Abstract
Control of centrosome duplication is tightly linked with the progression of the cell cycle. Recent studies suggest that the fundamental process of centriole duplication is evolutionally conserved. Here, we identified centrosomal P4.1-associated protein (CPAP), a human homologue of SAS-4, as a substrate of PLK2 whose activity oscillates during the cell cycle. PLK2 phosphorylates the S589 and S595 residues of CPAP in vitro and in vivo. This phosphorylation is critical for procentriole formation during the centrosome cycle. PLK4 also phosphorylates S595 of CPAP, but PLK4 phosphorylation is not a critical step in the PLK4 function in procentriole assembly. CPAP is phosphorylated in a cell cycle stage-specific manner, so that its phosphorylation increases at the G1/S transition phase and decreases during the exit of mitosis. Phosphorylated CPAP is preferentially located at the procentriole. Furthermore, overexpression of a phospho-resistant CPAP mutant resulted in the failure to form elongated centrioles. On the basis of these results, we propose that phosphorylated CPAP is involved in procentriole assembly, possibly for centriole elongation. This work demonstrates an example of how procentriole formation is linked to the progression of the cell cycle.
Keywords: centrosome duplication, CPAP, PLK2, PLK4, procentriole formation
Introduction
The centrosome consists of a pair of centrioles surrounded by amorphous pericentriolar material. Like chromosomes, the centrioles duplicate only once per cell cycle. During the exit from mitosis, the paired centrioles are disengaged by separase; this is considered to be a critical step for licensing centriole duplication (Tsou and Stearns, 2006a, 2006b; Tsou et al, 2009). A procentriole is generated next to the parental centriole just before, or at the onset of, S phase. The two pairs of mother and daughter centrioles then move apart to become spindle poles for mitosis. Thus, the centriole cycle must be tightly coupled to the progression of the cell cycle (Azimzadeh and Bornens, 2007; Bettencourt-Dias and Glover, 2007; Nigg, 2007).
Genomic and genetic analyses have identified five centrosome proteins that are required for centriole duplication in Caenorhabditis elegans: ZYG-1, a protein kinase, and SAS-4, SAS-5, SAS-6, and SPD-2, which contain coiled-coil domains (O'Connell et al, 2001; Leidel and Gonczy, 2003; Kemp et al, 2004). During centriole biogenesis, these proteins are sequentially recruited to the centrioles (Delattre et al, 2006; Pelletier et al, 2006). SPD-2 is responsible for the recruitment of centrosomal components, including ZYG-1 (Kemp et al, 2004; Pelletier et al, 2004). ZYG-1 is important for the recruitment of the complex that is formed by SAS-5 and SAS-6, which is necessary for the formation of the central tube (Dammermann et al, 2004; Delattre and Gonczy, 2004; Leidel et al, 2005). SAS-4 then has a function in tethering singlet microtubules to the central tube, the final step of centriole biogenesis (Pelletier et al, 2006).
Centrioles in vertebrates share a structural homology with those in C. elegans, but they are not identical. For example, human centrioles are composed of nine triplet microtubules, whereas the centrioles in Drosophila melanogaster and C. elegans are mostly composed of doublet and singlet microtubules, respectively (Delattre and Gonczy, 2004). During centriole biogenesis, a cartwheel structure, rather than a central core, is formed within the vertebrate procentriole (Azimzadeh and Bornens, 2007; Strnad and Gonczy, 2008). Nonetheless, recent studies propose a unified model of centriole duplication among animal cells (Bettencourt-Dias and Glover, 2007). First, SPD-2, SAS-4, and SAS-6 have homologues in human cells named CEP192, centrosomal P4.1-associated protein (CPAP), and HsSAS-6, respectively (Nigg, 2007). Second, CEP192, CPAP, and HsSAS-6 are required for centriole duplication in human cells (Leidel et al, 2005; Zhu et al, 2008; Kohlmaier et al, 2009). There are no obvious homologues of ZYG-1 in the human genome, but PLK4 is speculated to be its functional homologue for the initiation of centriole duplication (Bettencourt-Dias et al, 2005; Kleylein-Sohn et al, 2007; Nigg, 2007).
CPAP was initially identified as an interacting partner of Protein 4.1 R-135, and was reported to serve as a transcriptional coactivator that enhances both Stat-5-mediated and TNF-α-induced NF-κB-mediated transcriptional activity (Peng et al, 2002; Koyanagi et al, 2005). However, CPAP is best known for maintaining centrosome integrity and normal spindle morphology during cell division (Cho et al, 2006). A study using immunodepletion suggested that CPAP may regulate microtubule nucleation at the centrosomes (Hung et al, 2000). Mutations of the CPAP gene are linked to autosomal recessive primary microcephaly, suggesting that CPAP is involved in the cell division of neuronal precursors to produce the proper number of neurons during brain development (Bond et al, 2005). As a human orthologue of SAS-4, the importance of CPAP in procentriole assembly is evident (Kleylein-Sohn et al, 2007; Kohlmaier et al, 2009; Tang et al, 2009). Overexpression of CPAP induces centriole elongation, suggesting that CPAP has a function in tethering microtubules for procentriole formation (Kohlmaier et al, 2009; Schmidt et al, 2009; Tang et al, 2009). However, it is still poorly understood how the biological activity of CPAP is regulated during procentriole assembly.
In addition to PLK4, several human protein kinases are critical for centriole duplication and assembly (Habedanck et al, 2005; Kleylein-Sohn et al, 2007). For example, CDK2 is necessary for the initiation of centrosome duplication (Hinchcliffe and Sluder, 2002). A few candidate substrates have been identified as being necessary for the function of CDK2 in centrosome duplication. For example, CDK2 phosphorylates nucleophosmin/B23, MPS1, and CP110 (Okuda et al, 2000; Fisk and Winey, 2001; Chen et al, 2002). Phosphorylation of nucleophosmin/B23 results in its dissociation from the centrosome before the initiation of centrosome duplication (Okuda et al, 2000). MPS1 and NDR kinases are centrosomal kinases that are critical for centrosome duplication (Fisk et al, 2003; Hergovich et al, 2007). PLK2 is also crucial for centriole assembly, as knockdown of PLK2 results in defects in centriole duplication (Warnke et al, 2004).
In this study, we tested the hypothesis in which the biological activities of centriole assembly components are controlled by protein kinases, whose activities oscillate during the cell cycle. In this manner, centriole assembly is coupled to the progression of the cell cycle. We report that PLK2 phosphorylates CPAP and controls its biological activity for centriole elongation.
