Abstract
T lymphocytes develop into two major lineages characterized by expression of the αβ and γδ T cell receptor (TCR) heterodimers. Within each major lineage, further specialization occurs, resulting in distinct subsets that differ in TCR specificity, phenotype and functional attributes. Thus, in the murine thymus, two distinct subsets of mature (CD24−) γδ cells have been identified, that is NK1.1+ cells, which are enriched for Vγ1.1 usage and selectively produce IFNγ on stimulation, and CCR6+ cells, which are enriched for Vγ2 usage produce IL17. The upstream signals and transcriptional pathways that promote development of these distinct γδ subsets remain relatively poorly understood. Here, we show that the Zn-finger transcription factor ThPOK has a critical function in the development of γδ thymocytes. Thus, lack of functional ThPOK causes a marked reduction in the percentage and absolute number of mature γδ thymocytes, and a particularly severe reduction of NK1.1+ cells. Conversely, constitutive ThPOK expression leads to a striking increase in mature NK1.1+ γδ thymocytes. Further, we show that ThPOK induction in γδ thymocytes is induced by strong TCR signals mediated by engagement with antibody or high-affinity endogenous ligands, and that an important ThPOK cis-acting element, the distal regulatory element (DRE), is sufficient for this TCR-dependent induction. These results show that ThPOK expression in γδ thymocytes is regulated in part by the strength of TCR signalling, identify ThPOK as an important mediator of γδ T cell development/maturation, and lend strong support to the view that development of a significant fraction of γδ T cells depends on TCR engagement/signalling.
Keywords: T lymphocyte development, ThPOK transcription factor, γδ T lymphocyte
Introduction
Most T lymphocytes undergo development in the thymus, in which they diverge into several lineages distinguished by the type and specificity of the T cell receptors (TCRs) they express, as well as their functional specializations. Developing T cells (thymocytes) progress through four stages defined according to their expression of the CD4 and CD8 surface markers, that is in order of maturity, CD4−CD8– (double negative or DN), CD4+CD8+ (double positive or DP) and CD4+CD8− or CD4−CD8+ (single positive or SP). Important lineage branch points occur at the DN and DP stages. At the DN stage, thymocytes diverge into alternate γδ and αβ lineages, depending on whether they express and transmit signals through the γδTCR or preTCR (an immature precursor of the αβTCR). Cells that adopt the γδ lineage remain DN, whereas cells committed to the αβ lineage progress to the DP stage. At the DP stage, αβ thymocytes diverge again into alternate CD4 and CD8 lineages, depending on the ability of their αβTCR to recognize major histocompatibility complex (MHC) class II or class I ligands, respectively, which are expressed by antigen-presenting cells in the thymus.
Within the DN thymocyte compartment, development proceeds through additional stages defined according to the expression of the surface markers CD44 and CD25, that is in order of maturity, CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3) and CD44−CD25– (DN4). γδTCR surface expression is initiated at the DN3>DN4 transition. Final maturation of γδ DN4 thymocytes is marked by re-expression of CD44 and downmodulation of the maturation marker CD24, so that mature and immature γδ thymocytes are defined by CD44+CD24− and CD44−CD24+ phenotypes, respectively. Consistent with functional maturity, CD44+CD24− γδ thymocytes can proliferate and secrete cytokines in response to γδTCR engagement, whereas CD44−CD24+ γδ thymocytes cannot (Haks et al, 2005; Haas et al, 2009). Mature γδ thymocytes can be further subdivided into CCR6+ (NK1.1−) and NK1.1+ (CCR6−) subsets, which differ in their capacity to produce either IL17 or IFNγ cytokines, respectively (Haas et al, 2009; Do et al, 2010).
There is considerable accumulating evidence that TCR signalling has an important function in the development of γδ thymocytes. First, almost all mature γδ thymocytes express high levels of CD44 and many express NK1.1, both of which are activation markers that are inducible on γδ thymocytes by antibody-mediated TCR crosslinking (Kreslavsky et al, 2009). Second, mature CD24− γδ thymocytes display a skewed TCR V region repertoire, in particular a larger proportion of CD24− than CD24+ cells express Vγ1.1, suggesting that they have undergone selection based on their TCR specificity for intrathymic ligands (Lees et al, 2001). Third, thymocytes expressing a transgenic γδTCR of defined specificity, the KN6 TCR, undergo maturation to the CD24− stage only in the presence of their specific intrathymic ligand, whereas in its absence, they undergo alternative development to the αβ lineage (Pereira et al, 1992; Haks et al, 2005). TCR signal strength seems to influence γδ development in at least two respects: (1) as mentioned, strong TCR signals seem to promote commitment to the γδ lineage at the expense of αβ commitment (Hayes et al, 2003, 2005; Haks et al, 2005; Lauritsen et al, 2006) and (2) within the γδ lineage, differentiation into specialized effector subsets seems to be controlled by differences in TCR signal strength. In particular, strong engagement by intrathymic ligands seems to promote development of IFNγ-producing γδ cells (Jensen et al, 2008). Although TCR signalling thus has an important function in γδ development, the downstream pathways and transcription factors that mediate alternate developmental signals remain to be defined.
Recently, we and others identified the Zn-finger transcription factor ThPOK as an important regulator of CD4/CD8-lineage choice by αβ thymocytes (He et al, 2005; Sun et al, 2005). Thus, functional deficiency or constitutive expression of ThPOK perturbs normal lineage choice and causes αβ thymocytes to develop exclusively to the CD8 or CD4 lineages, respectively (Keefe et al, 1999; He and Kappes, 2006). Given that both CD4 and γδ development has been postulated to be controlled by quantitative differences in TCR signalling (Matechak et al, 1996; Haks et al, 2005), we reasoned that the downstream transcription pathways used in these processes might be partly overlapping. Therefore, we have explored whether ThPOK might also have a function in the development of γδ thymocytes. In this study, we report that ThPOK is indeed expressed in a subset of developing γδ thymocytes, and that its expression is promoted by TCR stimulation in vitro and correlates with TCR signal strength in vivo. Second, we show that ThPOK is important for development of mature (CD24−) γδ thymocytes, particularly the NK1.1+ subset. These results provide strong support for the view that development of a substantial fraction of γδ thymocytes depends on TCR engagement and signalling, and indicate that ThPOK is an important downstream effector of this process.
