Summary
Background
In response to stress- or tissue damage-induced apoptosis, unaffected epithelial cells undergo compensatory proliferation to maintain the integrity of the epithelium. Proximal signals regulating this response are not fully appreciated, but JNK activity appears to be critical for both apoptosis and compensatory proliferation. Since disruption of epithelial cell apical-basal polarity, as can occur in early cancer development and is correlated with increased proliferation by means not fully characterized, we considered whether disruption of the various polarity complexes could provide signals identifying damaged epithelial cells, and thus lead to apoptosis-induced compensatory proliferation.
Results
We identify the Cdc42/Par6/aPKC Par polarity complex as uniquely and specifically regulating apoptosis-induced compensatory proliferation in Drosophila epithelia. Genetic depletion of individual components or disruption of complex formation and localization, but not other polarity complexes, induces JNK-dependent apoptosis and JNK-dependent compensatory proliferation following radiation injury. When apoptosis execution is blocked, by P35 expression, Cdc42/Par6/aPKC depleted tissues uniquely hyperproliferate leading to tissue/organ overgrowth. Disruption of Cdc42/Par6/aPKC leads to activation of JNK through increased Rho1-Rok activity, and Rok’s capacity to activate Myosin, but not F-actin.
Conclusions
We show that the Cdc42/Par6/aPKC polarity complex influences both a physiologic compensatory proliferation response after irradiation injury as well as a contrived compensatory non-cell autonomous hyperproliferation response when cell autonomous apoptosis, resulting from Cdc42/Par6/aPKC disruption, is inhibited. These results suggest the possibility that in cancer where apoptotic regulation is disrupted, loss of the Cdc42/Par6/aPKC polarity complex organization or localization could contribute to tumor hyperproliferation and explain how polarity disruption contributes to tumor development.
Keywords: Cdc42, apoptosis, compensatory proliferation, polarity regulation, epithelia
Introduction
Epithelial cells exhibit apical-basal polarity and major insight into proteins involved in epithelial apical-basal polarity establishment, maintenance, and function has been gained from studies of Drosophila epithelia during morphogenesis [1]. Three protein complexes have emerged as key regulators in establishing and maintaining epithelial polarity. These include the Cdc42/Par6/Par3/aPKC containing Par polarity complex, the Scribble (Scrib)/Discs large (Dlg)/Lethal giant larvae (Lgl) containing Scribble polarity complex, and the Crumbs/Pals/PatJ containing Crumbs polarity complex. While these three complexes all function in epithelial polarity, they do so by different mechanisms, and likely also exhibit unique functional properties in epithelia [2].
Maintenance of epithelial apical-basal polarity is not only critical for epithelial cell function, but loss of epithelial polarity contributes to carcinoma development [3]. Loss of epithelial polarity markers is associated with early stage tumors before metastasis [4, 5], and studies in Drosophila and mammalian systems have demonstrated that disruption of polarity complexes often results in increased epithelial proliferation [1, 6]. Despite these observations, how epithelial polarity regulation is coupled to proliferation is not well understood.
During development and adult homeostasis, epithelia replenish those cells that are damaged and shed during normal physiological conditions. When epithelia are exposed to insults, either environmental or genetic, that lead to increased cell death epithelia have a remarkable capacity to compensate for this cell loss. For example, Drosophila larval imaginal discs, a monolayer epithelial tissues, subjected to irradiation or tissue ablation causing loss of up to 60% of cells from the tissue compensate by stimulating proliferation of surrounding cells resulting in the development of normal sized adult tissue [7]. In the mouse intestine, loss of Mdm2 or Mdm4 induces p53-mediated cell death, but compensatory, increased proliferation helps maintain intestinal morphology and function [8, 9]. A similar phenomenon has recently been demonstrated to also occur in regeneration of hydra [10].
The ability of epithelial tissues to compensate for cell loss resulting from physical damage, irradiation, or genetically induced apoptosis has been termed apoptosis-induced compensatory proliferation [11]. In general, this model states that when apoptosis is initiated in epithelial cells these dying cells secrete morphogens to promote proliferation of the surrounding cells, which leads to replacement of the dying cells and maintenance of tissue size. In proliferating epithelial cells, activation of the pro-apoptotic genes Reaper and Hid lead to degradation of the apoptosis inhibitor Diap1, thereby releasing the initiator caspase Dronc and ultimately upregulation and secretion of the morphogens Decapentaplegic (Dpp; Drosophila TGF-β) and Wingless (Wg; Drosophila Wnt) via JNK and, or p53 [12, 13]. How Dronc activity leads to JNK activation is not clear, and recently, the requirement for Dpp and Wg secreted by apoptotic cells for compensatory proliferation has been questioned [14]. While stress- or injury-induced apoptosis initiates compensatory proliferation, developmentally regulated apoptosis that occurs during normal development does not; suggesting that activation of apoptosis alone is not sufficient for compensatory proliferation. Why this difference exists and how epithelial cells undergoing apoptosis signal to initiate compensatory proliferation in response to cellular stress and tissue damage is not clear.
Here we demonstrate that Cdc42, the Rho family GTPase important for polarity responses in forming epithelia, is a critical and novel negative regulator of apoptosis-induced compensatory proliferation. It does so by properly assembling and localizing the Par polarity complex. When the Cdc42/Par6/aPKC complex is depleted or mislocalized from adherens junctions, dying cells activate Rho1/Rok, which activates Myosin. This Rho1/Rok/Myosin cascade is required for JNK activation, leading to compensatory proliferation, independent of Rho1/Rok’s effects upon actin dynamics. Therefore, loss of epithelial cell polarity, as a result of cellular damage or during cancer development, provides an upstream signal for cells to undergo compensatory proliferation. These results also suggest that in cancer states where apoptotic regulation is disrupted, loss of the Cdc42/Par6/aPKC polarity complex organization or localization could contribute to tumor hyperproliferation and explain how polarity disruption contributes to tumor development.
Results
Cdc42 and aPKC depletion promote compensatory proliferation following irradiation-induced epithelial damage
Drosophila larval wing imaginal discs undergo apoptosis induced compensatory proliferation following tissue injury, and while JNK activity is critical for this response, precisely how JNK is regulated and leads to compensatory proliferation is unclear. One possibility is that injury-induced disruption of polarity complexes, whose correct membrane localization and activity are associated with properly organized epithelial cells, could provide signals identifying abnormal cells to be deleted. To test this possibility, we determined whether genetic depletion of central components from each of the three polarity complexes regulated compensatory proliferation of Drosophila larval imaginal discs, a proliferating epithelial monolayer, in response to irradiation [7]. Exposure of wild type larvae imaginal discs to irradiation causes cell cycle arrest allowing for DNA damage repair. Following apoptosis of cells with irreparable DNA damage, the remaining, unaffected imaginal disc cells undergo compensatory proliferation about 8 hours after irradiation exposure [15].
