Abstract
Despite extensive investigations of Cbl-interacting protein of 85 kDa (CIN85) in receptor trafficking and cytoskeletal dynamics, little is known about its functions in vivo. Here, we report the study of a mouse deficient of the two CIN85 isoforms expressed in the central nervous system, exposing a function of CIN85 in dopamine receptor endocytosis. Mice lacking CIN85 exon 2 (CIN85Δex2) show hyperactivity phenotypes, characterized by increased physical activity and exploratory behaviour. Interestingly, CIN85Δex2 animals display abnormally high levels of dopamine and D2 dopamine receptors (D2DRs) in the striatum, an important centre for the coordination of animal behaviour. Importantly, CIN85 localizes to the post-synaptic compartment of striatal neurons in which it co-clusters with D2DRs. Moreover, it interacts with endocytic regulators such as dynamin and endophilins in the striatum. Absence of striatal CIN85 causes insufficient complex formation of endophilins with D2DRs in the striatum and ultimately decreased D2DR endocytosis in striatal neurons in response to dopamine stimulation. These findings indicate an important function of CIN85 in the regulation of dopamine receptor functions and provide a molecular explanation for the hyperactive behaviour of CIN85Δex2 mice.
Keywords: CIN85, dopamine receptor, endocytosis, hyperactivity, synapse
Introduction
Cbl-interacting protein of 85 kDa (CIN85)/SH3-domain kinase binding protein 1 (SH3KBP1) forms, together with CMS (p130Cas ligand with multiple SH3 domains)/CD2-associated protein (CD2AP), a family of adaptor molecules with established functions in coordinating the spatial and temporal assembly of protein complexes during receptor endocytosis, formation of kidney glomeruli and organization of the immunological synapse in T cells (Dikic, 2002). Common to both CIN85/SH3KBP1 (alias CIN85) and CMS/CD2AP is a core structural organization comprising three N-terminal SH3 domains, followed by a centrally located proline-rich region and a C-terminal coiled coil (Dikic, 2002). In addition, in a functional perspective, both family members share certain characteristics, given that their SH3 domains specifically recognize a consensus Px(P/A)xxR motif, present in Cbl (Petrelli et al, 2002; Soubeyran et al, 2002; Kurakin et al, 2003; Kobayashi et al, 2004; Jozic et al, 2005; Moncalian et al, 2006) and other proteins involved in endocytosis, including synaptojanin, Huntingtin-interacting protein 1-related, Alix and Dab2 (Chen et al, 2000; Kowanetz et al, 2004), as well as regulators of the actin cytoskeleton, such as cortactin and the actin-capping protein CAPZ (Kirsch et al, 2001; Welsch et al, 2001; Cormont et al, 2003; Hutchings et al, 2003; Lynch et al, 2003). Indeed, a function of CIN85 and CMS/CD2AP in the regulation of actin dynamics is emphasized by their ability to directly interact with F-actin and induce bundling of actin filaments (Gaidos et al, 2007). The multitude of verified interaction partners has placed CIN85 as a central adaptor molecule involved in the recruitment of the endocytic machinery required for the internalization of a variety of cell surface receptors, including growth factor receptors (such as EGFR, Met and VEGFR) (Petrelli et al, 2002; Soubeyran et al, 2002; Kobayashi et al, 2004), immunoglobulin IgE receptors in mast cells (Molfetta et al, 2005), as well as during the infectious internalization of the bacterial pathogen Listeria monocytogenes (Veiga and Cossart, 2005).
Despite sharing many overlapping molecular functions in cultured cells, investigations in mice deficient of CD2AP have highlighted that CIN85 and CD2AP may have distinct functions in vivo. In agreement with its abundance in the kidney, mice lacking CD2AP suffer from a nephrotic syndrome caused by defective podocyte functionality during the formation of the glomerular slit diaphragm, eventually resulting in lethality at 6–8 weeks of age (Shih et al, 1999). An important function for CD2AP in spermatocyte production has furthermore been established, given that podocyte-specific reintroduction of transgenic CD2AP in knockout mice is sufficient to rescue animals into adulthood, but renders male mice infertile (Grunkemeyer et al, 2005). Interestingly, the podocyte and the cells of the basal seminiferous tubule are both cell types in which CIN85 is poorly expressed.
