Abstract
Functional imaging studies of psychopathy have demonstrated reduced activity in the anterior cingulate, yet it is unclear whether this region is structurally impaired. In this study, we used structural MRI to examine whether volumetric differences exist in the anterior cingulate between psychopathic (n=24) and control (n=24) male participants. We found no group differences in the volume of the anterior cingulate or its dorsal and ventral subregions. Our findings call into question whether the anterior cingulate is impaired in psychopathy, or whether previous findings of reduced activity may result from reduced input from other deficient regions.
Keywords: psychopathy, structural MRI, antisocial, emotion, cognition
1. Introduction
The anterior cingulate cortex (ACC) is a well-studied brain region that has been implicated in a many aspects of emotion and cognition. Its dorsal subdivision is involved in processes such as effortful control, self regulation, and signaling conflict or error, while its ventral/rostral subdivision is involved in affective processing, including empathy-related functions (Bush et al., 2000; Shirtcliff et al., 2009).
Given these functions, it is perhaps not surprising that the ACC has been implicated in studies of psychopathy, a personality disorder involving a pronounced lack of empathy, as well as poor self-regulation and decision-making. Two studies have observed reduced activity in the dorsal ACC (Kiehl et al., 2001; Muller et al., 2003), and several studies have found adult psychopathy to be associated with reduced activity in the ventral ACC during a variety of tasks, including affective memory (Kiehl et al., 2001), fear conditioning (Viet et al., 2002; Birbaumer et al., 2005), negative picture viewing (Muller et al., 2003), and during defection in the Prisoner’s dilemma (Rilling et al., 2007), although see Vollm et al. (in press).
Although these findings appear to suggest that abnormality in the ACC contributes to the deficits observed in psychopathy, it is unclear whether this is the case. Given its role as a form of relay station of information (Shirtcliff et al., 2009), the ACC is densely connected to regions such as the amygdala and orbitofrontal cortex (OFC), both of which are widely implicated in psychopathy. Because of this, it is unclear whether findings of reduced ACC during emotion-related tasks reflect a deficit within the ACC itself, or whether these findings reflect reduced input from associated regions.
One way to test this is to assess whether structural differences can be observed in the ACC, a hypothesis that has not been explored previously (Blair, 2009). An observable structural difference would indicate that the region is indeed impaired, and that reduced activity is not simply a result of reduced input from regions such as the amygdala. In the present study, we assessed the structure of the functionally distinct dorsal and ventral subregions of the ACC, in addition to total volume. Based on previous findings from the fMRI literature, we hypothesized that the volume of the ventral, but not dorsal, subregion would be reduced in psychopathic individuals.
2. Methods
A community sample of males (n=72) between ages 21 and 45 years were recruited from five temporary employment agencies. The only exclusion criteria were nonfluency in English, history of epilepsy, claustrophobia, pacemaker, and metal implants; participants were not screened for psychopathy or criminal behavior prior to entry in the study. The University of Southern California Institutional Review Board approved the study. Participants were informed that the purpose of the study was to examine various correlates of antisocial behavior. After complete description of the study to the subjects, written informed consent was obtained.
Psychopathy was assessed using the Psychopathy Checklist – Revised (PCL-R; Hare, 1991), supplemented by five sources of collateral data – the Interpersonal Measure of Psychopathy (Kosson et al., 1997), self-reported crime as assessed by an adult extension of the National Youth Survey self-report delinquency measure (Elliot et al., 1983), official criminal records and data derived from the Structured Clinical Interview for the DSM-IV mental disorders (SCID-I and SCID-II; First et al., 1996; First et al., 1997). To maximize confidentiality and minimize denial of self-report crime, a certificate of confidentiality was obtained from the Secretary of Health and Human Service under section 303 (a) of the Public Health Act 42. Scores on the two overarching factors of the PCL-R were also calculated to determine whether there may be volumetric differences specific to one factor or the other. Factor 1 represents the interpersonal and affective characteristics of psychopathy (e.g., glibness, superficial charm, pathological lying, shallow affect, lack of guilt, or remorse). Factor 2 represents the antisocial traits and behaviors (e.g., impulsivity, stimulation seeking, juvenile delinquency).