We genetically depleted Discs Large (Dlg) of the Scribble complex, Crumbs (Crb) of the Crumbs complex, or Cdc42 of the Par complex in the posterior compartment of wings discs by expressing respective RNAi’s with engrailed-gal4 (en-gal4) and analyzed the compensatory proliferation response in the posterior compartment compared to internally controlled, wild type anterior compartment 6 hours after irradiation, a time when compensatory proliferation is beginning [15]. Depletion of Discs large (Dlg) or Crumbs (Crb) did not promote compensatory proliferation (Figure 1B, 1C), despite efficient knockdown of respective protein levels (Figure S1A, S1E) and despite Dlg depletion activating JNK and inducing apoptosis (Figure S1A and S1B).
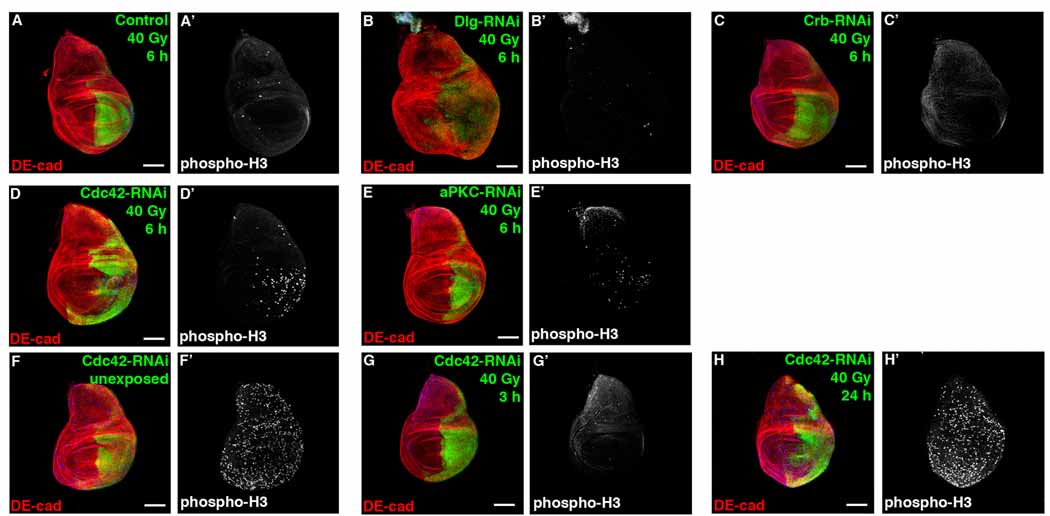
Figure 1. Cdc42/aPKC depletion accelerates compensatory proliferation following irradiation.
Confocal immunofluorescent localization of DE-cadherin (DE-cad) (A–H) and phospho-Histone H3 (phospho-H3) (A’–H’) 6 hours after exposure to 40 Gy of irradiation, in larval wing discs expressing GFP alone (A, A’), or co-expressing GFP with Dlg-RNAi (B, B’), Crb-RNAi (C, C’), Cdc42-RNAi (D, D’), and aPKC-RNAi (E, E’) using en-gal4. These images are representative of 24 wing discs (A), 12 wing discs (B), 10 wing discs (C), 17 wing discs (D), and 9 wing discs (E) analyzed. Larval wing discs co-expressing GFP and Cdc42-RNAi using en-gal4 unexposed to irradiation (F, F’), and 3 hours (G, G’) or 24 hours (H, H’) after exposure to 40 Gy of irradiation. These images are representative of 12 wing discs (F), 12 wing discs (G), and 11 wing discs (H) analyzed. Scale bars represent 100 µm (A–H) and 10 µm (I, L).
In stark contrast, wing imaginal disc cells depleted of Cdc42 exhibited increased proliferation following irradiation (Figure 1D). Depletion of Cdc42 in wing discs did not significantly alter proliferation before irradiation damage or 24 hours after irradiation and did not block the initial cell cycle arrest following irradiation (Figure 1F–H), suggesting that depletion of Cdc42 accelerated the compensatory proliferation response following irradiation. Genetic depletion of aPKC, another component of the Cdc42/Par6/aPKC polarity complex, also appeared to promote compensatory proliferation following irradiation (Figure 1E), but to an extent less than observed following Cdc42 depletion, indicating that the Cdc42/Par6/aPKC/Par3 polarity complex uniquely functions to negatively regulate apoptosis-induced compensatory proliferation following radiation induced cell damage.
The Cdc42/Par6/aPKC polarity complex regulates tissue overgrowth when cell death is inhibited
To determine how disruption of the Cdc42/Par6/aPKC/Par3 polarity complex in dying cells induced compensatory proliferation, we employed a well-utilized, genetically defined system in Drosophila [12, 15, 16]. The caspase inhibitor P35 [17], when expressed in apoptotic cells, blocks effector caspase execution of cell death. Sustained signals from these “undead” cells exaggerate the compensatory proliferation response leading to hyperproliferation or overgrowth of epithelial tissues. If depletion/disruption of the Cdc42/Par6/aPKC polarity complex promotes apoptosis-induced compensatory proliferation, then this should lead to apoptosis, and blocking apoptosis with P35 expression should result in tissue overgrowth.
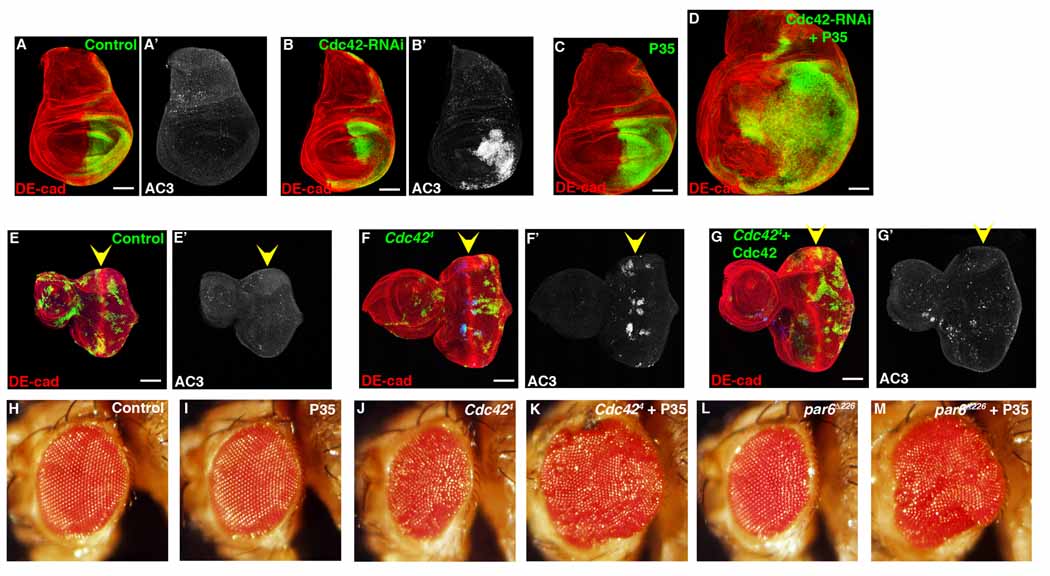
Depletion of Cdc42 (Cdc42 RNAi or Cdc424 LOF allele) in both wing and eye imaginal discs resulted in cell autonomous apoptosis (Figure 2A, 2B, 2E, 2F). When apoptosis was blocked in these clones, by expressing P35, expansion and enlargement of the imaginal discs as a whole (i.e., non-cell autonomous) was observed (Figure 2D). In controls, expression of P35 alone had little effect on larval wing epithelium (Figure 2C), and importantly, expression of wild type Cdc42 within Cdc424 clones rescued apoptosis (Figure 2G). Finally adult eyes containing Cdc424 clones and P35 were overgrown (Figure 2H–K). These same results were also seen with Par6 and aPKC LOF clones expressing P35 (Figure 2M, and data not shown).