Results
CPAP is phosphorylated by PLK2 in vitro
To explore the function of PLK2 in centriole assembly, we carried out in vitro kinase assays with selected centrosomal proteins and identified CPAP as a specific substrate. Subsequent in vitro kinase assays with truncated forms of GST-CPAP narrowed down the PLK2 phosphorylation site(s) to within 563–613 residues (Figure 1A; Supplementary Figure S1A–D). To pinpoint the PLK2 phosphorylation site(s), we prepared point mutant proteins of GST-CPAP563–613 in which each serine or threonine was substituted to alanine. The phosphorylated form of wild-type GST-CPAP563–613 produced two radioactive bands of different sizes, suggesting that there are at least two phosphorylation sites in CPAP563–613 (Figure 1B). All GST-CPAP563–613 point mutants, except the S589A and S595A mutants, had two bands (Figure 1B). In fact, the S589A mutant showed only a lower band, whereas the S595A mutant showed only an upper band (Figure 1B and C). Furthermore, the double mutant (CPAPSSAA), in which both serine residues at positions 589 and 595 were substituted with alanine residues, was not phosphorylated (Figure 1C). We also confirmed that full-length PLK2 did not phosphorylate GST-CPAP563–613,SSAA in vitro (Supplementary Figure S1E). These results indicate that PLK2 phosphorylates the S589 and S595 residues of CPAP in vitro.
Figure 1.
Phosphorylation of CPAP S589 and S595 is critical for centriole duplication. (A) A summary of the in vitro kinase assays that are presented in Supplementary Figure S1. In vitro kinase assays of PLK2 were performed to determine specific phosphorylation sites within the CPAP protein. As substrates, CPAP was truncated into small pieces. The red lines indicate the truncated CPAP proteins that were strongly phosphorylated by PLK2. The relative phosphorylation intensities are indicated in the right panel. (B) In vitro kinase assays of PLK2 were performed with the mutated GST-CPAP563–613 proteins. The wild type (WT) or kinase dead (KD) forms of GST-PLK21–390-kido (kinase domain) were used for kinases. The phosphorylation activity was examined by autoradiogram. The amounts of the proteins were determined by Coomassie blue staining. (C) In vitro kinase assays of PLK2 were performed with WT or point mutants (S589A, S595A, and S589A/S595A (SSAA)) of GST-CPAP563–613. The red and blue arrowheads indicate the phosphorylation of S589 and S595, respectively. (D) HeLa cells were transfected with pMyc-CPAP or pMyc-CPAPSSAA. Forty-eight hours later, the cells were coimmunostained with antibodies specific to Myc (red) and CP110 (green), and centriole duplication was determined by counting the CP110 dots. Where, 1 or 2 CP110 dot(s), unduplicated centrioles; and 3 or 4 CP110 dots, duplicated centrioles. (E) HeLa cells were transfected with pMyc-CPAP, pMyc-CPAPS589A, pMyc-CPAPS595A, or pMyc-CPAPSSAA. Forty-eight hours later, the cells were coimmunostained with antibodies specific to Myc (green) and centrobin (red), and centriole duplication was determined by counting the centrobin dots. Where, 0 or 1 centrobin dot, unduplicated centrioles; and 2 or 3 centrobin dots, duplicated centrioles. (F) HeLa cells were transfected with siCTL or siCPAP-U and cultured for 48 h. The cell lysates were then subjected to immunoblot analysis with antibodies specific to CPAP and α-tubulin. (G) The siCTL- or siCPAP-U-transfected HeLa cells were coimmunostained with antibodies specific to CPAP (green) and γ-tubulin (red). (H) HeLa cells were transfected with siCTL or siCPAP-U and subsequently with pMyc-CPAP or pMyc-CPAPSSAA. The cells were coimmunostained with antibodies specific to Myc (red) and CP110 (green) to determine centriole duplication. The scale bar represents 10 μm. For statistical analysis, over 50 cells were counted and the experiments were repeated three times. The results are presented as means and standard errors. *P<0.05, analysed with the paired t-test. Insets are magnified views of the centrosomes.
Specific phosphorylation of CPAP S589 and S595 is critical for procentriole formation
CPAP associates with microtubules through a specific-binding domain at the 423–607 residues (Hung et al, 2004; Hsu et al, 2008). To examine whether the alanine substitution mutations at S589 and S595 of CPAP affects its binding to microtubules, we performed the microtubule-binding assays. GST-CPAP563–613 was coprecipitated with polymerized microtubules irrespective of the S589A and S595A mutations (Supplementary Figure S2A; Hsu et al, 2008). Furthermore, both Myc-CPAPWT and Myc-CPAPSSAA were associated with microtubules in nocodazole (NZ)- or taxol (TX)-treated cells (Supplementary Figure S2B; Hsu et al, 2008). These results indicate that the S589A and S595A mutations do not affect the microtubule-binding activity of CPAP.
To examine whether phosphorylation of S589 and S595 of CPAP is critical for procentriole assembly, we counted the centriole numbers of the cells in which the Myc-CPAPSSAA mutant protein was ectopically expressed. Immunostaining results showed that both Myc-CPAPWT and Myc-CPAPSSAA were localized at the centrosomes and the excess proteins were detected at the cytoplasm (Figure 1D). Centriole duplication in the cells expressing moderate levels of transfected CPAP at the centrosome was determined. Approximately 75% of the Myc-CPAPWT-expressing cells, as well as control cells, had four centrioles that were immunostained using the CP110 antibody. In contrast, only 35% of the Myc-CPAPSSAA-expressing cells contained four centrioles, suggesting that CPAPSSAA inhibited procentriole assembly in a dominant-negative manner. To determine which serine is crucial for centriole duplication, we counted the number of procentrioles in the CPAPS589A- or CPAPS595A-expressing cells by staining centrobin, which specifically localizes to newly assembled procentrioles (Zou et al, 2005; Jeong et al, 2007). These results indicate that double phosphorylations at S589 and S595 are required for procentriole formation (Figure 1E).
It was reported that CPAP is critical for procentriole formation (Kleylein-Sohn et al, 2007; Kohlmaier et al, 2009; Tang et al, 2009). We also observed that suppression of CPAP using two different siRNAs resulted in a reduction in the number of centrioles in hydroxyurea (HU)-treated U2OS cells, as well as in mitotic HeLa cells (Supplementary Figure S3). To confirm the importance of the phosphorylation of CPAP for procentriole formation, we carried out knockdown-rescue experiments. The knockdown efficiency of siCPAP-U was confirmed using immunoblot and immunostaining analyses (Figure 1F and G). Twenty-four hours after siCTL or siCPAP-U transfection, pMyc-CPAPWT or pMyc-CPAPSSAA was introduced into HeLa cells and the number of centrioles was counted. In the siCTL-transfected cells, Myc-CPAPSSAA suppressed centriole assembly, as shown earlier (Figure 1H; Supplementary Figure S4). Centriole assembly was significantly impaired in the CPAP-depleted cells, with no obvious G1 arrest of the cells (Figure 1H; Supplementary Figure S5). In the siCPAP-U-transfected cells, Myc-CPAPWT rescued the impaired centriole assembly, whereas Myc-CPAPSSAA did not (Figure 1H; Supplementary Figure S4). These results collectively indicate that phosphorylation of S589 and S595 is critical for the function of CPAP in centriole assembly.