As a result of alternative splicing and differential promoter usage, the mouse CIN85 genomic locus gives rise to multiple isoforms, including CIN85-xl, CIN85-l (CIN85), CIN85-ΔA, CIN85-m, CIN85-s, CIN85-t and CIN85-h (Supplementary Figure S1) (Buchman et al, 2002; Finniss et al, 2004). The different isoforms display specific patterns of expression, among which CIN85-xl and CIN85-l are most abundant in brain and CIN85-l and CIN85-ΔA in thymus and spleen (Figure 2D; Supplementary Figure S1; Buchman et al, 2002). Lower levels of various isoforms can also be detected in kidney, muscle, skin, heart, lung and testis (Buchman et al, 2002 and unpublished observations). CD2AP is on the other hand predominantly expressed in spleen, thymus, heart, kidney, lung, muscle and liver, but seems to be lacking in neuronal cells (Dustin et al, 1998; Shih et al, 1999; Li et al, 2000).
In this study, we report a novel function of CIN85 in the regulation of post-synaptic dopamine receptor endocytosis in striatal neurons. In wild-type mice, CIN85 resides post-synaptically and associates with endocytic regulators, such as dynamin and endophilins. In mouse striatal neurons, absence of brain-specific CIN85 expression results in inefficient complex formation between dopamine receptors and endophilins, as well as reduced internalization of stimulated dopamine receptors. Mice deficient of brain-specific CIN85 expression show hyperactive phenotypes, which in many ways resemble the behavioural aberrations displayed in human beings affected by attention deficit hyperactivity disorder (ADHD), a disorder strongly associated with abnormal dopamine signalling.
Results
Specific isoforms of CIN85 localize post-synaptically in neurons
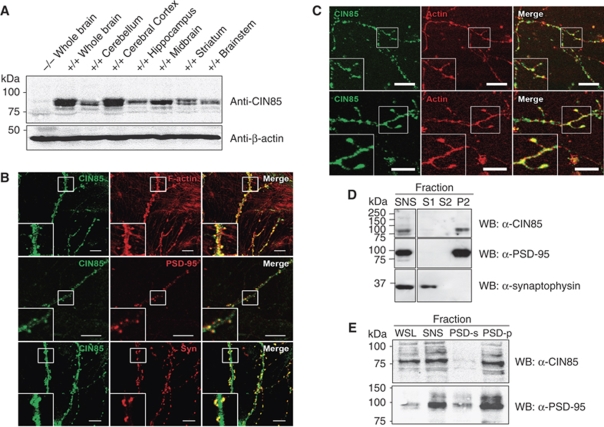
As CIN85 is highly expressed in the brain (Buchman et al, 2002), we were interested to investigate the function of CIN85 in the central nervous system (CNS). We initially analysed the expression pattern of CIN85 in various brain regions prepared from both mouse and rat (Figure 1A; Supplementary Figure S2 and data not shown) and found both of the major isoforms expressed in brain, CIN85-xl and CIN85-l, to be abundant in most brain regions tested, in line with earlier reported observations (Bian et al, 2008). To refine our survey of CIN85 localization on a sub-cellular level, we subsequently performed immunohistochemical analysis of endogenous CIN85 in cultured primary rat hippocampal neurons with mature synapses. In agreement with earlier reported observations (Kawata et al, 2006), and as shown in Figure 1B, we found high levels of CIN85 in the somatodendritic compartment, in which it frequently clustered in dendritic shafts, as well as within dendritic spines. Dendritic spines are small protrusions extending from the surface of dendrites, which are believed to be the main sites of excitatory synapses and thus vital centres for synaptic transmission in the brain (Segal, 2005).
Figure 1.
CIN85 is highly expressed in neurons in which it localizes to post-synaptic sites. (A) Western blot showing CIN85 expression in different mouse brain regions. Lysates (15 μg protein/lane) of the indicated brain regions from wild-type (+/+) and CIN85Δex2 knockout (−/−) mice were separated by 7% SDS–PAGE and immunoblotted with antibodies against CIN85 (CT). Equal protein loading was confirmed by re-blotting with anti-β-actin antibodies. (B) In primary hippocampal neurons derived from wild-type rat, CIN85 (SETA antibody, green) is localized to dendritic spines, in which it is accumulated in post-synaptic compartments, co-localizing with F-actin (red, upper panel) and PSD-95 (red, middle panel), closely juxtaposing the pre-synaptic synaptophysin protein (red, lower panel). Scale bars: 10 μm. (C) CIN85 (green) localizes to actin-positive (red), spine-like structures in dendrites of primary rat striatal neurons. Cells were fixed and stained with antibodies against CIN85 (SETA antibody, green) and with rhodamine phalloidin (red). Scale bars: 10 μm. (D) CIN85 is present in post-synaptic compartments in mouse brains. Synaptosome (SNS) fractions were isolated using the Percoll-step-gradient method from whole brain lysates as described in the Supplementary data. The western blot shows that CIN85, PSD-95 and synaptophysin are present in SNS fractions and that extraction with Triton X-100 solubilizes pre-synaptic synaptophysin into supernatant 1 (S1), whereas CIN85 and PSD-95 are present in post-synaptic fractions (pellet 2, P2, after a second Triton X-100 extraction). Supernatant 2 (S2) is the supernatant after the second Triton X-100 extraction. (E) CIN85 localizes to post-synaptic compartments in the striatum. Synaptosomes from mouse striata were prepared using a sucrose density-gradient method as described in the Supplementary data and immunoblotted with antibodies against CIN85 and PSD-95. WSL, whole striatal lysates; SNS, synaptosomes; PSD-s, supernatant of PSD fraction; PSD-p, pellet of PSD fraction. Each lane contains 15 μg of total protein.
