Abstract
Objectives To review the evidence for an association of white matter hyperintensities with risk of stroke, cognitive decline, dementia, and death.
Design Systematic review and meta-analysis.
Data sources PubMed from 1966 to 23 November 2009.
Study selection Prospective longitudinal studies that used magnetic resonance imaging and assessed the impact of white matter hyperintensities on risk of incident stroke, cognitive decline, dementia, and death, and, for the meta-analysis, studies that provided risk estimates for a categorical measure of white matter hyperintensities, assessing the impact of these lesions on risk of stroke, dementia, and death.
Data extraction Population studied, duration of follow-up, method used to measure white matter hyperintensities, definition of the outcome, and measure of the association of white matter hyperintensities with the outcome.
Data synthesis 46 longitudinal studies evaluated the association of white matter hyperintensities with risk of stroke (n=12), cognitive decline (n=19), dementia (n=17), and death (n=10). 22 studies could be included in a meta-analysis (nine of stroke, nine of dementia, eight of death). White matter hyperintensities were associated with an increased risk of stroke (hazard ratio 3.3, 95% confidence interval 2.6 to 4.4), dementia (1.9, 1.3 to 2.8), and death (2.0, 1.6 to 2.7). An association of white matter hyperintensities with a faster decline in global cognitive performance, executive function, and processing speed was also suggested.
Conclusion White matter hyperintensities predict an increased risk of stroke, dementia, and death. Therefore white matter hyperintensities indicate an increased risk of cerebrovascular events when identified as part of diagnostic investigations, and support their use as an intermediate marker in a research setting. Their discovery should prompt detailed screening for risk factors of stroke and dementia.
Introduction
As magnetic resonance imaging has become widely available and brain magnetic resonance imaging is increasingly being carried out in various clinical settings, clinicians often have to deal with the incidental discovery of white matter lesions, appearing as hyperintensities on T2 weighted images (fig 1). In the general population the prevalence of white matter hyperintensities ranges from 11-21% in adults aged around 64 to 94% at age 82.1 2 Pathological findings in regions of white matter hyperintensity include myelin pallor, tissue rarefaction associated with loss of myelin and axons, and mild gliosis.3 4 These lesions are located in the deep white matter, typically sparing subcortical U-fibres, and are often seen together with vessels affected by small vessel disease.5 The affected vessels are presumed to induce the lesions in deep white matter through chronic hypoperfusion of the white matter and disruption of the blood-brain barrier, leading to chronic leakage of plasma into the white matter.3 6 7 White matter hyperintensities are more common and extensive in patients with cardiovascular risk factors and symptomatic cerebrovascular disease.8 White matter hyperintensities can be measured quantitatively and non-invasively on large population samples and have been proposed as an intermediate marker, which could be used for the identification of new risk factors and potentially as a surrogate end point in clinical trials.9
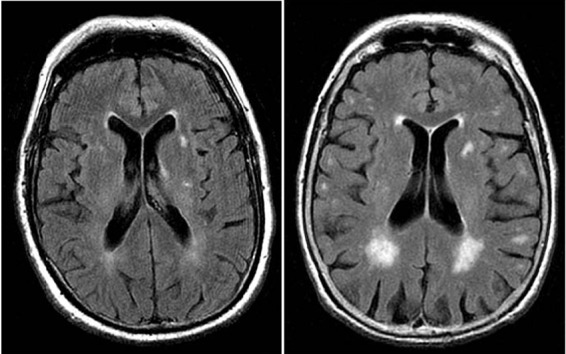
Fig 1 White matter hyperintensities on magnetic resonance imaging (axial fluid attenuated inversion recovery sequence) in two 80 year old patients: (left) minor white matter hyperintensities; (right) extensive white matter hyperintensities predominating in periventricular region. White matter lesions are considered present if hyperintense on T2 weighted, fluid attenuated inversion recovery, and proton density images, without prominent hypointensity on T1 weighted images
Several studies have assessed the relation between white matter hyperintensities and risk of stroke, dementia, and death, with partly conflicting results. We systematically reviewed and meta-analysed all published longitudinal studies that tested the association of white matter hyperintensities with risk of stroke, dementia, and death, both in the general population and in a hospital based setting.
Methods
Using predefined search terms we identified references through searches of PubMed from 1966 to 23 November 2009 (see web extra methods 1). We also identified papers by reviewing the reference lists of relevant articles.
Study selection
Studies were limited to those in adults. We included only prospective studies with longitudinal data on the association of white matter hyperintensities with risk of stroke, dementia, cognitive decline, and death. We excluded studies if they evaluated white matter lesions only by computed tomography (because of the lower sensitivity of this method for measuring white matter lesions compared with magnetic resonance imaging10); carried out magnetic resonance imaging at the end of follow-up instead of at baseline; or had a sample size of fewer than 50 patients. For studies with more than one publication describing results among overlapping groups of participants and with the same outcome measure, we included only the dataset with the longest follow-up. If the follow-up period was identical we included only the dataset with the largest number of patients (see web extra methods 2).
Data extraction
From the studies we extracted data on number of participants, general population versus high risk participants, mean age, duration of follow-up, characteristics of magnetic resonance imaging and definition of white matter hyperintensities, outcome definition (all strokes versus intracerebral haemorrhage or ischaemic stroke; all types of dementia versus Alzheimer’s disease, vascular dementia, mixed vascular and Alzheimer’s dementia; and neuropsychological measurements used to assess cognitive decline), and number of events that occurred during follow-up. For the measure of the association between white matter hyperintensities and the outcome we recorded, when available, hazard ratios, odds ratios, and adjustment variables when applicable. Both authors extracted the information from each study, resolving any disagreements by discussion.