Figure 2. Depletion of Cdc42 causes apoptosis. Blocking apoptosis in Cdc42-depleted cells results in overgrowth.
Confocal immunofluorescent localization of DE-cadherin (A, B) and activated Caspase 3 (AC3) (A’, B’) in control (A) and en>Cdc42-RNAi (B) larval wing discs. Confocal immunofluorescent localization of DE-cadherin (C, D) in larval wing discs expressing P35 (C) and P35 with Cdc42-RNAi (D) with en-gal. Confocal immunofluorescent localization of DE-cadherin (E–G) and activated Caspase 3 (E’–G’) in GFP-labeled control clones (E), Cdc424 clones (F), and Cdc424 clones expressing wild type Cdc42 (G) in larval eye discs. Arrowheads identify the morphogenetic furrow. Adult eyes resulting from generation of control clones (H), clones expressing P35 (I), Cdc424 clones (J), Cdc424 clones expressing P35 (K), par6Δ226 clones (L), par6Δ226 clones expressing P35 (M). Scale bars represent 100 µm.
Promotion of compensatory proliferation following irradiation and tissue overgrowth when apoptosis is blocked was specific to depletion of the Cdc42/Par6/aPKC polarity complex and not simply due to induction of apoptosis by loss of polarity control or activation of JNK. Following irradiation of Cdc42 or Dlg depleted imaginal discs, apoptosis was apparent in both (Figure S1C, S1D), however only Cdc42, not Dlg depletion, accelerated compensatory proliferation (Figure 1D vs 1B). In larval eye imaginal discs, LOF clones of Cdc42, Dlg or Scrib all underwent apoptosis (Figure 2F, S2A) [18–20]; however, when P35 was co-expressed, tissue overgrowth was only apparent in Cdc42 depleted tissue (Figure 2K and S2C, S2D) [19, 20]. In addition, several other genetic manipulations (e.g., depletion of Rho1 or slingshot (Cofilin phosphatase) result in apoptosis but not tissue overgrowth when apoptosis was blocked (Figure 6B, S2E, S2F), further supporting that apoptosis induction alone was not sufficient to promote compensatory proliferation. Taken together, these experiments indicated that the Cdc42/Par6/aPKC polarity complex was a specific and novel regulator of apoptosis-induced compensatory proliferation, and that compensatory proliferation was not simply a response to apoptosis induction.
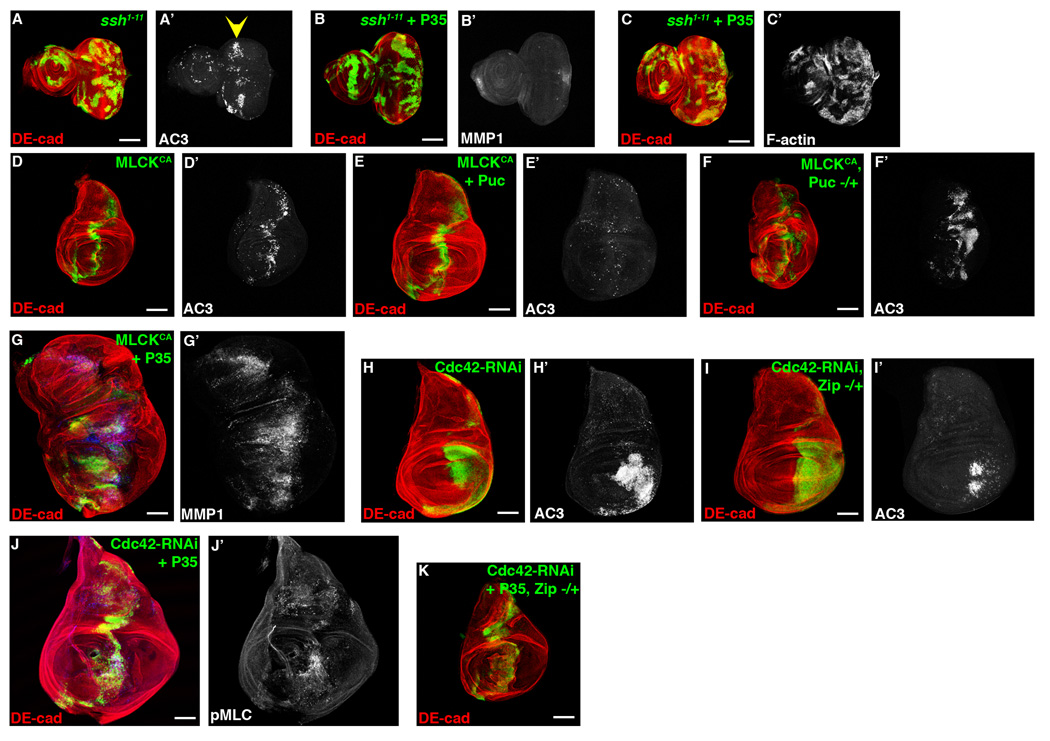
Figure 6. Activation of Myosin, but not increased F-actin, induces overgrowth downstream of Cdc42 depletion.
Confocal immunofluorescent localization of DE-cadherin (A–C), activated Caspase 3 (A’) and MMP1 (B’), and phalloidin staining (C’) in larval eye discs with GFP-labeled ssh1–11 clones alone (A) and ssh1–11 clones expressing P35 (B, C). Confocal immunofluorescent localization of DE-cadherin (D–G), activated Caspase 3 (D’–F’), and MMP1 (G’) in larval wing discs expressing constitutively active MLCK (MLCKCA) alone (D), MLCKCA with Puc (E), MLCKCA in a heterozygous pucE69 background (F), and MLCKCA with P35 (G) using ptc-gal4. Confocal immunofluorescent localization of DE-cadherin (H, I) and activated Caspase 3 (H’, I’) in larval wing discs expressing Cdc42-RNAi alone (H) and Cdc42-RNAi in a Zip1 heterozygous background (I) using en-gal4. Confocal immunofluorescent localization of DE-cadherin (J, K) and phospho-MLC (J’) in larval wing discs expressing Cdc42-RNAi with P35 (J) and Cdc42-RNAi with P35 in a Zip1 heterozygous background (K) using ptc-gal4. Scale bars represent 100 µm.
