Abstract
Huntington's disease (HD) is a fatal neurodegenerative disorder causing selective neuronal death in the brain. Dysfunction of the ubiquitin–proteasome system may contribute to the disease; however, the exact mechanisms are still unknown. We report here a new pathological mechanism by which mutant huntingtin specifically interferes with the degradation of β-catenin. Huntingtin associates with the β-catenin destruction complex that ensures its equilibrated degradation. The binding of β-catenin to the destruction complex is altered in HD, leading to the toxic stabilization of β-catenin. As a consequence, the β-transducin repeat-containing protein (β-TrCP) rescues polyglutamine (polyQ)-huntingtin-induced toxicity in striatal neurons and in a Drosophila model of HD, through the specific degradation of β-catenin. Finally, the non-steroidal anti-inflammatory drug indomethacin that decreases β-catenin levels has a neuroprotective effect in a neuronal model of HD and in Drosophila and increases the lifespan of HD flies. We thus suggest that restoring β-catenin homeostasis in HD is of therapeutic interest.
Keywords: β-TrCP, indomethacin, neurodegeneration, polyglutamines
Introduction
Huntington's disease (HD) is a devastating neurodegenerative disorder caused by an abnormal polyglutamine (polyQ) tract in the N-terminal part of the huntingtin protein (Young, 2003; Borrell-Pages et al, 2006). HD develops when this expansion exceeds 35 glutamine residues, and there is a strong inverse correlation between the number of residues and age at onset. HD is characterized by the preferential dysfunction and death of striatal and cortical neurons in the brain and the presence of neuritic and intranuclear inclusions in neurons. These events may result from a gain of toxic functions conferred by the expanded polyglutamine tract and from a loss of the beneficial properties of huntingtin. There is currently no effective treatment for preventing or delaying disease progression, and death usually occurs within 10–20 years after the appearance of the first clinical symptoms.
The Wnt signal transduction cascade has a central function in development, in adults and in disease processes (Clevers, 2006; Gordon and Nusse, 2006; Polakis, 2007; Angers and Moon, 2009). This pathway controls the levels of cytosolic β-catenin that are tightly regulated in cells, which constantly synthesize and degrade this protein. In the presence of Wnt ligands, β-catenin degradation is inhibited, it accumulates in the cytoplasm, translocates in the nucleus and activates Wnt-responsive genes. Under unstimulated conditions, a multiprotein complex known as the destruction complex is required for the processing of β-catenin through the generation of ubiquitinated β-catenin (Kimelman and Xu, 2006). Within this complex, which includes the two scaffolding proteins—axin and the adenomatous polyposis coli protein APC—β-catenin is phosphorylated sequentially at serine 45 by casein kinase 1 (CKI) and at positions 41, 37 and 33 by the glycogen synthase kinase GSK3-β (Liu et al, 2002). These two phosphorylation events create a consensus recognition site for the β-transducin repeat-containing protein (β-TrCP), which serves as the substrate recognition subunit for the SCFβ-TrCP E3 ubiquitin ligase complex (Jiang and Struhl, 1998; Hart et al, 1999; Latres et al, 1999; Winston et al, 1999; Wu et al, 2003; Kimelman and Xu, 2006). SCFβ-TrCP is an SCF (Skp1-Cul1-F-box protein) complex consisting of three invariable components—Rbx1, Cul1 and Skp1—together with the F-box protein β-TrCP. Ubiquitinated β-catenin is subsequently degraded by the 26S proteasome (Aberle et al, 1997; Orford et al, 1997).
Earlier studies have provided evidence that misfolded toxic polyQ-huntingtin protein induces a global impairment of the ubiquitin–proteasome system (UPS) that is pathogenic in HD (Bennett et al, 2005). However, other studies suggest an accumulation of polyubiquitinated proteins in HD in the absence of a general UPS impairment (Bett et al, 2006, 2009; Maynard et al, 2009). This suggests that the UPS is generally functional in HD, but that pathogenic huntingtin may impair selectively the ubiquitination process of specific substrates. However, the identity of such substrates remains to be defined. Furthermore, how mutant huntingtin regulates the degradation of specific substrates has not been addressed.
We report here the accumulation of β-catenin in its phosphorylated form in HD. This accumulation of β-catenin does not lead to transcriptional activation of c-myc and axin2, two endogenous targets of β-catenin, and is toxic for striatal neurons. β-Catenin stabilization results from disruption of the β-catenin destruction complex, which normally ensures the controlled degradation of this molecule. The restoration of physiological β-catenin levels therefore has a neuroprotective effect in HD. In particular, the anti-inflammatory drug indomethacin, which reduces β-catenin levels, inhibits the toxic effects of mutant huntingtin in vitro and in vivo.
Results
β-catenin accumulates in HD
Conflicting results on β-catenin levels have been reported in cells expressing fragments or full-length mutant huntingtin (Carmichael et al, 2002; Gines et al, 2003). We thus analysed the effect of polyQ-huntingtin on β-catenin, by expressing N-terminal fragments of huntingtin containing the first 480 amino acids with 17Q (wild-type, htt-480-17Q) or 68Q (mutant, polyQ, htt-480-68Q) in MDCK and HEK 293 cells, and assessing β-catenin levels by immunoblotting (Figure 1A and B). Wild-type huntingtin had no effect on β-catenin levels, whereas the htt-480-68Q fragment resulted in significantly higher levels of total β-catenin in two different cell types.
Figure 1.
β-Catenin accumulates in HD. (A, B) β-Catenin accumulates in cells expressing polyQ-huntingtin. MDCK (A) and HEK 293 (B) cells are transfected with htt-480-17Q or htt-480-68Q constructs or empty vector (pcDNA) and analysed by immunoblotting for the presence of β-catenin, α-tubulin and huntingtin (htt). Quantifications show an increase in β-catenin level. (C) Armadillo is stabilized in a Drosophila model of HD. Armadillo (arm) levels are analysed in (gmr-Gal4/UAS-htt548aa-Q0; UAS-LacZ/+) and (gmr-Gal4/UAS-htt548aa-Q128; UAS-LacZ/+) flies by immunoblotting. Quantification reveals a strong increase of armadillo level. (D–H) β-Catenin is upregulated in CAG140 knock-in mice and HD brains. (D) Cortical extracts from 8-month-old wild-type (WT, samples 1 and 2) and CAG140 (CAG140, samples 1–3) knock-in mice are analysed by immunoblotting for the presence of β-catenin, GFAP, βIII-tubulin and α-tubulin used as a loading control. Quantification shows an increase in β-catenin levels in HD. (E) Cortical extracts from 22-month-old wild-type (WT, samples 1–4) and CAG140 (CAG140, samples 1–3) knock-in mice are analysed by immunoblotting for the presence of β-catenin, its phosphorylated forms at positions 33–37–41 (P33–37–41) and 41–45 (P41–45) and α-tubulin. (F) Quantification shows that β-catenin accumulates under its phosphorylated form. (G) Protein extracts are prepared from post-mortem striatum of control (CT samples 1 and 2) and HD individuals (HD grade 3, sample 3; HD grade 4, sample 4) and analysed by immunoblotting for β-catenin, its phosphorylated forms at positions 33–37–41 (P33–37–41) and 41–45 (P41–45), and β-actin (actin). (H) Quantification of the western blot shows an increase in the protein level of phosphorylated β-catenin in HD samples compared with control samples. (A–H) Values refer to band densities. The results are expressed as ratios between β-catenin and α-tubulin or β-actin intensity. (I, J) PolyQ-huntingtin does not regulate downstream targets of β-catenin. Total RNA is extracted from cortices of 22-month-old wild-type (WT) and CAG140 knock-in mice. The mRNA levels of c-myc (I) and axin2 (J) are determined by quantitative PCR analysis. (NS, not significant; *P<0.05; **P<0.01; see Supplementary data for detailed statistical analyses and number of measures).