Variable definition
We defined studies as being in high risk populations if they were carried out in people selected for the presence of prevalent disease such as mild cognitive impairment, stroke, or other vascular events, or for the presence of a high risk factor profile. (See web extra tables 1-5 for the inclusion criteria for each study.) Stroke was defined as an acute onset focal neurological deficit of presumed vascular cause lasting at least 24 hours or interrupted by death within 24 hours. Web extra tables 1-5 show the number of events by stroke type (ischaemic versus haemorrhagic), when available. Unless specified differently (see web extra tables 1-5) we defined dementia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, third or fourth editions11 12; Alzheimer’s disease based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for definite, probable, or possible Alzheimer’s disease13; and vascular dementia according to the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria.14 Cognitive decline was defined as worsening performance on repeated neuropsychological tests. In the absence of consensus on which neuropsychological test was the most appropriate, we did not apply restrictions on type of test used. We examined separately tests of global cognitive function and tests assessing performances in specific cognitive domains (see web extra tables 1-5 for types of test used in each study).
Web extra table 6 details study quality criteria, including loss to follow-up, surveillance carried out for the diagnosis of incident events, description of the definition used for these events, and event subtypes. We also report the availability of effect estimates and whether the analysis took into account time to event.
Statistical analyses
Cochrane RevMan version 4·2 software (www.cc-ims.net/RevMan/current.htm) was used to carry out meta-analysis of the outcomes dementia, stroke, and death. Graphs were designed using the rmeta package in R (http://cran.r-project.org/web/packages/rmeta/index.html). We calculated pooled hazard ratios using the random effects inverse variance method. In a few studies only odds ratios were available despite the longitudinal design,15 16 17 therefore we considered the odds ratios as approximations of hazard ratios.18 Significant heterogeneity was defined by a P value <0.05 or I2 >50%. We carried out meta-analysis when at least three studies were available for the same outcome; we included only studies that used a categorical measure of white matter hyperintensities. When more than two categories were present we used the hazard ratio for the highest category compared with the lowest category. Studies for which the association of white matter hyperintensities with the outcome was studied solely in a continuous fashion (per unit increase or standard deviation increase in quantitative volume of white matter hyperintensities or semiquantitative grade for white matter hyperintensities) were not included in the meta-analysis. Indeed, white matter hyperintensities were measured on completely different scales in the various cohorts, using either automated quantitative volume measures or different types of visual semiquantitative non-linear volume measures. Although a sample size weighted meta-analysis could be done, this would not provide a pooled effect estimate. When both adjusted and unadjusted values were available for the hazard ratio, we included the value adjusted for vascular risk factors. For studies that measured deep white matter hyperintensities and periventricular hyperintensities separately and did not provide a global risk estimate for white matter hyperintensities, we meta-analysed the results for periventricular hyperintensities (see web extra methods 3). In a secondary analysis we reran the meta-analysis replacing the results for periventricular hyperintensities with those for deep white matter hyperintensities. All meta-analyses were done separately for population based studies and studies in a high risk population, and overall.
Results
The initial search identified 958 articles, of which 53 met the inclusion criteria.15 16 17 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 This included 14 studies for stroke,15 16 19 20 21 22 23 24 25 26 27 28 29 30 20 for dementia,29 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 21 for cognitive decline,17 22 23 29 39 46 48 50 51 52 53 54 55 56 57 58 59 60 61 62 63 and 11 for mortality.15 16 23 25 28 29 64 65 66 67 68 Some studies contained data on more than one outcome. Because of an overlap with another larger study carried out in the same population on the same outcome, seven studies were excluded: two on stroke,27 28 three on dementia,43 44 45 two on cognitive decline,51 59 and one on mortality.28 Hence a total of 46 studies was included in this systematic review15 16 17 19 20 21 22 23 24 25 26 29 30 31 32 33 34 35 36 37 38 39 40 41 42 46 47 48 49 50 52 53 54 55 56 57 58 60 61 62 63 64 65 66 67 68; 12 for stroke,15 16 19 20 21 22 23 24 25 26 29 30 17 for dementia,17 29 31 32 33 34 35 36 37 38 39 40 41 42 47 48 49 19 for cognitive decline,17 22 23 29 39 46 48 50 52 53 54 55 56 57 58 60 61 62 63 and 10 for mortality.15 16 23 25 29 64 65 66 67 68
White matter hyperintensities and incident stroke
Incident stroke overall
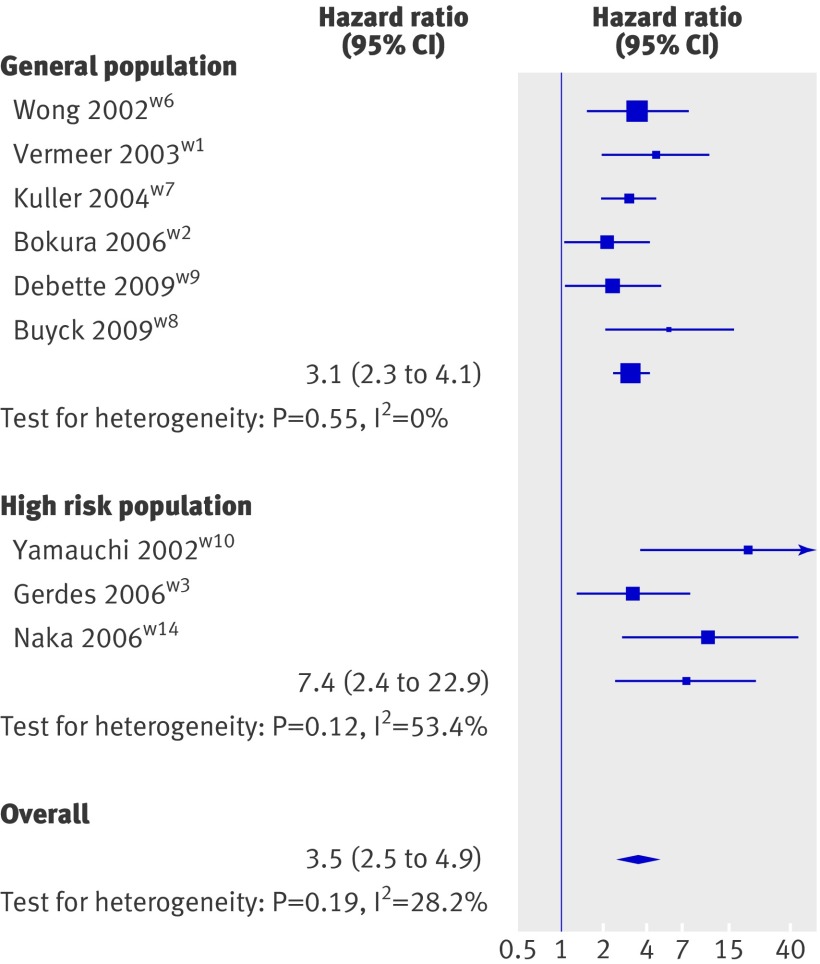
Six population based studies15 19 20 21 29 30 and six studies in high risk populations16 22 23 24 25 26 examined the relation between white matter hyperintensities and incident stroke (see web extra table 1). All six population based studies could be included in the meta-analysis (fig 2), which showed a significant association of white matter hyperintensities with risk of stroke (hazard ratio 3.1, 95% confidence interval 2.3 to 4.1, P<0.001). Three studies done in high risk populations could be included in the meta-analysis (fig 2), showing a significant association of white matter hyperintensities with the risk of stroke (7.4, 2.4 to 22.9, P=0.001).16 24 26 Three studies in high risk populations could not be included in the meta-analysis, two because white matter hyperintensities were studied continuously only,23 25 and a third because only intracerebral haemorrhage was studied as an outcome (see web extra table 1).22 All three found a significant association of white matter hyperintensities with incident stroke.