Results
ThPOK is expressed in the γδ T cell lineage
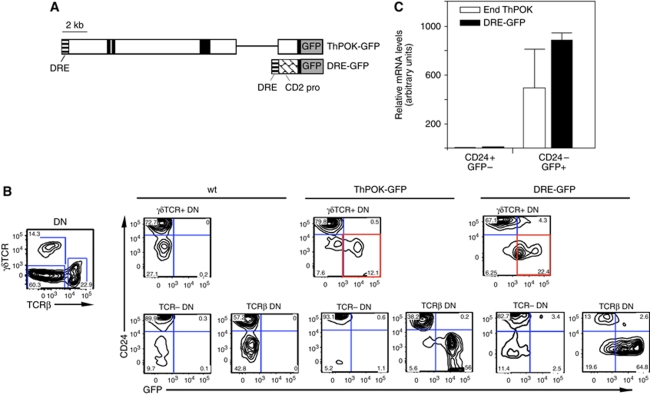
We and others have earlier reported that ThPOK is selectively induced during development to the SP CD4 stage, and is not expressed by SP CD8 cells or by their common DP precursor (He et al, 2005; Sun et al, 2005). ThPOK expression in the earlier DN subset has not been specifically examined. We, therefore, used transgenic reporter lines in which GFP is expressed under the control of ThPOK regulatory elements to assess ThPOK expression in DN thymocytes at the single-cell level. DN thymocytes can be divided into three categories, according to their TCR surface expression, that is TCRlow/− cells, which either express no TCR or express the preTCR (which is hard to detect by surface staining), αβTCR+ cells, which are mostly NKT cells, a sublineage of αβ cells that are derived from DP precursors, and γδTCR+ thymocytes, a distinct lineage, which diverges from the predominant αβ lineage at the DN stage. There is mounting evidence that development to the γδ lineage requires relatively strong TCR signals, compared with the development to the alternate αβ lineage (Haks et al, 2005; Hayes et al, 2005; Kreslavsky et al, 2008). Our initial analysis of ThPOK expression used the ThPOK-GFP transgene, in which reporter expression is controlled by a 12 kb ThPOK genomic fragment, including all earlier identified ThPOK control elements and promoters (Figure 1A) (He et al, 2008). FACS analysis of thymocytes from ThPOK-GFP reporter mice showed specific GFP expression in the CD4, but not CD8 lineages, as earlier reported (data not shown). More importantly, this analysis also revealed GFP expression in a significant fraction of DN thymocytes (Figure 1B). Most GFP+ DN thymocytes exhibit an αβTCR+ CD44+ phenotype, indicating that they likely belong to the αβNKT subset. Development and function of iNKT cells is severely perturbed in mice lacking functional ThPOK, indicating an important function for ThPOK in their differentiation (Engel et al, 2010). In addition, a significant fraction of γδTCR+ thymocytes also expresses GFP (Figure 1B). Real-time RT–PCR analysis showed that endogenous ThPOK transcripts were expressed in GFP+, but not GFP− γδ subsets from ThPOK-GFP mice, showing that reporter expression provides an accurate reflection of ThPOK transcriptional activity in the γδ lineage and is not a transgenic artefact (data not shown). Earlier, we and others have shown that a 500 bp distal regulatory element (DRE) located at the 5′ end of the ThPOK locus is necessary and sufficient for CD4-lineage-specific expression of ThPOK (He et al, 2008; Setoguchi et al, 2008). To assess whether the DRE element is also sufficient for reporter expression in γδTCR+ DN thymocytes, we used a transgenic DRE-GFP reporter line in which GFP expression is controlled by the DRE element in conjunction with a minimal hCD2 promoter (which by itself is insufficient for expression in thymocytes) (Figure 1A) (He et al, 2008). Significantly, we found that most mature (CD24−) γδTCR+ DN thymocytes in these DRE-GFP reporter mice express the reporter, similar to ThPOK-GFP mice (Figure 1B). The fact that the DRE-GFP reporter mediates stage-specific ThPOK expression in mature γδ thymocytes suggests that this small 500 bp DRE element is an important target of upstream factors that induce ThPOK in γδ thymocytes. Real-time RT–PCR analysis of sorted DN thymocyte subsets from DRE-GFP mice showed that GFP+ γδTCR+ cells express endogenous ThPOK mRNA (in addition to the expected GFP transcripts), whereas GFP− γδTCR+ thymocytes do not (Figure 1C). Hence, DRE-GFP similar to ThPOK-GFP reporter expression is regulated coordinately with endogenous ThPOK transcription. ThPOK levels in mature γδ TCR+ thymocytes are about four-fold less than for mature (CD69−) SP CD4 thymocytes (Supplementary Figure 1A). Of note, neither reporter construct includes ThPOK coding exons (the region detected by our ThPOK RT–PCR assay), so that ThPOK transcripts are unambiguously derived from the endogenous ThPOK locus rather than the reporter transgene. Finally, we examined whether DN cells lacking αβTCR or γδTCR expression, as well as markers of other haematopoietic lineages (Lin− cells) showed any reporter expression. Only some cells within the Lin− DN1 fraction showed GFP expression (see Supplementary Figure 1B). These GFP+ DN1 cells are negative for ckit (CD117) expression, and so do not belong to the CD117+ DN1a/b subsets that are believed to be the major canonical T cell precursors (Porritt et al, 2004). Instead, they seem to belong to the DN1d/e subsets, which apparently do not contribute significantly to canonical T cell development, and whose lineage potential is, therefore, unclear and potentially heterogenous (Porritt et al, 2004; Benz et al, 2008). Although it is formally possible that these Lin− GFP+ DN1d/e cells represent precursors of γδTCR+ GFP+ DN3/DN4 cells, this seems unlikely given that we detect no transitional GFP+ DN2 cells. Of note, because of the very low number of GFP+ DN1d/e cells, we were unable to perform RT–PCR to confirm that these cells also express endogenous ThPOK mRNA, so that it cannot be excluded that they represent a transgenic artefact.
Figure 1.
ThPOK expression by DN thymocyte subsets. (A) Diagrams of ThPOK-GFP and DRE-GFP reporter constructs. White bar represents ThPOK gene, with exons and DRE indicated in solid black and horizontal stripes, respectively. (B) FACS analysis of GFP expression by indicated gated DN thymocyte subsets from ThPOK-GFP or DRE GFP transgenic mice (left-hand panels). Note distinct population of GFP+ CD24− cells in γδTCR+ thymocytes from reporter lines, which are absent in control mice. (C) Real-time RT–PCR analysis of GFP and endogenous ThPOK expression in sorted γδTCR+ thymocyte subsets from DRE-GFP transgenic mice (right panel). Note the correlation between GFP and endogenous mRNA expression. Data is derived from three DRE-GFP mice, and error bars represent s.d.