In order to compare individuals scoring higher and lower in psychopathy, we formed high and low groups. A cutoff score of 23 and above on the PCL-R was used for membership into the high-scoring (psychopathic) group (n = 24), maintaining consistency with prior studies (Ishikawa et al., 2001; Raine et al., 2003; Yang et al., 2005). The low-scoring group (controls) was designated as individuals scoring in the bottom third (0–14) on the measure (n = 24). Descriptive statistics for the two groups are provided in Table 1.
Table 1.
Comparisons Between the Control and Psychopathic Groups on Demographic and Psychopathy Measures
| Control Group (n = 24) |
Psychopathic Group (n = 24) |
Statistic | P Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 29.2 | 6.1 | 32.7 | 6.8 | t = 1.8 | 0.07 |
| Ethnicity, % white | 50 | 46 | χ2= 0.08 | 0.77 | ||
| Whole brain volume | 1171.5 | 121.4 | 1712.3 | 183.4 | t =0.03 | 0.98 |
| Handedness, % right | 83.0 | 83.0 | χ2=0.00 | 1.00 | ||
| Psychopathy Scores | ||||||
| Total | 11.5 | 2.3 | 28.0 | 5.0 | t = 14.6 | <.001 |
| Factor 1 | 3.6 | 2.0 | 10.8 | 2.7 | t = 10.3 | <.001 |
| Factor 2 | 5.5 | 1.7 | 12.4 | 3.1 | t = 9.5 | <.001 |
For MRI acquisition and processing, 128 three-dimensional T1-weighted gradient-echo 1.7 mm coronal slices were obtained using a 1.5-T scanner (model S15/ACS; Phillips, Shelton, Conn.), with matrix 256 × 256, field of view= 24 cm, TR= 34 ms, TE = 12.4 ms, flip angle= 35°, taken directly orthogonal to the anterior – posterior commissure line.
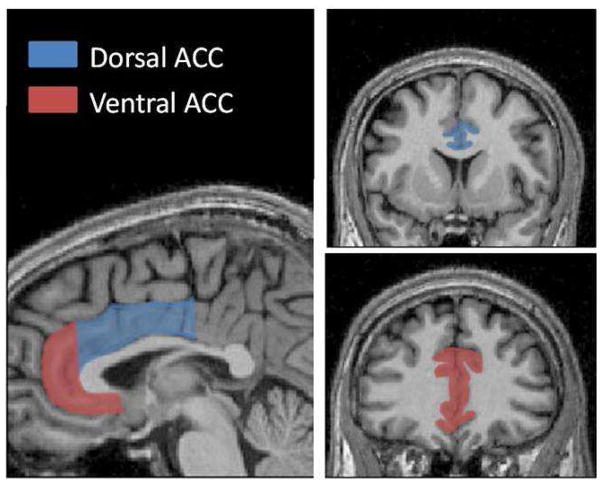
The anterior cingulate and its subregions were measured manually by one of the authors (ALG) blind to group status, by summing the total area measurements across all slices and multiplying by slice thickness. All volumetric measures were obtained in the coronal plane. Boundaries were based on the protocol by the Laboratory of Neuro Imaging (LONI) at the University of California, Los Angeles (http://www.loni.ucla.edu/~esowell/edevel/MedialLinesProtocol.htm) and by Rademacher et al. (1992). The division between dorsal and ventral ACC was based on Bush et al. (2000). Boundaries for defining the dorsal anterior cingulate segmentation were as follows and are depicted in Figure 1: posterior – the point at which the paracentral sulcus interrupts the cingulate sulcus; anterior – the coronal slice in which the most anterior part of the corpus callosum becomes visible; inferior – callosal sulcus; superior – cingulate sulcus. Boundaries for defining the ventral anterior cingulate were as follows: posterior boundary – the first coronal slice in which the anterior part of the corpus callosum can no longer be seen; anterior boundary – the cingulate sulcus; inferior boundary – the cingulate gyrus, in the event that it extends to the region inferior to the genu of the corpus callosum (if not, the first full gyrus ventral to the corpus callosum is the inferior boundary and is connected to the superior boundary); superior boundary – the cingulate sulcus. The anterior cingulate gyrus (dorsal and ventral) is bounded laterally by white matter. The test-retest reliability for the rater was found to be 0.94 using ten different scans.