Cdc42/Par6/aPKC polarity complex depletion increases JNK activity
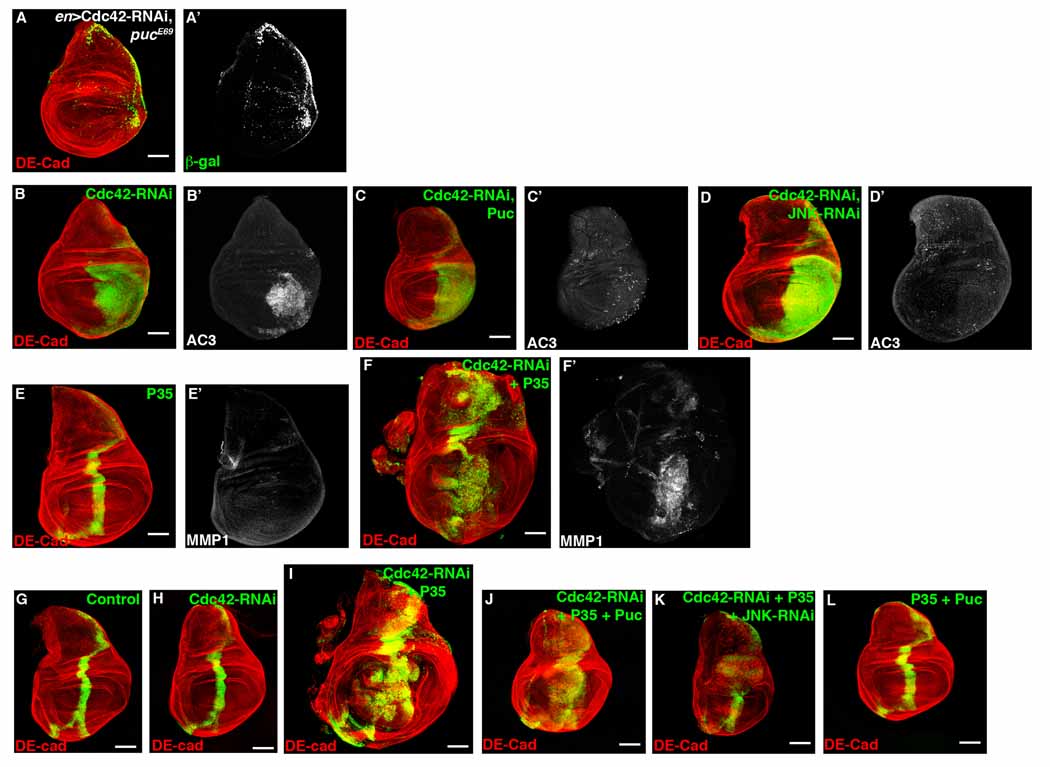
A key component in the apoptosis-induced compensatory proliferation pathway is JNK [12, 14]. Therefore, we asked whether disruption of the Cdc42/Par6/aPKC polarity complex influenced JNK activity. We expressed Cdc42-RNAi in larval wing imaginal discs with en-gal4 in the background of a puckered-lacZ transcriptional reporter (pucE69). Since puckered (puc) is a transcriptional target regulated indirectly by JNK, lacZ expression can be considered a functional readout of JNK activity [21]. En>Cdc42-RNAi increased transcription of puc (Figure 3A). Cdc42 and Par6 LOF clones also resulted in increased puc-lacZ expression in larval eye discs (Figure S3A, data not shown).
Figure 3. Cdc42 negatively regulates JNK activity to influence compensatory proliferation.
Confocal immunofluorescent localization of DE-cadherin (A) and β-galactosidase (A’) in larval wing disc expressing Cdc42-RNAi with en-gal4 in a heterozygous background of pucE69 (puc-lacZ). Confocal immunofluorescent localization of DE-cadherin (B–D) and activated Caspase 3 (B’–D’) in larval wing discs expressing Cdc42-RNAi alone (B), with Puc overexpression (C), and with JNK-RNAi expression (D) using en-gal4. Confocal immunofluorescent localization of DE-cadherin (E–F) and MMP1 (E’–F’) in larval wing discs expressing P35 alone (E) and Cdc42-RNAi with P35 (F) using ptc-gal4. Confocal immunofluorescent localization of DE-cadherin (G–L) in larval wing discs GFP alone (G), Cdc42-RNAi (H), Cdc42-RNAi with P35 (I), Cdc42-RNAi with P35 and Puc (J), Cdc42-RNAi with P35 and JNK-RNAi (K), and P35 with Puc (L) using ptc-gal4.
Next, we determined if increased JNK activation, as a result of Cdc42/Par6/aPKC polarity complex depletion, was responsible for the ensuing apoptosis. Blocking JNK activity by co-expressing Cdc42-RNAi with the JNK phosphatase Puckered (Puc) using en-gal4 substantially rescued apoptosis seen in en>Cdc42-RNAi alone (Figure 3C). Moreover, depletion of JNK itself with JNK-RNAi rescued apoptosis from en>Cdc42-RNAi (Figure 3D). These data indicated that Cdc42/Par6/aPKC polarity complex depletion resulted in apoptosis through activation of JNK.
Does the tissue overgrowth that resulted by blocking apoptosis with P35 in Cdc42/Par6/aPKC polarity complex depleted cells involve JNK activity? MMP1 is another transcriptional target downstream of JNK [22], therefore, increased MMP1 proteins levels indirectly reflect increased JNK activity. Whereas P35 expression alone did not affect MMP1 levels (Figure 3E, S3B), MMP1 protein levels were increased in imaginal disc cells expressing P35 and depleted of Cdc42, Par6, or aPKC (Figure 3F, S3C–E), consistent with increased JNK activity.
To determine if increased JNK activity in cells depleted of Cdc42/Par6/aPKC polarity complex components was responsible for hyperproliferation we inhibited JNK activity by overexpressing Puc or depleting JNK, in cells expressing Cdc42-RNAi and P35. To quantify tissue overgrowth and control for asynchronous development or pupation delay that can occur when genetically manipulating imaginal disc proliferation, the area of GFP-positive cells in a ptc expression domain was divided by the total wing disc area [14]. Because “undead” cells hyperproliferate in response to secreted morphogens from neighboring cells [14], hyperproliferation response should result in an increased area of GFP-positive, “undead” cells in the ptc domain relative to the whole wing disc area. Compared to ptc>Cdc42-RNAi + P35 wing discs (enlarged ptc expression domain), ptc>Cdc42-RNAi + P35 + Puc and ptc>Cdc42-RNAi + P35 + JNK-RNAi wing discs had ptc expression domains that were smaller relative to the whole wing disc (Figure 3G–K and Table S1). As controls, ptc>P35 + Puc and ptc>P35 + JNK-RNAi wing discs had ptc expression domains similar to wild type wing discs (Figure 3L, data not shown).
Components of the apoptotic pathway have been implicated with JNK in regulating apoptosis-induced compensatory proliferation [12, 15, 16]. Therefore we determined whether two upstream components of the apoptotic pathway, Reaper and Diap1, could influence the apoptosis-induced compensatory proliferation following Cdc42 depletion. Both LOF and overexpression analysis with Reaper and Diap1 suggested that these components did not significantly contribute to the compensatory proliferation initiated following Cdc42 depletion and P35 expression (Table S1).
The secreted morphogens Dpp and Wg, the Drosophila homologues of TGF-β and Wnt, respectively, have been demonstrated to be upregulated downstream of JNK in the apoptosis-induced compensatory proliferation response in proliferating epithelium [12, 14]. Wg protein level was increased in cells depleted of Cdc42 and expressing P35 (Figure S3F), as well as evidence of increased phospho-MAD levels (Figure S3G, S3H), indicative of increased Dpp signaling.