ThPOK expression in γδ thymocytes marks mostly mature/activated cells
The fact that GFP+ γδ thymocytes are mostly CD24−, indicated that ThPOK expression by γδ thymocytes was developmentally regulated. To explore this further, we carried out a detailed analysis of GFP expression by γδ thymocyte subsets using additional cell surface markers. γδ thymocytes can be divided into developmental stages based on differential surface expression of the CD24, CD44 and CD25 markers. The most immature DN thymocytes that express surface γδTCR belong to the CD24+CD44−CD25+ (DN3) stage, which then gives rise to the CD24+CD44−CD25− (DN4) stage, and finally to the CD24−CD25− (mature γδ) stage. The majority of mature CD24−CD25− γδ thymocytes re-express CD44 and so resemble the earlier DN1 (CD44+CD25−) stage, but can be readily distinguished by expression of γδTCR and absence of CD24. Eight-colour FACS analysis of γδ thymocytes from DRE-GFP reporter mice showed that the highest level of GFP expression and the highest proportion of GFP+ cells occurred within the mature CD24−CD44+ fraction, although a significant fraction of immature CD24+CD44− cells also showed reporter expression, albeit mostly at lower levels (Figure 2A, bottom left panel). This suggests that immature GFP+ thymocytes may be precursors of mature GFP+ cells. Although most mature (CD24−) cells express the ThPOK reporter, they fall into distinct GFPint and GFPhi subsets (Figure 2A). Mature γδ thymocytes have been separated into distinct CCR6+ and NK1.1+ lineages, which possess mutually exclusive capacities to produce IL17 and IFNγ, respectively (Haas et al, 2009). NK1.1 is induced on antibody-mediated TCR crosslinking of γδTCR+ DN3 cells, suggesting that NK1.1+ γδ thymocytes have received a particularly strong TCR signal in vivo (Kreslavsky et al, 2008). Interestingly, CCR6+ (NK1.1−) and NK1.1+ (CCR6−) γδ thymocyte subsets exhibit different GFP expression levels, that is they are predominantly GFPint (intermediate) and GFPhi (high), respectively (Figure 2A). RT–PCR analysis indicates that both GFPint and GFPhi mature γδ subsets express endogenous ThPOK mRNA, but that mRNA expression is somewhat lower in GFPhi cells (Figure 2B). This difference is most likely explained by the long half-life of GFP protein, which may persist/accumulate even if mRNA production levels are low or declining. We postulate that NK1.1+ cells or their immediate precursors transiently express high levels of ThPOK, which then decline, whereas CCR6+ cells express moderate levels, which remain stable.
Figure 2.
ThPOK expression by γδ thymocytes increases with maturation. (A) FACS analysis of γδTCR+ DN thymocytes from DRE-GFP mice stained with anti-γδTCR, -TCRβ, -CD24, -CD44 and -NK1.1. GFP expression is shown for indicated γδTCR+ subsets (left-hand panels). Note that GFP expression is evident in a minority of immature (CD24+) γδTCR+ thymocytes, but a majority of mature (CD24+) γδTCR+ cells. (B) Real-time RT–PCR analysis of endogenous ThPOK expression for indicated sorted γδTCR+ thymocyte subsets from DRE-GFP transgenic mice (right panel). (C) FACS analysis of γδTCR+ DN thymocytes from DRE-GFP mice stained with anti-CD24, -CD44, -CD25 and -Vγ1.1. DN3, DN4 and mature (CD24−CD44+) fractions were gated and reanalysed for GFP expression. Vγ1.1 expression is plotted for gated GFP+ and GFP− subsets from each stage (right panels). Shaded histograms represent background fluorescence of equivalent gated thymocyte subsets from non-transgenic control mice.
To assess whether ThPOK expression also marks γδ thymocytes expressing a distinct TCR repertoire, we compared Vγ usage by GFP+ and GFP− γδ subsets. As NK1.1+ γδ cells are reported to be enriched for Vγ1.1 usage (Azuara et al, 1997; Lees et al, 2001), we specifically examined whether GFP+ γδ thymocytes also showed such a preference. Indeed, among mature (CD24−) γδTCR+ thymocytes from adult mice, ∼50% of GFP+ cells used Vγ1.1, compared with ∼20% of equivalent mature GFP− γδ cells, indicating a significant divergence in TCR repertoire between the GFP+ and GFP− fractions (Figure 2C, bottom right and centre panels). Increased Vγ1.1 usage among ThPOK-expressing cells suggests the possibility that ThPOK transcription is triggered by γδTCR engagement, as Vγ1.1+ cells are thought to be enriched for self-reactive specificities (Lees et al, 2001; Lauritsen et al, 2009). Interestingly, enrichment for Vγ1.1 usage was evident in GFP+ cells even at the DN3 stage, the first stage at which γδTCR surface expression can be detected, consistent with a function for ThPOK early in γδ development (Figure 2C, top right panel). The correlation between ThPOK expression and Vγ1.1 usage at the DN3 stage suggests that ThPOK transcription may be triggered by γδTCR engagement.
ThPOK is induced in γδ thymocytes by strong TCR signals
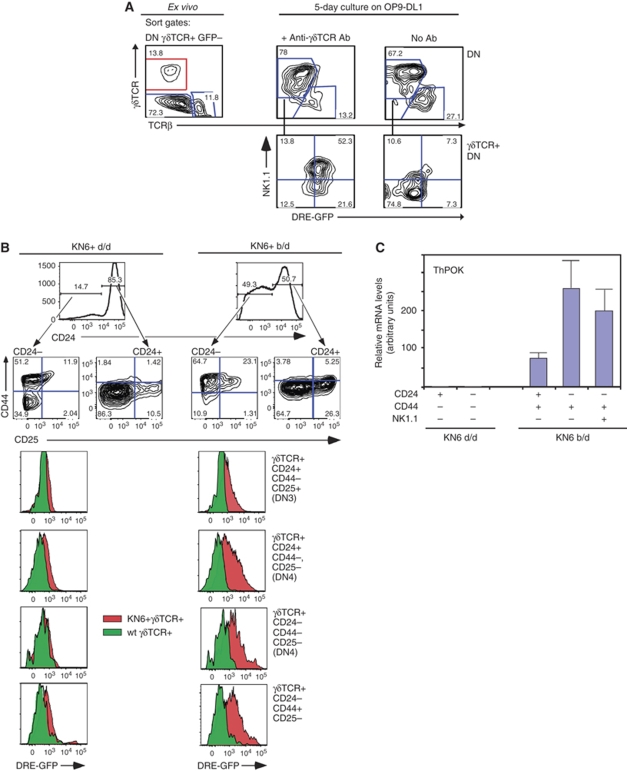
As outlined above, the predominantly activated (CD44+) phenotype of ThPOK+ γδ thymocytes as well as their increased Vγ1.1 usage both suggest that ThPOK transcription by these cells may be induced by strong TCR engagement. To directly test this hypothesis, we stimulated GFP− γδTCR+ DN3 and DN4 thymocytes from DRE-GFP mice with plate-bound anti-γδTCR antibody for 5 days. Significantly, stimulated but not untreated control cultures exhibited substantial GFP upregulation, strongly supporting a causal relationship between TCR engagement and ThPOK induction (Figure 3A). Nevertheless, it might be argued that TCR stimulation in these experiments leads to outgrowth of a minor contaminating population of GFP+ cells, rather than de novo generation of such cells from GFP− precursors. To exclude this possibility, we carried out similar antibody stimulation experiments using an immature DN thymic lymphoma cell line, Scid.adh (Carleton et al, 1999), transfected with the KN6 γδTCR (Bonneville et al, 1989). Antibody-mediated TCR stimulation of these KN6.Scid.adh cells leads to induction of the activation marker CD69 and downmodulation of CD25 consistent with partial differentiation, as earlier reported (Carleton et al, 1999). Importantly, these cells also exhibit dose-dependent upmodulation of ThPOK (Supplementary Figure 2). As KN6.Scid.adh cells are clonally derived and, therefore, unlikely to exhibit any pre-existing heterogeneity in ThPOK expression, this result provides compelling evidence for de novo induction of ThPOK in response to TCR stimulation. To test whether ThPOK expression in vivo also depends on TCR signalling, we used transgenic mice expressing the KN6 γδTCR, on either H-2d or H-2b backgrounds. The KN6 γδTCR represents one of the few examples of γδTCRs for which the ligand is known and in which ligand affinity can be