miller, 2003). CXCR4 is widely expressed across brain regions, such as the hippocampus, cerebellum and cortex, and can be found in all major cell types in the brain including neurons, astrocytes and microglia (Li and Ransohoff, 2008, Stumm and Hollt, 2007). A second CXCL12 receptor, CXCR7, has been identified and partially characterized in recent years. However, CXCR7, which also binds the chemokine ITAC, appears to signal primarily in a G-protein independent manner (Rajagopal et al., 2010) and may also act as a CXCL12 scavenger (Boldajipour et al., 2008). The impact of CXCR7 in the CNS, particularly in its ability to modulate the function of CXCR4 in neurons, has yet to be determined. Importantly, studies using CXCL12, CXCR4 or CXCR7 knockout animals have shown the primary role of CXCR4 in mediating the major physiological effects of CXCL12 in the brain (Zou et al., 1998, Lu et al., 2002, Sierro et al., 2007).
Opioid receptors are also GPCRs, classified into three groups (μ, δ, κ) (Waldhoer et al., 2004). Like chemokine receptors, opioid receptors are expressed in the CNS and immune system (Peterson et al., 1998, Patel et al., 2006, Burbassi et al., 2008). The interactions between the opioid and chemokine systems have been intensely studied in the immune system and peripheral nervous system giving rise to our understanding of opiate-induced immune suppression. Specifically, via a mechanism of bi-directional heterologous desensitization, chemokine and opioid receptors can reciprocally influence each others (Law et al., 2000, Wei and Loh, 2002, Cartier et al., 2005, Szabo et al., 2002, Steele et al., 2002). However, the interactions between opioid and chemokine receptors in the CNS is less well established, though recent studies have identified new mechanisms that may account for alteration of neuronal CXCR4 signaling by opiates, as discussed later.
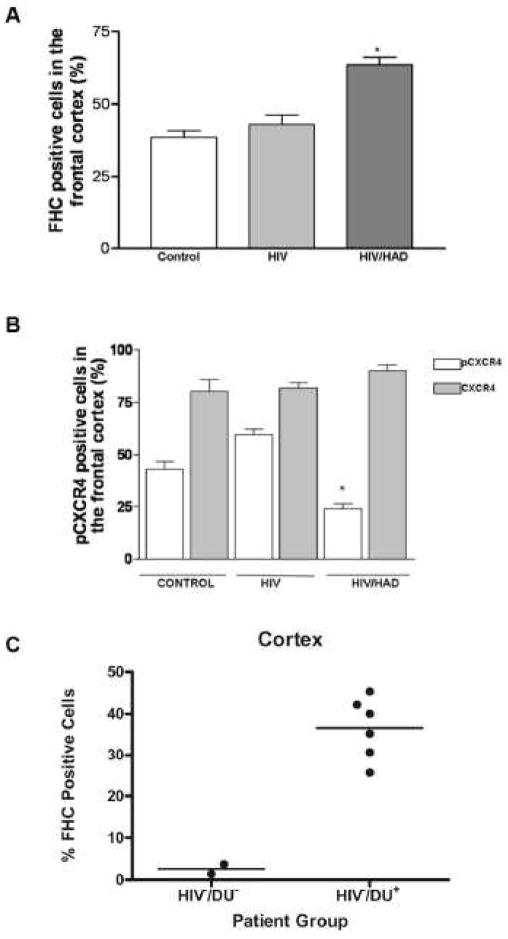
The functions of CXCR4 examined most intensively within neurons concern its ability to promote migration/differentiation of neuronal progenitors and neuronal survival within a toxic environment, as in the case of neuroAIDS. Several data show that stimulation of neuronal CXCR4 by its natural ligand promotes phosphorylation (i.e. activation) of ERK and Akt – thus supporting cell survival and function (Meucci et al., 1998, Khan et al., 2004). Interestingly, our recent studies demonstrate that Mu opioid receptor (MOR) agonists, such as morphine or DAMGO, inhibit CXCL12-induced activation of CXCR4 and stimulation of downstream signaling through ERK and Akt (Sengupta et al, 2009). The opiate-induced inhibition of CXCR4 requires de novo protein synthesis and up-regulation, within neurons, of the protein ferritin heavy chain (FHC) – a negative regulator of CXCR4 (Li et al., 2006). These data show that neuronal levels of FHC are augmented by MOR stimulation both in vitro (i.e. neuronal cultures) and in vivo (i.e. rat brain) leading to CXCR4 impairment (Sengupta et al., 2009). Recent findings in post mortem human brain are consistent with these results (Figure 1). Briefly, formalin fixed, paraffin embedded tissue from the frontal cortex of HIV patients were obtained from the National NeuroAIDS Tissue Consortium (NNTC) and sectioned to 6–10 microns. Patients were groups based on HIV status and documented neurological impairment (Control: HIV negative and no neurological impairment, HIV: HIV positive status with no neurological impairment, HIV/HAD: HIV positive status with significant neurological impairment). Adjacent tissue sections were stained for FHC or CXCR4 as briefly reported in the figure’s legend and previously described (Sengupta et al., 2009, Shimizu et al., 2007). In addition, an