PLK2 phosphorylation of S589 and S595 in CPAP is a critical step for procentriole formation
Knockdown of PLK2 suppresses centrosome overduplication in S phase-arrested U2OS cells (Warnke et al, 2004). We decided to re-examine the involvement of PLK2 in procentriole assembly in dividing cells. We confirmed the knockdown of the endogenous PLK2 protein in siPLK2-transfected cells (Figure 2A and B). When PLK2 expression was suppressed in asynchronous HeLa cells, the proportion of cells with two centrioles significantly increased with no G1 phase arrest, confirming that PLK2 is required for procentriole assembly, to some extent, during the cell cycle (Figure 2C; Supplementary Figure S5). To examine the functional significance of PLK2 phosphorylation at specific sites of CPAP, we prepared phospho-mimetic mutants of CPAP in which S589 and/or S595 were substituted to glutamate residues (CPAPS589E, CPAPS595E, and CPAPSSEE); these mutants were then expressed in PLK2-suppressed cells (Figure 2D). The results showed that procentriole assembly was significantly repressed in PLK2-suppressed cells. The CPAPSSEE mutant effectively rescued the impaired procentriole assembly, whereas the wild-type and single-point mutants did not (Figure 2D). None of the phospho-mimetic CPAP mutant proteins affected centriole duplication in a dominant-negative manner, and only the SSEE mutant restored the procentriole assembly in PLK2-depleted cells (Figure 2D; Supplementary Figure S6). Taken together, these results strongly suggest that PLK2 phosphorylation of S589 and S595 in CPAP is a critical step for procentriole assembly.
Figure 2.
PLK2 phosphorylation of CPAP is essential for centriole duplication. (A) HeLa cells were transfected with either siCTL or siPLK2 and cultured for 72 h. The lysates were subjected to immunoblot analysis with antibodies specific to PLK2 and α-tubulin. (B) The siCTL- or siPLK2-transfected U2OS cells were coimmunostained with antibodies specific to PLK2 (green) and γ-tubulin (red). (C) The siCTL- or siPLK2-transfected HeLa cells were coimmunostained with antibodies specific to CP110 (green) and γ-tubulin (red). The centrioles in the interphase cells were counted. (D) HeLa cells were transfected with siRNAs (siCTL or siPLK2) and subsequently with the CPAP constructs (pMyc-CPAP, pMyc-CPAPS589E, pMyc-CPAPS595E, or pMyc-CPAPSSEE). The cells were coimmunostained with antibodies specific to Myc and centrobin, and centriole duplication was determined. The scale bar represents 10 μm. For statistical analysis, over 50 cells were counted and the experiments were repeated three times. The results are presented as means and standard errors. *P<0.05; **P<0.01; ***P<0.001, analysed with the paired t-test. Insets are magnified views of the centrosomes.
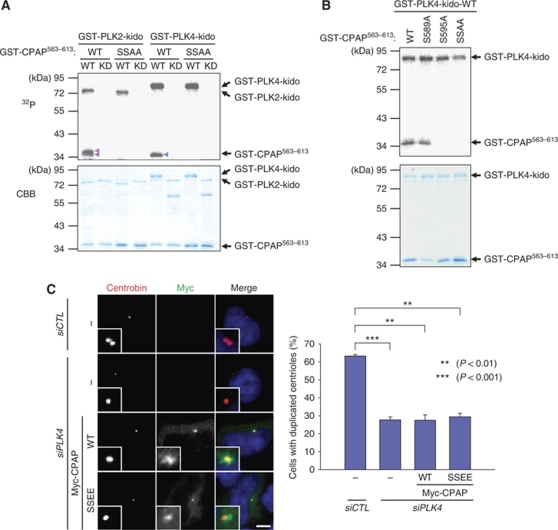
CPAP S595 is also phosphorylated by PLK4
PLK4 is a functional homologue of C. elegans ZYG-1 and has a crucial function in the initiation of centriole duplication (Kleylein-Sohn et al, 2007). Overexpression of PLK4 induces the formation of multiple procentrioles from a single parental centriole (Habedanck et al, 2005; Kleylein-Sohn et al, 2007; Cunha-Ferreira et al, 2009; Rogers et al, 2009). A preliminary result of ours revealed that CPAP is also phosphorylated by PLK4 in vitro (data not shown). We carried out in vitro kinase assays to examine whether S589 and S595 of CPAP are specific phosphorylation sites for PLK4. The results showed that PLK4 phosphorylates S595 but not S589 of CPAP (Figure 3A and B). Involvement of PLK4 phosphorylation of CPAP S595 in procentriole assembly was examined using knockdown-rescue experiments. As reported earlier, PLK4 suppression significantly impaired centriole duplication (Figure 3C; Habedanck et al, 2005). However, neither WT nor SSEE mutants of CPAP rescued the impaired centriole duplication in PLK4-depleted cells (Figure 3C). These results suggest that PLK4 phosphorylation at S595 of CPAP is not a critical step in PLK4 function in procentriole assembly.
Figure 3.
CPAP is also phosphorylated by PLK4. (A) In vitro kinase assays of PLK2 and PLK4 were performed with the mutated GST-CPAP563–613 proteins. The wild type (WT) and kinase dead (KD) forms of GST-PLK21–390-kido (kinase domain) or GST-PLK41–390-kido were used for kinases. The phosphorylation activity was determined by autoradiogram. The amounts of the proteins were determined by Coomassie blue staining. Blue and red arrowheads indicate the S589 and S595 phosphorylated bands, respectively. (B) In vitro kinase assays of PLK4 were performed with the WT or phospho-resistant mutants (S589A, S595A, and S589A/S595A (SSAA)) of GST-CPAP563–613. (C) HeLa cells were transfected with siPLK4 and subsequently with pMyc-CPAP or pMyc-CPAPSSEE. Forty-eight hours later, the cells were coimmunostained with antibodies specific to Myc (green) and centrobin (red), and centriole duplication was determined. The scale bar represents 10 μm. For statistical analysis, over 50 cells were counted and the experiments were repeated three times. The results are presented as means and standard errors. **P<0.01; ***P<0.001, analysed with the paired t-test. Insets are magnified views of the centrosomes.