modulated. In particular, the KN6 γδTCR recognizes the non-classical MHC class Ib products T10d and T10b with relatively low and high affinity, respectively (Adams et al, 2008). FACS and RT–PCR analysis of KN6+ thymocytes shows striking differences in ThPOK expression between the H-2d and H-2b backgrounds. In the presence of the weak T10d ligand, the majority of KN6+ thymocytes, including mature (CD24−) thymocytes express low or undetectable levels of ThPOK, as assessed by DRE-GFP reporter expression or by RT–PCR analysis of endogenous ThPOK mRNA (Figure 3B and C). Only a minor NK1.1+ CD44+CD24− population, comprising <0.5% of total γδ cells, shows substantial ThPOK expression by RT–PCR (data not shown). In contrast, in the presence of the strong T10b ligand, most KN6+ thymocytes exhibit GFP reporter expression, at both immature (DN3, DN4) and mature stages, showing a clear correlation between high affinity of γδTCR for endogenous ligand and ThPOK induction (similar results were obtained with both DRE-GFP and ThPOK-GFP mice) (Figure 3B and data not shown). To conversely test the consequences of impaired TCR signalling on ThPOK induction in γδ thymocytes, we used mice lacking Id3 (Pan et al, 1999). Id3 is an inhibitor of E proteins that is activated by TCR signalling and promotes thymocyte survival and proliferation (Bain et al, 2001; Xi et al, 2006). We have earlier shown that Id3 is important for mediating strong TCR signals that promote γδ development (Haks et al, 2005; Lauritsen et al, 2009). RT–PCR analysis of sorted γδ thymocyte subsets from Id3−/− versus wt mice, showed that ThPOK mRNA levels were significantly reduced in mature γδ thymocytes from Id3−/− mice, supporting a function for Id3 in ThPOK induction in γδ cells (Supplementary Figure 3A). As earlier shown, the Vγ repertoire in Id3−/− mice is skewed dramatically towards usage of Vγ1.1, which may reflect rescue of these putatively autoreactive cells from negative selection (Lauritsen et al, 2009; Ueda-Hayakawa et al, 2009) (Supplementary Figure 3B). Overall, the above results provide compelling evidence that ThPOK induction in DN thymocytes is mediated by TCR engagement, and that it requires relatively strong stimuli.
Figure 3.
ThPOK is induced in γδ thymocytes by TCR signalling. (A) γδTCR+ GFP− DN thymocytes were isolated from DRE-GFP mice, according to sort gate at left, cultured for 5 days on OP9-DL1 cells in the presence or absence of anti-γδTCR and reanalysed by FACS for expression of GFP, γδTCR and -NK1.1 (right-hand panels). GFP expression is shown for gated γδTCR+ subsets after culture. (B) Analysis of ThPOK expression by γδTCR+ DN thymocytes from H-2d/d or b/d mice carrying the KN6 γδTCR transgene. Thymocytes from DRE-GFP+ mice were stained with anti-γδTCR, -TCRβ, -CD24, -CD44 and -CD25, and GFP expression was assessed in the indicated gated subsets (left-hand panels). Red and green histograms represent equivalent gated populations from transgenic and non-transgenic mice, respectively. (C) Levels of endogenous ThPOK mRNA were assessed in indicated sorted γδTCR+ subsets from KN6+ mice H-2d/d or b/d mice (not carrying the DRE-GFP transgene) by real-time RT–PCR analysis (right panel).
Function of ThPOK in γδ development
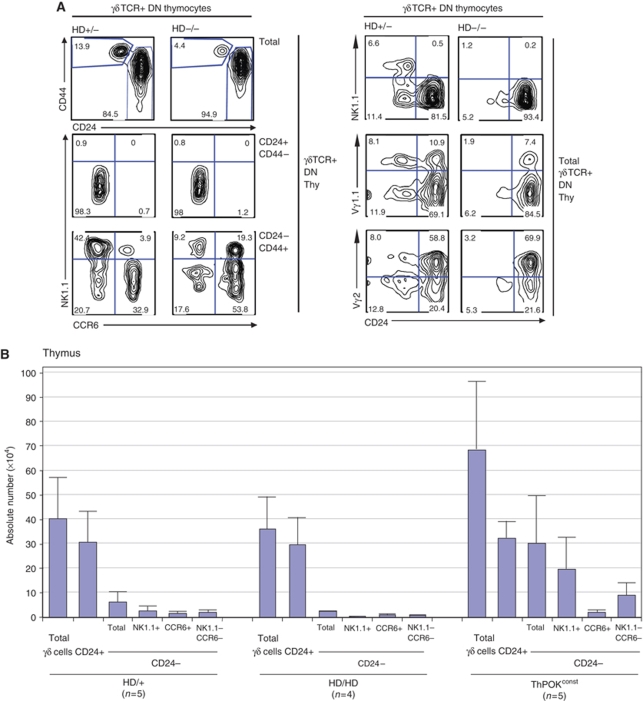
To investigate whether ThPOK has any function in γδ development, we next assessed γδ thymocyte proportions and surface marker expression in mice lacking functional ThPOK (ThPOKHD/HD or HD/HD mice) (Davé et al, 1998). Note that HD/HD mice are congenic for the HD mutation (backcrossed for 20 generations to the C57BL/6 background) and are maintained through HD/HD × HD/+ intercrosses to produce age-matched HD/HD and HD/+ littermates of a uniform genetic background. FACS analysis of adult HD/HD and HD/+ mice shows that proportions and absolute number of total γδ thymocytes are similar, but that HD/HD mice exhibit a marked three-fold reduction of mature (CD24−) γδ thymocytes (Figure 4A, top left panels). As mentioned, mature CD24− γδ thymocytes have been subdivided into distinct CCR6+ and NK1.1+ lineages (Lees et al, 2001; Haas et al, 2009). Absolute numbers of both subsets are reduced in HD/HD mice, but the NK1.1+ subset is affected most severely, such that the ratio of CCR6+ versus NK1.1+ cells is reversed (Figure 4A and B). As noted above, both CCR6+ and NK1.1+ γδ subsets express ThPOK, as measured by DRE-GFP reporter expression, but the NK1.1+ subset is enriched for cells expressing highest GFP levels. The GFPhi subset was severely depleted in mature γδ thymocytes from HD/HD mice, consistent with the preferential loss of NK1.1+ thymocytes (Supplementary Figure 4). Analysis of Vγ region usage at the mature (CD24−) stage showed a significant decrease in Vγ1.1 usage in HD/HD mice, indicative of a shift in the TCR repertoire (20% Vγ1.1+ cells in HD/HD mice, compared with 45% in HD/+ littermates) (Figure 4A, right panels). By comparison, the relative usage of Vγ2 by mature γδ thymocytes is slightly increased in HD/HD mice (45% of mature γδ thymocytes in HD/HD mice, compared with 38% in HD/+ littermates), although the absolute number of mature Vγ2+ cells is also significantly decreased (Supplementary Figure 5). We further examined the effect of ThPOK deficiency on γδ development early in ontogeny, that is in neonates. Consistent with adults, there was a severe reduction in the overall number of mature γδ thymocytes. Most γδ thymocytes in wt neonates express the Vγ2 segment, and ∼40% of Vγ2+ thymocytes are mature. Similarly, in HD/HD neonates, most γδ thymocytes express Vγ2, but only ∼20% of these cells belong to the mature CD24− fraction (Supplementary Figure 6). Hence, the effect of ThPOK deficiency on γδ development is already apparent early in ontogeny, when there has been little/no opportunity for post-maturation outgrowth of particular γδ subsets. Finally, we examined the effect of ThPOK deficiency on development of thymocytes expressing a single γδTCR specificity, that is the KN6 γδTCR on the H-2b background (in which ThPOK is highly induced). A high proportion of KN6+ H-2b HD/+ thymocytes develop to the mature (CD24−) stage, and a significant number accumulate in the periphery, consistent with earlier studies (Supplementary Figure 7, right panels) (Pereira et al, 1992). In the absence of functional ThPOK, both the proportions of mature KN6+ thymocytes as well as peripheral KN6+ cells are greatly reduced, indicating a severe defect in development (Supplementary Figure 7, left panels). Overall, these results show that ThPOK has an important physiological function in promoting development of diverse γδ subsets including endogenous Vγ1.1+ and Vγ2+ subsets, as well as KN6 transgenic thymocytes.