Fig 2 Inverse variance meta-analysis of studies testing association of white matter hyperintensities with incident stroke
The meta-analysis combining the data from population based and high risk populations yielded a significant association of white matter hyperintensities with incident stroke (3.5, 2.5 to 4.9, P<0.001; see web extra fig 2).
Incident stroke types
Few data existed on the association of white matter hyperintensities with specific stroke types (intracerebral haemorrhage, ischaemic stroke). One community based study21 found a significant association of white matter hyperintensities with incident ischaemic stroke; another study did not observe any association of white matter hyperintensities with recurrent intracerebral haemorrhage in patients with lobar haemorrhage (see web extra table 1).22
Progression of white matter hyperintensities and incident stroke
Only one study assessed the relation between progression of white matter hyperintensities and incident stroke, in 89 patients with a history of lacunar stroke, headache, or dizziness: a significantly increased risk of stroke was observed in patients with progression of white matter hyperintensities after 4.3 years compared with those with no progression.16
White matter hyperintensities and incident dementia
Incident dementia overall
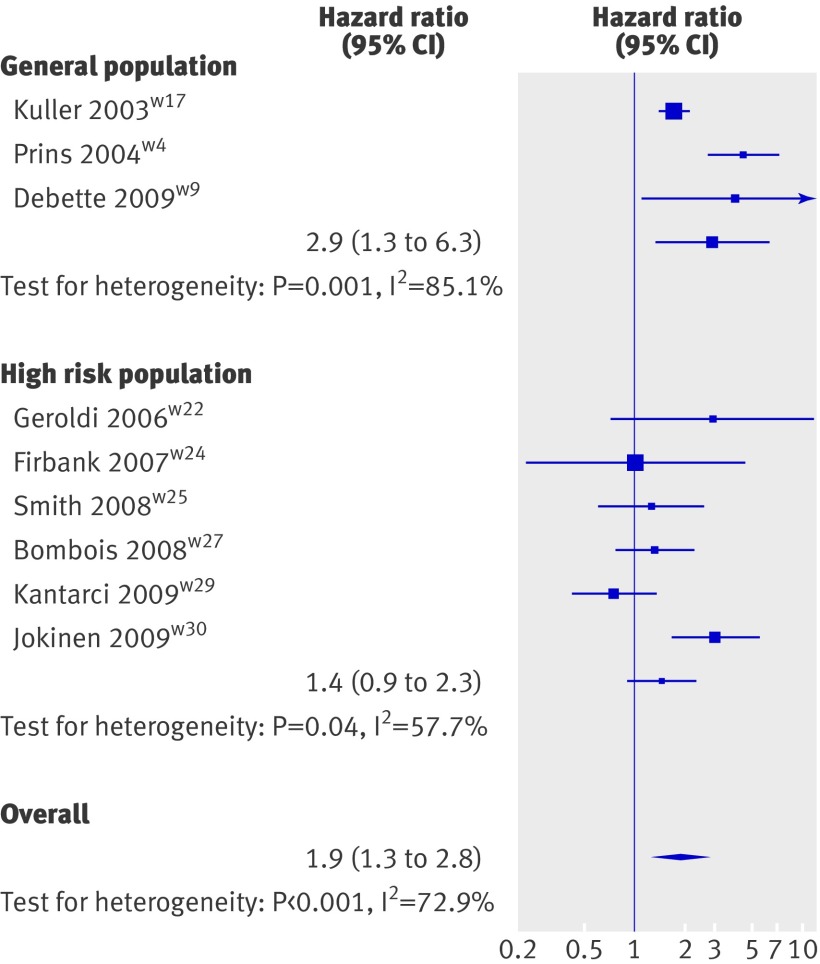
Three population based studies29 31 32 and 11 studies in high risk populations17 34 35 36 37 38 39 40 41 47 48 examined the relation between white matter hyperintensities and incident dementia overall (see web extra table 2). Meta-analysing the three population based studies yielded a significant association of white matter hyperintensities with the occurrence of all types of dementia (2.9, 1.3 to 6.3, P=0.008; fig 3).29 31 32 Six of the 11 studies done in high risk populations could be included in the meta-analysis,17 37 39 41 47 48 yielding no significant association of white matter hyperintensities with incident dementia (1.4, 0.9 to 2.3, P=0.14). Four of these six studies included patients with mild cognitive impairment37 39 41 47: meta-analysing these separately also did not yield any significant association (1.1, 0.8 to 1.6, P=0.46; see web extra fig 2). Five studies in high risk populations could not be included in the meta-analysis, three because white matter hyperintensities were studied continuously only,35 36 40 and two because the authors reported the absence of association without indicating numbers (see web extra table 2).34 38 None of them identified a significant association of white matter hyperintensities with incident dementia. Not all quality criteria were satisfied for several of the studies (see web extra table 6).34 37 38 48
Fig 3 Inverse variance meta-analysis of studies testing association of white matter hyperintensities with incident dementia
When combining data from population based studies and high risk populations the risk of dementia associated with white matter hyperintensities was increased overall (1.9, 1.3 to 2.8, P=0.002; fig 3, also see web extra fig 3).