antibody that recognizes a ligand-induced phosphorylated form of CXCR4 (Woerner et al., 2005, Sengupta et al., 2009) was used as indication of the receptor activation by CXCL12. The percentage of stain-positive cells per total cells was counted for each subject and averaged within each group. As reported in Figure 1A, an increase in FHC-positive cells was found in the frontal cortex of HIV patients affected by neurological deficits (HIV/HAD). Additionally, the levels of phospho-CXCR4 (pCXCR4) are significantly reduced in patients with a history of HAD, while total levels of CXCR4 within the cortex are unchanged (Figure 1B). Taken together, these preliminary data suggest that FHC is induced in HIV neuropathology and associated with a disruption in CXCR4 signaling.
Figure 1. Ferritin heavy chain (FHC) expression within the human brain impairs CXCR4 activation and is correlated with drug use and HAD.
A) Percentage of FHC+ cells in the frontal cortex of control, HIV, and HIV/HAD patients (n=3/3/4) was determined by IHC (FHC Ab, Santa Cruz 1:50) *p<0.001. B) The same groups of individuals were used to study expression of CXCR4 (12G5, 1:50) and pCXCR4 in adjacent sections, (Ser 339, 1:60) as reported in (Woerner et al., 2005) and (Sengupta et al., 2009); *p<0.001. C) Percentage of FHC+ cells in frontal cortex of control subjects, i.e. HIV negative, no drug use (HIV−/DU−), and HIV negative opiate users (HIV−/DU+) as determined by IHC (FHC Ab, Abcam 1:100; n=2 and 6, respectively, for HIV−/DU− and HIV−/DU+). Each point represents the average of about 10 microscopic fields.
4: Changes of Ferritin Heavy Chain in drug users
Ferritin is a widely expressed iron binding protein important for the sequestration and storage of free iron within cells (Torti and Torti, 2002). Two components, ferritin heavy chain and ferritin light chain, assemble in varying proportions to create the 24 subunit protein. Translation of FHC and FLC mRNA are regulated by iron and cytokines, whereas transcription of the two genes is selectively modulated by hormones and drugs, and during cell differentiation (Torti and Torti, 2002). Within the CNS, FHC can be found in all cell types but is predominately expressed by oligodendrocytes and microglia, whereas FLC is not expressed in neurons (Cheepsunthorn et al., 1998, Connor et al., 1990). While initially used as a sensitive index of systemic iron levels, ferritin has been shown to have abnormal expression with numerous neuroinflammatory conditions including Parkinson’s disease, Alzheimer’s disease, restless leg syndrome and neuroAIDS (Verde Mendez et al., 2003, Deisenhammer et al., 1997, Knovich et al., 2009). Our recent work, examining the role of FHC in opiate abuse and neuroAIDS, has focused on a few different methodological approaches to assess the expression of FHC within the human cortex, in the context of both opiate abuse and neuroAIDS, and its ability to disrupt CXCR4 signaling within cortical neurons in vivo. Initial studies in post mortem human tissue suggest that opiate abuse increases FHC levels within the frontal cortex and that FHC is found within neurons. Specifically, immmunohistochemistry using a FHC specific antibody, showed a marked increase in the percentage of FHC-positive cells within the cortex of patients with a history of drug abuse (Figure 1C). As reported in the figure, patients with a history of drug abuse (mainly opiates) had approximately 10 fold greater FHC expression (~3% in controls vs ~30% in drug use). Interestingly, the increase in FHC expression occurs in multiple cell types, most notably oligodendrocytes and neurons (presented at The 9th Meeting of the International Society of Neurovirology, Pitcher et al., 2009).