Cdc42, Par6, and aPKC regulate tissue overgrowth as a complex
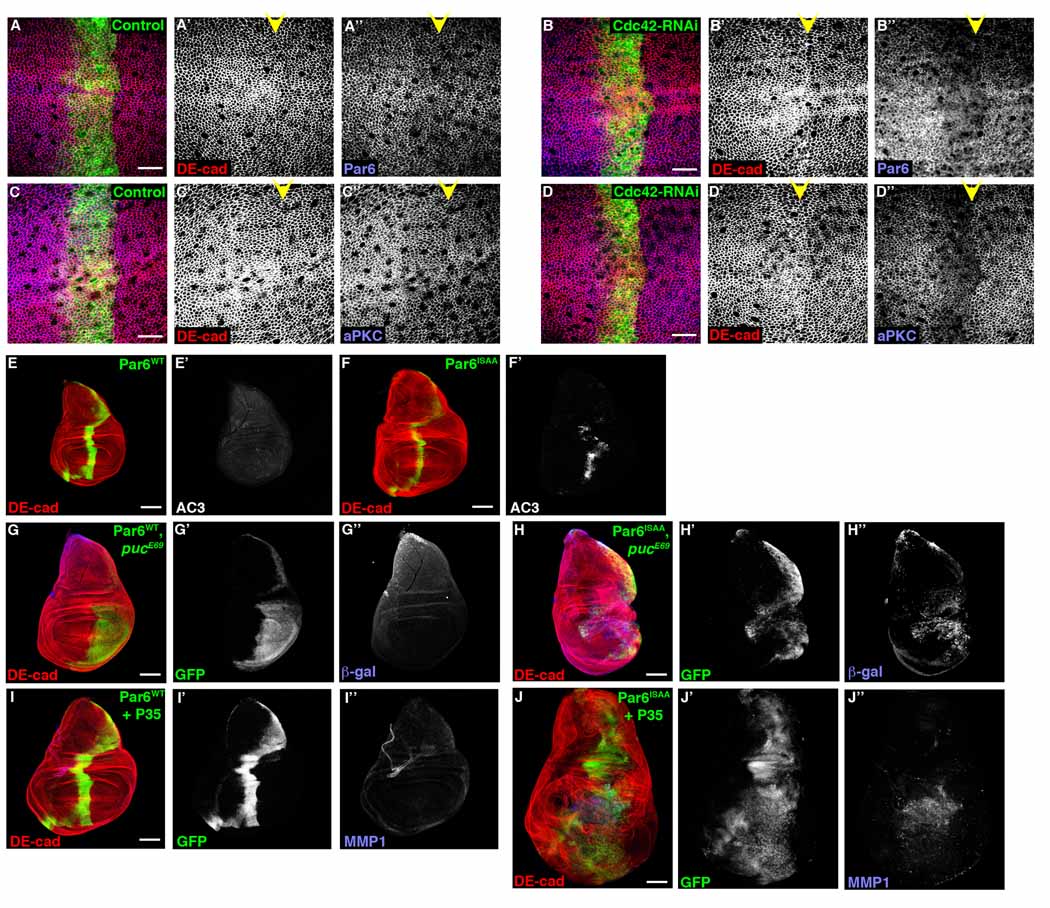
Having demonstrated that Cdc42, Par6, and aPKC can function individually to regulate hyperproliferation of “undead” cells, we asked whether assembly of the Cdc42/Par6/aPKC complex was important for this function. Par6 binds to active Cdc42 thereby recruiting Par3 (Baz) and aPKC to the complex [23]. Par6 and aPKC, but not Baz, levels at cell junctions in larval wing imaginal disc cells expressing Cdc42-RNAi was reduced (Figure 4A–D, S4C), suggesting that junctional mislocalization of Par6/aPKC may be critical for Cdc42-mediated regulation of compensatory proliferation and hyperproliferation of “undead” cells. When a Cdc42 binding mutant of Par6 (Par6ISAA) was expressed in larval wing imaginal discs, using ptc-gal4, similar to LOF analysis with Cdc42, Par6, and aPKC, apoptosis resulted (Figure 4F), activation of JNK was evident (Figure 4G), and aPKC level at AJs was decreased (Figure S4B). When Par6ISAA was co-expressed with P35, tissue overgrowth and upregulation of MMP1 resulted (Figure 4J). In contrast, overexpression of wt Par6 did not induce apoptosis (Figure 4E), activate JNK (Figure 4G), alter junctional aPKC level (Figure S4A), or result in tissue overgrowth following P35 expression (Figure 4I). These data suggested that the assembly of the Cdc42/Par6/aPKC complex, at cell junctions, was critical for regulating JNK activity and hyperproliferation of “undead” cells.
Figure 4. Cdc42, Par6, and aPKC function as a complex to regulate tissue overgrowth.
Confocal immunofluorescent localization of DE-cadherin (A–D, A’–D’), Par6 (A, A’’, B, B’’), and aPKC (C, C’’, D, D’’) in larval wing disc expressing GFP alone (A, C) or Cdc42-RNAi (B, D) with ptc-gal4. Confocal immunofluorescent localization of DE-cadherin (E–J), activated Caspase 3 (E’, F’), β-galactosidase (G’’, H’’), and MMP1 (I’’, J’’) in larval wing discs expressing either wild type Par6 (Par6WT) (E, G, I) or Cdc42 binding mutant Par6 (Par6ISAA) (F, H, J) alone (E, F), in a pucE69 heterozygous background (G, H), and with P35 (I, J) using ptc-gal4 (E, F, I, J) and en-gal4 (G, H). Scale bars represent 10 µm (A–D) and 100 µm (E–J).
Cdc42 depletion-induced tissue overgrowth requires Rho1-Rok activation
Members of the Rho GTPase family can affect JNK activity [24, 25]. Specifically RhoA-ROCK activate JNK in mammalian cells [26], but precisely how is not known [26]. Other work has identified opposing crosstalk between Cdc42 and Rho1 activity in the regulation of post-mitotic Drosophila epithelial morphogenesis [27, 28]. Specifically, depletion of Cdc42 and mislocalization of Par6 and aPKC results in increased Rho1 activity at AJs [27]. This raised the possibility that depletion of Cdc42 regulates JNK and apoptosis-induced compensatory proliferation in proliferating larval epithelia through crosstalk with Rho1 signaling.