Incident subtypes of dementia
Three population based studies31 32 33 and three studies in high risk populations41 42 49 investigated the relation between white matter hyperintensities and incident Alzheimer’s disease (see web extra table 2). Only one population based study31 and two studies in high risk populations41 49 fulfilled our predefined criteria for meta-analysis. Meta-analysis of these yielded a significant association of white matter hyperintensities with the risk of Alzheimer’s disease (see web extra fig 4).
Two population based studies31 33 and one study in a high risk population41 tested the relation between white matter hyperintensities and incident vascular dementia (see web extra table 2). We did not carry out a meta-analysis as only two studies fulfilled our predefined criteria for meta-analysis,31 41 but all three studies found a significant association of white matter hyperintensities with incident vascular dementia (see web extra table 2).
Progression of white matter hyperintensities and incident dementia
One study observed that increasing volume of white matter hyperintensities during a two year follow-up was associated with incident dementia in depressed patients.38 Another study, in people with white matter hyperintensities of varying degree and mild neurological problems, found that increasing degrees of progression of white matter hyperintensities over a three year period were associated with a higher rate of conversion to dementia.48
White matter hyperintensities and cognitive decline
Global cognitive decline
Five population based studies assessed the relation between volume of white matter hyperintensities and global cognitive decline (see web extra table 3).29 39 50 53 63 Three studies observed that white matter hyperintensities were associated with an increased risk of low global cognitive test scores,50 conversion to mild cognitive impairment, or persistent cognitive impairment at the end of follow-up.39 63 In one study, white matter hyperintensities were associated with an increased risk of incident amnestic mild cognitive impairment in participants aged over 60,29 but did not predict incident mild cognitive impairment overall. Two studies found that increasing periventricular hyperintensities only predicted a higher rate of annual decline in mini-mental state examination scores,53 or of conversion to persistent cognitive impairment.63
Eight studies carried out in high risk populations examined the relation of white matter hyperintensities with global cognitive decline (see web extra table 3).17 22 23 46 48 54 55 58 Five of them found that increasing baseline volume of white matter hyperintensities was associated with a more noticeable decline in global cognitive function.23 46 48 55 58 The other three studies observed no association.17 22 54
Decline in domain specific cognitive functions
Two population based studies evaluated the relation of volume of white matter hyperintensities with decline in domain specific cognitive functions52 53: one found a significant association of periventricular hyperintensities (but not deep white matter hyperintensities) with decline in processing speed and executive function,53 whereas the other study observed no association of baseline burden of white matter hyperintensities with decline in any cognitive domain.52
Four studies in high risk populations assessed the relation between white matter hyperintensities and decline in specific cognitive domains48 56 57 58: all found an association of baseline white matter hyperintensities with more rapid change in executive function or processing speed. In two of these studies the association was assessed separately for periventricular hyperintensities and deep white matter hyperintensities and was significant for periventricular hyperintensities only.57 58
Progression of white matter hyperintensities and global cognitive decline
Two population based studies examined the relation of progression of white matter hyperintensities with decline in global cognitive functions (see web extra table 4).60 62 Both found that increasing progression of white matter hyperintensities was associated with a greater deterioration in global cognitive score; in one study this association was significant for periventricular hyperintensities only.62
Progression of white matter hyperintensities and decline in domain specific cognitive functions
Four population based studies tested the association of progression of white matter hyperintensities with decline in domain specific cognitive scores (see web extra table 4).52 60 61 62 One study observed a significant association of progression of white matter hyperintensities with decline in memory performance, conceptualisation, and visuopractical skills.52 The three other studies found an association of progression of white matter hyperintensities with a faster decline of executive functions or processing speed.60 61 62 In one study this association was significant for periventricular hyperintensities only.62