We then investigated whether this accumulation of β-catenin occurred in vivo. We first used a Drosophila model, in which N-terminal 548-amino acid fragments of human huntingtin containing 0 (upstream activator sequence (UAS)-htt548aa-Q0) or 128 (UAS-htt548aa-Q128) glutamines were inserted into the genome under the control of UAS recognized by the Gal4 transcription activator (Lee et al, 2004). This model reproduces a number of the hallmarks of HD (Lee et al, 2004). The UAS-htt548aa-Q0 and UAS-htt548aa-Q128 forms of huntingtin were expressed under the control of the eye-specific gmr-Gal4 driver, and the amount of armadillo (arm)—the Drosophila homolog of β-catenin—was analysed by immunoblotting (Figure 1C). We found that armadillo levels in UAS-htt548aa-Q128 flies were twice those in UAS-htt548aa-Q0 flies. We next analysed the levels of β-catenin in CAG140 knock-in HD mice (Menalled et al, 2003). This mouse line carries a CAG expansion inserted into the endogenous mouse huntingtin gene. As revealed by immunoblotting, β-catenin levels were significantly higher in cortical extracts from 8-month-old CAG140 mice compared with cortical extracts from wild-type littermates (Figure 1D). As described earlier (Hickey et al, 2008), at this stage, brain extracts did not show profound modifications of the neuronal marker βIII-tubulin or the glial marker glial fibrillary acidic protein (GFAP). Similar observations were made in the striata of both CAG140 mice and HdhQ111/Q111 mice, a different knock-in model of HD (Wheeler et al, 2000) (data not shown and Supplementary Figure S1A). We also performed immunohistochemical staining of brain sections from 12-month-old CAG140 mice (Supplementary Figure S1B). We found β-catenin almost exclusively in the neuropil in the striatum. β-Catenin staining appeared to surround NeuN staining. In the thalamus, β-catenin was nuclear. This localization agrees with an earlier study (Lucas et al, 1999) and negates the possibility that β-catenin accumulation only results from neuronal loss or gliosis.
To establish the physiological relevance of this accumulation in HD, we assessed the levels of β-catenin in post-mortem striatal samples from grades 3 and 4 HD patients by immunoblotting (Figure 1G). We found that β-catenin levels were much higher in HD patients than in control individuals (Figure 1G and H). Thus, β-catenin accumulates in cellular, murine and Drosophila models of HD and in post-mortem samples from patients.
The β-catenin that accumulates is phosphorylated and this accumulation does not result in transcriptional activation
Phosphorylation of β-catenin by CKI and GSK3-β is a prerequisite step to its ubiquitination and degradation by the proteasome (Liu et al, 2002). We investigated the phosphorylation status of β-catenin that accumulates in HD with specific antibodies recognizing forms of β-catenin phosphorylated either at positions 41 and 45 or at positions 33, 37 and 41 (Figure 1E and G). Phosphorylated β-catenin was elevated in brain extracts from 22-month-old CAG140 mice and in post-mortem striatal samples from HD patients (Figure 1E–H). Similar observations were made in 8-month-old CAG140 mice (data not shown). Therefore, the stabilization of β-catenin in HD occurs subsequent to its phosphorylation by CKI and GSK3-β.
On Wnt signalling, β-catenin accumulates in the cytoplasm and translocates to the nucleus, where it acts as a transcriptional coactivator to modulate the expression of target genes such as those encoding c-myc and axin2 (He et al, 1998; Yan et al, 2001; Jho et al, 2002). We analysed the levels of c-myc (Figure 1I) and axin2 (Figure 1J) transcripts by quantitative real-time RT–PCR in cortical extracts from 22-month-old CAG140 knock-in mice. We did not find changes in axin2 or c-myc gene expression in HD mice as compared with their wild-type littermates. We conclude that polyQ-huntingtin-induced β-catenin accumulation does not result in transcriptional activation. This agrees with our observation that β-catenin accumulates under its phosphorylated form.
Huntingtin associates with the β-catenin destruction complex
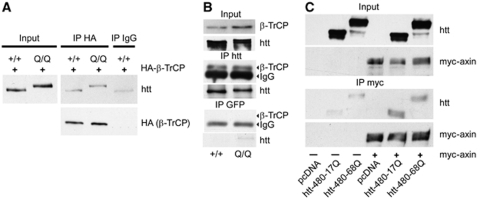
As β-catenin was found to accumulate in HD, we investigated whether huntingtin interacted with the β-TrCP and axin components of the destruction complex, and the possible consequence of the abnormal polyQ expansion in mutant huntingtin. The F-box protein β-TrCP is the substrate-recognizing subunit of the SCFβ-TrCP E3 ubiquitin ligase complex, which is required for β-catenin degradation, and axin is a core component of the destruction complex (Kimelman and Xu, 2006). We used primary cultures of striatal neurons from wild-type mice or HdhQ111/Q111 knock-in mice, which have a CAG expansion inserted into the endogenous mouse huntingtin gene (Wheeler et al, 2000). We electroporated these neurons with haemagglutinin (HA)-tagged β-TrCP and carried out immunoprecipitation experiments with an anti-HA antibody. HA-β-TrCP bound to endogenous huntingtin (Figure 2A). Moreover, an endogenous interaction between these proteins occurred, as demonstrated by experiments in which huntingtin was immunoprecipitated from wild-type and HdhQ111/Q111 mouse brain extracts (Figure 2B). The polyQ expansion did not affect the interaction of huntingtin with β-TrCP, as no obvious differences were observed in HA and huntingtin immunoprecipitation experiments in wild-type and HdhQ111/Q111 conditions (Figure 2A and B).
Figure 2.
Huntingtin associates with the β-catenin destruction complex. (A) Huntingtin and β-TrCP interact in a polyQ-independent manner. Primary culture of cortical neurons from wild-type mice (+/+) or HdhQ111/Q111 knock-in mice (Q/Q) are electroporated with HA-tagged β-TrCP. β-TrCP immunoprecipitation (HA antibody; IP HA) experiments show an interaction of β-TrCP with endogenous huntingtin (htt). No difference in the interaction is observed between wild-type and polyQ-huntingtin. Immunoprecipitation with mouse IgG (IP IgG) is used as a control. (B) Similar results are obtained doing the converse immunoprecipitation (4C8; IP htt) on whole brain extracts from wild-type mice (+/+) or HdhQ111/Q111 knock-in mice (Q/Q). Anti-GFP antibody is used to perform the control immunoprecipitation (IP GFP). (C) Huntingtin interacts with axin and this binding is not modified by the polyQ expansion as revealed by a myc immunoprecipitation (IP myc). HEK 293 cells are transfected with htt-480 constructs or the corresponding empty vector (pcDNA) and myc-axin.
We next used an anti-myc antibody to study the interaction between huntingtin and axin in HEK 293 cells cotransfected with a myc-tagged axin construct and fragments of huntingtin (htt-480-17Q or -68Q) or an empty vector (pcDNA). Huntingtin interacted with axin, and this interaction was not modulated by the polyQ expansion (Figure 2C). In conclusion, huntingtin interacts with the β-catenin destruction complex, but this interaction is unmodified by the presence of mutated huntingtin.