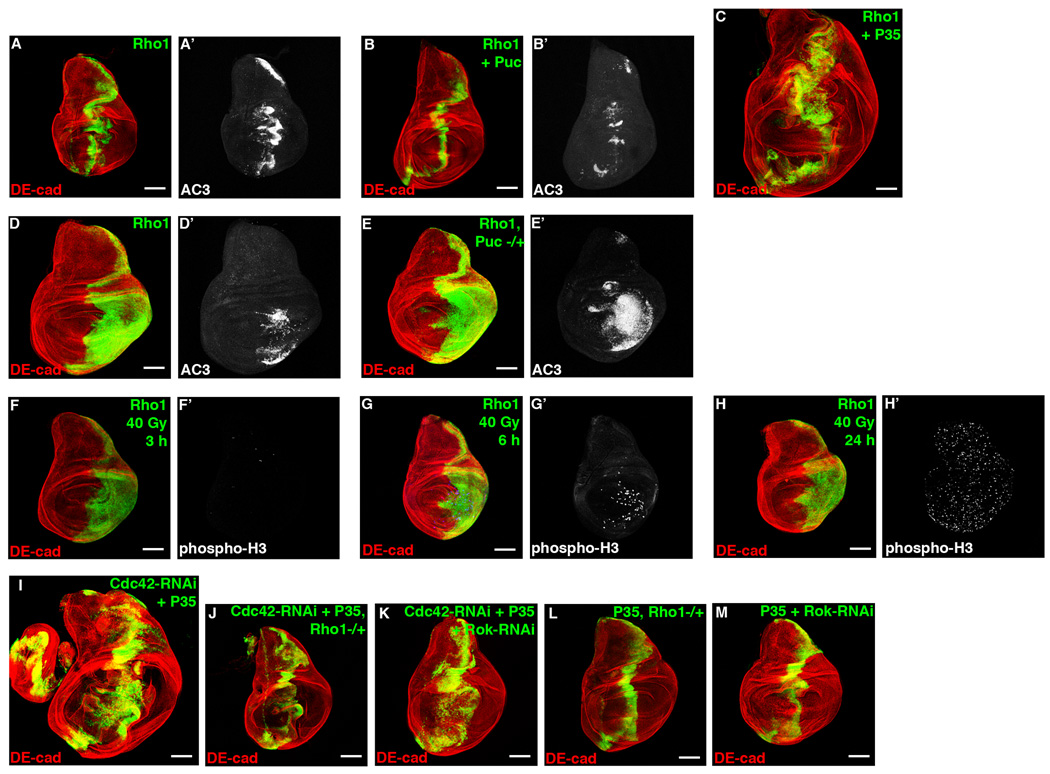
We first determined if increased Rho1 could induce apoptosis in larval wing discs. Similar to Cdc42-RNAi expression, overexpression of Rho1 induced ectopic apoptosis (Figure 5A, 5D), which was inhibited by Puc (Figure 5B), and enhanced by removing a genomic copy of puc (Figure 5E), indicating that it too was JNK dependent. When Rho1 and P35 were co-expressed, hyperproliferation resulted (Figure 5C). Following irradiation damage, overexpression of Rho1 accelerated compensatory proliferation (Figure 5F–5H). These data indicated that increased Rho1 expression induced JNK-dependent apoptosis and compensatory proliferation. Consistent with data from mammalian systems, expression of an active form of Rok (Rok-CAT) also induced apoptosis (Figure S5A), and when Rok-CAT and P35 were co-expressed, hyperproliferation and upregulation of MMP1 resulted (Figure S5B), indicative of increased JNK activity.
Figure 5. Rho1 promotes compensatory proliferation downstream of Cdc42 depletion.
Confocal immunofluorescent localization of DE-cadherin (A–C) and activated Caspase 3 (A’, B’) in larval wing discs overexpressing Rho1 (A), Rho1 and Puc (B), and Rho1 and P35 (C) using ptc-gal4. Confocal immunofluorescent localization of DE-cadherin (D, E) and activated Caspase 3 (D’, E’) in larval wing discs overexpressing Rho1 (D) and overexpressing Rho1 in a pucE69 heterzygous background (E) using en-gal4. Confocal immunofluorescent localization of DE-cadherin (F–H) and phospho-Histone H3 (F’–H’) in larval wing discs overexpressing Rho1 using en-gal4 3 hours (F), 6 hours (G), and 24 hours (H) after exposure to 40 Gy of irradiation. Confocal immunofluorescent localization of DE-cadherin in larval wing discs expressing Cdc42-RNAi with P35 (I), Cdc42-RNAi with P35 in a Rho172F heterozygous background (J), Cdc42-RNAi with P35 and Rok-RNAi (K), P35 in a Rho172F heterozygous background (L), and Rok-RNAi with P35 (M) using ptc-gal4. Scale bars represent 100 µm.
Having demonstrated that increased Rho1-Rok signaling activated JNK and compensatory proliferation, we asked whether the hyperproliferation response in ptc>Cdc42-RNAi + P35 tissue depended on Rho1-Rok activity. We depleted Rho1 or Rok from ptc>Cdc42-RNAi + P35 cells by removing either a genomic copy of Rho1 or expressing Rok-RNAi. By either approach, the hyperproliferation response due to expression of Cdc42-RNAi and P35 was attenuated (Figure 5I–K, Table S1). As controls, wing discs depleted of Rho1 or Rok and co-expressing P35 alone did not affect the ptc expression domain size (Figure 5L and 5M).
Rho1/Rok-induced Myosin activity controls JNK mediated hyperproliferation
How then does Rok activate JNK in proliferating epithelium? Two main functions downstream of Rok are to promote F-actin assembly by inhibiting Cofilin activity and to activate Myosin through direct or indirect phosphorylation of the Myosin Light Chain (MLC). Slingshot (Ssh) is a cofilin phosphatase that promotes Cofilin’s F-actin severing activity [29]. We used Ssh LOF analysis to inhibit Cofilin and promote F-actin assembly, similar to what can occur downstream of Rok. Larval eye imaginal disc clones with ssh1–11 resulted in apoptosis near the morphogenetic furrow, similar to Cdc42 depletion (Figure 6A). However, when P35 was expressed in ssh1–11 clones, no hyperproliferation or upregulation of MMP1 occurred (Figure 6B). As a control, F-actin assembly was clearly increased in these clones (Figure 6C). Overexpression of Serum Response Factor (SRF), a transcription factor regulated by levels of polymerized and monomeric actin downstream of Cofilin [30], also did not cause hyperproliferation when co-expressed with P35 in larval wing imaginal discs (data not shown). These results suggested that Rok did not regulate JNK and hyperproliferation through F-actin regulation.
When a constitutively active form of Myosin Light Chain Kinase was expressed in larval wing imaginal discs, apoptosis ensued (Figure 6D), like Cdc42 depletion or Rho1-Rok activation. This apoptosis was attenuated by blocking JNK activity with Puc overexpression (Figure 6E) and enhanced by activating JNK through removing a genomic copy of puc (Figure 6F). In contrast to actin manipulation, blocking MLCK-CA-induced apoptosis with P35, led to hyperproliferation and upregulation of MMP1 (Figure 6G). This indicated that activation of Myosin, a known downstream target of Rok, is sufficient to induce JNK-dependent apoptosis and hyperproliferation of undead cells.