Two studies tested the association of progression of white matter hyperintensities with decline in domain specific cognitive scores in high risk populations.(see web extra table 4).56 57 One study, in patients with vascular risk and no pre-existing cognitive impairment, found an association of progression in volume of periventricular hyperintensities with decline in executive function.57 The other study, in patients attending memory clinics who were already cognitively impaired, found no association of progression of white matter hyperintensities with memory or executive function.56
White matter hyperintensities and mortality
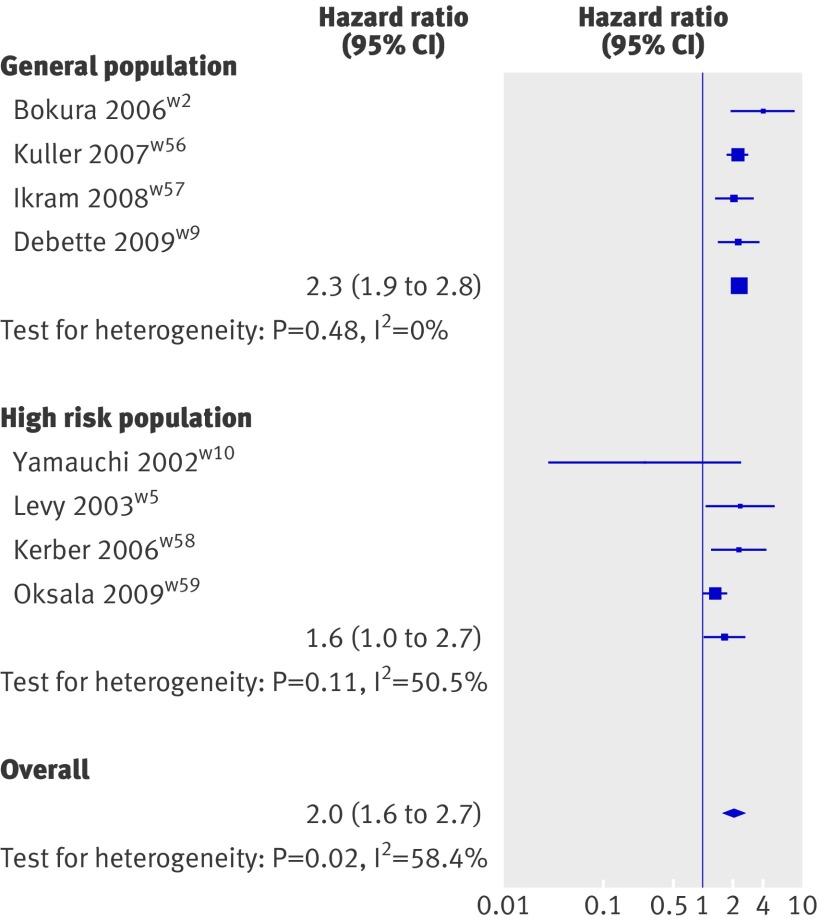
Four population based studies15 29 64 65 and six studies in high risk populations16 23 25 66 67 68 prospectively investigated the relation between white matter hyperintensities and mortality (see web extra table 5). All four population based studies could be included in the meta-analysis (fig 4), which showed a significant association of white matter hyperintensities with risk of death (2.3, 1.9 to 2.8, P<0.001). Four studies done in high risk populations could also be meta-analysed (fig 4); this showed an association of white matter hyperintensities with mortality (1.6, 1.01 to 2.7, P=0.04). Two studies could not be included because white matter hyperintensities were studied continuously only,23 25 both found a significant association of white matter hyperintensities with mortality (see web extra table 5). When studies from both population based samples and high risk populations were pooled, white matter hyperintensities were associated with an increased risk of death during follow-up (2.0, 1.6 to 2.7, P<0.001; fig 4, also see web extra fig 5).
Fig 4 Inverse variance meta-analysis of studies testing association of white matter hyperintensities with mortality
Discussion
This systematic review of 46 studies, and meta-analysis, provides strong evidence that white matter hyperintensities are an important indicator of future risk of disease, being associated with an increased risk of stroke, cognitive decline (especially in the executive function and processing speed domains), dementia, and death.
Incident stroke
White matter hyperintensities were significantly associated with an increased risk of stroke, both in the general population and in high risk populations with a history of stroke or vascular disease. It could be hypothesised that this association merely reflects the relation of vascular risk factors with stroke. Indeed, age and hypertension are the main predictors of white matter hyperintensities,69 70 and other vascular risk factors such as smoking, diabetes, and history of vascular disease were also shown to be associated with lesions in white matter.71 In all studies, however, the association of white matter hyperintensities with stroke remained significant after adjustment for vascular risk factors. Although some residual confounding may exist, this suggests that white matter hyperintensities reflect either the overall effect of uncontrolled vascular risk factors better than the mere presence or absence of each individual factor or that other, yet unknown, factors play a role in the association of white matter hyperintensities with stroke.
Incident dementia and cognitive decline
White matter hyperintensities were associated with an increased risk of dementia in the general population, but not in studies on high risk populations (mainly with mild cognitive impairment or a history of stroke). When subtypes of dementia were assessed, the three studies that investigated the relation of white matter hyperintensities with incident vascular dementia found a significant association, both in the general population and in patients with mild cognitive impairment.31 33 41 Conversely, although the meta-analysis testing the relation of white matter hyperintensities with incident Alzheimer’s disease yielded an overall significant association, this association was driven by the large population based study,31 whereas the two smaller studies on patients with mild cognitive impairment did not identify any association.41 49 Finally, our systematic review suggests that, in most studies looking at the association of white matter hyperintensities with decline in cognitive performance, white matter hyperintensities were associated with a faster decline in global cognitive performance as well as in executive function and processing speed. This was true both in the general population and in high risk patients with mild cognitive impairment, memory problem, or cerebrovascular disease.