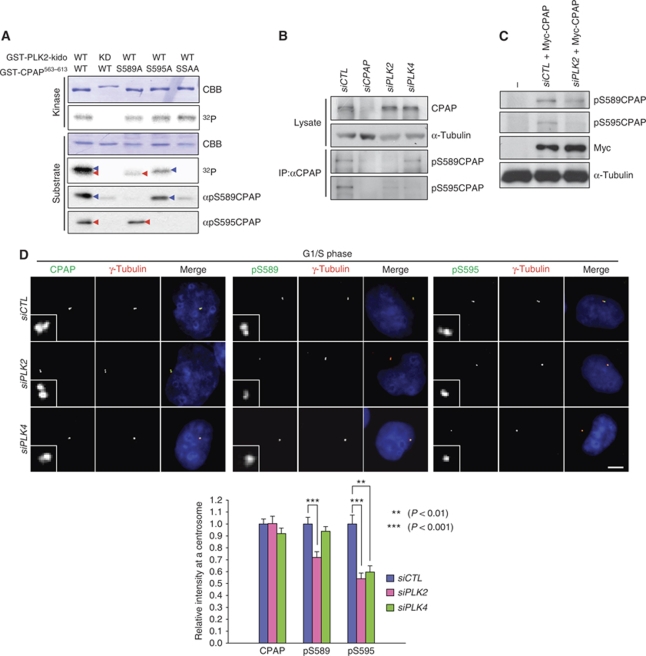
Cell cycle stage-specific phosphorylation of CPAP
We generated phospho-antibodies specific to pS589 and pS595 of CPAP. Specificity of the phospho-antibodies was determined using immunoblot analysis with the various GST-CPAP563–613 point mutant proteins that had been phosphorylated by PLK2 in vitro (Figure 4A). The pS589CPAP antibody detected wild-type and S595A mutant of GST-CPAP563−613 phosphorylated by PLK2 in vitro, but not the S589A and SSAA mutants (Figure 4A). On the contrary, the pS595CPAP antibody detected the phosphorylated wild-type and S589A mutant of GST-CPAP563−613, but not the S595A and SSAA mutant (Figure 4A). Both phospho-antibodies were not able to detect the unphosphorylated GST-CPAP563−613 at all. These results revealed that the antibodies are specific to the phospho-CPAP proteins.
Figure 4.
CPAP phosphorylation in vivo. (A) In vitro kinase assay of PLK2 was performed with GST-CPAP563−613 point mutants (S589A, S595A, and SSAA) as substrates. The kinase activity of PLK2 was determined with an autoradiogram. The same blot was subjected to immunoblot analysis with antibodies specific to phospho-S589 (αpS589CPAP) or phospho-S595 (αpS595CPAP) of CPAP. Blue and red arrowheads indicate the S589 and S595 phosphorylated bands, respectively. (B) HeLa cells were transfected with siCTL, siCPAP, siPLK2 or siPLK4. Total lysates were immunoprecipitated with the CPAP antiserum to concentrate the endogenous CPAP proteins, followed by immunoblotting with the phospho-CPAP antibodies. One hundredth of the total lysates were subjected to immunoblot analysis with CPAP and α-tubulin antibodies, (C) HeLa cells were transfected with siRNAs (siCTL or siPLK2) and subsequently with the Myc-CPAP. The lysates were subjected to immunoblot analysis with phospho-CPAP (pS589CPAP and pS595CPAP), Myc and α-tubulin antibodies. (D) HeLa cells were transfected with siCTL, siPLK2, or siPLK4. The cells were then synchronized at G1/S phase by a double thymidine block. The cells were immunostained with the CPAP or phospho-CPAP (pS589CPAP and pS595CPAP) antibodies (green) and with the γ-tubulin antibody (red). Immunostaining intensities of the centrosomal CPAP and phospho-CPAP proteins were determined densitometrically relative to the intensity of siCTL-transfected cells. The scale bar represents 10 μm. For statistical analysis, immunofluorescent intensities of at least 20 cells were determined, and the results are presented as means and standard errors. **P<0.01; ***P<0.001, analysed with the t-test. Insets are magnified views of the centrosomes.
It is unfortunate that our phospho-antibodies could not detect the endogenous CPAP protein in immunoblot analysis with whole-cell lysates (data not shown). Therefore, we decided to concentrate the endogenous CPAP protein by immunoprecipitation and performed immunoblot analysis. The results showed that both pS589CPAP and pS595CPAP antibodies detected specific bands in control cells but not in CPAP-depleted cells (Figure 4B). Furthermore, the pS589CPAP-specific band was diminished in PLK2-depleted cells (Figure 4B). The pS595CPAP-specific band intensity was reduced in both PLK2- and PLK4-depleted cells (Figure 4B). These observations are consistent with the conclusion that PLK2 and PLK4 phosphorylate specific residues of CPAP.
We also detected the phospho-CPAP-specific bands in cells overexpressing ectopic Myc-CPAP proteins (Figure 4C). The intensities of both phospho-CPAP-specific bands were reduced significantly in PLK2-depleted cells (Figure 4C). These results confirmed that our phospho-antibodies detect the phospho-CPAP proteins specifically. Furthermore, the results revealed that S589 is phosphorylated by PLK2 and S595 is phosphorylated by both PLK2 and PLK4 in vivo.
We performed immunocytochemistry with the phospho-CPAP antibodies (Supplementary Figure S7). As the phosphorylation levels at the centrosomes are heterogeneous in asynchronous cells, we arrested the cell cycle at G1/S transition phase when both PLK2 and PLK4 are active (Fode et al, 1996; Warnke et al, 2004). The results showed that both phospho-CPAP antibodies immunostained the centrosome specifically (Supplementary Figure S7A). The staining intensity of the phospho-antibodies was reduced in the CPAP-depleted cells, revealing the immunostaining specificity of the antibodies. It is somewhat unexpected that significant proportions of the phospho-CPAP signals were left at the centrosomes of the CPAP-depleted cells (Supplementary Figure S7A). It is possible that the phosphorylated form of CPAP may be preferentially concentrated at the centrosome.
The centrosomal levels of pS589CPAP was significantly reduced in the PLK2-depleted cells, and those of pS595CPAP was reduced in both PLK2- and PLK4-depleted cells (Figure 4D; Supplementary Figure S7B). These results are consistent with those of the in vitro kinase assays shown in Figures 1 and 3.
We then observed the phosphorylation of CPAP during the cell cycle. As the catalytic activity of PLK2 starts to increase during G1/S transition phase, when new procentrioles are assembled, and decreases again in G2 phase, it is reasonable to expect that S589 and S595 of CPAP are phosphorylated by PLK2 during the G1/S transition (Warnke et al, 2004). PLK4 levels also oscillate during the cell cycle, reaching a maximum during the early M phase (Fode et al, 1996). To examine the cell cycle stage-specific phosphorylation of CPAP, we treated the cells with nocodazole to arrest the cell cycle at M phase and then synchronously released the cells. Most of the cells exited from M phase within an hour and reached S phase from 8 h after release, as confirmed using immunoblot analysis (Figure 5A). Phosphorylation of S589 and S595 was minimal in cells at G1, but increased by three-fold when the cells entered S phase (Figure 5A). We also observed the levels of centrosomal phospho-CPAP during mitosis. The phosphorylation levels of S589 and S595 remained high until metaphase, but were dramatically reduced when the cells entered anaphase (Figure 5B). The levels of CPAP per centrosome remained constant as a whole, irrespective of the cell cycle stage (Figure 5). These results indicate that phosphorylation of CPAP S589 and S595 oscillates during the cell cycle, increasing at G1/S transition phase and decreasing during the exit from mitosis.
Figure 5.