To address whether increased Myosin activity was associated with hyperproliferation of undead cells larval wing imaginal disc epithelial cells co-expressing Cdc42-RNAi and P35 were found to exhibit increased levels of phospho-MLC (Figure 6J). Phospho-MLC levels were not affected by depletion of Dlg or Scrib (Figure S6A, data not shown). To determine if Myosin activity was necessary to induce JNK-dependent apoptosis and hyperproliferation of undead cells downstream of Cdc42 depletion, we reduced Myosin levels by removing a genomic copy of Zipper (Zip, Drosophila Myosin) in the context of Cdc42 depletion. Reducing Zip attenuated both apoptosis from Cdc42-RNAi expression (Figure 6H and 6I) and hyperproliferation from co-expression of Cdc42-RNAi with P35 (Figure 6K, Table S1). As a control, wing discs with Zip reduction and P35 expression alone had ptc expression domains similar in size to wild type wing discs (data not shown). These data indicated that Cdc42 depletion activated JNK in proliferating epithelial cells via increased Myosin activation downstream of Rho1/Rok signaling, independent of Rho1/Rok effects upon actin assembly.
Discussion
In proliferating epithelia, disruption of the Cdc42/Par6/aPKC polarity complex, specifically, led to increased Rho1-Rok-Myosin activity and JNK-dependent apoptosis, resulting in accelerated compensatory proliferation following irradiation and hyperproliferation of undead cells (Figure 7). This represents a novel upstream signal in the regulation of the apoptosis-induced compensatory proliferation response and suggests a contributing mechanism for how an epithelium maintains tissue homeostasis in response to injury or stress. Proper localization of Cdc42/Par6/aPKC at epithelial cell junctions is an indicator of a normal epithelium with correct apical-basal polarity, however, tissue damage that cause disruption of this polarity complex may identify abnormal cells that need to be removed by apoptosis. These cells can induce proliferation of surrounding, non-apoptotic cells so normal cells replace them.
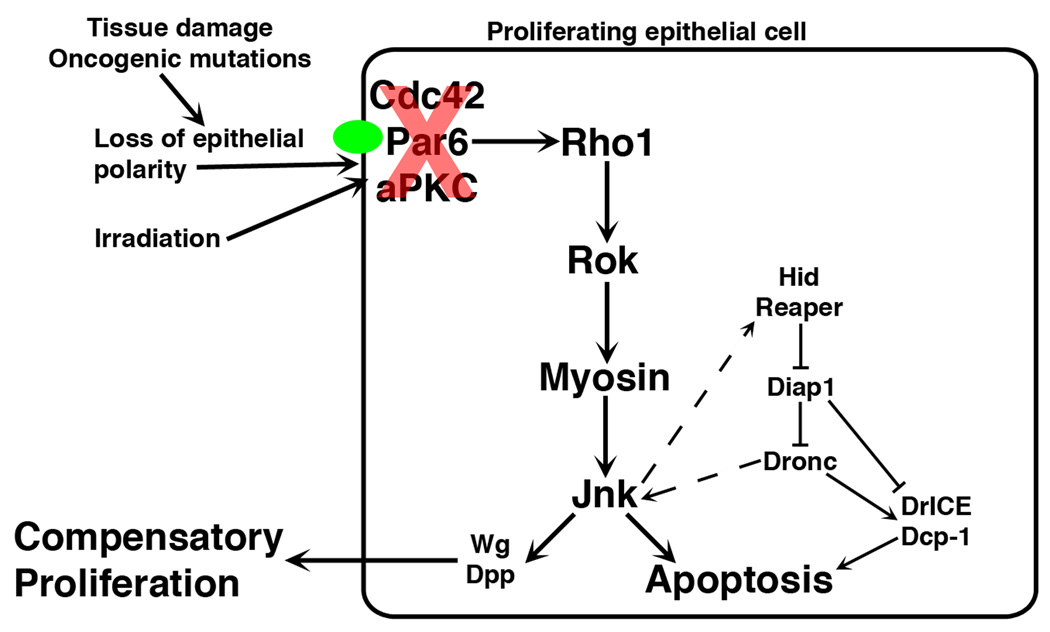
Figure 7. A working model for regulation of apoptosis-induced compensatory proliferation.
Tissue damage, oncogenic mutations that disrupt epithelial polarity, or irradiation leads to mislocalization of Cdc42/Par6/aPKC from junctions (green oval). This activates a Rho1/Rok/Myosin/JNK cascade, resulting in apoptosis and compensatory proliferation.
Disruption of the Scribble/Dlg polarity complex induced JNK-dependent apoptosis (Figure S1, data not shown) [18, 20, 31], however, in contrast to Cdc42/Par6/aPKC complex disruption, did not promote compensatory proliferation (Figure 1B and S1) [19, 20]. Possibly this reflects the fact that these two complexes activate JNK by different upstream signaling events. Disruption of the Cdc42/Par6/aPKC complex activates JNK through a Rho-Rok-Myosin axis while disruption of the Scribble complex activates JNK by increased endocytosis of Eiger (Drosophila tumor necrosis factor) [20]. The activation of Rho-Rok-Myosin following disruption of the Cdc42/Par6/aPKC complex may also provide additional signals required for compensatory proliferation. The Reaper-Diap1 apoptotic components did not appear to influence Cdc42/Par6/aPKC regulation of compensatory proliferation. Interestingly, Reaper-induced apoptosis can occur without JNK activation [35].
p53 has also been implicated in apoptosis-induced compensatory proliferation. The initiator caspase Dronc induces p53, which then induces pro-apoptotic genes Hid and Reaper, creating a positive feedback loop [32]. p53 is also required for JNK activation and apoptosis after irradiation exposure [33]. If and how the Cdc42/Par6/aPKC polarity complexes regulates p53, or requires p53 for apoptosis-induced compensatory proliferation remains to be determined.
Our data suggested that Cdc42, through a complex with Par6/aPKC localized to cell junctions, inhibits Rho1 activity in proliferating epithelial cells. Due to the disruption of tissue architecture in hyperproliferative imaginal discs we could not demonstrate direct evidence for activated Rho1 in Cdc42-depleted epithelia. However, in a post-mitotic, nonproliferating epithelium (pupal eye), depletion of Cdc42, which does not disrupt tissue architecture, increases level of the Rho1 effectors PKN and Diaphanous at AJs, indicative of increased Rho1 activation [27]. This and genetic data herein indicate that Rho functions downstream of Cdc42/Par6/aPKC polarity complex disruption to regulate apoptosis-induced compensatory hyperproliferation. Rho1 activates JNK and compensatory hyperproliferation through Rok’s regulation of Myosin activity but not F-actin assembly. Whether increased acto-myosin tension or some other function of active Myosin is necessary for JNK activation remains to be determined. JNK has been implicated in many other developmental processes, such as dorsal closure [34], however, signals upstream of JNK during dorsal closure are not well characterized. Possibly Cdc42 through Rho/Rok/Myosin regulates JNK activity during dorsal closure.