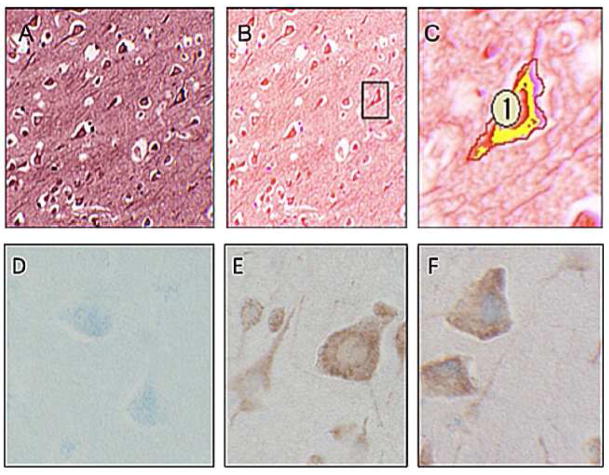
In an effort to better quantify changes in FHC in specific cell types, we have begun to employ an imaging technique (i.e. multispectral microscopy) designed to quantify FHC expression within particular cellular populations. Spectral un-mixing, or color deconvolution, is a digital imaging technique that isolates discrete absorption spectra from multiple overlapping chromogen dyes within a single slide preparation (Mansfield et al., 2008, Levenson, 2008). This method allows to determine the specific co-localization of multiple proteins with greatly enhanced specificity and reduced background, but also the quantification of co-localization and sub-cellular localization. In this context, we are able to stain a single tissue preparation with multiple markers or antibodies, each conjugated to a different chromogen, and determine the degree of co-localization. In this example (Fig 2), human brain tissue was stained with antibodies against the neuronal marker MAP2 and FHC. Using the specific absorption spectrum unique to each chromogen/marker, the FHC conjugated purple chromogen (VIP) and the MAP2 conjugated red chromogen (NovaRed) are isolated to create a digital composite of the first image (Fig. 2B). After identifying a neuron, based on morphology and positive MAP2 staining, it is digitally cropped (labeled as “1”) and the co-localization of both MAP2 and FHC in each pixel is quantified by the Nuance software (CRi, Woburn, MA). A 48% overlap of FHC and MAP2 was observed (Fig 2C). As expected, the area of overlap (indicated by yellow) reveals distinct cytoplasmic localization within the neuron, in line with predicted MAP2 expression; the consequence of this FHC subcellular localization remains to be elucidated. These preliminary data are an important proof of concept demonstrating FHC expression within human neurons and the utility of advanced imaging approaches like multispectral microscopy to study its expression in the diseased brain.
Figure 2. FHC co-localizes with neuronal marker, MAP2, in post mortem human brain tissue.
A) Standard red-green-blue (RGB) photomicrograph of formalin fixed paraffin embedded human brain stained with both neuronal marker (MAP2, Chemicon 1:250, conjugated to NovaRed chromogen, red) and FHC (Abcam 1:50, conjugated to VIP chromogen, purple). B) Un-mixing of chromogen absorbance spectra shows discrete staining pattern of both MAP2 and FHC with co-localization in neurons as indicated in boxed region C) Digital enhancement of boxed region shows co-localization of MAP2 and FHC, indicated by yellow. Co-localization is quantified on a per pixel basis with 48% of MAP2 positive pixels also FHC positive within the cropped region as indicated by the red boundary.