Figure 4.
ThPOK deficiency impairs γδ thymocyte maturation. (A) FACS analysis of γδTCR+ DN thymocytes from 1-month-old HD/HD and HD/+ littermates, showing expression of CD24, CD44, NK1.1, CCR6, Vγ1.1 and Vγ2 for indicated gated subsets. (B) Absolute numbers of indicated γδTCR+ thymocyte subsets from 6-week-old HD/HD (n=4), HD/+ (n=5) and ThPOKconst mice (n=5). Error bars indicate s.d.
Given that ThPOK deficiency impairs normal γδ development, we reasoned that ectopic ThPOK expression might conversely promote γδ maturation. To test this, we used a transgenic line expressing ThPOK constitutively during T cell development under the control of the CD4 promoter/enhancer (ThPOKconst mice). Of note, although the CD4 enhancer/promoter construct is predicted to initiate transcription at the DN4/DP transition, expression of this ThPOKconst transgene is already detected at the DN2 stage (the reason for this unexpectedly early expression pattern is unknown, but presumably depends on the founder-specific transgene integration site) (Supplementary Figure 8A). Significantly, ThPOKconst mice exhibit a five to seven-fold increase in the proportion of mature (CD24−CD44+) thymocytes most of which exhibit an NK1.1+ Vγ1.1+ phenotype, supporting an important function for ThPOK in development, proliferation and/or survival of mature γδ thymocytes, and of the NK1.1+ γδ subset in particular (Figures 4B and 5A–C). The proportion of γδ cells in the spleen and lymph nodes of ThPOKconst mice was also increased, although the absolute number of cells in the spleen was not (Figures 5C and 6C). Interestingly, some γδTCR+ thymocytes and a substantial proportion of peripheral γδ cells express CD4, suggesting that persistent/increased ThPOK expression can lead to adoption of some features of the CD4-lineage commitment programme by γδ cells (Supplementary Figure 8C). In ThPOKconst mice carrying the KN6 γδTCR transgene in the presence of the low-affinity T10d ligand, CD44 and NK1.1 expression was significantly upmodulated in both immature and mature γδ subsets, suggesting a function in thymocyte activation (data not shown). In view of the significant effects of ThPOK deficiency and ThPOK overexpression on γδ thymocyte proportions and Vγ usage, we conclude that ThPOK is, in fact, an important regulator of development, survival and/or proliferation of these subsets.
Figure 5.
Constitutive ThPOK expression promotes γδ thymocyte maturation. (A) FACS analysis of total DN or γδTCR+ DN thymocytes from 1-month-old ThPOKconst and wt littermates stained with anti-γδTCR, -TCRβ, -CD24, -CD44, -NK1.1 and -CCR6. (B) FACS analysis of γδTCR+ DN thymocytes from 6-week-old ThPOKconst and wt littermates, stained with anti-Vγ1.1, -NK1.1 and -CD24. (C) FACS analysis of total lymph node or spleen cells from ThPOKconst and wt littermates stained with anti-γδTCR and -TCRβ.
Figure 6.
Effect of ThPOK deficiency on peripheral γδ T cell subsets. (A) FACS analysis of total or gated γδTCR+ splenocytes, as indicated, from HD/HD, HD/+ and ThPOKconst mice, showing expression of NK1.1, CCR6 and DRE-GFP reporter. (B) FACS analysis of γδTCR+ splenocytes, showing Vγ2 expression for indicated gated NK1.1/CCR6 subsets. (C) Absolute numbers of indicated γδTCR+ splenic subsets from 1-month-old HD/HD (n=4), HD/+ (n=5) and ThPOKconst mice (n=5). Error bars indicate s.d.
To test whether ThPOK affects proliferation of γδ thymocytes, we carried out in vivo BrdU labelling of wt, HD/HD and ThPOKconst thymocytes. After a short BrdU pulse, the amount of incorporation by γδ thymocytes was found to correlate inversely with the level of maturity, that is γδTCR+ DN3 cells exhibited greatest incorporation, whereas γδTCR+ DN4 and mature (CD24−CD44+) γδTCR+ cells exhibited progressively less (Supplementary Figure 9A, left-hand panels). Only after a longer chase period did any label move into the mature fraction. Of note, there was no consistent difference in the proportion of BrdU-labelled γδTCR+ DN3 or DN4 cells between wt, HD/HD and ThPOKconst mice, suggesting that ThPOK has little effect on proliferation of these cells. The fact that mature γδTCR+ cells took up virtually no label in these short pulse experiments indicates that they proliferate very slowly. We tested whether extended BrdU treatment would eventually lead to BrdU incorporation by mature γδTCR+ cells because of recruitment of cells labelled at earlier stages. Indeed, after 7 days of continuous BrdU treatment, 15–20% of mature γδTCR+ cells became labelled (Supplementary Figure 9A, right-hand panels). Again, there was no difference in the percentage of labelled mature cells in HD/HD or ThPOKconst mice with respect to wt controls, suggesting that turnover, that is the balance between recruitment and egress of cells from the mature γδTCR+ thymocyte compartment, is not significantly altered in these mice. We infer that ThPOK exerts its effect on an early developmental checkpoint that controls the rate at which γδ cells progress to the mature stage, rather than at the level of mature γδ cell proliferation.