β-catenin binding to the destruction complex is altered in HD
We next analysed huntingtin binding to β-catenin. We transfected HEK 293 cells with constructs encoding 1301-amino acid N-terminal fragments of huntingtin containing 17 (wild-type, htt-1301-17Q) or 73 (polyQ, htt-1301-73Q) glutamines. These 1301-amino acid N-terminal fragments, like the htt-480-17Q or htt-480-68Q fragments, reproduce key features of HD (Saudou et al, 1998; Humbert et al, 2002; Anne et al, 2007). Similarly to the htt-480-17Q fragment (Supplementary Figure S2A), the htt-1301-17Q fragment bound β-catenin (Figure 3A). Furthermore, β-catenin did not interact with ataxin 3 and 7, whose mutations—abnormal polyQ expansion—are the cause of spinal and cerebellar ataxias (Supplementary Figure S2A and B). Therefore, the first 480 amino acids of huntingtin are sufficient for the huntingtin/β-catenin interaction, and this interaction is specific to huntingtin.
Figure 3.
PolyQ-huntingtin decreases β-catenin binding to the destruction complex. (A–C) The presence of an abnormal polyQ expansion in huntingtin alters the binding of huntingtin to β-catenin. (A) HEK 293 cells are transfected with YFP-fused htt-1301 variants and the corresponding empty vector (control, CT). A 4C8 immunoprecipitation (IP htt) shows a decreased interaction of huntingtin (htt) with β-catenin in polyQ-huntingtin compared with wild-type huntingtin-expressing cells. (B, C) A 4C8 immunoprecipitation (IP htt) from cortical extracts from wild-type mice (+/+) or HdhQ111/Q111 knock-in mice (Q/Q) demonstrates a decreased interaction of β-catenin with polyQ-huntingtin compared with wild-type huntingtin. Similar results are obtained doing the converse immunoprecipitation (IP β-catenin). Immunoprecipitations with mouse IgG (IP IgG) are used as controls. (D, E) β-Catenin binding to axin is impaired in polyQ context. HEK 293 cells are transfected with myc-tagged axin and htt-480-17Q or htt-480-68Q constructs or empty vector (pcDNA). Immunoprecipitation experiments using myc (D, IP myc) or β-catenin antibodies (E, IP β-catenin) reveal a strong decrease of β-catenin interaction with axin in the presence of polyQ-huntingtin compared with wild-type huntingtin. (F) PolyQ-huntingtin modifies β-catenin interaction with β-TrCP. β-Catenin immunoprecipitation (IP β-catenin) in HEK 293 cells expressing myc-β-TrCP and a fragment of huntingtin (htt-480-17Q or -68Q) shows a defect of β-catenin and β-TrCP binding when huntingtin encompasses an abnormal polyQ expansion. The graphs represent the quantitative assessment of the band densities of the coimmunoprecipitated versus immunoprecipitated proteins (*P<0.05; **P<0.01; see Supplementary data for detailed statistical analyses and number of measures).
We also tested the influence of the presence of the polyQ tract and found that the coimmunoprecipitated β-catenin was decreased in cells transfected with htt-1301-73Q (Figure 3A). We then showed that endogenous huntingtin and β-catenin interacted by performing a huntingtin immunoprecipitation from wild-type mouse cortical extracts (Figure 3B). This association was decreased when huntingtin contained an abnormal polyQ expansion in HdhQ111/Q111 cortical extracts. When performing the converse immunoprecipitation experiment using an anti-β-catenin antibody (Figure 3C), we consistently observed that β-catenin bound huntingtin in a polyQ-dependent manner.
Given this modification of the polyQ-huntingtin/β-catenin interaction, we investigated the possible effect of the polyQ expansion on the recruitment of β-catenin to the destruction complex. We thus analysed the interaction between β-catenin and axin in wild-type and polyQ-huntingtin conditions. We performed immunoprecipitation experiments with anti-myc antibodies on extracts from HEK 293 cells expressing myc-tagged axin and htt-480-17Q or htt-480-68Q constructs (Figure 3D). The binding of β-catenin to axin was significantly decreased in the polyQ context consistent with the impairment of β-catenin binding to the degradation complex in HD. Similar results were obtained in the converse experiment, using anti-β-catenin antibody (Figure 3E). We also asked whether mutant huntingtin influences the interaction between β-catenin and β-TrCP. We performed β-catenin immunoprecipitation experiments with HEK 293 cells expressing myc-β-TrCP and wild-type or polyQ-huntingtin constructs, and found that the interaction between β-catenin and β-TrCP was impaired by the presence of mutant huntingtin (Figure 3F).
Thus, the decreased interaction between β-catenin and polyQ-huntingtin reduces the binding of β-catenin to the core of the destruction complex and to the subunit with the ubiquitination activity.
β-TrCP protects striatal neurons against polyQ-huntingtin-induced cell death through β-catenin degradation
Having demonstrated the accumulation of β-catenin in HD and the impairment of its degradation complex, we investigated whether β-TrCP could restore the activity of the destruction complex, thereby conferring neuroprotection. We used a neuronal model of HD recapitulating several features of the disease (Saudou et al, 1998; Humbert et al, 2002; Anne et al, 2007). We evaluated neuronal death in primary cultures of rat striatal neurons transfected with the htt-480-17Q and htt-480-68Q plasmids in the presence of a construct encoding HA-tagged β-TrCP. As expected, transfection with the htt-480-68Q fragment resulted in significantly higher levels of neuronal death than observed with the htt-480-17Q construct (Figure 4A). β-TrCP decreased the levels of neuronal death induced by the htt-480-68Q fragment of huntingtin to levels similar to those observed with the wild-type protein (Figure 4A). These findings show that β-TrCP exerts a neuroprotective effect in a cellular model of HD.
Figure 4.
Preventing accumulation of β-catenin reduces polyQ-huntingtin-induced toxicity in striatal neurons. (A) Wild-type (htt-480-17Q) and polyQ-huntingtin (htt-480-68Q) are cotransfected with β-TrCP or the empty vector (pCS2) in striatal neurons. Data from six independent experiments reveal that β-TrCP significantly reduces polyQ-huntingtin-induced cell death. (B) Neuronal cell death is assessed in primary culture of striatal neurons transfected with huntingtin constructs (htt-480-17Q or -68Q) and chimeric F-box fusion proteins, F-box-βBDTcf4 or F-box-βBDEcad. Data from four independent experiments show a significant reduction of polyQ-huntingtin-induced toxicity in cells expressing F-box proteins that can only degrade β-catenin. (C) Striatal neurons are electroporated with siRNA directed against β-catenin (si-β-catenin) or scramble RNA and 48 h later transfected with htt-480-17Q or htt-480-68Q. Data from four independent experiments demonstrate a protective effect of β-catenin downregulation towards polyQ-huntingtin-induced toxicity. Immunoblotting using anti-β-catenin and anti-α-tubulin antibodies reveals a decrease in β-catenin levels in the si-β-catenin-treated neurons compared with scramble situation. Values refer to band densities. Results are expressed as a ratio between β-catenin and α-tubulin intensity. (D) Striatal neurons are electroporated with β-catenin or the corresponding empty vector (pCMV). Data from four independent experiments demonstrate a toxic effect of β-catenin expression (NS, not significant; *P<0.05; **P<0.01; ***P<0.001; see Supplementary data for detailed statistical analyses and number of measures).