Cell cycle stage-specific phosphorylation of CPAP. (A) HeLa cells were treated with nocodazole for 14 h to arrest the cell cycle at M phase and transferred to a fresh medium to synchronously resume the cell cycle. At the indicated time points, the cells were fixed and immunostained with the CPAP or phospho-CPAP (pS589CPAP and pS595CPAP) (green) antibodies, along with the γ-tubulin antibody (red). Immunostaining intensities of the centrosomal CPAP and phospho-CPAP proteins were determined densitometrically relative to the intensity of the 4 h-released cells. Cell cycle synchronization was confirmed by immunoblot analysis of cyclin A and phospho-histone H3 (pHH3). (B) Actively proliferating HeLa cells were immunostained with the CPAP or phospho-CPAP pS589CPAP and pS595CPAP) (green) antibodies and with the γ-tubulin antibody (red). Mitotic phases of the cells were determined by DAPI staining of the chromosomes. The immunostaining intensities of the centrosomal CPAP and phospho-CPAP proteins were determined densitometrically relative to the intensity of the prometaphase cells. Scale bar represents 10 μm. For statistical analysis, immunofluorescent intensities of at least 10 cells were determined and the results are presented as means and standard errors. Insets are magnified views of the centrosomes.
Phosphorylated CPAP is preferentially located at the procentrioles
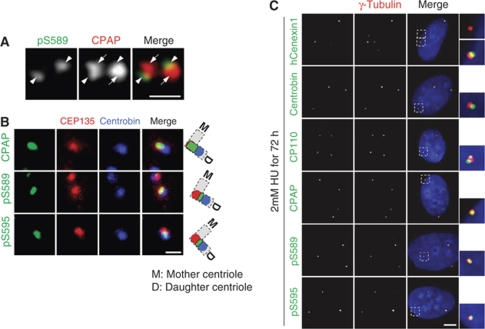
The specificity of the CPAP antibody that we had generated was determined using immunoblot and immunostaining analyses (Supplementary Figure S8A and B). The centrosomal localization of CPAP was also confirmed biochemically using a centrosome fractionation assay (Supplementary Figure S8C). CPAP was detected at the proximal end of the centriole, consistent with the immunoelectron microscopy results in the PLK4-induced cell line (Supplementary Figure S8D; Kleylein-Sohn et al, 2007). To determine the localization of the phosphorylated CPAP proteins, we coimmunostained HeLa cells with the CPAP and pS589CPAP antibodies. Both antibodies specifically immunostained the centrioles, but the pS589CPAP antibody immunostained only a portion of the area that was stained by the CPAP antibody (Figure 6A). These results suggest that the phospho-CPAP is concentrated at a specific site within a centriole pair.
Figure 6.
Phosphorylated CPAP localizes to the procentrioles. (A) Centrioles in U2OS cells were coimmunostained with CPAP (red) and pS589CPAP (green) antibodies. Arrows and arrowheads indicate the parental centrioles and procentriole, respectively. The scale bar represents 1 μm. (B) HeLa cells were accumulated at G2 phase by a thymidine block and release for 7 h for detection of the cells with separated centrosomes. The centrioles were triple stained with antibodies specific to CEP135 (red) and centrobin (blue), along with CPAP or phospho-CPAP (pS589CPAP and pS595CPAP, green). The scale bar represents 1 μm. (C) U2OS cells were treated with hydroxyurea (HU) for 72 h and coimmunostained with hCenexin1, centrobin, CP110, CPAP, or phospho-CPAP (pS589CPAP and pS595CPAP) (green) antibodies, along with the γ-tubulin antibody (red). The scale bar represents 10 μm. Insets are magnified views of the centrosomes.
To determine the exact location of phospho-CPAP within a centriole pair, we immunostained the centrioles with three different antibodies simultaneously. We used CEP135 and centrobin antibodies to mark the proximal end of parental centriole and procentriole, respectively. As expected, the CPAP signal was largely overlapped with the CEP135 signal at the proximal end of the parental centriole (Figure 6B). On the other hand, the phospho-CPAP proteins were localized between the CEP135 and centrobin signals (Figure 6B). We also obtained a similar result in double labelling of the centriole pairs with the CEP135 and centrobin antibodies (Supplementary Figure S9). These results are consistent with the conclusion that the phospho-CPAP proteins are predominantly localized at the proximal region of the procentriole.
An S phase-arrested U2OS cell contains amplified centrioles that are composed of two parental centrioles and multiple procentrioles. The parental centrioles and procentrioles can be distinguished with the hCenexin1 and centrobin antibodies, respectively (Zou et al, 2005; Soung et al, 2006; Jeong et al, 2007). As expected, the hCenexin1 antibody immunostained only two centrosomes in which the parental centrioles are included (Figure 6C). Conversely, the centrobin antibody immunostained all centrosomes, including those with parental centrioles, because the procentrioles are engaged with the parental centrioles in the same centrosome (Loncarek et al, 2008). When we immunostained the U2OS cells with the phospho-CPAP antibodies, we observed that all of the amplified centrosomes were immunostained (Figure 6C). These results suggest that phosphorylated CPAP is preferentially located at the procentriole.
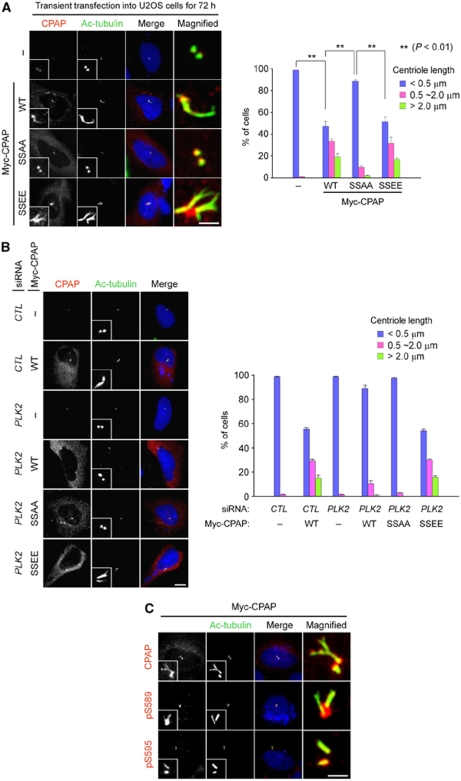
Specific phosphorylation of CPAP is required for centriole elongation
The stable overexpression of CPAP protein can cause the formation of centriole threads, also called procentriole-like structures, from both parental and nascent procentrioles (Kohlmaier et al, 2009; Schmidt et al, 2009; Tang et al, 2009). We repeated the same experiments with the CPAP proteins in which the specific phosphorylation sites were mutated. The ectopic expression of Myc-CPAPWT or Myc-CPAPSSEE efficiently induced the formation of centriole threads (Figure 7A). The ectopic CPAP proteins were detected throughout the elongated centrioles. Conversely, Myc-CPAPSSAA did not induce centriole elongation, even though it was detected at the centriole (Figure 7A). Ectopic CPAP proteins failed to induce centriole elongation in PLK2-depleted cells, but the phospho-mimetic SSEE mutant (Myc-CPAPSSEE) was able to elongate centrioles in PLK2-depleted cells (Figure 7B). Moreover, the phospho-specific CPAP antibodies, as well as the CPAP antibody, immunostained the elongated centriole threads (Figure 7C). These results suggest that PLK2 phosphorylation of CPAP S589 and S595 is critical for centriole elongation.