Figure 1.
The anterior cingulate and its subregions. Left: Sagittal slice depicting the division between the dorsal and ventral subregions. Right: Coronal slices were used for tracing the subregions.
We used analysis of variance (ANOVA) to test for volumetric differences between the two groups in total anterior cingulate volume, and the volume of the dorsal and ventral subregions. Univariate ANOVAs with whole brain gray matter as a covariate were used to determine whether this factor affected results. Group analyses were followed up by correlational analyses including all 72 participants. Multiple regression was used to enter age as a covariate to determine whether this affected results.
3. Results
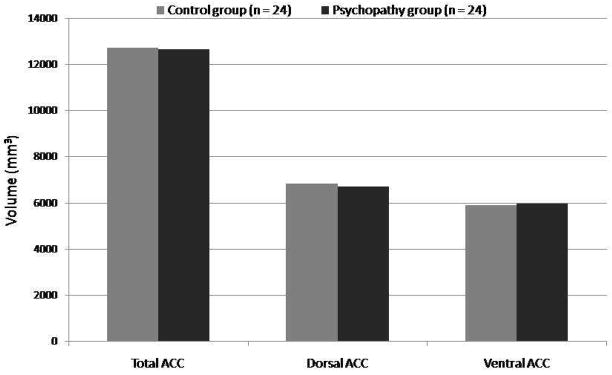
There were no significant differences between the two groups on volume of the total anterior cingulate (F(1,46)=0.005, P=0.95, d=0.02), dorsal anterior cingulate (F(1,46)= 0.07, p= 0.80, d= 0.07), or ventral anterior cingulate (F(1,46)=0.03, P=0.88, d=0.04) (Figure 2). This was true when controlling for whole brain gray matter volume (all P>0.55). Division of the subregions into right and left hemispheres also revealed no significant differences (all P>0.44).
Figure 2.
Mean volumes in the psychopathic and control groups.
Correlational analyses on the entire sample (N=72) revealed no significant associations (total ACC: r= −0.01, P= 0.92; dorsal ACC: r= −0.05, P=0.67; ventral ACC: r=0.03, P=0.78). Age was significantly associated with anterior cingulate volumes; however, when included in a regression analysis, psychopathy scores still did not significantly predict anterior cingulate volumes (all P>0.68).
We also examined correlations between anterior cingulate volumes and the two factors of psychopathy. No significant correlations were observed for Factor 1 (total ACC: r= −0.03, P=0.80; dorsal ACC: r= −0.05, P=0.65; ventral ACC: r=0.01, P=0.97) or Factor 2 (total ACC: r= −0.4, P=0.76; dorsal ACC: r= −0.08, P=0.53; ventral ACC: r=0.02, P=0.90).
To ensure that our lack of significant findings was not due to a lack of sufficient power, we conducted a power analysis using G*POWER (www.psycho.uni-duesseldorf.de/aap/projects/gpower/). We determined the effect sizes of several previous studies that have found volumetric differences in relation to psychopathy (Yang et al., 2005; Yang et al., 2009a; Yang et al., 2009b; Glenn et al., 2010). The smallest reported effect size from these studies was η2=0.13, reported by Glenn et al. (2010). Power analysis indicated that this effect size could be detected with power of 0.80 with a sample size of 55 (critical F(53)=4.02, delta=8.25). Thus, we concluded that our sample size of 72 was sufficient to have detected an effect of this size.