To unequivocally address whether β-TrCP rescued polyQ-huntingtin-induced toxicity through β-catenin degradation, we evaluated the possible neuroprotective properties of F-box proteins degrading only β-catenin in our neuronal model of HD. We cotransfected primary cultures of striatal neurons with htt-480-17Q or htt-480-68Q constructs and constructs encoding FLAG-tagged chimeric F-box fusion proteins, F-box-βBDTcf4 or F-box-βBDEcad (Figure 4B). In the FLAG-tagged constructs, the WD40 repeat of β-TrCP was replaced with the β-catenin-binding domain from Tcf4 (F-box-βBDTcf4) or E-cadherin (F-box-βBDEcad) (Liu et al, 2004). F-box-βBDTcf4 and F-box-βBDEcad greatly increased β-catenin ubiquitination and turnover (Liu et al, 2004). These chimeric F-box proteins had no effect on neuronal toxicity themselves, but they significantly reduced polyQ-huntingtin-induced toxicity to levels similar to those observed in the wild-type situation (Figure 4B). We conclude that the neuroprotective properties of β-TrCP in HD specifically depend on its capacity to degrade β-catenin.
Decreasing β-catenin levels by RNAi-mediated silencing is protective in HD
As β-TrCP exerts neuroprotective effects in HD by degrading β-catenin, we directly tested the effect on polyQ-induced toxicity of reducing β-catenin levels. Primary cultures of striatal neurons were electroporated with an siRNA directed against β-catenin or with a scramble RNA and were then transfected with htt-480-17Q or htt-480-68Q constructs (Figure 4C). Transfection with the htt-480-68Q construct resulted in significantly higher levels of neuronal death than transfection with the htt-480-17Q construct under control conditions (scramble RNA) (Figure 4C). Thus, the downregulation of β-catenin levels completely abolished the deleterious effects of polyQ-huntingtin.
Conversely, we addressed whether an abnormal accumulation of β-catenin was toxic in striatal neurons. For this, we examined the effect of expressing β-catenin in primary cultures of striatal neurons (Figure 4D). We found that the elevated levels of β-catenin resulted in striatal cell death as compared with the control situation, suggesting that abnormal accumulation of β-catenin in striatal neurons is toxic.
Slimb inhibits neurodegeneration in a Drosophila HD model and reduces armadillo levels
We evaluated the protective effect of β-TrCP in a more physiological situation, focusing on Slimb, the Drosophila homolog of β-TrCP in HD flies. We determined the phenotypes induced by mutant polyQ-huntingtin (UAS-htt548aa-Q128) in the presence and absence of Slimb (Figure 5A). Using a gmr-Gal4 line driving expression in the eyes, we showed that expression of the wild-type UAS-htt548aa-Q0 resulted in no particular adult eye phenotype. By contrast, the expression of mutant polyQ-huntingtin (UAS-htt548aa-Q128) in developing eyes resulted in a loss of eye pigmentation in adults (left panel) and a rough eye phenotype correlated with an abnormal ommatidial array, as shown by scanning electron microscopy (right panels) (Lee et al, 2004). This phenotype is related to progressive neurodegeneration of the retina, with a loss of photoreceptors (Lee et al, 2004). Expression of the UAS-Slimb transgene in UAS-htt548aa-Q128 flies decreased the eye depigmentation defect and improved ommatidial morphology, as shown by comparisons with UAS-htt548aa-Q128 flies expressing a neutral UAS-LacZ transgene. As a control, we checked that Slimb expression (gmr-Gal4,UAS-GFP/+; UAS-Slimb/+ flies) was not itself associated with a particular phenotype (Figure 5A). We then quantified rescue of the eye pigmentation defect. For polyQ-huntingtin-expressing flies (gmr-Gal4/UAS-htt548aa-Q128; UAS-LacZ/+) with maximal levels of depigmentation (100% of flies), we calculated the percentage of flies expressing both Slimb and mutant huntingtin and showing a loss of eye pigmentation phenotype (gmr-Gal4/UAS- htt548aa-Q128; UAS-Slimb/+). We found that Slimb overexpression decreased the proportion of flies displaying a loss of pigmentation by over 70% (Figure 5A, graph). Thus, Slimb inhibits loss of eye pigmentation and ommatidial morphology defects in Drosophila—two hallmarks of neuronal degeneration in this in vivo HD model.
Figure 5.
Slimb, the Drosophila homolog of β-TrCP, reduces polyQ-induced toxicity and β-catenin level in a Drosophila model of HD. (A) Left panels: Slimb regulates polyQ-huntingtin-induced eye depigmentation. Eye depigmentation is analysed in the following females at 10 days: (gmr-Gal4,UAS-GFP/+; UAS-LacZ/+) or (gmr-Gal4,UAS-GFP/+; UAS-Slimb/+) as controls; (gmr-Gal4/UAS-htt548aa-Q0; UAS-LacZ/+), (gmr-Gal4/UAS-htt548aa-Q128; UAS-LacZ/+) and (gmr-Gal4/UAS-htt548aa-Q128; UAS-Slimb/+). Eye-specific polyQ-huntingtin expression leads to a complete loss of pigmentation (compare line 4 to line 3). Expression of UAS-Slimb transgene in those flies inhibits toxicity (line 5). The graph represents the percentage of flies with >50% of eye depigmentation. Right panels: visualization of ommatidial morphology using scanning electron microscopy. PolyQ-huntingtin expression induces a strong desorganization of ommatidia that is compensated by expression of Slimb. (B) Slimb reverses armadillo accumulation in polyQ-huntingtin-expressing Drosophila. Immunoblotting analysis of (gmr-Gal4/UAS-htt548aa-Q0; UAS-LacZ/+), (gmr-Gal4/UAS-htt548aa-Q128; UAS-LacZ/+) and (gmr-Gal4/UAS-htt548aa-Q128; UAS-Slimb/+) flies shows a loss of armadillo (arm) accumulation in UAS-htt548aa-Q128 flies expressing Slimb as compared with flies expressing a neutral UAS-LacZ transgene. Quantification of four independent experiments shows a reduction of armadillo levels when Slimb is expressed. The results are expressed as a ratio between armadillo and α-tubulin band densities and are normalized to control (gmr-Gal4/UAS-htt548aa-Q0; UAS-LacZ/+). (NS, not significant; *P<0.05; **P<0.01; ***P<0.001; see Supplementary data for detailed statistical analyses and number of measures).
Having shown that the accumulation of β-catenin was toxic in HD (Figure 4D), we determined whether the protective effect of Slimb in HD flies was accompanied by a decrease in armadillo levels. We analysed the levels of total armadillo protein by immunoblotting head extracts of Drosophila expressing wild-type or polyQ-huntingtin in the eye in the presence or absence of Slimb. When Slimb was expressed, armadillo failed to accumulate in UAS-htt548aa-Q128-expressing flies (Figure 5B). In our experimental conditions, Slimb did not itself modify armadillo levels (data not shown). We also quantified polyQ-huntingtin in HD Drosophila with and without Slimb (Figure 5B) and found that the levels of this protein were similar in the presence and absence of Slimb. This strongly suggests that the neuroprotective effect of Slimb in HD is linked to its ability to degrade β-catenin/armadillo in vivo.
Indomethacin reduces polyQ-huntingtin-induced toxicity in neurons and in Drosophila
As it is not yet possible to target β-TrCP in treatment approaches for use in humans, we tested the possible neuroprotective properties of the anti-inflammatory drug indomethacin, an inhibitor of cyclooxygenase, in a neuronal model of HD. Indomethacin downregulates β-catenin mRNA and protein levels in cells (Dihlmann et al, 2001; Hawcroft et al, 2002; Kapitanovic et al, 2006). Immunoblotting experiments showed that the treatment of neurons with indomethacin decreased the levels of β-catenin protein (Figure 6A). We transfected primary cultures of striatal neurons with wild-type huntingtin and polyQ-huntingtin (htt-480-17Q or htt-480-68Q) constructs and treated neurons with indomethacin (0.6 mM) or 1% DMSO for 24 h. Consistent with the toxicity of β-catenin accumulation in HD, indomethacin treatment decreased polyQ-huntingtin-induced neuronal toxicity (Figure 6B).