We confirmed the localization of CIN85 in dendritic spines by showing a co-localization of CIN85 with F-actin, which is abundant in these structures (Figure 1B, upper panel). In addition, by counter-staining primary hippocampal neurons for CIN85 together with markers for pre-synaptic (synaptophysin) and post-synaptic (PSD-95) compartments, we found CIN85 to co-localize with PSD-95 at post-synaptic sites, closely juxtaposing synaptophysin (Figure 1B, middle and lower panel, respectively). In addition, in cultured primary striatal neurons, CIN85 was enriched in spine-like structures in dendrites (Figure 1C). Aiming at further characterizing the localization of CIN85 in neurons, we subsequently isolated synaptosomes, which are enriched in pre- and post-synaptic structures (Booth and Clark, 1978), from whole mouse brain preparations, as well as from isolated mouse striata, using conventional Percoll step-gradient or sucrose density-gradient centrifugations, respectively. Whereas CIN85, PSD-95 and synaptophysin were all present in crude synaptosomal isolates from whole mouse brains, further extraction with Triton X-100, which is known to specifically solubilize pre-synaptic compartments and thus enrich post-synaptic components, depicted an enrichment of CIN85 together with PSD-95 in the post-synaptic fractions (Figure 1D). Similar results were obtained in isolated mouse striata in which CIN85 co-fractionated with PSD-95 in post-synaptic density preparations (Figure 1E).
Mice lacking brain-specific CIN85 isoforms are hyperactive
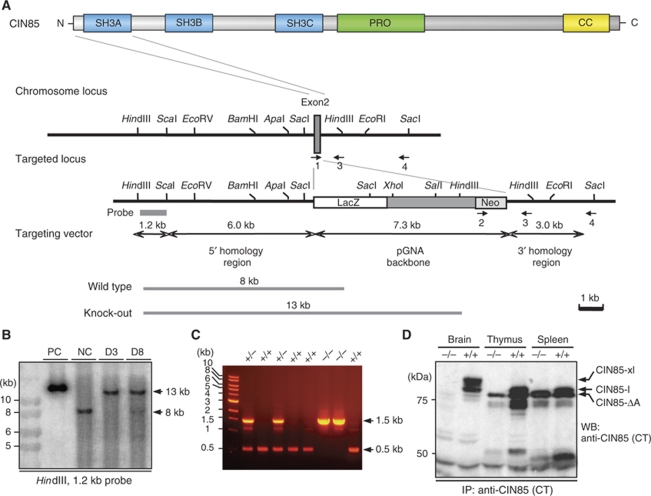
To investigate the function of CIN85 in the CNS, we next set out to generate mice deficient of the two major CIN85 isoforms expressed in the brain (CIN85-xl and CIN85-l). Using homologous recombination, we targeted exon 2 of the CIN85 genomic locus for deletion (Figure 2A) and could by a combinatorial approach based on Southern blot (Figure 2B) and PCR (Figure 2C; Supplementary Figure S3) confirm a successful integration of the targeting vector and absence of CIN85 exon 2 (CIN85Δex2). In agreement with the observed loss of CIN85Δex2 on the DNA level, we could furthermore confirm the absence of CIN85-xl and CIN85-l isoforms in brain, as well as CIN85-l in thymus and spleen, in CIN85Δex2 knockout mice by western blot analysis (Figure 2D). As expected, whereas all CIN85 protein variants encoded by transcripts initiated from promoter #1 (CIN85-xl, CIN85-l and the shorter CIN85-ΔCP) were abolished in CIN85Δex2 mice, none of the transcripts using alternative, downstream promoters, such as that of CIN85-ΔA (Figure 2D; Supplementary Figure S1; Buchman et al, 2002), were affected by the induced recombination event. Importantly, the neo-cassette, which as a result of the homologous recombination event replaces exon 2 in CIN85Δex2 animals, has been designed with a terminal translational stop codon, which should make ribosomal read-through and re-initiation highly unlikely. Moreover, given that exons 1 and 3 are out of frame, we anticipate that splicing events skipping the introduced LacZ/neo-cassette should result in inappropriate and prematurely terminated translation.