4. Discussion
Our findings of no difference in the volume of the ACC or its subregions call into question the notion that the ACC is compromised in psychopathy. These findings contrast results from several fMRI studies indicating reduced activity in the ACC of psychopathic individuals (Kiehl et al., 2001; Viet et al., 2002; Birbaumer et al., 2005; Rilling et al., 2007). One possible interpretation is that the anterior cingulate is not actually impaired in psychopathy. Rather, reduced functioning may be due to lack of input from other regions such as the amygdala and OFC. Others have suggested that the ACC may be hypoactive in psychopathic individuals because signals from other regions have not reached sufficient threshold to signal conflict within the ACC (Shirtcliff et al., 2009). Shirtcliff et al. (2009) note that most studies showing reduced ACC activation in individuals high on psychopathy also indicate reduced amygdala activation, suggesting that activity in the ACC may be dependent on sufficient input from the amygdala. The idea that the ACC is not in fact impaired supports the hypothesis of Blair (2009) that it is unlikely that abnormalities in the ACC contribute to psychopathic traits, as ACC lesions do not appear to give rise to the neuropsychological profile observed in this population (Blair, 2007).
An alternative explanation for the present results is that the functioning of the ACC is in fact impaired, but the impairment does not affect volume of the structure. For example, there may be altered connections between neurons within the cingulate, but not necessarily greater or fewer numbers. Thus, the present findings do not rule out the possibility that a deficit may exist.
Finally, the present findings in adult psychopathy differ from a recent structural imaging study that found increased volume of both the dorsal and ventral ACC in boys with callous-unemotional traits, which are thought to be precursors of adult psychopathic traits (De Brito et al., 2009). Future studies are necessary to clarify this discrepancy in child and adult populations, and to explore whether or not the ACC contributes to the development of psychopathy, as currently an articulated reason as to why it might be implicated has not yet been provided (Blair, 2009). Given its dense interconnections with regions that are consistently implicated in psychopathy, clarifying the role of the ACC in psychopathy is essential for furthering our understanding of the neurobiology of psychopathy.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health to Dr. Raine (Research Scientist Development Award No. K02 MH01114-01, Independent Scientist Award K02 MH01114-01, and Grant No. 5 R03 MH50940-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birbaumer N, Viet R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Too much of a good thing: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:831–832. doi: 10.1093/brain/awp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;46:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: Increased gray matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- Elliot DS, Ageton SS, Huizinga D, Knowles BA, Canter RJ. The prevalence and incidence of delinquent behavior: 1976–1980. Behavioral Research Institute; Boulder, CO: 1983. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I Clinician Version. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biological Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. Multi-Health Systems; Toronto: 1991. [Google Scholar]
- Ishikawa SS, Raine A, Lencz T, Bihrle S, Lacasse L. Autonomic stress reactivity and executive functions in successful and unsuccessful criminal psychopaths from the community. Journal of Abnormal Psychology. 2001;110:423–432. doi: 10.1037//0021-843x.110.3.423. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Steuwerald BL, Forth AE, Kirkhart KJ. A new method for assessing the interpersonal behavior of psychopathic individuals: Preliminary validation studies. Psychological Assessment. 1997;9:89–101. [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Schuierer G, Klein HE, Hajak G. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS. Human cerebral cortex; localization, parcellation, and morphometry. Journal of Cognitivie Neuroscience. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, Lee M, Ishikawa SS, Colletti P. Corpus Callosum Abnormalities in Psychopathic Antisocial Individuals. Archives of General Psychiatry. 2003;60:1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural Correlates of Social Cooperation and Non-Cooperation as a Function of Psychopathy. Biological Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MM, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27:131–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viet R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, McKie S, Reniers R, Elliot R, Anderson IM, Williams S, Dolan M, Deakin B. Neuronal correlates and serotonergic modulation of behavioral inhibition and reward in healthy and antisocial individuals. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2009.07.005. in press. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular Psychiatry. 2009a;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, Lacasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;15:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009b;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]