Figure 7.
Phosphorylation of CPAP S589 and S595 is required for centriole elongation. (A) U2OS cells were transfected with pMyc-CPAP, pMyc-CPAPSSAA, or pMyc-CPAPSSEE. Seventy-two hours later, the cells were placed on ice to depolymerize unstable microtubules, fixed, and coimmunostained with antibodies specific to CPAP (red) and acetylated tubulin (green). The scale bar represents 2 μm. For statistical analysis, over 100 cells were counted and the experiments were repeated three times. The results are presented as means and standard errors. **P<0.01, analysed with the paired t-test. (B) U2OS cells were transfected with siRNAs (siCTL or siPLK2) and subsequently with the CPAP constructs (pMyc-CPAP, pMyc-CPAPSSAA, or pMyc-CPAPSSEE). The cells were coimmunostained with antibodies specific to CPAP (red) and acetylated tubulin (green). The scale bar represents 10 μm. For statistical analysis, over 50 cells were counted and the experiments were repeated three times. (C) U2OS cells were transfected with pMyc-CPAP. Seventy-two hours later, the cells were coimmunostained with antibodies specific to CPAP or phospho-CPAP (pS589CPAP and pS595CPAP) (red) antibodies, along with the acetylated-tubulin antibody (green). The scale bar represents 2 μm. Insets are magnified views of the centrosomes.
Discussion
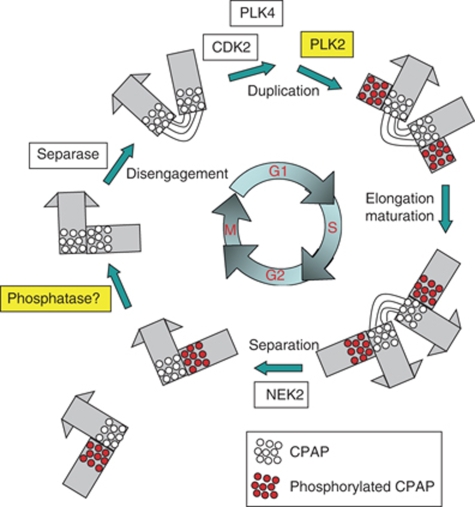
In this study, we revealed that CPAP is a substrate of PLK2 and PLK4. PLK2 phosphorylation of CPAP S589 and S595 was critical for procentriole assembly, whereas PLK4 phosphorylation of S595 was not. On the basis of the results, we propose a model in which PLK2 phosphorylation is critical for CPAP function in procentriole assembly during the cell cycle (Figure 8). CPAP may be recruited to the centrosome as it is synthesized; however, to participate in procentriole formation, CPAP must be phosphorylated by PLK2, the activity of which is induced at G1/S transition phase (Warnke et al, 2004). CPAP is dephosphorylated during the exit from mitosis, immediately before the procentriole is disengaged from the mother centriole. It remains unknown whether the CPAP located at the mother centriole is critical for procentriole formation. Such behaviour of CPAP is reminiscent of the proposed mechanism of SAS-4 in C. elegans. As SAS-4 is recruited to the centrosome earlier than its activation, it was proposed that SAS-4 is regulated at two different steps (Pelletier et al, 2006; Dammermann et al, 2008). Once SAS-4 is recruited to the centrosome, it should be stabilized at the nascent procentriole (Dammermann et al, 2008). In human cells, CPAP may be stably incorporated into procentrioles by its phosphorylation.
Figure 8.
Model. A number of protein kinases control the centriole cycle in a close association with the cell cycle. PLK2 phosphorylation is important for the function of CPAP in procentriole formation at G1/S transition phase. The phosphorylated CPAP (red dots) is located at the proximal end of the procentriole. CPAP is dephosphorylated by an unknown phosphatase once the cell enters anaphase (blank dots).
A function for CPAP in procentriole assembly was recently proposed. On the basis of the observation of centriole elongation in CPAP-overexpressing cells, it was proposed that CPAP has an important function in assembling procentriolar microtubules, possibly by recruiting tubulin dimers (Tang et al, 2009). This observation is consistent with the function of SAS-4 in C. elegans that is responsible for tethering singlet microtubules surrounding a central tube, the core structure of centrioles in C. elegans (Delattre et al, 2006; Pelletier et al, 2006). In this study, we observed that the ectopic expression of Myc-CPAPWT or Myc-CPAPSSEE efficiently induced the formation of centriole threads, whereas Myc-CPAPSSAA did not. Furthermore, phosphorylated CPAP is detected at the elongated centrioles. These results suggest that the specific phosphorylation of CPAP is a prerequisite for the participation of CPAP in centriole elongation. It is possible that phosphorylation of CPAP may increase its efficiency to recruit tubulin dimers into the procentriole.
PLK2 and PLK4 are known to have distinct structural properties, especially at the polo-box domains, and to have their own substrate specificities (Leung et al, 2002; Elia et al, 2003; Johnson et al, 2007). Therefore, it is of interest that S595 of CPAP can be phosphorylated by both PLK2 and PLK4. It is possible that S595 of CPAP is phosphorylated by these enzymes at different stages of the cell cycle. Nonetheless, PLK4 phosphorylation of this residue is not sufficient for the completion of procentriole assembly, as evidenced by the observation that Myc-CPAPSSEE could not rescue the knockdown phenotype of PLK4. It is likely that additional PLK4 phosphorylation sites in other molecules are required for procentriole assembly. In the case of PLK2, phosphorylation of CPAP S589 and S595 is sufficient for its involvement in procentriole formation.
Although centriole assembly is controlled by a highly conserved mechanism throughout species, PLK2 is not conserved in invertebrates, whereas PLK4 has an obvious structural and functional homologue in D. melanogaster (Bettencourt-Dias et al, 2005; Peel et al, 2007). Moreover, depletion of PLK2 suppresses centriole duplication less effectively than PLK4. Here, we demonstrated that PLK2 positively regulates centriole assembly through the phosphorylation of CPAP. In this manner, procentriole formation is linked to the progression of the cell cycle. It is likely that biogenesis of the centriole is controlled by additional protein kinases whose activities oscillate in a cell cycle stage-specific manner.
Materials and methods
cDNA and plasmid preparation
The CPAP, PLK2, and PLK4 clones were purchased from ImaGenes (http://www.imagenes-bio.de) and the CP110 cDNA clone was purchased from the Kazusa DNA Research Institute (http://www.kazusa.or.jp). Amplified PCR products from the cDNAs were subcloned into pGEX (Amersham Biosciences) for GST-fusion protein purification and into pCMV-Tag3 (Stratagene) for expression in mammalian cells. All point mutant clones were prepared by PCR-based site-directed mutagenesis.