ThPOK deficiency alters peripheral γδ subset representation
Comparison of splenocytes from HD/+ and HD/HD mice revealed similar absolute numbers of γδ cells, virtually all of which express low CD24 levels (Figure 6C; data not shown). However, the distribution of γδ subsets, as defined by expression of NK1.1 and CCR6 markers, was markedly altered. In particular, the absolute number of CCR6+ γδ cells was increased two to three-fold in HD/HD mice, whereas the number of CCR6− NK1.1− γδ cells was reduced (Figure 6B and C). Splenic CCR6+ γδ cells, such as CCR6+ γδ thymocytes, exhibit almost exclusive usage of Vγ2 on both HD/+ and HD/HD backgrounds (Figure 6B). It, therefore, seems unlikely that the increase in CCR6+ γδ splenocytes in HD/HD mice is due to diversion of cells from the NK1.1+ or NK1.1− CCR6− fractions, as the latter populations include much lower proportions of Vγ2+ cells. We also examined representation of CCR6+ and NK1.1+ γδ subsets in the spleen of ThPOKconst mice. Interestingly, the number of NK1.1+ γδ splenocytes was markedly reduced compared with wt mice, suggesting that constitutive expression of ThPOK may inhibit survival/expansion of these cells in the spleen (Figure 6C). Most NK1.1+ γδ splenocytes from wt mice are GFPlo/−, indicating that they normally downregulate ThPOK, consistent with the possibility that continued ThPOK expression is deleterious to persistence of NK1.1+ γδ splenocytes (Figure 6A). Given that NK1.1+ and CCR6+ γδ subsets have been shown to preferentially secrete IFNγ or IL17, respectively, on stimulation (Haas et al, 2009; Martin et al, 2009), we assessed whether this was also the case in HD/HD and ThPOKconst mice. In both kinds of mice, IFNγ and IL17 production remained largely restricted to NK1.1+ and CCR6+ γδ subsets, respectively, indicating that acquisition of these effector functions is not regulated by ThPOK (Supplementary Figure 9B). In addition, the fraction of cells expressing each cytokines and the levels of expression were similar to wt mice.
CD122 and CD25 have also been used to distinguish different functional γδ subsets in the periphery, including in the peritoneal cavity (Jensen et al, 2008; Shibata et al, 2008). CD122 and CD25 seem to identify similar, though not necessarily completely overlapping, γδ subsets to those marked by NK1.1 and CCR6 expression, respectively. Thus, NK1.1+ and CCR6+ γδ thymocytes have been shown to be largely CD122+ and CD122−, respectively (Haas et al, 2009), and peripheral CD122+ and CD122− (CD25+) subsets preferentially produce IFNγ and IL17A, respectively (Jensen et al, 2008; Shibata et al, 2008), similar to NK1.1+ and CCR6+ subsets. Analysis of peritoneal cavity γδ cells in HD/HD mice revealed that the relative proportion and absolute number of CD122+ γδ cells was selectively reduced, paralleling the reduction in NK1.1+ γδ thymocytes in these mice (Supplementary Figure 10). These changes could reflect developmental or post-developmental effects occurring in the periphery.
Discussion
Although TCR engagement and signalling are widely acknowledged to have a critical function in αβ T cell development, that is in thymic selection and CD4/CD8-lineage commitment, it remains controversial whether this is also true for γδ development. Direct evidence that TCR engagement and signalling can promote γδ T cell development comes from studies showing that thymocytes expressing a transgenic γδTCR of defined specificity become functionally mature only in the presence of specific ligand (Haks et al, 2005). However, as endogenous ligands have not been identified for most γδ thymocytes, it is unclear whether these findings are applicable to γδ thymocytes in general. Furthermore, the transcriptional pathways that control γδ development remain poorly characterized. Here, we provide evidence that the Zn-finger transcription factor ThPOK is induced by TCR signalling and promotes γδ maturation in the thymus, and thus represents an important effector of TCR-mediated γδ development.
We present two observations in support of a mechanistic link between TCR engagement on γδ thymocytes and ThPOK induction. First, ThPOK is induced in vitro by strong antibody-mediated TCR stimuli both in primary γδ thymocytes and in a γδTCR-expressing DN cell line. Second, ThPOK is expressed in vivo by almost all KN6 γδTCR transgenic thymocytes in the presence of the high-affinity T10b ligand. The fact that KN6 thymocytes express ThPOK only in the presence of the high-affinity T10b, but not the lower-affinity T10d ligand, implies that ThPOK induction by γδ thymocytes requires relatively strong TCR signals. This resembles control of ThPOK during αβ development, in which ThPOK is induced preferentially in cells expressing class II-restricted TCRs, which are believed to transmit stronger TCR signals than class I-restricted TCRs (He et al, 2005). Our results indicate a direct causal relationship between TCR signalling and ThPOK induction in γδ thymocytes, although it cannot be excluded that other non-TCR-mediated signals also contribute to ThPOK induction.
Our results further show that ThPOK has a critical function in controlling γδ development, particularly of cells with high reactivity to thymic self-ligands. First, the proportion of mature (CD24−) γδ thymocytes is severely reduced in HD/HD mice, and conversely greatly increased in ThPOKconst mice. Particularly affected in both kinds of mice are NK1.1+ cells, which are believed to be enriched for self-reactive specificities (Lees et al, 2001). Second, the Vγ repertoire of mature γδ thymocytes in both kinds of mice is significantly altered compared with wt littermates. In particular, the proportion of Vγ1.1+ cells within the mature γδ thymocyte compartment is severely reduced in adult HD/HD mice and conversely increased in ThPOKconst mice. In DRE-GFP mice, the γδ repertoire is already skewed towards Vγ1.1 usage in ThPOK-expressing DN3 cells, consistent with the idea that ThPOK affects γδ selection from the earliest point at which surface γδTCR is expressed.
In the light of our evidence that ThPOK is induced in response to strong TCR signals, it may seem counter-intuitive that ThPOK is still expressed by γδ thymocytes from Id3−/− mice, even though Id3 deficiency severely impairs development of KN6 thymocytes and DETC γδ cells (Lauritsen et al, 2009). Significantly, mature γδ thymocytes from Id3−/− mice almost all express the Vγ1.1 segment. The same phenomenon occurs in CD3δ−/−mice in which TCR signalling is impaired by diminishing TCR surface expression (Park K and Kappes DJ, unpublished data). We suspect that preferential development of Vγ1.1+ cells in these situations reflects a selective advantage for strongly self-reactive specificities, which seem to be disproportionately represented within the Vγ1.1+ fraction. High-affinity γδTCRs could compensate for downstream signalling deficiencies, for instance, by sustaining longer signals, and thus still promote ThPOK induction. Although no self-ligands for Vγ1.1+ cells have yet been identified, there is circumstantial evidence that cellular selection controls their development (Azuara et al, 1998). Furthermore, many Vγ1.1 thymocytes exhibit an activated CD44+ NK1.1+ phenotype, resembling αβ NKT cells, which are well known to be selected by thymic self-antigens (Kronenberg and Engel, 2007). Hence, one might speculate that Vγ1.1+ cells developing on a signalling deficient background are particularly high-affinity cells, perhaps including cells that would normally undergo negative selection.