Various potential mechanisms could account for the association of white matter hyperintensities with dementia. Firstly, subcortical neural networks could be damaged directly. Consistent with this hypothesis is the predominant association with decline in executive functions (believed to depend on cortical-subcortical circuits passing through the white matter), which could be disrupted by damage to white matter.56 72 Secondly, white matter hyperintensities could interact with pathological changes related to Alzheimer’s disease and thereby accelerate their clinical expression, or vice versa.73 Thirdly, white matter hyperintensities could be a confounder reflecting the association of vascular risk factors74 or of concomitant ischaemic damage to the cortex with dementia. Several studies have adjusted for vascular risk factors, for prevalent or incident stroke, or for concomitant magnetic resonance imaging defined brain infarcts, and found similar results,29 31 32 suggesting that vascular risk factors are unlikely to be a major mechanism, although undetected cortical microinfarcts cannot be excluded.75 Finally, reverse causation through amyloid angiopathy or wallerian degeneration as a result of cortical atrophy has been hypothesised.76 77 However, data from the Austrian Stroke Prevention Study suggest that progression of lesions in white matter precedes loss in parenchymal volume in elderly people without dementia, implying that this reverse causation is unlikely to play a predominant role.52
The finding that white matter hyperintensities were associated with an increased risk of dementia and Alzheimer’s disease mainly in healthy populations and not in high risk patients who are already cognitively impaired, needs to be interpreted cautiously. Studies in high risk populations were carried out in small samples and had several methodological flaws. If this lesser association in higher risk and cognitively impaired populations is confirmed, however, it could suggest that once the neurodegenerative lesions become clinically evident, the effect of white matter lesions may be less important.78 As has been suggested by others, the presence of white matter hyperintensities may increase the risk that an individual will develop mild cognitive impairment or have declining performances on cognitive tests but may not be enough to facilitate progression from mild cognitive impairment to dementia, the latter being overwhelmingly driven by neurodegenerative lesions.36 41 47 An exception could be the rare cases of pure vascular dementia, where diffuse white matter hyperintensities could be important also at later stages of cognitive decline and conversion.41
Mortality
White matter hyperintensities were significantly associated with an increased risk of death both in the general population and in high risk populations with a history of stroke, depression, or minor neurological symptoms. This association could be mediated by an increased risk of stroke and dementia. However, both in the Rotterdam and the Framingham Offspring Study the association of white matter hyperintensities with mortality did not disappear after accounting for incident dementia or stroke.29 65 The association was more noticeable for cardiovascular mortality,29 65 67 which could reflect that white matter hyperintensities are a surrogate for an important burden of vascular risk factors and a marker of diffuse vascular damage involving not only the arteries supplying the brain but also peripheral and coronary arteries.24 79
Effect of progression of white matter hyperintensities
Only three studies have assessed the impact of progression of white matter hyperintensities on incident stroke and dementia, suggesting a significant association of increasing progression of white matter hyperintensities with these outcomes.16 38 48 Progression of white matter hyperintensities has been more widely studied in association with cognitive decline, with increasing progression predicting a more rapid decline in global cognitive performance and executive function.52 56 57 60 61 62
Limitations of the review
Although we corrected for statistical heterogeneity in the meta-analyses, the measurement and analysis of white matter hyperintensities varied substantially (see web extra tables 1-5). One important source of heterogeneity was the automated quantitative versus visual semiquantitative measurement of white matter hyperintensity volume, although visual semiquantitative scales were shown to correlate well with volumetric measures.80 Furthermore, total intracranial volume was unequally included in the analyses to account for differences in head size, and not all studies explicitly distinguished white matter hyperintensities from lacunar infarcts. Consensus recommendations on the measurement of white matter hyperintensities and their progression, as have been published for instance for carotid intima-media thickness,81 would be useful to improve the comparability of future studies. Another limitation is that some studies could not be included in the meta-analysis because white matter hyperintensity was studied only as a continuous variable using different scales. All the results, including those that were not incorporated in the meta-analysis, are available in web extra tables 1-5. The pooled hazard ratios for dichotomous measures of burden of white matter hyperintensities also need to be interpreted with caution, given the heterogeneous definitions of these measures across studies. They should therefore not be extrapolated to estimate the individual risk of stroke, dementia, or death in a patient with white matter hyperintensities on magnetic resonance imaging. To be as comprehensive as possible in this review we included studies using different tests or criteria for the diagnosis of cognitive decline and dementia, which is an additional source of heterogeneity. Finally, given the strong association of vascular risk factors both with burden of white matter hyperintensities and with the clinical outcomes we studied, it would be interesting to assess the modifying effect of these risk factors on the associations we reported. This was not feasible in the present paper, however, as the nature and definition of vascular risk factors included in the analyses varied substantially across studies.
Implications of the review
The findings of this review have several implications. For the clinician, they emphasise the clinical importance of white matter hyperintensities, even when these are found as incidental findings on brain imaging. In such cases doctors might consider detailed screening for risk factors for stroke and dementia, even though the benefit of doing so is not formally proved. Although data showing that treatment of risk factors reduces the progression of white matter hyperintensities are limited, more aggressive antihypertensive treatment was associated with reduced progression in patients after stroke, as well as with a reduction in stroke itself.82
In terms of research, white matter hyperintensities could constitute a potentially useful intermediate marker for the identification of new risk factors for stroke and dementia. Indeed, besides being strongly associated with the risk of stroke and dementia, white matter hyperintensities can be quantified reliably and non-invasively on large samples and can be measured as a continuous trait, thus providing increased statistical power to detect an association. White matter hyperintensities could prove particularly interesting for the identification of novel genetic risk factors, as their heritability was shown to be as high as 55-73%.83 84
White matter hyperintensities may also have a role as a surrogate marker to assess treatment efficacy. Despite the importance of treatment, few have been shown to be specifically effective in the small vessel disease subtype of stroke. Treatment trials for small vessel disease face major challenges, including relatively slow progression of the disease necessitating large patient populations.85 Neuropathological studies have shown that white matter hyperintensities usually represent pathological small vessel disease,3 5 therefore white matter lesions may be useful to screen treatments before evaluation in much larger clinical studies, using stroke and dementia as clinical end points.9 The European Task Force on Age-Related White Matter Changes estimated that 195 people with confluent lesions would be required per treatment arm to show a 20% reduction in the rate of disease progression over three years.9 Criteria have been proposed for the use of imaging markers as surrogate markers for treatment trials.86 The first criterion was that the marker must be able to predict the natural course of the disease and should correlate with relevant clinical features in both cross sectional and longitudinal studies. Our meta-analysis provides strong evidence that this applies to white matter hyperintensities. A second criterion was that therapeutic interventions can delay progression of surrogate markers and that this correlates with reduced occurrence of clinical end points. Support for this is available from the magnetic resonance imaging substudy of the Perindopril Protection against Recurrent Stroke Study, which revealed a less noticeable progression of white matter hyperintensities with aggressive antihypertensive treatment, particularly in the subgroup of patients with a higher volume of white matter hyperintensities at study entry82; an effect on clinical end points (stroke and cognitive decline) was also found in the main study.87
Conclusions
In conclusion, our systematic review and meta-analysis provides strong evidence that white matter hyperintensities predict the risk of stroke, dementia, and mortality. This emphasises that white matter hyperintensities indicate increased cerebrovascular risk when identified clinically as part of diagnostic investigations and supports their use as an intermediate marker in a research setting. Further studies assessing the impact of progression of white matter hyperintensities on stroke and dementia are needed to help design therapeutic trials incorporating progression of white matter hyperintensities as an intermediate end point.