Cell culture, transfections, and RNA interference experiments
HeLa and 293T cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. U2OS cells were cultured in McCoy's 5A medium supplemented with 10% FBS at 37°C and 5% CO2. Transfection into HeLa or U2OS cells was performed using Lipofectamine Plus (Invitrogen) or polyethylenimine (Sigma) for plasmid DNA, and Oligofectamine or RNAiMAX (Invitrogen) for siRNA according to the manufacturer's instructions. siCTL (control) (5′-GCA AUC GAA GCU CGG CUA CTT-3′), siCPAP (5′-GGA CUG ACC UUG AAG AGA ATT-3′), siCPAP-U (5′-UGU AAA UGC UGC GGA GAU UTT-3′), siPLK2 (5′-GGA CAU GGC UGU GAA UCA GTT-3′), and siPLK4 (5′-CUA UCU UGG AGC UUU AUA ATT-3′) were purchased from Samcully Pharm and were used for RNA interference experiments.
Antibodies
Anti-CPAP and anti-CP110 rabbit polyclonal antibodies were generated and affinity purified against GST-CPAP970–1338 and GST-CP1101–334, respectively. Phospho-S589 peptides (EQAADEIpSFSSNSSF) and phospho-S595 peptides (SNSpSFVLKILERDQQ; Anygen) were prepared, and the generated rabbit polyclonal antibodies were pre-cleared with unphosphorylated GST-CPAP563–613 to increase phospho-specificity. PLK2, centrobin, and CEP135 antibodies were described earlier (Warnke et al, 2004; Jeong et al, 2007; Kim et al, 2008). The hCenexin1 antibody was a generous gift from Kyung S Lee (National Institutes of Health, Bethesda, MD). Myc (Cell Signaling), γ-tubulin (Sigma for mouse or Santa Cruz for goat), β-tubulin (Sigma), α-tubulin (Abcam), acetylated tubulin (Sigma), cyclin A2 (Abcam), phospho-histone H3 (Upstate), and PCNA (DakoCytomation) antibodies were purchased. For coimmunostaining of two anti-rabbit IgG-containing primary antibodies, one antibody was labelled with Zenon Alexa Fluor 555 (Molecular Probes) and the other was detected with a secondary antibody.
Immunoblot analysis
Immunoblot analysis was performed as described earlier (Kim et al, 2008). In brief, protein samples were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a nitrocellulose or a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked using a blocking solution (Tris-buffered saline (TBS), 0.1% Triton X-100, 5% skimmed milk) for 30 min, incubated with the primary antibody for 2 h, washed three times with TBST (TBS, 0.1% Triton X-100), incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, washed three times with TBST, and the signal was developed on an X-ray film. To detect the endogenous CPAP proteins with the phospho-CPAP antibodies, we performed immunoblotting with the CPAP immunoprecipitates. HeLa cells were lysed with the RIPA buffer (50 mM Tris–HCl at pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, and protease inhibitors) and immunoprecipitated with the CPAP antiserum. The immunoprecipitates were subjected to immunoblotting with the phospho-CPAP antibodies.
Immunocytochemistry and image processing
For immunocytochemistry, HeLa or U2OS cells cultured on coverslips were briefly washed with phosphate-buffered saline (PBS), fixed with cold methanol for 10 min, washed with PBS and PBST (PBS, 0.1% Triton X-100), and blocked with blocking solution (PBS, 0.3% Triton X-100, 3% BSA) for 15 min. In some cases, cytoplasmic proteins were briefly pre-extracted with 0.3% PBST before fixation to stain the centrioles more clearly. The cells were incubated with primary antibodies for 1 h, washed three times with PBST, incubated with secondary antibodies for 1 h, and washed with PBST. DNA was stained with DAPI solution and the coverslip was mounted on a glass slide. The immunostained cells were observed using a fluorescence microscope (Olympus IX51). Images were acquired using a CCD (Qicam fast 1394, Qimaging) camera, and the images were analysed and processed using ImagePro 5.0 (Media Cybernetics, Inc.). For triple immunostaining of the centriole, CEP135 and centrobin antibodies were directly labelled with Zenon Alexa Fluor 594 and 647 (Molecular Probes), respectively. The CPAP or phospho-CPAP antibodies were detected with the Alexa Fluor 488 secondary antibody. Images were acquired by confocal laser scanning microscope (Carl Zeiss-LSM510). For measurement of the staining level, immunofluorescent images were processed using Adobe Photoshop (Adobe Systems, Inc.). The intensities were determined by subtracting the background intensity from the sum fluorescent intensity. All statistical data were analysed with SigmaPlot (Systat Software, Inc.).
Cell synchronization
For the centrosome overduplication assay, U2OS cells were arrested in S phase by treatment with 2 mM HU for 72 h. To synchronize cells in specific interphase stages, cells were arrested in prometaphase by treatment with 100 ng/ml nocodazole for 14 h. The cells were replated and released by mitotic shake-off. To examine the phosphorylation levels in G1/S phase, cells were arrested using a double thymidine block with 2 mM thymidine.
In vitro kinase assay
All kinases and substrates were prepared using the GST Gene Fusion System (Amersham Biosciences) in bacteria according to the manufacturer's instructions. Phosphorylation reactions were performed for 20 min at 30°C in kinase buffer (50 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 10 μM MnCl2, 10 mM β-glycerophosphate) supplemented with 10 μM ATP, 1 mM dithiothreitol, and 5 μCi [γ-32P]ATP in a volume of 20 μl. The reaction was stopped by adding SDS sample buffer and heating for 10 min at 95°C. The protein samples were subjected to SDS–PAGE, transferred onto a PVDF membrane, and the membrane was exposed to a BAS plate or an X-ray film to obtain an autoradiograph image, and then stained with Coomassie brilliant blue solution.
Supplementary Material
Acknowledgments
We thank Dr K Lee (NIH) who kindly provided us with the hCenexin1 antibody. This study was supported by grants from the BioImaging Research Center at GIST, the Basic Research Program (R01-2007-000-20116-0), and the SRC Program (R11-2005-009-03005-0). J Chang was supported by the second stage of the Brain Korea 21 Project in 2007.
Footnotes
The authors declare that they have no conflict of interest.