Although ThPOK+ (GFP+) γδ thymocytes are enriched for Vγ1.1 usage (∼50 versus ∼30% among total γδ cells), a large proportion of ThPOK+ cells express Vγ2 or other Vγ segments, and in the absence of functional ThPOK, both mature Vγ1.1+ and Vγ2+ subsets are diminished. In contrast, another POK family factor found in γδ thymocytes, Plzf, seems to be expressed almost exclusively by Vγ1.1+ thymocytes (Kreslavsky et al, 2009; Alonzo et al, 2010). Although the proportion of mature (CD24−) γδ thymocytes is dramatically reduced in the absence of functional ThPOK, a significant number of mature γδ thymocytes still develop, indicating that ThPOK is not absolutely required for γδ cell development. Furthermore, KN6 thymocytes developing in the presence of the lower-affinity T10d ligand do not express detectable ThPOK. Taken together, these observations suggest that a subset of γδ thymocytes that recognizes ligand with lesser affinity does not require ThPOK for maturation. This raises the interesting question of why higher-affinity γδ thymocytes would require ThPOK for their development. One possibility is that ThPOK expression protects these cells from negative selection.
Although, representation of mature γδ thymocytes is drastically impaired by ThPOK deficiency, there is no corresponding decrease in total peripheral γδ cell numbers in HD/HD mice. There are at least two possible, non-mutually exclusive, explanations for this observation: (1) although the rate of export of mature γδ thymocytes is likely reduced in HD/HD mice, remaining emigrants may undergo homeostatic expansion in the periphery to fill available niches, and (2) immature γδ thymocytes, which are not diminished in HD/HD mice, may be exported from the thymus and undergo post-thymic development in the periphery. Regarding the latter possibility, it remains unclear whether peripheral γδ cells are descended mostly from mature or immature thymic precursors. In vivo labelling studies indicate that the majority of γδ thymocytes that have recently entered the periphery (recent thymic emigrants or RTEs) exhibit a CD24+ phenotype, and undergo CD24 downmodulation only after arriving in the periphery (Kelly et al, 1993; Tough and Sprent, 1998), in contrast to αβ RTEs, which are mostly CD24− (Kelly and Scollay, 1990). Further, it has been argued that the rate of production of CD24− γδ thymocytes is too low to account for the appearance of new γδ cells in the periphery (Zorbas and Scollay, 1993). Nevertheless, as γδ RTEs are not uniformly CD24+ and, in fact, contain a substantial CD24−/lo fraction (Kelly et al, 1993; Zorbas and Scollay, 1993), and as a high proportion of CD24+ RTEs seem to die off soon after reaching the periphery (Tough and Sprent, 1998), the relative contribution of CD24+ and CD24− RTEs to the long-lived peripheral γδ pool remains difficult to estimate. Regardless, of the precise developmental route by which peripheral γδ cells arise, they exhibit significant differences in subset distribution in ThPOK-deficient mice, consistent with an important function for ThPOK in development of these cells as well. In particular, they display a marked increase in the proportion of CCR6+ cells. Although CCR6+ cells from HD/HD mice are essentially all Vγ2+, as in wt mice, it will be interesting to assess in future whether they exhibit more subtle changes in TCR repertoire at the level of junctional diversity and/or Vδ usage.
The present results considerably expand the known functions of ThPOK in controlling T lymphoid development and function. Three distinct functions have now been reported: (1) promoting CD4-lineage commitment at the expense of CD8 commitment (He et al, 2005; Sun et al, 2005), (2) promoting clonal expansion and functional responses of CD8 T cells during acute viral infection (Setoguchi et al, 2009), and (3) promoting maturation of a subset of γδ thymocytes with high affinity for intrathymic ligands, as shown here. In all three situations, it seems that ThPOK induction occurs in response to a relatively strong TCR stimulus, as mimicked by antibody-mediated TCR crosslinking (He et al, 2008; Setoguchi et al, 2009), arguing that this is a general mechanism governing ThPOK expression in T lymphocytes. Strikingly, the DRE element is sufficient to mediate appropriate stage-specific ThPOK induction both during CD4 and γδ development (He et al, 2008), arguing that ThPOK transcriptional control is regulated similarly in these two situations. Clearly, however, downstream outcomes in terms of lineage-specific gene expression programme are quite different for each subset. This could reflect different kinetics/levels of ThPOK induction, cell-intrinsic differences in transcriptional regulatory networks and/or differences in environmental cues and co-stimulatory signals.
During the submission process of this manuscript, a report appeared, which also showed expression of ThPOK in a significant fraction of γδ thymocytes and splenocytes (Alonzo et al, 2010). This study focused primarily on development of a small subset of γδ cells defined by expression of the Vγ1.1/Vδ6.3 heterodimer. Although many Vγ1.1+Vδ6.3+ splenocytes showed high or intermediate expression levels of ThPOK, the frequency of such cells was apparently not significantly diminished by the absence of ThPOK. This is consistent with our analysis, which shows that ThPOK deficiency primarily affects maturation and subset distribution of γδ thymocytes, but has more subtle effects on peripheral γδ cells.
The precise function of ThPOK in γδ development remains to be elucidated. The present data suggests that ThPOK acts early in the development of γδ thymocytes to drive their maturation and/or effector differentiation, rather than late in the development to promote survival/expansion of mature γδ thymocytes. Future experiments to compare the developmental potential of immature γδTCR+ thymocytes from ThPOK deficient and wt thymocytes at the single-cell level may clarify these questions.
Materials and methods
Mice
HD/HD (Davé et al, 1998), ThPOK-GFP (He et al, 2008) (referred to as F2F3), DRE-GFP (He et al, 2008) (referred to as F2c-pCD2), KN6 transgenic (Ishida et al, 1990) and Id3−/− mice (Pan et al, 1999) have been earlier described. ThPOKconst mice were generated by inserting wild-type ThPOK cDNA into a pCD4 vector consisting of the CD4 promoter and proximal enhancer (Ellmeier et al, 1999). Animal care was in accordance with National Institutes of Health (NIH) guidelines.
TCR stimulation assays
For in vitro stimulation of thymocytes, 2–5 × 105 sorted thymocytes were cultured on OP9-DL1 monolayers in DMEM with 10% FSC on 96-well plates, as described (Haks et al, 2005). Plates were untreated or precoated with 0.02 μg/ml anti-γδTCR (UC7-13D5; BD-Pharmingen), as indicated. For in vitro stimulation of KN6.Scid.adh cells, 1 × 106 cells were cultured overnight in 96-well plates that were untreated or precoated with anti-CD3e (2C11) or -γδTCR (UC7-13D5; BD Pharmingen) at the indicated concentration. The KN6.Scid.adh cell line was generated by retroviral transduction of Scid.adh cells with the KN6 TCRγ and TCRδ subunits connected by the 2A Tescovirus linker peptide, as described (Lauritsen et al, 2009).
Flow cytometry
Cells were prepared from thymus, and other lymphoid organs, and analysed by flow cytometry according to standard procedures. All antibodies were obtained commercially (from BioLegend, eBioscience, BD-PharMingen, or R&D Systems), except for anti-Vγ1.1 (kindly provided by R O'Brien, NJH, Denver).
Intracellular cytokine assays
For measurement of intracellular cytokine levels, sorted CCR6+ and NK1.1+ γδ subsets were cultured for 4 h in Iscove's MDM (with 10% FCS and 1000 IU/ml human rIL2) in the presence of 50 ng/ml PMA and 2 μg/ml ionomycin, and for an additional 3 h in the presence of GolgiStop protein transport inhibitor (BD). Treated cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD), and stained with either anti-IFNγ or -IL17A antibodies.