Bottom row of RGB photomicrographs show primary antibody controls for FHC and MAP2. Samples include human cortex stained with FHC (Abcam, 1:50) conjugated to VectorBlue chromogen (VB), blue, and/or MAP2 (Chemicon, 1:250) conjugated to NovaRed (NR) chromogen, red. D) MAP2 negative control: Tissue preparation includes FHC Ab, FHC 2°Ab, VB & MAP2 2°Ab and NR but no MAP2 Ab. E) FHC negative control: Tissue preparation includes FHC 2°Ab, VB & MAP2 Ab, MAP2 2°Ab and NR but no FHC Ab. F) FHC & MAP2 dual stain: Neurons show strong cytoplasmic MAP2 staining with some overlapping of FHC. Tissue preparation includes FHC Ab, FHC 2°Ab, VB & MAP2 Ab, MAP2 2°Ab and NR.
5: Molecular consequences of CXCR4 alteration
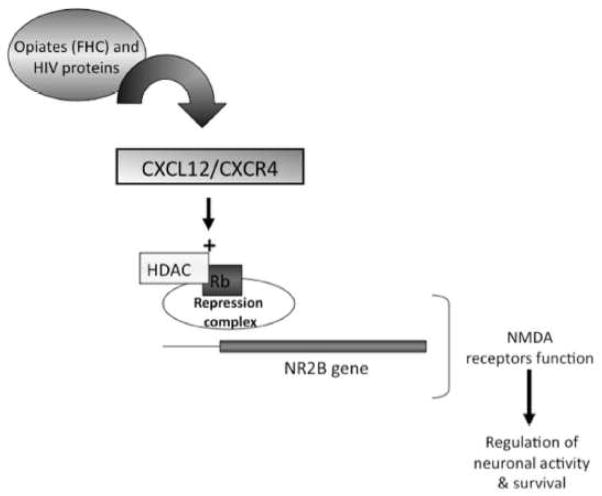
Different studies have shown that CXCR4 can regulate cell cycle proteins in both neuronal and non-neuronal cells (Khan et al., 2005, Khan et al., 2003, Khan et al., 2008, Furukawa, 1998, Cashman et al., 2002, Lataillade et al., 2002, Brandimarti et al., 2004, Castedo et al., 2002). In post-mitotic neurons cell cycle proteins, such as the retinoblastoma gene product Rb and its downstream target E2F1, play crucial roles in neuronal differentiation and survival (Khan et al., 2008, McClellan and Slack, 2007). Rb contributes to the assembly of repressor complexes and inhibits the pro-apoptotic transcription factor E2F1 (Brandimarti et al., 2004, Qiu and Ghosh, 2008). Rb also regulates expression of crucial components of synaptic transmission, such as the NMDA receptor regulatory subunit NR2B (Qiu and Ghosh, 2008). Interestingly, functional loss of Rb is associated with neuronal deficits in vitro and in vivo (Clarke et al., 1992, Jacks et al., 1992, Lee et al., 1992) and several studies, including ours, have shown alteration of the Rb/E2F1 pathway in neuroAIDS (Khan et al., 2008, Jordan-Sciutto et al., 2002, Shimizu et al., 2007) - suggesting a link between altered CXCR4 signaling and neurological deficits. Indeed, CXCL12 is able to elevate Rb RNA and protein in rat cortical neurons and induce Rb-mediated transcriptional repression (Khan et al., 2008). Furthermore, the Rb expression following CXCL12 stimulation is required for the neuroprotective effect of CXCL12 in NMDA neurotoxicity assays (Khan et al., 2008). These data are consistent with previous reports suggesting that a functional CXCL12/CXCR4 axis is required for normal neuronal health (Guyon and Nahon, 2007). In line with these findings, a recent study suggested novel functional consequences due to disruption of CXCR4/Rb signaling, through the modulation of the NMDA receptor (Nicolai et al., 2010). The NMDA receptor, is one of the glutamate receptors critical for normal neuronal function and, therefore, important in physiological neurotransmission as well as excitotoxicity (Burnashev, 1996, Sattler and Tymianski, 2001). The structure of the receptor follows a common paradigm with two classes of subunits, one pair, the NR1 subunits (critical but not susceptible to modulation) and a second pair of subunits, regulatory subunits termed NR2A–D and NR3 A–B. These regulatory subunits are inducible, depending upon multiple factors including extracellular stimulation. The subunit composition of the NMDA receptor directly alters the biophysical properties of