What is already known on this topic
Studies have assessed the relation between white matter lesions and risk of stroke, cognitive decline, dementia, and mortality
The results are, however, partly conflicting
Heterogeneity of study designs, imaging types, setting, sample size, and follow-up makes the interpretation of published data difficult
What this study adds
White matter hyperintensities are associated with an increased risk of stroke, dementia, and death
For stroke and death the association was present in both community based and high risk populations, whereas for dementia the association was significant for community based patients only
We thank for their collaboration F Pasquier, S Bombois, and D Leys (Department of Neurology, Lille University Hospital, France); PA Wolf, S Seshadri, A Beiser, and R Au (Department of Neurology, Boston University School of Medicine), and C DeCarli (University of California at Davis).
Contributors: SD did the literature search, wrote the first draft, and prepared the tables and some figures. HSM did the literature search, revised the draft manuscript, and prepared some of the figures. SD and HSM are guarantors.
Funding: SD was supported by a grant from the European Neurological Society, a Fulbright grant, and an award from the Bettencourt-Schueller and the Lilly foundations.
Competing interests: All authors have completed the unified competing interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: (1) SD was supported by a grant from the European Neurological Society, a Fulbright grant, and an award from the Bettencourt-Schueller and the Lilly foundations; (2) SD and HSM have no relationship with companies that might have an interest in the submitted work in the previous 3 years; (3) that their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) that they have no non-financial interests that may be relevant to the submitted work.
Ethical approval: Not required.
Data sharing: Additional data not available.
Cite this as: BMJ 2010;341:c3666
Web Extra. Extra material supplied by the author
Supplemental tables, figures, and references of included studies
Study quality criteria
References
- 1.Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995;26:1171-7. [DOI] [PubMed] [Google Scholar]
- 2.Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 2000;356:628-34. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652-9. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683-9. [DOI] [PubMed] [Google Scholar]
- 5.Van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 1991;114:761-74. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002;59:321-6. [DOI] [PubMed] [Google Scholar]
- 7.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192-7. [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging 2004;15:365-7. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, et al. White matter lesion progression: a surrogate endpoint for trials in cerebral small-vessel disease. Neurology 2004;63:139-44. [DOI] [PubMed] [Google Scholar]
- 10.Lechner H, Schmidt R, Bertha G, Justich E, Offenbacher H, Schneider G. Nuclear magnetic resonance image white matter lesions and risk factors for stroke in normal individuals. Stroke 1988;19:263-5. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. APA, 1987.
- 12.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. APA, 1994.
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939-44. [DOI] [PubMed] [Google Scholar]
- 14.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250-60. [DOI] [PubMed] [Google Scholar]
- 15.Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis 2006;15:57-63. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatry 2002;72:576-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firbank MJ, Burton EJ, Barber R, Stephens S, Kenny RA, Ballard C, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging 2007;28:1664-9. [DOI] [PubMed] [Google Scholar]
- 18.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002;55:893-9. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67-74. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126-9. [DOI] [PubMed] [Google Scholar]
- 21.Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821-5. [DOI] [PubMed] [Google Scholar]
- 22.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004;63:1606-12. [DOI] [PubMed] [Google Scholar]
- 23.Appelros P, Samuelsson M, Lindell D. Lacunar infarcts: functional and cognitive outcomes at five years in relation to MRI findings. Cerebrovasc Dis 2005;20:34-40. [DOI] [PubMed] [Google Scholar]
- 24.Gerdes VE, Kwa VI, ten Cate H, Brandjes DP, Buller HR, Stam J. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis 2006;186:166-72. [DOI] [PubMed] [Google Scholar]
- 25.Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry 2005;76:793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naka H, Nomura E, Takahashi T, Wakabayashi S, Mimori Y, Kajikawa H, et al. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. AJNR Am J Neuroradiol 2006;27:830-5. [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997;28:1932-9. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT Jr, Diehr P, Manolio TA, Beauchamp NJ, Jungreis CA, Lefkowitz D. Cluster analysis and patterns of findings on cranial magnetic resonance imaging of the elderly: the Cardiovascular Health Study. Arch Neurol 2001;58:635-40. [DOI] [PubMed] [Google Scholar]
- 29.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia and mortality: the Framingham Offspring Study. Stroke 2010;41:600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyck JF, Dufouil C, Mazoyer B, Maillard P, Ducimetiere P, Alperovitch A, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3-City Dijon Study. Stroke 2009;40:2327-31. [DOI] [PubMed] [Google Scholar]
- 31.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22:13-22. [DOI] [PubMed] [Google Scholar]
- 32.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004;61:1531-4. [DOI] [PubMed] [Google Scholar]
- 33.Meguro K, Ishii H, Kasuya M, Akanuma K, Meguro M, Kasai M, et al. Incidence of dementia and associated risk factors in Japan: the Osaki-Tajiri Project. J Neurol Sci 2007;260:175-82. [DOI] [PubMed] [Google Scholar]
- 34.Steffens DC, MacFall JR, Payne ME, Welsh-Bohmer KA, Krishnan KR. Grey-matter lesions and dementia. Lancet 2000;356:1686-7. [DOI] [PubMed] [Google Scholar]
- 35.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 2004;63:94-100. [DOI] [PubMed] [Google Scholar]
- 36.DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004;63:220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geroldi C, Rossi R, Calvagna C, Testa C, Bresciani L, Binetti G, et al. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry 2006;77:1219-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry 2007;15:839-49. [DOI] [PubMed] [Google Scholar]