Figure 2.
Generation of CIN85Δex2 knockout mice. (A) Gene targeting of the CIN85 locus by removal of exon 2 by homologous recombination. The targeting vector consisted of a 6.0 kb 5′ homology region, the pGNA backbone containing a LacZ/neomycin cassette followed by a 3.0 kb 3′ homology region. The wild-type and targeted loci with their respective restriction sites are indicated. The arrows (1, 2, 3, 4) represent the primers used for genotyping of ES cells and CIN85Δex2 knockout mice (see Supplementary Figure S3 for primer sequences). The 1.2 kb probe used for Southern blot detects an 8 kb product in wild-type mice and a 13 kb product in CIN85Δex2 knockout mice after HindIII digestion. (B) Confirmation of recombinant targeting events in ES cell clones by Southern blot. Genomic DNA from wild-type ES cells and targeted ES cell clones (D3 and D8) was digested with HindIII and analysed by Southern blotting using the probe indicated in (A). The two targeted ES cell clones showed a 13 kb mutant band, indicating successful integration of the targeting vector by homologous recombination, whereas the wild-type ES cells, as expected, showed the 8 kb band of the wild-type locus (NC). An extended form of a linearized targeting vector was used as a positive control (PC). (C) Confirmation of the targeting event in CIN85Δex2 knockout mice by PCR, using a mixture of primers 1, 2 and 3. CIN85Δex2 knockout mice (−/−) give rise to the expected 1.5 kb product (primers 2 and 3), wild-type mice (+/+) the expected 0.5 kb product (primers 1 and 3) and heterozygous mice (+/−) to both products (primers 1, 2 and 3). Primer sequences are found in Supplementary Figure S3A. (D) Whole tissue lysates from mouse brain, thymus and spleen (200 μg/lane) from wild-type (+/+) and CIN85Δex2 knockout mice (−/−) were subjected to immunoprecipitation with anti-CIN85 (CT) antibodies. Subsequent western blot analysis with antibodies against CIN85 confirms the removal of CIN85-xl and CIN85-l in the brain and of CIN85-l in thymus and spleen in CIN85Δex2 knockouts. CIN85-ΔA remains in thymus and spleen. Some smaller isoforms present in thymus and spleen are also removed in CIN85Δex2 knockouts.
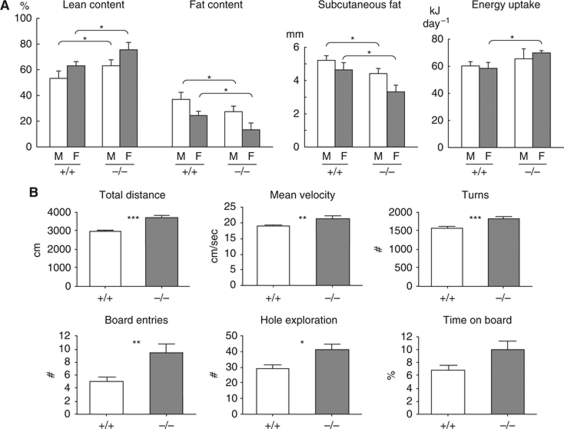
Homozygous CIN85Δex2 mice are viable and fertile, and display no obvious abnormalities. However, as neurological phenotypes may not be evident at first glance and often progress with age, we subjected the CIN85Δex2 knockouts to extensive analyses of a broad range of parameters according to the physiological screens defined by the German Mouse Clinic, summarized in Supplementary Figure S4A and the Supplementary data. Among the parameters tested, the screen exposed a clear knockout-specific phenotype in energy metabolism, behaviour (see below) and gene expression profiles (data not shown). When comparing CIN85Δex2 knockout and wild-type animals, we found that mice deficient of CIN85-xl and CIN85-l showed deviations in several metabolic parameters, including increased energy uptake, higher lean mass and lower fat content (Figure 3A; Supplementary Figure S4B), characteristics that in many ways resemble the phenotypes earlier reported for c-Cbl knockout mice (Molero et al, 2004).
Figure 3.