Real-time RT–PCR
Real-time RT–PCR analysis for ThPOK and EGFP was carried out according to the probe-based method and analysed by the comparative Ct method (compared with β-actin). Primer and probe sequences are available on request.
Supplementary Material
Acknowledgments
We thank J Oesterling for expert flow cytometry, Emmanuelle Nicolas for real-time RT–PCR analysis and Dai Zhongping and Yi Zhang for technical assistance. This research was supported by grants from the NIH grants to DJK (AI42915), DLW (AI081314), Fox Chase Cancer Center (PO1CA06927), and by an appropriation from the Commonwealth of Pennsylvania.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams EJ, Strop P, Shin S, Chien YH, Garcia KC (2008) An autonomous CDR3delta is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by gammadelta T cells. Nat Immunol 9: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant'Angelo DB (2010) Development of promyelocytic zinc finger and ThPOK-expressing innate γδ T cells is controlled by strength of TCR signaling and Id3. J Immunol 184: 1268–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara V, Lembezat MP, Pereira P (1998) The homogeneity of the TCRdelta repertoire expressed by the Thy-1dull gammadelta T cell population is due to cellular selection. Eur J Immunol 28: 3456–3467 [DOI] [PubMed] [Google Scholar]
- Azuara V, Levraud JP, Lembezat MP, Pereira P (1997) A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol 27: 544–553 [DOI] [PubMed] [Google Scholar]
- Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C (2001) Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol 2: 165–171 [DOI] [PubMed] [Google Scholar]
- Benz C, Martins VC, Radtke F, Bleul CC (2008) The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med 205: 1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M, Ito K, Krecko EG, Itohara S, Kappes D, Ishida I, Kanagawa O, Janeway CA, Murphy DB, Tonegawa S (1989) Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci USA 86: 5928–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton M, Ruetsch NR, Berger MA, Rhodes M, Kaptik S, Wiest DL (1999) Signals transduced by CD3epsilon, but not by surface pre-TCR complexes, are able to induce maturation of an early thymic lymphoma in vitro. J Immunol 163: 2576–2585 [PubMed] [Google Scholar]
- Davé VP, Allman D, Keefe R, Hardy RR, Kappes DJ (1998) HD mice: a novel mouse mutant with a specific defect in the generation of CD4(+) T cells. Proc Natl Acad Sci USA 95: 8187–8192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J-S, Fink PJ, Li L, Spllski R, Robinson J, Leonard WL, Letterio JL, Min B (2010) Spontaneous development of IL-17-producing γδ T cells in the thymus occurs via a TGF-b1-dependent mechanism. J Immunol 184: 1675–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmeier W, Sawada M, Littman DR (1999) The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Ann Rev Immunol 17: 523–554 [DOI] [PubMed] [Google Scholar]
- Engel I, Hammond K, Sullivan BA, He X, Taniuchi I, Kappes D, Kronenberg M (2010) Co-receptor choice by Vα14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection. J Exp Med 207: 1015–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Malinarich González FH, Schmitz S, Chennupati V, Föhse L, Kremmer E, Förster R, Prinz I (2009) CCR6 and NK1.1 distinguish between IL-17A and IFN-γ producing effector T cells. Eur J Immunol 39: 3488–3497 [DOI] [PubMed] [Google Scholar]
- Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL (2005) Attenuation of gammadelta TCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 22: 595–606 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Li L, Love PE (2005) TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity 22: 583–593 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Shores EW, Love PE (2003) An architectural perspective on signaling by the pre-, alphabeta and gammadelta T cell receptors. Immunol Rev 191: 28–37 [DOI] [PubMed] [Google Scholar]
- He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ (2005) The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433: 826–833 [DOI] [PubMed] [Google Scholar]
- He X, Kappes DJ (2006) CD4/CD8 lineage commitment: light at the end of the tunnel? Curr Opin Immunol 18: 135–142 [DOI] [PubMed] [Google Scholar]
- He X, Park K, Wang H, Zhang Y, Hua X, Li Y, Kappes DJ (2008) CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 28: 346–358 [DOI] [PubMed] [Google Scholar]
- Ishida I, Verbeek S, Bonneville M, Itohara S, Berns A, Tonegawa S (1990) T-cell receptor gamma delta and gamma transgenic mice suggest a role of a gamma gene silencer in the generation of alpha beta T cells. Proc Natl Acad Sci USA 87: 3067–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH (2008) Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 29: 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe R, Dave V, Allman D, Wiest D, Kappes DJ (1999) Regulation of lineage commitment distinct from positive selection. Science 286: 1149–1153 [DOI] [PubMed] [Google Scholar]
- Kelly KA, Pearse M, Lefrancois L, Scollay R (1993) Emigration of selected subsets of γδ+ T cells from the adult murine thymus. Int Immunol 5: 331–335 [DOI] [PubMed] [Google Scholar]
- Kelly KA, Scollay R (1990) Analysis of recent thymic emigrants with subset- and maturity-related markers. Int Immunol 2: 419–425 [DOI] [PubMed] [Google Scholar]
- Kreslavsky T, Garbe AI, Krueger A, von Boehmer H (2008) T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med 205: 1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H (2009) TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci USA 106: 12453–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Engel I (2007) On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol 19: 186–193 [DOI] [PubMed] [Google Scholar]
- Lauritsen JP, Haks MC, Lefebvre JM, Kappes DJ, Wiest DL (2006) Recent insights into the signals that control alphabeta/gammadelta-lineage fate. Immunol Rev 209: 176–190 [DOI] [PubMed] [Google Scholar]
- Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zuniga-Pflucker JC, Wiest DL (2009) Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity 31: 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees RK, Ferrero I, MacDonald HR (2001) Tissue-specific segregation of TCRgamma delta+ NKT cells according to phenotype TCR repertoire and activation status: parallels with TCR alphabeta+NKT cells. Eur J Immunol 31: 2901–2909 [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M (2009) Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31: 321–330 [DOI] [PubMed] [Google Scholar]
- Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ (1996) MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity 4: 337–347 [DOI] [PubMed] [Google Scholar]
- Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y (1999) Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol 19: 5969–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S (1992) Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. EMBO J 11: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT (2004) Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity 20: 735–745 [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, Okuda T, Taniuchi I (2008) Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science 319: 822–825 [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Taniuchi I, Bevan MJ (2009) ThPOK derepression is required for robust CD8 T cell responses to viral infection. J Immunol 183: 4467–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y (2008) Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol 181: 5940–5947 [DOI] [PubMed] [Google Scholar]
- Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R (2005) The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol 6: 373–381 [DOI] [PubMed] [Google Scholar]
- Tough DF, Sprent J (1998) Lifespan of γ/δ T cells. J Exp Med 187: 357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda-Hayakawa I, Mahlios J, Zhuang Y (2009) Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J Immunol 182: 5306–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Schwartz R, Engel I, Murre C, Kersh GJ (2006) Interplay between RORγt, Egr3, and E proteins controls proliferation in response to Pre-TCR signals. Immunity 24: 813–826 [DOI] [PubMed] [Google Scholar]
- Zorbas M, Scollay R (1993) Development of γδT cells in the adult murine thymus. Eur J Immunol 23: 1655–1660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.