the channel and thus, the influx of ions (including calcium) into mature neurons following glutamatergic stimulation. Hence, changes in the subunit composition of the receptor directly affect neuronal function and survival. Our recent data (Nicolai et al., 2010) show that the chemokine CXCL12 modulates expression of the NR2B subunit of the NMDA receptor, which may have different implications on both neuronal signaling and survival. These studies indicate that CXCL12 specifically inhibits neurotoxic signaling via extrasynaptic NR2B-containing NMDA receptor, without impairing physiological activity of the synaptic receptors. Importantly, the mechanism by which this modulation takes place is contingent, at least in part, on epigenetic mechanisms, specifically the regulation of histone deacetylase (HDAC) in response to CXCR4 stimulation (Nicolai et al., 2010). By reducing the acetylation of histone tails in nucleosomes, HDAC favor chromatin condensation and thus transcriptional repression (Kouzarides, 2007). Consequently, the above findings mechanistically link, a stepwise, sequential series of interactions whereby CXCL12 signaling is able to impact neuronal survival and neuroprotection, possibly through its ability to regulate HDAC (Figure 3). Therefore, the CXCL12/CXCR4 signaling axis appears to have the potential for broad, sweeping influences across neuronal pathways and neural networks including cell-to-cell communication and cortical information processing through epigenetic modulation. Ongoing studies in our lab are currently testing these hypotheses.
Figure 3. Model of CXCL12/CXCR4 signaling regulating NR2B expression.
Activation of the CXCL12/CXCR4 axis, and its subsequent regulation of the NMDA receptor subunit NR2B is critical for neuronal activity and survival. This regulation is partially dependent upon the regulation of the gene repressor protein Rb and its recruitment of the chromatin modifying protein, histone deacetylase (HDAC). Additionally, both gp120 and ferritin heavy chain have been shown to disrupt the CXCL12/CXCR4 axis through aberrant signaling via CXCR4 and heterologous desensitization via the μ opioid receptor, respectively. Taken together, these data suggest the neuronal CXCL12/CXCR4 axis as a complex system of regulation influenced by host, viral and epigenetic mechanisms.
6: Conclusions
Neurons are vulnerable to the dysregulation of CXCR4 by viral proteins and other factors, including drug abuse. Both of these factors may contribute to the neuropathology and cognitive decline seen in neuroAIDS. Several lines of studies suggest that opiates can regulate FHC in the brain of HIV patients thus interfering with CXCR4 function. Ongoing studies in our laboratory aim to test this hypothesis in order to establish the role of opiate abuse in neuroAIDS and confirm the involvement of FHC in the modulation of the CXCL12/CXCR4 axis within human neurons. Our current working model, schematically reported in Figure 3, outlines a mechanism whereby the CXCL12/CXCR4 signaling axis is able to promote neuronal function and survival through epigenetic regulation of the NR2B subunit. We propose that disruption of this signaling by gp120 and/or FHC contributes to the neuropathology associated with the opiate using HIV positive population.