CIN85Δex2 knockout mice display metabolic and behavioural phenotypes. (A) CIN85Δex2 mutant mice display abnormalities in several metabolic parameters. Both male (M) and female (F) CIN85Δex2 knockout mice (−/−) showed a leaner body mass, as well as a decrease in total and subcutaneous fat content as compared with their wild-type littermates (+/+). Female CIN85Δex2 mice also showed a significantly increased energy uptake compared with their wild-type littermates (+/+). The graphs are based on the values in the table presented in Supplementary Figure S4B. *P<0.05. (B) CIN85Δex2 knockout mice are hyperactive. Behavioural analysis of 8–10-week-old CIN85Δex2 (−/−) mice and wild-type (+/+) littermate controls (n=22–26 per genotype) by the modified hole-board test (described in detail in the Supplementary data). The CIN85Δex2 knockout mice showed significantly increased forward locomotor activity, speed, turning frequency, board entry frequency and hole exploration frequency, as compared with wild-type controls; *P<0.05; **P<0.01, ***P<0.001, +/+ versus −/−.
In agreement with a function of CIN85 in the CNS, CIN85Δex2 mice also displayed obvious aberrations in behaviour (Figure 3B). When subjected to the modified hole-board test, which assays spontaneous behaviour such as forward and vertical locomotor activity, speed of movement and exploratory behaviour in a novel environment (Supplementary Figure S4C), CIN85Δex2 mice showed significantly increased activities, as compared with wild-type littermates (Figure 3B). Specifically, CIN85Δex2 animals exhibited enhanced forward locomotor activity, manifested by an increase in total distance travelled, number of line crossings, mean and maximum velocity, as well as turning frequency (Figure 3B; Supplementary Figure S4C). In addition, CIN85Δex2 knockout mice showed enhanced exploratory behaviour—entering the board more frequently and exploring a larger number of holes on the board, than wild-type animals (Figure 3B). Increase in total time spent on the board just missed a significant genotype effect limit (P=0.06, NS; Figure 3B). The increased physical activity displayed by the CIN85Δex2 animals should naturally be accompanied by an increased energy demand and indeed these mice show an increased energy intake (Figure 3A). However, given the lean body composition phenotype of these animals, the increased energy intake cannot completely compensate for the increased energy requirements. In summary, CIN85Δex2 mice are characterized by hyperactive behaviour, as evidenced by the increased locomotor and exploratory activities.
No obvious alterations in long-term potentiation, short-term synaptic plasticity or learning and memory in CIN85Δex2 knockout mice
The similarity of the metabolic phenotypes displayed by CIN85Δex2 and c-Cbl knockout mice is interesting in the light of a recent report indicating brain-specific activities of another member of the Cbl protein family, Cbl-b, which is involved in the regulation of long-term memory as well as short-term synaptic plasticity (Tan et al, 2006). This connection, together with the localization of CIN85 in dendritic spines of hippocampal neurons, led us to hypothesize that deficits in synaptic plasticity may be an underlying cause of the behavioural alterations observed in CIN85Δex2 mice. To address this question, we implemented established electrophysiological stimulation protocols (Freudenthal et al, 2004) to study synaptic plasticity in CIN85Δex2 mutants in vivo. Initially, we recorded field excitatory post-synaptic potentials (fEPSPs) in the dentate gyrus as a read-out of synaptic strength. In this assay, we found the synaptic response to be equally potentiated by tetanic stimulation in wild-type and CIN85Δex2 mice, thus indicating long-term potentiation (LTP) to be normal in the absence of CIN85 (Supplementary Figure S5A). Importantly, the stimulus–response curves of fEPSP slopes and population spikes, depicting synaptic strength and granule cell firing, respectively, were also unaltered in the mutants (Supplementary Figure S5B). Subsequently, we also analysed presynaptic short-term plasticity and GABAergic network inhibition by performing paired-pulse stimulation tests. However, in none of these assays did CIN85Δex2 knockout mice show any significant differences as compared with wild-type animals (Supplementary Figure S5C). We furthermore exposed the CIN85Δex2 mutants to learning and memory tests, and in line with the normal LTP and short-term plasticity, CIN85Δex2 knockout mice showed normal spatial learning in a water-maze place navigation task (Supplementary Figures S6A–C), as well as normal associative and contextual memory in a fear-conditioning task (Supplementary Figures S6A, D and E). Taken together, absence of CIN85 in the CNS does not cause any obvious abnormalities in learning and memory.