Figure 6.
Indomethacin reduces polyQ-huntingtin-induced toxicity in neurons and in Drosophila. (A) Indomethacin treatment of striatal neurons reduces the level of β-catenin. Primary culture of striatal neurons are treated with increasing concentration of indomethacin (IndoM) or 1% DMSO as a control (CT) for 24 h. Immunoblotting reveals a reduction of β-catenin level on indomethacin treatment. (B) Primary cultures of striatal neurons are transfected with htt-480-17Q or htt-480-68Q and treated with indomethacin (IndoM, 0.6 mM) or 1% DMSO (CT) for 24 h. Data from six independent experiments reveal that indomethacin treatment significantly reduces polyQ-huntingtin-induced cell death. (C) Wild-type (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q0) and polyQ-huntingtin (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q128) expressing flies are fed with indomethacin or ethanol (CT). Expressing polyQ-huntingtin in developing eyes leads to adults with a loss of eye pigmentation. Indomethacin reduces the loss of eye pigmentation induced by mutant huntingtin in the developing eyes of flies in a statistically significant manner. (D) Armadillo accumulation in polyQ-huntingtin-expressing Drosophila is blocked by indomethacin. (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q0) and (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q128) flies are treated with indomethacin or ethanol (CT) and analysed by immunoblotting for armadillo level (arm). (E) The lifespan of polyQ-huntingtin flies is significantly prolonged by indomethacin. Elav-Gal4/UAS-htt548aa-Q0 and elav-Gal4/UAS-htt548aa-Q128 flies are raised on standard media containing indomethacin or ethanol (CT). Lifespan of the progeny is analysed. (F) Huntingtin binds to β-catenin and to the destruction complex by interacting with β-TrCP and axin. The presence of an abnormal polyQ expansion in mutant huntingtin (htt) leads to a decreased binding to β-catenin therefore impairing the binding of β-catenin to the destruction complex and subsequently resulting in β-catenin accumulation. WD: WD40 repeat of β-TrCP (NS, not significant; **P<0.01; ***P<0.001; see Supplementary data for detailed statistical analyses and number of measures).
We investigated the possibility of inducing neuroprotection by treatment with indomethacin in a more physiological situation. We analysed the effect of indomethacin on flies expressing wild-type (UAS-htt548aa-Q0) and polyQ- (UAS-htt548aa-Q128) huntingtin. As shown above (Figure 5A, left panel), expression of the mutant polyQ-huntingtin construct in developing eyes resulted in a loss of eye pigmentation in adults (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q128 flies) (Figure 6C). This defect was decreased by indomethacin treatment. We quantified this effect in polyQ-huntingtin-expressing flies with maximal depigmentation (100% of flies) and found that indomethacin significantly reduced the loss of pigmentation (Figure 6C, graph). Immunoblotting experiments also revealed a lack of armadillo accumulation after the treatment of polyQ-huntingtin-expressing flies with indomethacin (Figure 6D), demonstrating a correlation between the neuroprotective effect of indomethacin and the decrease in armadillo levels.
We analysed the effect of indomethacin on the survival of adult flies with HD. The expression of UAS-htt548aa-Q128 under the control of the pan neuronal elav-GAL4 driver resulted in fully viable adults with uncoordinated movement and abnormal grooming behaviour (elav-Gal4/UAS-htt548aa-Q128 flies) (Lee et al, 2004). These behavioural defects worsened with age and the animals died prematurely. Indomethacin treatment increased the mean survival of UAS-htt548aa-Q128 flies from 5.9 to 7.5 days, a 27% fold increase with respect to untreated UAS-htt548aa-Q128 flies (Figure 6E). Together, these data show that indomethacin rescues degeneration in primary cultures of striatal neurons and in a Drosophila HD model, and increases the survival of flies expressing polyQ-huntingtin.
Discussion
In this study, we found that β-catenin levels were upregulated by either N-terminal 480- and 548-amino acid fragments or full-length forms of huntingtin in cell lines, primary cultures of striatal neurons, two knock-in HD mouse models, Drosophila and patients. Our data thus reveal an impairment in β-catenin turnover, and we have now unequivocally demonstrated that specifically decreasing β-catenin levels is neuroprotective in HD. Indeed, chimeric F-box proteins that only recognize β-catenin and facilitate its degradation (Liu et al, 2004), reduce the deleterious effects of polyQ-huntingtin in striatal neurons. In vivo, we used genetic and pharmacological approaches. Expression of β-TrCP/Slimb prevented the loss of eye pigmentation and ommatidial morphology defects in a Drosophila model of HD. In support, β-catenin/armadillo knockdown has been shown to abolish the toxic effects of polyQ-huntingtin in a similar Drosophila model (Kaltenbach et al, 2007). Finally, indomethacin both decreased the eye pigmentation defect and increased the lifespan of HD flies.
We found that polyQ-huntingtin impaired the correct formation of the β-catenin destruction complex. Huntingtin interacts with β-catenin, β-TrCP and axin. We suggest that, in normal conditions, huntingtin may act as a scaffold protein, promoting β-catenin degradation by facilitating the recognition of β-catenin by β-TrCP within the destruction complex (Figure 6F). Consistent with this hypothesis, Wu et al (2003) showed that the interaction between β-catenin and β-TrCP is weak and suggested that other proteins may stabilize this interaction. Furthermore, several SCF components in addition to β-catenin, including cullins 2 and 5 and the ring finger 20 and 40 proteins, have been shown to interact with huntingtin in yeast two-hybrid system (Kaltenbach et al, 2007). In pathological conditions, polyQ-huntingtin interacts less strongly with β-catenin than wild-type huntingtin, so this effect is lost and β-catenin subsequently accumulates.
The UPS has an important function in HD, due to the involvement of this system in the degradation of misfolded toxic polyQ-huntingtin protein (Ortega et al, 2007). The observation of an abnormal enrichment of HD inclusion bodies with ubiquitin and proteasome subunits provided the first evidence that the dysfunction in UPS may contribute to the pathogenesis of the disease. Indeed, a global inhibition of the UPS caused by the inhibition of the proteasome activity by polyQ-huntingtin has been suggested in disease (Jana et al, 2001; Venkatraman et al, 2004; Bennett et al, 2005; Diaz-Hernandez et al, 2006). Furthermore, the age-dependent decrease in proteasome activity contributes to the accumulation of short fragments of polyQ-huntingtin (Zhou et al, 2003). However, other studies suggest the presence of a largely operative UPS in HD (Bett et al, 2006, 2009; Maynard et al, 2009). Finally, as many diverse proteins are degraded by the proteasome, the UPS may not be considered an appropriate target for treatment purposes. We describe here a new pathogenic pathway in which polyQ-huntingtin interferes with the degradation of a single protein, β-catenin. This function is specific to huntingtin and does not necessarily extend to other proteins whose polyQ mutations cause neurodegenerative disorders. Indeed, β-catenin does not bind to ataxin 7 or ataxin 3 even though ataxin 3 is known to be involved in the UPS (Chai et al, 2004). We provide the proof-of-principle that the restoration of β-catenin levels to normal levels may be of therapeutic value. This restoration may be achieved by targeting β-TrCP, through the use of β-catenin-specific chimeric F-box proteins, or by indomethacin treatment.