Acknowledgments
The authors thank previous and current members of the Meucci Lab for sharing data and for helpful discussion and the NIH for generous support (R01-DA19808 and R01-DA15014 to OM). Jonathan Pitcher is a fellow of the “Interdisciplinary and Translational Research Training in neuroAIDS” (T32-MH078795); thus, this investigation was supported in part by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5T32MH079785. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ANTINORI A, ARENDT G, BECKER JT, BREW BJ, BYRD DA, CHERNER M, CLIFFORD DB, CINQUE P, EPSTEIN LG, GOODKIN K, GISSLEN M, GRANT I, HEATON RK, JOSEPH J, MARDER K, MARRA CM, MCARTHUR JC, NUNN M, PRICE RW, PULLIAM L, ROBERTSON KR, SACKTOR N, VALCOUR V, WOJNA VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARANGO JC, SIMMONDS P, BRETTLE RP, BELL JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18(Suppl 1):S69–74. [PubMed] [Google Scholar]
- BELL JE, ARANGO JC, ANTHONY IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- BOLDAJIPOUR B, MAHABALESHWAR H, KARDASH E, REICHMAN-FRIED M, BLASER H, MININA S, WILSON D, XU Q, RAZ E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- BRANDIMARTI R, KHAN MZ, FATATIS A, MEUCCI O. Regulation of cell cycle proteins by chemokine receptors: A novel pathway in human immunodeficiency virus neuropathogenesis? J Neurovirol. 2004;10(Suppl 1):108–12. doi: 10.1080/753312761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURBASSI S, ALOYO VJ, SIMANSKY KJ, MEUCCI O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3:26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNASHEV N. Calcium permeability of glutamate-gated channels in the central nervous system. Curr Opin Neurobiol. 1996;6:311–7. doi: 10.1016/s0959-4388(96)80113-9. [DOI] [PubMed] [Google Scholar]
- CARTIER L, HARTLEY O, DUBOIS-DAUPHIN M, KRAUSE KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- CASHMAN J, CLARK-LEWIS I, EAVES A, EAVES C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–9. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- CASTEDO M, ROUMIER T, BLANCO J, FERRI KF, BARRETINA J, TINTIGNAC LA, ANDREAU K, PERFETTINI JL, AMENDOLA A, NARDACCI R, LEDUC P, INGBER DE, DRUILLENNEC S, ROQUES B, LEIBOVITCH SA, VILELLA-BACH M, CHEN J, ESTE JA, MODJTAHEDI N, PIACENTINI M, KROEMER G. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 2002;21:4070–80. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC; U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, C. F. D. C. A. P. HIV/AIDS Surveillance Report. Atlanta: 2009. [Google Scholar]
- CHEEPSUNTHORN P, PALMER C, CONNOR JR. Cellular distribution of ferritin subunits in postnatal rat brain. J Comp Neurol. 1998;400:73–86. [PubMed] [Google Scholar]
- CLARKE AR, MAANDAG ER, VAN ROON M, VAN DER LUGT NM, VAN DER VALK M, HOOPER ML, BERNS A, TE RIELE H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–30. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- CONNOR JR, MENZIES SL, ST MARTIN SM, MUFSON EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res. 1990;27:595–611. doi: 10.1002/jnr.490270421. [DOI] [PubMed] [Google Scholar]
- DAVIES J, EVERALL IP, WEICH S, GLASS J, SHARER LR, CHO ES, BELL JE, MAJTENY C, GRAY F, SCARAVILLI F, LANTOS PL. HIV-associated brain pathology: a comparative international study. Neuropathol Appl Neurobiol. 1998;24:118–24. doi: 10.1046/j.1365-2990.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- DEISENHAMMER F, MILLER RF, BRINK NS, HARRISON MJ, THOMPSON EJ. Cerebrospinal fluid ferritin in HIV infected patients with acute neurological episodes. Genitourin Med. 1997;73:181–3. doi: 10.1136/sti.73.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONAHOE RM, VLAHOV D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- ELLIS R, LANGFORD D, MASLIAH E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- ERNST T, CHANG L, JOVICICH J, AMES N, ARNOLD S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–9. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- FURUKAWA Y. Cell cycle regulation of hematopoietic stem cells. Hum Cell. 1998;11:81–92. [PubMed] [Google Scholar]