Dopamine receptor endocytosis is impaired in the absence of CIN85
The genetics behind behavioural traits such as locomotor activity and exploratory ambition is undoubtedly highly complex and involves a multitude of pathways, among which serotonergic, dopaminergic and noradrenergic signalling are frequently quoted (Pattij and Vanderschuren, 2008). On the basis of multiple studies reporting involvement of dopaminergic signalling in the regulation of movement, learning, reward-seeking behaviour and motivation (Yao et al, 2008), together with the rich clustering of dopamine receptors in dentritic spines (Missale et al, 1998; Gainetdinov et al, 2004; Viggiano et al, 2004; Zhu et al, 2004; Yao et al, 2008), we next investigated whether CIN85 could be involved in the regulation of dopamine signalling.
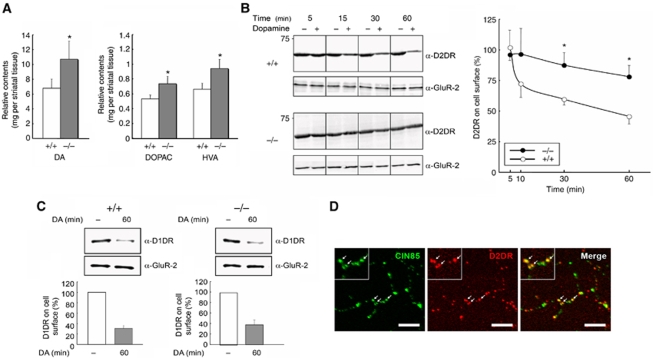
On the basis of the fact that increased levels of dopamine in the striatum have been correlated with locomotor hyperactivity phenotypes (Gainetdinov et al, 2004; Viggiano et al, 2004; Zhu et al, 2004), we first set out to analyse dopamine levels in the striatum of CIN85Δex2 knockout mice, using HPLC electro-chemical detection. Interestingly, we found that CIN85Δex2 knockout animals displayed significantly increased levels of dopamine in the striatum compared with wild-type animals (approximately 65% increase, Student's t-test, P<0.05) (Figure 4A). As elevation of dopamine levels should convey accumulation also of its metabolites, we additionally quantified the striatal concentrations of the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). In the process of dopamine degradation, dopamine is first converted into DOPAC and finally, after a series of subsequent events, into HVA, the end product in the dopamine metabolic pathway. The amounts of striatal DOPAC and HVA were higher in CIN85Δex2 knockout mice compared with wild-type littermates, consistent with the observed increase in dopamine levels (Figure 4A). Augmentation of dopamine levels may have multiple explanations, but in the light of earlier studies reporting important functions for CIN85 in the endocytic regulation of multiple cell surface receptors (see Introduction and Petrelli et al, 2002; Soubeyran et al, 2002; Kobayashi et al, 2004; Molfetta et al, 2005; Veiga and Cossart, 2005), we reasoned that the accumulation of dopamine in CIN85Δex2 mutant striata could be caused by defective internalization of dopamine receptors. Mammalian genomes have been reported to encode at least five different dopamine receptor sub-types, all belonging to the seven transmembrane G protein-coupled receptor (GPCR) superfamily (Yao et al, 2008). The receptors are entitled D1–D5 and fall into two distinct classes based on sequence and functional similarities, the D1-like (D1 and D5) and D2-like (D2, D3, D4) dopamine receptors. Whereas D1-like receptors are highly expressed in the prefrontal cortex, D2-like receptors, in particular the D2 dopamine receptor (D2DR) (Missale et al, 1998; Gainetdinov et al, 2004), show stronger expression in the striatum and nucleus accumbens (Arias-Carrion and Poppel, 2007). Even though D1 dopamine receptors (D1DRs) are also expressed in the striatum, D1DR and D2DR are in general segregated into distinct striatal neurons. Neurons expressing both receptor types are rare (Missale et al, 1998). Given the intriguing expression pattern of dopamine receptors, we were interested to analyse whether removal of CIN85-l and -xl isoforms in the striatum affects the endocytosis of D1DR and/or D2DR dopamine receptors. For this purpose, we used biotinylated D1 and D2 dopamine receptors to perform internalization assays in cultured primary striatal neurons from wild-type and CIN85Δex2 knockout mice, allowing us to follow the uptake of either D1DR or D2DR after dopamine stimulation. Interestingly, we detected diverging effects on the endocytosis of D1DR versus D2DR. When stimulating the neurons with dopamine for 5, 15, 30 and 60 min, neurons derived from wild-type animals rapidly decreased the levels of D2DRs dopamine receptors on the cell surface, whereas the levels of cell surface-associated D2DRs in neurons from CIN85Δex2 knockout mice remained high throughout the time course, depicting a function for CIN85 in D2DR internalization (Figure 