Huntingtin may modulate the degradation of β-catenin only or may also affect the degradation process of other substrates. Indeed, β-TrCP has other substrates (Kimelman and Xu, 2006) including REST, the calcineurin inhibitor RCAN1, IκBα and p53, all of which are known to be either upregulated or downregulated in HD (Zuccato et al, 2003; Khoshnan et al, 2004; Bae et al, 2005; Westbrook et al, 2008; Ermak et al, 2009). As the huntingtin/β-TrCP interaction is not modified by the presence of an abnormal polyQ expansion, the way in which huntingtin and its mutant form interact with these proteins may be responsible for the observed changes in degradation, with weaker interactions in pathological conditions favouring degradation and conversely. Consistent with our data, one recent study reported an accumulation of polyubiquitinated proteins in HD in the absence of a general UPS impairment (Maynard et al, 2009). This suggests that the UPS system is generally functional in HD, but, as for β-catenin and β-TrCP, polyQ-huntingtin may induce the impairment of ubiquitination processes for specific substrates.
Several molecular mechanisms are involved in the physiopathology of HD. These mechanisms include mitochondrial, transcriptional and transport alterations, oxidative stress, inflammation and apoptosis. It may be necessary to interfere with several of these mechanisms to treat HD successfully. Indomethacin is a multitarget drug that was initially studied as an inhibitor of several isoforms of cyclooxygenase (Hawcroft et al, 2002; Kapitanovic et al, 2006). It increases the cellular levels of heat shock proteins in mammalian cells (Ishihara et al, 2004). We show here that at least some of the neuroprotective effects of indomethacin in HD could be mediated by the β-catenin pathway. However, indomethacin may be beneficial due to its inhibitory effects on cyclooxygenase. Indomethacin could also act by suppressing protein aggregation, as it was shown in a cellular model of polyglutamine disease (Ishihara et al, 2004). Finally, anti-inflammatory compounds, such as celecoxib, acetylsalicylate and rofecoxib, did not show beneficial effects in transgenic HD mice expressing short fragments of polyQ-huntingtin to model HD (Norflus et al, 2004; Schilling et al, 2004). These fragments may not be sufficient to interact with β-catenin and/or β-TrCP and therefore such models may not be relevant to study molecules counteracting the toxic effect of this pathway.
An association between β-catenin and cancer has been clearly demonstrated, particularly for colorectal cancer (Polakis, 2007). The accumulation of β-catenin in the nucleus leads to excessive cell proliferation and tumorigenesis (Polakis, 2007). In neurodegenerative conditions, the situation is less clear. Both increases and decreases in β-catenin levels have been reported for Alzheimer's disease (Boonen et al, 2009). Mutations in the presenilin 1 gene account for a large proportion of cases of familial early onset Alzheimer's disease, in which β-catenin stabilization occurs (Kang et al, 1999). In PC12 cells expressing mutant presenilin 1, the increase in β-catenin levels is associated with a defect in neuronal differentiation (Teo et al, 2005). In adult mice, mutation of the presenillin 1 gene and the resulting accumulation of β-catenin lead to a transient increase in proliferation, followed by an increase in apoptosis in the dentate gyrus (Chevallier et al, 2005). In HD, we show an accumulation of phosphorylated β-catenin. Indeed, the impairment leading to this accumulation occurs downstream of the phosphorylations by CKI and GSK3-β (Figure 6F). Non-phosphorylated β-catenin activates Wnt-responsive genes. In agreement, the observed accumulation of β-catenin in HD did not lead to a global increased transcriptional activity. Treatments targeting β-catenin are neuroprotective in HD, but further studies are required to identify the precise mechanisms by which an aberrant stabilization of phosphorylated β-catenin ultimately leads to dysfunction and toxicity.
Materials and methods
Statistical analyses
Statview 4.5 software (SAS Institute, Cary, NC) was used for statistical analyses. All data herein described were performed at least in duplicate. Data are expressed as means±s.e.m. Complete statistical analyses are available in Supplementary data.
DNA constructs, siRNA and antibodies
Constructs encoding β-galactosidase, myc- or HA-tagged β-TrCP and axin, FLAG-F-box-βBDTcf4, FLAG-F-box-βBDEcad and phCMV2-human-β-catenin were described elsewhere (Zeng et al, 1997; Margottin et al, 1998; Li et al, 1999; Liu et al, 2004; Bouteille et al, 2009). The wild-type and polyQ-huntingtin constructs htt-480-17Q, htt-480-68Q, YFP-htt-1301-17Q and YFP-htt-1301-73Q were described earlier (Saudou et al, 1998; Anne et al, 2007). The siRNA sequence targeting rat β-catenin was described earlier (Gu et al, 2008). The scramble RNA (scRNA, control; Eurogentec, Seraing, Belgium) used had a unique sequence, which did not match with any sequence in the genome of interest. The following antibodies were used: mouse monoclonal anti-huntingtin (htt, clone 1HU-4C8 (Trottier et al, 1995), WB 1:5000, IF 1:200), anti-β-catenin (BD Bioscience, San Jose, CA, WB 1:5000), anti-α-tubulin (clone DM1A, Sigma, St Louis, MO, WB 1:3000), anti-GFAP (Sigma, WB 1:1000), anti-βIII-tubulin (Millipore, Bedford, MA, WB 1:1000), anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA, WB 1:2000), anti-β-galactosidase (Promega, Madison, WI, IF, 1:100), anti-β-TrCP (clone 1B1D2, Zymed, San Fransisco, CA, WB 1:500), anti-β-actin (Sigma, WB 1:5000), anti-GFP (Roche, Mannheim, Germany), anti-armadillo (clone N27A1, ascites, Developmental Studies Hybridoma Bank, Iowa city, IA, WB 1:500) and anti-mouse IgG (Upstate, Charlottesville, VA), polyclonal rabbit anti-phosphorylated-S33/S37/T41-β-catenin (Upstate, WB 1:1000) and anti-phosphorylated-T41/S45-β-catenin (Upstate, WB 1:1000) and monoclonal rat anti-HA (clone 3F10, Roche, WB 1:5000). Anti-mouse and anti-rat secondary antibodies conjugated to HRP were purchased, respectively, from Jackson Immunoresearch Laboratories (West Grove, PA) and Beckman coulter (Fullerton, CA). Indomethacin was purchased from Sigma.
Mouse tissues
HdhQ111/Q111 and CAG140 knock-in mice have been described earlier (Wheeler et al, 2000; Menalled et al, 2003). To collect brain samples, mice were deeply anaesthetized in a CO2 chamber, and their cortices were dissected out on ice and rapidly frozen using CO2 pellets. All experimental procedures were performed in strict accordance with the recommendations of the European Community (86/609/EEC) and the French National Committee (87/848) for care (HdhQ111/Q111 mice) and in accordance with the US Public Health Service Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCLA (CAG140 knock-in mice).
Brain tissues
Tissues were obtained from the Harvard Brain Tissue Resource Center (HBTRC; Belmont, MA): two controls (samples 1–2; mean±s.e.m., age: 56.0±3.0 years; post-mortem delay: 23.5±3.4 h), one HD grade 3 (HD3, sample 3), and one grade 4 (HD4, sample 4) patients (age: 63.5±18.5 years; post-mortem delay: 24.0±2.0 h). Samples correspond, respectively, to brain numbers 4741, 4744, 4797 and 4680 as numbered by HBTRC. Samples were homogenized in NP-40 lysis buffer and cleared by centrifugation at 6000 g (15 min; 4°C). Western blot analysis was performed on 10 μg of total extracts.
Drosophila stocks and experiments
UAS-htt548aa-Q0 and UAS-htt548aa-128Q were obtained from J Troy Littleton (Lee et al, 2004) and UAS-Slimb (on III) from B Limbourg-Bouchon (Grima et al, 2002). gmr-Gal4, UAS-GFP recombinant flies (on II) were provided by F Juge. UAS-LacZ (on III), gmr-Gal4 (on II), elav-Gal4 (on II) strains were obtained from the National Drosophila Stock Center (Bloomington, IN). Using those stocks, we generated the following homozygous strains: gmr-Gal4;UAS-LacZ and gmr-Gal4;UAS-Slimb. All flies were raised on standard medium at 25°C. As polyQ-huntingtin-induced phenotypes are particularly sensitive to its level of expression (Mugat et al, 2008), flies always contain the same number of UAS transgenes. Neutral transgenes can be either UAS-LacZ or UAS-GFP, and do not provide any eye phenotype as heterozygous.