- 39.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008;65:94-100. [DOI] [PubMed] [Google Scholar]
- 40.Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hanninen T, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging 2008;29:31-8. [DOI] [PubMed] [Google Scholar]
- 41.Bombois S, Debette S, Bruandet A, Delbeuck X, Delmaire C, Leys D, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke 2008;39:2046-51. [DOI] [PubMed] [Google Scholar]
- 42.Van Straaten EC, Harvey D, Scheltens P, Barkhof F, Petersen RC, Thal LJ, et al. Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol 2008;255:1302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215-22. [DOI] [PubMed] [Google Scholar]
- 44.Rosano C, Aizenstein HJ, Wu M, Newman AB, Becker JT, Lopez OL, et al. Focal atrophy and cerebrovascular disease increase dementia risk among cognitively normal older adults. J Neuroimaging 2007;17:148-55. [DOI] [PubMed] [Google Scholar]
- 45.Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 2005;64:1548-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dufouil C, Godin O, Chalmers J, Coskun O, MacMahon S, Tzourio-Mazoyer N, et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 2009;40:2219-21. [DOI] [PubMed] [Google Scholar]
- 47.Kantarci K, Weigand SD, Przybelski SA, Shiung MM, Whitwell JL, Negash S, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009;72:1519-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS Study. Cerebrovasc Dis 2009;27:384-91. [DOI] [PubMed] [Google Scholar]
- 49.Staekenborg SS, Koedam EL, Henneman WJ, Stokman P, Barkhof F, Scheltens P, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 2009;40:1269-74. [DOI] [PubMed] [Google Scholar]
- 50.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998;29:388-98. [DOI] [PubMed] [Google Scholar]
- 51.De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335-41. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol 2005;58:610-6. [DOI] [PubMed] [Google Scholar]
- 53.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034-41. [DOI] [PubMed] [Google Scholar]
- 54.Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology 2002;59:867-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Flier WM, van der Vlies AE, Weverling-Rijnsburger AW, de Boer NL, Admiraal-Behloul F, Bollen EL, et al. MRI measures and progression of cognitive decline in nondemented elderly attending a memory clinic. Int J Geriatr Psychiatry 2005;20:1060-6. [DOI] [PubMed] [Google Scholar]
- 56.Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology 2005;65:565-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 2006;77:149-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debette S, Bombois S, Bruandet A, Delbeuck X, Lepoittevin S, Delmaire C, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007;38:2924-30. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 1999;53:132-9. [DOI] [PubMed] [Google Scholar]
- 60.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56-61. [DOI] [PubMed] [Google Scholar]
- 61.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 2007;21:412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 2008;39:2712-9. [DOI] [PubMed] [Google Scholar]
- 63.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology 2009;73:120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuller LH, Arnold AM, Longstreth WT Jr, Manolio TA, O’Leary DH, Burke GL, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging 2007;28:1307-15. [DOI] [PubMed] [Google Scholar]
- 65.Ikram MA, Vernooij MW, Vrooman HA, Hofman A, Breteler MM. Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiol Aging 2009;30:450-6. [DOI] [PubMed] [Google Scholar]
- 66.Kerber KA, Whitman GT, Brown DL, Baloh RW. Increased risk of death in community-dwelling older people with white matter hyperintensities on MRI. J Neurol Sci 2006;250:33-8. [DOI] [PubMed] [Google Scholar]
- 67.Oksala NK, Oksala A, Pohjasvaara T, Vataja R, Kaste M, Karhunen PJ, et al. Age related white matter changes predict stroke death in long term follow-up. J Neurol Neurosurg Psychiatry 2009;80:762-6. [DOI] [PubMed] [Google Scholar]
- 68.Levy RM, Steffens DC, McQuoid DR, Provenzale JM, MacFall JR, Krishnan KR. MRI lesion severity and mortality in geriatric depression. Am J Geriatr Psychiatry 2003;11:678-82. [DOI] [PubMed] [Google Scholar]
- 69.Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996;27:1274-82. [DOI] [PubMed] [Google Scholar]
- 70.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology 2001;56:921-6. [DOI] [PubMed] [Google Scholar]
- 71.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 2004;35:1857-61. [DOI] [PubMed] [Google Scholar]
- 72.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 2006;18:418-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 2005;28:202-8. [DOI] [PubMed] [Google Scholar]
- 74.Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009;80:1194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke 2004;35:410-4. [DOI] [PubMed] [Google Scholar]
- 76.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke 2004;35:2616-9. [DOI] [PubMed] [Google Scholar]
- 77.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006;67:2192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol 2006;60:677-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bots ML, van Swieten JC, Breteler MM, de Jong PT, van Gijn J, Hofman A, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet 1993;341:1232-7. [DOI] [PubMed] [Google Scholar]
- 80.Kapeller P, Barber R, Vermeulen RJ, Ader H, Scheltens P, Freidl W, et al. Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003;34:441-5. [DOI] [PubMed] [Google Scholar]
- 81.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75-80. [DOI] [PubMed] [Google Scholar]
- 82.Dufouil C, Chalmers J, Coskun O, Besancon V, Bousser MG, Guillon P, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644-50. [DOI] [PubMed] [Google Scholar]
- 83.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, et al. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 2004;35:1609-13. [DOI] [PubMed] [Google Scholar]
- 84.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 1998;29:1177-81. [DOI] [PubMed] [Google Scholar]
- 85.Aharon-Peretz J, Daskovski E, Mashiach T, Tomer R. Natural history of dementia associated with lacunar infarctions. J Neurol Sci 2002;203-4:53-5. [DOI] [PubMed]
- 86.Fazekas F, Ropele S, Schmidt R. Can small-vessel disease-related cerebral abnormalities be used as a surrogate marker for vascular dementia trials? J Neural Transm Suppl 2002:61-7. [DOI] [PubMed]
- 87.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003;163:1069-75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables, figures, and references of included studies
Study quality criteria