References
- Azimzadeh J, Bornens M (2007) Structure and duplication of the centrosome. J Cell Sci 120 (Pt 13): 2139–2142 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM (2007) Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 8: 451–463 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM (2005) SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 15: 2199–2207 [DOI] [PubMed] [Google Scholar]
- Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, Costa SM, Baralle D, Raponi M, Karbani G, Rashid Y, Jafri H, Bennett C, Corry P, Walsh CA, Woods CG (2005) A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet 37: 353–355 [DOI] [PubMed] [Google Scholar]
- Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell 3: 339–350 [DOI] [PubMed] [Google Scholar]
- Cho JH, Chang CJ, Chen CY, Tang TK (2006) Depletion of CPAP by RNAi disrupts centrosome integrity and induces multipolar spindles. Biochem Biophys Res Commun 339: 742–747 [DOI] [PubMed] [Google Scholar]
- Cunha-Ferreira I, Rodrigues-Martins A, Bento I, Riparbelli M, Zhang W, Laue E, Callaini G, Glover DM, Bettencourt-Dias M (2009) The SCF/Slimb ubiquitin ligase limits centrosome amplification through degradation of SAK/PLK4. Curr Biol 19: 43–49 [DOI] [PubMed] [Google Scholar]
- Dammermann A, Maddox PS, Desai A, Oegema K (2008) SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. J Cell Biol 180: 771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A, Muller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K (2004) Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell 7: 815–829 [DOI] [PubMed] [Google Scholar]
- Delattre M, Canard C, Gonczy P (2006) Sequential protein recruitment in C. elegans centriole formation. Curr Biol 16: 1844–1849 [DOI] [PubMed] [Google Scholar]
- Delattre M, Gonczy P (2004) The arithmetic of centrosome biogenesis. J Cell Sci 117(Pt 9): 1619–1630 [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB (2003) The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115: 83–95 [DOI] [PubMed] [Google Scholar]
- Fisk HA, Mattison CP, Winey M (2003) Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc Natl Acad Sci USA 100: 14875–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk HA, Winey M (2001) The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106: 95–104 [DOI] [PubMed] [Google Scholar]
- Fode C, Binkert C, Dennis JW (1996) Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol Cell Biol 16: 4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA (2005) The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 7: 1140–1146 [DOI] [PubMed] [Google Scholar]
- Hergovich A, Lamla S, Nigg EA, Hemmings BA (2007) Centrosome-associated NDR kinase regulates centrosome duplication. Mol Cell 25: 625–634 [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Sluder G (2002) Two for two: Cdk2 and its role in centrosome doubling. Oncogene 21: 6154–6160 [DOI] [PubMed] [Google Scholar]
- Hsu WB, Hung LY, Tang CJ, Su CL, Chang Y, Tang TK (2008) Functional characterization of the microtubule-binding and -destabilizing domains of CPAP and d-SAS-4. Exp Cell Res 314: 2591–2602 [DOI] [PubMed] [Google Scholar]
- Hung LY, Chen HL, Chang CW, Li BR, Tang TK (2004) Identification of a novel microtubule-destabilizing motif in CPAP that binds to tubulin heterodimers and inhibits microtubule assembly. Mol Biol Cell 15: 2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LY, Tang CJ, Tang TK (2000) Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol 20: 7813–7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Lee J, Kim K, Yoo JC, Rhee K (2007) Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J Cell Sci 120(Pt 12): 2106–2116 [DOI] [PubMed] [Google Scholar]
- Johnson EF, Stewart KD, Woods KW, Giranda VL, Luo Y (2007) Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry 46: 9551–9563 [DOI] [PubMed] [Google Scholar]
- Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O'Connell KF (2004) Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell 6: 511–523 [DOI] [PubMed] [Google Scholar]
- Kim K, Lee S, Chang J, Rhee K (2008) A novel function of CEP135 as a platform protein of C-NAP1 for its centriolar localization. Exp Cell Res 314: 3692–3700 [DOI] [PubMed] [Google Scholar]
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA (2007) Plk4-induced centriole biogenesis in human cells. Dev Cell 13: 190–202 [DOI] [PubMed] [Google Scholar]
- Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gonczy P (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 19: 1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Hijikata M, Watashi K, Masui O, Shimotohno K (2005) Centrosomal P4.1-associated protein is a new member of transcriptional coactivators for nuclear factor-kappaB. J Biol Chem 280: 12430–12437 [DOI] [PubMed] [Google Scholar]
- Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P (2005) SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol 7: 115–125 [DOI] [PubMed] [Google Scholar]
- Leidel S, Gonczy P (2003) SAS-4 is essential for centrosome duplication in C. elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell 4: 431–439 [DOI] [PubMed] [Google Scholar]
- Leung GC, Hudson JW, Kozarova A, Davidson A, Dennis JW, Sicheri F (2002) The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat Struct Biol 9: 719–724 [DOI] [PubMed] [Google Scholar]
- Loncarek J, Hergert P, Magidson V, Khodjakov A (2008) Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol 10: 322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA (2007) Centrosome duplication: of rules and licenses. Trends Cell Biol 17: 215–221 [DOI] [PubMed] [Google Scholar]
- O'Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG (2001) The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105: 547–558 [DOI] [PubMed] [Google Scholar]
- Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, Fukasawa K (2000) Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103: 127–140 [DOI] [PubMed] [Google Scholar]
- Peel N, Stevens NR, Basto R, Raff JW (2007) Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 17: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, O'Toole E, Schwager A, Hyman AA, Muller-Reichert T (2006) Centriole assembly in Caenorhabditis elegans. Nature 444: 619–623 [DOI] [PubMed] [Google Scholar]
- Pelletier L, Ozlu N, Hannak E, Cowan C, Habermann B, Ruer M, Muller-Reichert T, Hyman AA (2004) The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr Biol 14: 863–873 [DOI] [PubMed] [Google Scholar]
- Peng B, Sutherland KD, Sum EY, Olayioye M, Wittlin S, Tang TK, Lindeman GJ, Visvader JE (2002) CPAP is a novel stat5-interacting cofactor that augments stat5-mediated transcriptional activity. Mol Endocrinol 16: 2019–2033 [DOI] [PubMed] [Google Scholar]
- Rogers GC, Rusan NM, Roberts DM, Peifer M, Rogers SL (2009) The SCF Slimb ubiquitin ligase regulates Plk4/Sak levels to block centriole reduplication. J Cell Biol 184: 225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA (2009) Control of centriole length by CPAP and CP110. Curr Biol 19: 1005–1011 [DOI] [PubMed] [Google Scholar]
- Soung NK, Kang YH, Kim K, Kamijo K, Yoon H, Seong YS, Kuo YL, Miki T, Kim SR, Kuriyama R, Giam CZ, Ahn CH, Lee KS (2006) Requirement of hCenexin for proper mitotic functions of polo-like kinase 1 at the centrosomes. Mol Cell Biol 26: 8316–8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P, Gonczy P (2008) Mechanisms of procentriole formation. Trends Cell Biol 18: 389–396 [DOI] [PubMed] [Google Scholar]
- Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK (2009) CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 11: 825–831 [DOI] [PubMed] [Google Scholar]
- Tsou MF, Stearns T (2006a) Controlling centrosome number: licenses and blocks. Curr Opin Cell Biol 18: 74–78 [DOI] [PubMed] [Google Scholar]
- Tsou MF, Stearns T (2006b) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442: 947–951 [DOI] [PubMed] [Google Scholar]
- Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell 17: 344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, Hoffmann I (2004) Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol 14: 1200–1207 [DOI] [PubMed] [Google Scholar]
- Zhu F, Lawo S, Bird A, Pinchev D, Ralph A, Richter C, Muller-Reichert T, Kittler R, Hyman AA, Pelletier L (2008) The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr Biol 18: 136–141 [DOI] [PubMed] [Google Scholar]
- Zou C, Li J, Bai Y, Gunning WT, Wazer DE, Band V, Gao Q (2005) Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J Cell Biol 171: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.