- GHAFOURI M, AMINI S, KHALILI K, SAWAYA BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-SCARANO F, MARTIN-GARCIA J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- GUYON A, NAHON JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38:365–76. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- HARRISON KM, SONG R, ZHANG X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53:124–30. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- HAUSER KF, EL-HAGE N, BUCH S, BERGER JR, TYOR WR, NATH A, BRUCE-KELLER AJ, KNAPP PE. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSER KF, EL-HAGE N, BUCH S, NATH A, TYOR WR, BRUCE-KELLER AJ, KNAPP PE. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1:98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- HU S, SHENG WS, LOKENSGARD JR, PETERSON PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191:886–9. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- JACKS T, FAZELI A, SCHMITT EM, BRONSON RT, GOODELL MA, WEINBERG RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- JONES GJ, BARSBY NL, COHEN EA, HOLDEN J, HARRIS K, DICKIE P, JHAMANDAS J, POWER C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN-SCIUTTO KL, WANG G, MURPHEY-CORB M, WILEY CA. Cell cycle proteins exhibit altered expression patterns in lentiviral-associated encephalitis. J Neurosci. 2002;22:2185–95. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN MZ, BRANDIMARTI R, MUSSER BJ, RESUE DM, FATATIS A, MEUCCI O. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9:300–14. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN MZ, BRANDIMARTI R, PATEL JP, HUYNH N, WANG J, HUANG Z, FATATIS A, MEUCCI O. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retroviruses. 2004;20:1063–71. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN MZ, BRANDIMARTI R, SHIMIZU S, NICOLAI J, CROWE E, MEUCCI O. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 2008;15:1663–72. doi: 10.1038/cdd.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN MZ, SHIMIZU S, PATEL JP, NELSON A, LE MT, MULLEN-PRZEWORSKI A, BRANDIMARTI R, FATATIS A, MEUCCI O. Regulation of neuronal P53 activity by CXCR 4. Mol Cell Neurosci. 2005;30:58–66. doi: 10.1016/j.mcn.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHURDAYAN VK, BUCH S, EL-HAGE N, LUTZ SE, GOEBEL SM, SINGH IN, KNAPP PE, TURCHAN-CHOLEWO J, NATH A, HAUSER KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–82. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOVICH MA, STOREY JA, COFFMAN LG, TORTI SV, TORTI FM. Ferritin for the clinician. Blood Rev. 2009;23:95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUZARIDES T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- LATAILLADE JJ, CLAY D, BOURIN P, HERODIN F, DUPUY C, JASMIN C, LE BOUSSE-KERDILES MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117–29. doi: 10.1182/blood.v99.4.1117. [DOI] [PubMed] [Google Scholar]
- LAW PY, WONG YH, LOH HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- LEE EY, CHANG CY, HU N, WANG YC, LAI CC, HERRUP K, LEE WH, BRADLEY A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–94. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- LEVENSON R. Putting the “more” back in morphology: spectral imaging and image analysis in the service of pathology. Arch Pathol Lab Med. 2008;132:748–57. doi: 10.5858/2008-132-748-PTMBIM. [DOI] [PubMed] [Google Scholar]
- LI M, RANSOHOFF RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI R, LUO C, MINES M, ZHANG J, FAN GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616–27. doi: 10.1074/jbc.M607266200. [DOI] [PubMed] [Google Scholar]
- LU M, GROVE EA, MILLER RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSFIELD JR, HOYT C, LEVENSON RM. Visualization of microscopy-based spectral imaging data from multi-label tissue sections. Curr Protoc Mol Biol. 2008;Chapter 14(Unit 14):19. doi: 10.1002/0471142727.mb1419s84. [DOI] [PubMed] [Google Scholar]
- MARTINEZ AJ, SELL M, MITROVICS T, STOLTENBURG-DIDINGER G, IGLESIAS-ROZAS JR, GIRALDO-VELASQUEZ MA, GOSZTONYI G, SCHNEIDER V, CERVOS-NAVARRO J. The neuropathology and epidemiology of AIDS. A Berlin experience. A review of 200 cases. Pathol Res Pract. 1995;191:427–43. doi: 10.1016/S0344-0338(11)80730-2. [DOI] [PubMed] [Google Scholar]
- MCCLELLAN KA, SLACK RS. Specific in vivo roles for E2Fs in differentiation and development. Cell Cycle. 2007;6:2917–27. doi: 10.4161/cc.6.23.4997. [DOI] [PubMed] [Google Scholar]