In other examples of crosstalk between Rho family proteins, upstream regulators of GTPase activity have been implicated. These include Rho guanine nucleotide exchange factors (RhoGEFs), which promote Rho activity, and Rho GTPase-activating proteins (RhoGAPs) and Rho-GDP dissociation inhibitors (RhoGDIs), which inhibit Rho activity. While mammalian RhoGDIs have been shown to mediate crosstalk between Cdc42 and Rac [35] and RhoA and RhoB [36], Drosophila deleted of the only RhoGDI gene were viable with no gross external defects (unpublished results). Likewise, mammalian p190RhoGAP has been implicated mediating crosstalk between Rac and Rho [37] and Par6/aPKC and Rho [38], but Drosophila deleted of the sole p190RhoGAP gene were also viable and exhibited no gross external defects (unpublished results). Thus, if Cdc42/Par6/aPKC regulate Rho1 activity through other RhoGAPs or RhoGEFs, these remain to be determined. Alternatively, Cdc42 and Rho1 may communicate through mechanisms independent of these upstream Rho GTPase regulators. For example, aPKC may directly or indirectly affect Rho activity.
Epithelial tumor cells are often resistant to apoptosis [39, 40] and have disrupted polarity [3]. Therefore, that mislocalization of the Par polarity complex in the presence of inhibited apoptosis results in epithelial hyperproliferation parallels early carcinoma development. This raises the interesting possibility that a significant component of carcinoma development could be misregulation of the apoptosis-induced compensatory proliferation response, which normally is used to remove damaged cells with altered polarity. However, when apoptosis is blocked (e.g., cancer) epithelial hyperproliferation results.
Methods
Drosophila stocks and genetics
All crosses and staging were performed at 25°C unless otherwise noted. w1118 was used as wild type. Stocks are described in Flybase (http://flybase.bio.indiana.edu). GMR-gal4, ey-gal4, tubulin-gal80ts, cdc424 FRT19A, UAS-GFP, pak16 FRT82B, ssh1–11 FRT82B, pucE69, UAS-P35, UAS-Reaper, thread1, UAS-Diap1, UAS-JNK, UAS-Rho1, and UAS-aPKC-RNAi were kindly provided by the Bloomington Drosophila Stock Center, patched-gal4, UAS-Puc, and scrib1 FRT82B by R. Cagan (Mount Sinai, New York, NY), Zip1 by T. Wolff (Washington University, St. Louis, MO), en-gal4 (J. Skeath, Washington University, St. Louis, MO), baz4 FRT19A, par6Δ226 FRT19A, apkck06403 FRTG13, UAS-aPKCWT, and UAS-aPKCCaax by C. Doe (University of Oregon, Eugene, OR), UAS-MLCKCA by M. VanBerkum (Wayne State University, Detroit, MI), UAS-Dlg-RNAi and UAS-Crb-RNAi by the Vienna Drosophila RNAi Center (Vienna, Austria), UAS-Scrib-RNAi and UAS-JNK-RNAi by the National Institute of Genetics (Shizuoka, Japan). Rho1-RNAi and Cdc42-RNAi were previously described [28]. GFP-labeled clones in larval eye discs were generated using the following stocks: tub-gal80, FRT19A; ey-FLP, act>y+>gal4, UAS-GFP (19A Tester); yw, ey-FLP; act>y+>gal4, UAS-GFP; tub-gal80, FRT82B (82B Tester) (both provided by T. Xu, Yale Univeristy, New Haven, CT); and yw, ey-FLP; tub-gal80, FRTG13; act>y+>gal4, UAS-GFP. Expression of UAS-MLCKCA using patched-gal4 was early larval lethal, so these crosses were performed using patched-gal4, tub-gal80ts and progeny were shifted from 18°C to 29°C five days after egg laying.
Irradiation treatment
Wandering third-instar larvae were exposed to 40 Gy of irradiation and dissected at the indicated time points after exposure.
Immunofluorescence
Wing or eye imaginal discs from wandering third-instar larvae were dissected and processed as previously described [28]. Antibodies used were rat anti-DE-cadherin (1:20), mouse anti-Discs large (1:50), mouse anti-MMP1 (all from the Developmental Studies Hybridoma Bank at the University of Iowa), rabbit anti-β-galactosidase (1:2000, ICN/Cappel), rabbit anti-cleaved Caspase 3 (1:100, Cell Signaling), rabbit anti-phospho-Histine H3 (1:1000, Upstate Laboratories, Syracuse, NY), rabbit anti-Bazooka (1:500, from A. Wodarz, University of Göttingen, Germany), guinea pig anti-Scrib (1:500, from D. Bilder, University of California, Berkeley, CA), rabbit anti-Par6 (1:500, from J. Knoblich, IMBA, Vienna, Austria), rabbit anti-aPKC (C-20) (1:200, Santa Cruz Biotechnology), and rabbit anti-phospho-Myosin Light Chain 2 (Ser19) (1:20, Cell Signaling). Rhodamine-phalloidin (1:500, Invitrogen) was added in the primary and secondary antibody incubations to visualize F-actin. Secondary antibodies were Alexa 488 and 568 (Invitrogen) and Cy5 (Jackson ImmunoResearch). Immunofluorescence was analyzed on a Zeiss 510 LSM.
Quantification and statistics
Quantification of hyperproliferation in ptc expression domain was performed by outlining the area of GFP-positive cells in the ptc expression domain and the area of the total wing disc, determining the number of pixels within each outline, and calculating the ratio of pixels in the ptc expression domain relative to the total wing disc. Quantifications were performed using ImageJ v1.38. P-values were calculated using an unpaired, two-sided Student’s t-test.
Supplementary Material
Acknowledgements
We thank J. Skeath, T. Wolff, C. Doe, M. VanBerkum, R. Cagan, T. Xu, A. Wodarz, D. Bilder, J. Knoblich, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, the National Institute of Genetics, and the Developmental Studies Hybridoma Bank for reagents. This work was supported by grants NIH CA85839 and GM080673 to GDL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 2.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 4.Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 5.Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal disc of Drosophila melanogaster. Rouxs Arch. Dev. Biol. 1977;183:85–100. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- 8.Valentin-Vega YA, Box N, Terzian T, Lozano G. Mdm4 loss in the intestinal epithelium leads to compartmentalized cell death but no tissue abnormalities. Differentiation. 2009;77:442–449. doi: 10.1016/j.diff.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008;15:1772–1781. doi: 10.1038/cdd.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136:1169–1177. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 18.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henrique D, Schweisguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr Opin Genet Dev. 2003;13:341–350. doi: 10.1016/s0959-437x(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 24.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 25.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 26.Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell. 2004;14:29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 27.Warner SJ, Longmore GD. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J Cell Biol. 2009 doi: 10.1083/jcb.200906047. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol. 2009;185:1111–1125. doi: 10.1083/jcb.200901029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 30.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 31.Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci U S A. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- 34.Jacinto A, Woolner S, Martin P. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev Cell. 2002;3:9–19. doi: 10.1016/s1534-5807(02)00208-3. [DOI] [PubMed] [Google Scholar]
- 35.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–127. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Ho TT, Merajver SD, Lapiere CM, Nusgens BV, Deroanne CF. RhoA-GDP regulates RhoB protein stability. Potential involvement of RhoGDIalpha. J Biol Chem. 2008;283:21588–21598. doi: 10.1074/jbc.M710033200. [DOI] [PubMed] [Google Scholar]
- 37.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajra KM, Liu JR. Apoptosome dysfunction in human cancer. Apoptosis. 2004;9:691–704. doi: 10.1023/B:APPT.0000045786.98031.1d. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.