For rescue experiments, we crossed UAS-htt548aa-Q0 or UAS-htt548aa-Q128 flies with gmr-Gal4;UAS-Slimb or gmr-Gal4;UAS-LacZ flies. As control, gmr-Gal4,UAS-GFP flies were crossed with UAS-LacZ and UAS-Slimb flies. All crosses were done at the same time and under the same conditions: flies were allowed to mate for 3 days at 25°C, and the eggs were transferred to 29°C. Phenotypes were analysed between 10 and 12 days after adults eclosion, when the eye depigmentation is maximal for (gmr-Gal4/UAS-htt548aa-128Q; UAS-LacZ/+) flies. As a rescue, we considered fly with over than 50% of remaining pigmentation. For analysis of ommatidial morphology, flies were frozen on dry ice, and kept at −80°C until used. Before use, all samples were dehydrated and coated with gold/palladium. Structure of ommatidia was then analysed by scanning electron microscopy. For western blotting experiments, crosses were performed at 25°C and 1-day-old adult were collected. Drosophila were frozen in dry ice, heads were isolated and homogenized in lysis buffer (25 mM Tris–HCl, 5 mM EDTA, 250 mM NaCl and 1% triton supplemented with protease and phosphatases inhibitors cocktail (Sigma, Steinhelm, Germany)), left for 20 min on ice and centrifuged at 11 000 g for 20 min at 4°C. Equal amount of proteins were loaded onto SDS–PAGE and subjected to western blot analysis.
For indomethacin rescue experiment (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q0) or (gmr-Gal4,UAS-GFP/UAS-htt548aa-Q128), flies were raised on standard drosophila food containing either indomethacin (250 mg/l) or ethanol as a control. Flies were allowed to mate for 3 days at 25°C, and the eggs were then transferred to 29°C. Adults were isolated everyday and transferred to a fresh tube every other day. Phenotypic analyses were conducted as described above.
For lifespan analysis (elav-Gal4/UAS-htt548aa-Q0) or (elav-Gal4/UAS-htt548aa-Q128), flies were grown on standard drosophila food containing either indomethacin (250 mg/l) or ethanol as control. Flies were allowed to mate for 3 days at 25°C, and the eggs were transferred to 29°C. Newly eclosed flies were collected and reared on either indomethacin or control media. The flies were transferred to fresh media every other day, and the number of dead flies was counted daily. Survival curves were generated and data were analysed by Kaplan–Meier survival analysis method; statistical significance was examined using the logrank test.
Measurement of neuronal survival
Three days after plating, primary cultures of striatal neurons were transfected by a modified calcium phosphate technique (Saudou et al, 1998), with the same amount of wild-type or polyQ-huntingtin and β-TrCP or β-catenin construct. Cytomegalovirus-β-galactosidase plasmid (10:1 ratio) was also added to identify the transfected cells. Forskolin (10 μM; Sigma) and IBMX (100 μM; Sigma) were added to the cultures 2 h after transfection. One day after transfection, cells were fixed with 4% paraformaldehyde for 20 min and immunostained with anti-huntingtin (4C8) and anti-β-galactosidase antibodies. When stated, neurons were electroporated the day of plating with si-β-catenin or scramble RNA and, 2 days after electroporation, transfected by lipofectamine (Invitrogen, Carlsbad, CA) with the different huntingtin constructs. For indomethacin experiments, cells were transfected with lipofectamine 2 days after plating. Conditioned media containing either indomethacin or DMSO was added to cells 6 h after transfection for 24 h. Neurons were fixed and immunostained with anti-huntingtin 4C8 antibody. 4C8-positive neurons of similar intensities were scored under fluorescence microscopy in a blinded manner. Neuronal degeneration was assessed by neurite loss and nuclear shrinkage (Anne et al, 2007). Cell death was expressed as a fold increase in neuronal cell death relative to the death induced by the htt-480-17Q construct. Each graph represents four to six independent experiments performed in duplicate. Each bar in a given graph corresponds to the scoring of ∼2000 neurons. Data were submitted to complete statistical analysis.
Real-time RT–PCR
Dissected frozen sample (cortices) from CAG140 and wild-type mice were lysed in TRIzol (Invitrogen Corp.). Total RNA was extracted, and samples were retrotranscribed using the First-Strand cDNA Synthesis Kit (Invitrogen). cDNAs were then diluted 1:10 and submitted to RT–PCR (iQ SYBR Green Supermix; Bio-Rad) with the following c-myc (5′-CACCAGCAGCGACTCTGAA-3′ and 5′-GCCCGACTCCGACCTCTTG-3′) and axin2 (5′-GATTCCCCTTTGACCAGGTGG-3′ and 5′-CCATTACAAGCAAACCAGAAGT-3′) oligonucleotides. β-Actin gene was used as an internal control and quantified with the following oligonucleotides: 5′-AGGTGACAGCATTGCTTCTG-3′ and 5′-GCTGCCTCAACACCTCAAC-3′. Results were analysed using the ICycler apparatus (Bio-Rad).