4B). Specifically, after 15, 30 and 60 min of stimulation, striatal neurons from CIN85Δex2 knockout mice still maintained high levels of surface D2DRs (96.5, 87.5 and 78.1%, respectively), as compared with wild-type neurons, which displayed significantly reduced levels (72.3, 59.7 and 45.1%, respectively) (P<0.05) (Figure 4B). In contrast to D2DRs, internalization of D1DRs occurred at comparable levels in striatal neurons from wild-type and CIN85Δex2 knockout mice (Figure 4C). In both experiments, the level of an unrelated receptor (Glutamate receptor-2) was used as an internal control and did not change throughout the time course. To confirm the involvement of CIN85 in D2DR internalization, we additionally performed a receptor-ligand-binding assay, in which the binding of [3H]spiperone, a tritium-labelled D2DR antagonist, to membrane fractions of striatal extracts from wild-type and CIN85Δex2 knockout mice was analysed. In agreement with the earlier experiment, striatal neurons from CIN85Δex2 knockout mice displayed increased levels of cell surface-associated D2DRs after dopamine stimulation (Supplementary Figure S7). Whereas wild-type neurons showed a 50% reduction of D2DR at the cell surface after 60 min of dopamine treatment, CIN85Δex2 knockout neurons retained 80% of cell surface-associated D2DR. Taken together, these results strongly indicate that lack of CIN85-xl and CIN85-l leads to impaired D2DR internalization, and in extension that CIN85 specifically regulates D2DR, but not D1DR, endocytosis in striatal neurons. Consistent with these findings, we found that CIN85 and D2DRs co-clustered in punctuate, synapse-like structures in dendrites of primary striatal neurons (Figure 4D).
Figure 4.
Impaired endocytic internalization of D2 dopamine receptors in mice lacking CIN85 expression in the CNS. (A) The levels of dopamine (DA) and its metabolites (3,4-dihydroxyphenylacetic acid, DOPAC and homovanillic acid, HVA) are increased in the striatum of CIN85Δex2 knockout mice. The contents of total striatal dopamine, DOPAC and HVA were determined by high performance liquid chromatography using an electro-chemical (HPLC-EC) detector as described in the Supplementary data. Differences between wild-type (+/+) and CIN85Δex2 knockout mice (−/−) were analysed using a Student's t-test, with the level of significance set at *P<0.05. +/+: n=4; −/−: n=4. (B) D2 dopamine receptor endocytosis is impaired in striatal neurons deficient of CIN85. Representative immunoblots (left panel) and quantification (right panel) showing the amount of surface-associated D2 dopamine receptors (D2DR) in cultured striatal neurons after dopamine stimulation. Striatal neurons were treated with dopamine hydrochloride (3-hydroxytyramine, 20 μM) for the indicated times before biotinylation. The remaining cell surface proteins were subsequently isolated with avidin and analysed by western blotting. The quantification of surface-associated D2DRs after dopamine stimulation is normalized to and depicted as the reduction of band intensity as compared with non-stimulated cells at the indicated times. Differences between wild type (+/+) and CIN85Δex2 knockout (−/−) were analysed by ANOVA and Duncan multiple range test for post hoc between group comparisons, with the level of significance set at *P<0.05. Glutamate receptor-2 (GluR-2) was used as an internal control. +/+: n=3; −/−: n=3. (C) Endocytosis of D1 dopamine receptors (D1DR) is not affected by loss of CIN85. Representative immunoblots and quantification showing the amount of surface D1DR in cultured striatal neurons before and after dopamine stimulation. Striatal neurons were treated with dopamine hydrochloride (20 μM) for 1 h before biotinylation. The remaining cell surface proteins were subsequently isolated with avidin and analysed by western blotting. D1DRs of cultured striatal neurons from wild-type (+/+) and CIN85Δex2 knockout mice (−/−) were internalized 69±5.7% and 63±8.8%, respectively. Glutamate receptor-2 (GluR-2) was used as an internal control. +/+: n=3; −/−: n=3. (D) CIN85 co-clusters with D2 dopamine receptors (D2DRs) at punctuate synapse-like structures in primary rat striatal neurons. Cells were fixed and stained with antibodies against CIN85 (SETA antibody, green) and D2DRs (red). Scale bars: 5 μm.