- MEUCCI O, FATATIS A, SIMEN AA, BUSHELL TJ, GRAY PW, MILLER RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER RJ, MEUCCI O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–9. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- MILLER RJ, ROSTENE W, APARTIS E, BANISADR G, BIBER K, MILLIGAN ED, WHITE FA, ZHANG J. Chemokine action in the nervous system. J Neurosci. 2008;28:11792–5. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE JP, KITCHEN SG, PUGACH P, ZACK JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–26. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- NICOLAI J, BURBASSI S, RUBIN J, MEUCCI O. CXCL12 regulates expression of the NMDA receptor’s NR2B subunit through a Histone deacetylase-dependent pathway contributing to neuronal survival. Cell Death and Disease. doi: 10.1038/cddis.2010.10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL JP, SENGUPTA R, BARDI G, KHAN MZ, MULLEN-PRZEWORSKI A, MEUCCI O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON PK, MOLITOR TW, CHAO CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–9. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- PITCHER J, SHIMIZU S, BURBASSI S, SENGUPTA R, MEUCCI O. Role of ferritin heavy chain and opiates in neuroAIDS; The 9th Meeting of the International Society of Neurovirology; June 2–7, 2009; Miami, FL. 2009. [Google Scholar]; Journal of Neurovirology. 2009;15(s1):74. [Google Scholar]
- QIU Z, GHOSH A. A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron. 2008;60:775–87. doi: 10.1016/j.neuron.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJAGOPAL S, KIM J, AHN S, CRAIG S, LAM CM, GERARD NP, GERARD C, LEFKOWITZ RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107:628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANSOHOFF RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–21. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROM I, DESHMANE SL, MUKERJEE R, KHALILI K, AMINI S, SAWAYA BE. HIV-1 Vpr deregulates calcium secretion in neural cells. Brain Res. 2009;1275:81–6. doi: 10.1016/j.brainres.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSTENE W, KITABGI P, PARSADANIANTZ SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- SATTLER R, TYMIANSKI M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–29. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- SENGUPTA R, BURBASSI S, SHIMIZU S, CAPPELLO S, VALLEE RB, RUBIN JB, MEUCCI O. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–44. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU S, KHAN MZ, HIPPENSTEEL RL, PARKAR A, RAGHUPATHI R, MEUCCI O. Role of the transcription factor E2F1 in CXCR4-mediated neurotoxicity and HIV neuropathology. Neurobiol Dis. 2007;25:17–26. doi: 10.1016/j.nbd.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIERRO F, BIBEN C, MARTINEZ-MUNOZ L, MELLADO M, RANSOHOFF RM, LI M, WOEHL B, LEUNG H, GROOM J, BATTEN M, HARVEY RP, MARTINEZ AC, MACKAY CR, MACKAY F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE AD, SZABO I, BEDNAR F, ROGERS TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13:209–22. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- STUMM R, HOLLT V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol. 2007;38:377–82. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- STUMM R, KOLODZIEJ A, SCHULZ S, KOHTZ JD, HOLLT V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 2007;502:382–99. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- SZABO I, CHEN XH, XIN L, ADLER MW, HOWARD OM, OPPENHEIM JJ, ROGERS TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99:10276–81. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIVERON MC, CREMER H. CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol. 2008;18:237–44. doi: 10.1016/j.conb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- TORTI FM, TORTI SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–16. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- TRAN PB, MILLER RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–55. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- VALCOUR V, SHIKUMA C, SHIRAMIZU B, WATTERS M, POFF P, SELNES O, HOLCK P, GROVE J, SACKTOR N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–7. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDE MENDEZ CM, DIAZ-FLORES JF, SANUDO RI, RODRIGUEZ RODRIGUEZ EM, DIAZ ROMERO C. Haematologic parameters in opiate addicts. Nutr Hosp. 2003;18:358–65. [PubMed] [Google Scholar]
- WALDHOER M, BARTLETT SE, WHISTLER JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–90. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- WEI LN, LOH HH. Regulation of opioid receptor expression. Curr Opin Pharmacol. 2002;2:69–75. doi: 10.1016/s1471-4892(01)00123-0. [DOI] [PubMed] [Google Scholar]
- WOERNER BM, WARRINGTON NM, KUNG AL, PERRY A, RUBIN JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–9. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- ZOU YR, KOTTMANN AH, KURODA M, TANIUCHI I, LITTMAN DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]