Supplementary Material
Acknowledgments
We greatly acknowledge A Brice, MF Chesselet, F Constantini, B Grima, S Gutkind, F Juge, F Lallemand, B Limbourg-Bouchon, T Littleton, J Liu, F Margottin-Goguet, G Peignon, L Pereira de Almeida, F Rouyer, R White, D Wu and the Bloomington Drosophila stock centre for reagents, flies and/or discussions; I LeDisquet (electronic microscopy facility IFR83), S Domenichini (electronic microscopy facility IFR87) and FP Cordelières (Institut Curie imaging facility) for help in image acquisition and treatment; Harvard Brain Tissue Resource Center (Belmont, MA, MH/NS 31862) for providing human brain tissue; members of the Saudou/Humbert's laboratories for helpful comments and F Saudou for continuous support and invaluable discussions. Our work is supported by grants from Agence Nationale pour la Recherche (ANR-09-BLAN-0080, SH), Association pour la Recherche sur le Cancer (ARC subvention libre no3188, SH). JG was supported by Region Ile de France and ARC; SH is an INSERM/AP-HP investigator.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) beta-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477 [DOI] [PubMed] [Google Scholar]
- Anne SL, Saudou F, Humbert S (2007) Phosphorylation of huntingtin by cyclin-dependent kinase 5 is induced by DNA damage and regulates wild-type and mutant huntingtin toxicity in neurons. J Neurosci 27: 7318–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A (2005) p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron 47: 29–41 [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Bence NF, Jayakumar R, Kopito RR (2005) Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17: 351–365 [DOI] [PubMed] [Google Scholar]
- Bett JS, Cook C, Petrucelli L, Bates GP (2009) The ubiquitin-proteasome reporter GFPu does not accumulate in neurons of the R6/2 transgenic mouse model of Huntington's disease. PLoS One 4: e5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett JS, Goellner GM, Woodman B, Pratt G, Rechsteiner M, Bates GP (2006) Proteasome impairment does not contribute to pathogenesis in R6/2 Huntington's disease mice: exclusion of proteasome activator REG{gamma} as a therapeutic target. Hum Mol Genet 15: 33–44 [DOI] [PubMed] [Google Scholar]
- Boonen RA, van Tijn P, Zivkovic D (2009) Wnt signaling in Alzheimer's disease: up or down, that is the question. Ageing Res Rev 8: 71–82 [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Zala D, Humbert S, Saudou F (2006) Huntington's disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci 63: 2642–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F (2009) Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 28: 2569–2580 [DOI] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC (2002) Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem 277: 33791–33798 [DOI] [PubMed] [Google Scholar]
- Chai Y, Berke SS, Cohen RE, Paulson HL (2004) Poly-ubiquitin binding by the polyglutamine disease protein ataxin-3 links its normal function to protein surveillance pathways. J Biol Chem 279: 3605–3611 [DOI] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH (2005) Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol 167: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Valera AG, Moran MA, Gomez-Ramos P, Alvarez-Castelao B, Castano JG, Hernandez F, Lucas JJ (2006) Inhibition of 26S proteasome activity by huntingtin filaments but not inclusion bodies isolated from mouse and human brain. J Neurochem 98: 1585–1596 [DOI] [PubMed] [Google Scholar]
- Dihlmann S, Siermann A, von Knebel Doeberitz M (2001) The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 20: 645–653 [DOI] [PubMed] [Google Scholar]
- Ermak G, Hench KJ, Chang KT, Sachdev S, Davies KJ (2009) Regulator of calcineurin (RCAN1-1L) is deficient in Huntington disease and protective against mutant huntingtin toxicity in vitro. J Biol Chem 284: 11845–11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines S, Ivanova E, Seong IS, Saura CA, MacDonald ME (2003) Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington's disease knock-in striatal cells. J Biol Chem 278: 50514–50522 [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281: 22429–22433 [DOI] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F (2002) The F-box protein slimb controls the levels of clock proteins period and timeless. Nature 420: 178–182 [DOI] [PubMed] [Google Scholar]
- Gu W, Wells AL, Pan F, Singer RH (2008) Feedback regulation between zipcode binding protein 1 and beta-catenin mRNAs in breast cancer cells. Mol Cell Biol 28: 4963–4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P (1999) The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 9: 207–210 [DOI] [PubMed] [Google Scholar]
- Hawcroft G, D'Amico M, Albanese C, Markham AF, Pestell RG, Hull MA (2002) Indomethacin induces differential expression of beta-catenin, gamma-catenin and T-cell factor target genes in human colorectal cancer cells. Carcinogenesis 23: 107–114 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF (2008) Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience 157: 280–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert S, Bryson EA, Cordelieres FP, Connors NC, Datta SR, Finkbeiner S, Greenberg ME, Saudou F (2002) The IGF-1/Akt pathway is neuroprotective in Huntington's disease and involves Huntingtin phosphorylation by Akt. Dev Cell 2: 831–837 [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yamagishi N, Hatayama T (2004) Suppression of heat- and polyglutamine-induced cytotoxicity by nonsteroidal anti-inflammatory drugs. Eur J Biochem 271: 4552–4558 [DOI] [PubMed] [Google Scholar]
- Jana NR, Zemskov EA, Wang G, Nukina N (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet 10: 1049–1059 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Struhl G (1998) Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391: 493–496 [DOI] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, Cha GH, Ukani L, Chepanoske CL, Zhen Y, Sahasrabudhe S, Olson J, Kurschner C, Ellerby LM, Peltier JM, Botas J et al. (2007) Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet 3: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH (1999) Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci 19: 4229–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitanovic S, Cacev T, Antica M, Kralj M, Cavric G, Pavelic K, Spaventi R (2006) Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp Mol Pathol 80: 91–96 [DOI] [PubMed] [Google Scholar]
- Khoshnan A, Ko J, Watkin EE, Paige LA, Reinhart PH, Patterson PH (2004) Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. J Neurosci 24: 7999–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Xu W (2006) beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25: 7482–7491 [DOI] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M (1999) The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18: 849–854 [DOI] [PubMed] [Google Scholar]
- Lee WC, Yoshihara M, Littleton JT (2004) Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease. Proc Natl Acad Sci USA 101: 3224–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH III, Sussman DJ, Jonkers J, Kimelman D, Wu D (1999) Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J 18: 4233–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens J, Matsunami N, White RL (2004) Targeted degradation of beta-catenin by chimeric F-box fusion proteins. Biochem Biophys Res Commun 313: 1023–1029 [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Avila J (1999) Nuclear localization of beta-catenin in adult mouse thalamus correlates with low levels of GSK-3beta. Neuroreport 10: 2699–2703 [DOI] [PubMed] [Google Scholar]
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R (1998) A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell 1: 565–574 [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Bottcher C, Ortega Z, Smith R, Florea BI, Diaz-Hernandez M, Brundin P, Overkleeft HS, Li JY, Lucas JJ, Dantuma NP (2009) Accumulation of ubiquitin conjugates in a polyglutamine disease model occurs without global ubiquitin/proteasome system impairment. Proc Natl Acad Sci USA 106: 13986–13991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF (2003) Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol 465: 11–26 [DOI] [PubMed] [Google Scholar]
- Mugat B, Parmentier ML, Bonneaud N, Chan HY, Maschat F (2008) Protective role of engrailed in a Drosophila model of Huntington's disease. Hum Mol Genet 17: 3601–3616 [DOI] [PubMed] [Google Scholar]
- Norflus F, Nanje A, Gutekunst CA, Shi G, Cohen J, Bejarano M, Fox J, Ferrante RJ, Hersch SM (2004) Anti-inflammatory treatment with acetylsalicylate or rofecoxib is not neuroprotective in Huntington's disease transgenic mice. Neurobiol Dis 17: 319–325 [DOI] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW (1997) Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem 272: 24735–24738 [DOI] [PubMed] [Google Scholar]
- Ortega Z, Diaz-Hernandez M, Lucas JJ (2007) Is the ubiquitin-proteasome system impaired in Huntington's disease? Cell Mol Life Sci 64: 2245–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17: 45–51 [DOI] [PubMed] [Google Scholar]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95: 55–66 [DOI] [PubMed] [Google Scholar]
- Schilling G, Savonenko AV, Coonfield ML, Morton JL, Vorovich E, Gale A, Neslon C, Chan N, Eaton M, Fromholt D, Ross CA, Borchelt DR (2004) Environmental, pharmacological, and genetic modulation of the HD phenotype in transgenic mice. Exp Neurol 187: 137–149 [DOI] [PubMed] [Google Scholar]
- Teo JL, Ma H, Nguyen C, Lam C, Kahn M (2005) Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci USA 102: 12171–12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier Y, Devys D, Imbert G, Saudou F, An I, Lutz Y, Weber C, Agid Y, Hirsch EC, Mandel JL (1995) Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat Genet 10: 104–110 [DOI] [PubMed] [Google Scholar]
- Venkatraman P, Wetzel R, Tanaka M, Nukina N, Goldberg AL (2004) Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of polyglutamine-containing proteins. Mol Cell 14: 95–104 [DOI] [PubMed] [Google Scholar]
- Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ (2008) SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452: 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li XJ, Li SH, Yi H, Vonsattel JP, Gusella JF, Hersch S, Auerbach W, Joyner AL, MacDonald ME (2000) Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet 9: 503–513 [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW (1999) The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev 13: 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP (2003) Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell 11: 1445–1456 [DOI] [PubMed] [Google Scholar]
- Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT (2001) Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA 98: 14973–14978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB (2003) Huntingtin in health and disease. J Clin Invest 111: 299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
- Zhou H, Cao F, Wang Z, Yu ZX, Nguyen HP, Evans J, Li SH, Li XJ (2003) Huntingtin forms toxic NH2-terminal fragment complexes that are promoted by the age-dependent decrease in proteasome activity. J Cell Biol 163: 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet 35: 76–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.