CIN85Δex2 mice show altered behavioural responses to dopamine agonists and antagonists
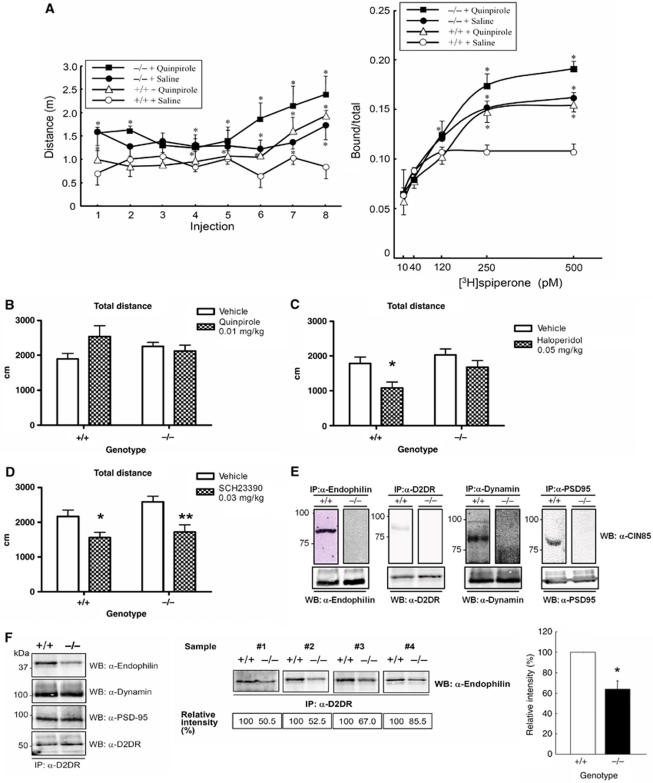
To investigate the connection between CIN85 and the D2DR under physiological conditions, we continued our studies by treating animals with the selective D2DR agonist quinpirole (or saline, as control). In both wild-type and CIN85Δex2 knockout mice repeated injections of quinpirole (0.5 mg/kg, s.c.) induced increased physical activity, manifested as an almost two-fold increase in distance travelled after eight injections of quinpirole compared with saline-injected control animals of both genotypes (Figure 5A, left panel). Interestingly, wild-type animals increased their locomotor activity after quinpirole treatment to similar levels as CIN85Δex2 knockout mice under saline-treated conditions (Figure 5A, left panel). In the light of earlier reports, this effect may be caused by a quinpirole-induced behavioural sensitization and increased dopamine receptor density in the striatum of treated mice (Culver et al, 2008). To test this hypothesis, we monitored the binding of [3H]spiperone to D2DRs in striatal extracts prepared from mice after the final treatment and completion of the locomotor activity recordings. Indeed, the [3H]spiperone binding was 36% higher in quinpirole-sensitized wild-type mice compared with saline-injected controls (P<0.05). In CIN85Δex2 knockout mice, the [3H]spiperone binding was 45 and 73% higher in saline- and quinpirole-treated animals, respectively, when compared with wild-type mice treated with saline (P<0.05) (Figure 5A, right panel). These data show that the D2DR levels in the striatum are increased in CIN85Δex2 knockout mice per se and that sensitization to the D2DR agonist quinpirole increases the D2DR levels even further. In all cases, the level of striatal D2DR was thus strongly correlated with the intensity of the locomotor activity.
Figure 5.
Defective complex formation between endophilins and D2 dopamine receptors in CIN85Δex2 knockouts. (A) Locomotor responses (left panel) and [3H]spiperone binding (right panel) of quinpirole-treated mice. Mice of the indicated genotypes were repeatedly injected with quinpirole (0.5 mg/kg, s.c.) or saline in 48 h intervals until a total of eight injections were completed as described in the Supplementary data. Immediately after each injection, the locomotor activity was measured for 30 min using an actimeter. The left panel shows the distance travelled (m) during the last 5 min of the 30-min testing period. Data are presented as the mean±s.e.m. (n=3). The difference between saline-injected wild types and the other genotypes/treatments were analysed by ANOVA and Student's t-test, with the level of significance set at *P<0.05. Right panel: After the final measurement of locomotor activity, the membrane fraction of the striatum (12.5 μg/reaction) was prepared and incubated with [3H]spiperone. Concentrations of [3H]spiperone ranging from 10 to 500 pM were used, and non-specific binding was determined in the presence of D-butaclamol. Data are presented as the mean±s.e.m. (n=3). The differences between saline-injected wild types and the other genotypes/treatments were analysed by ANOVA and Duncan multiple range test for post hoc between group comparisons, with the level of significance set at *P<0.05. (B) Total distance travelled after a threshold dose of quinpirole; 19–26-week-old CIN85Δex2 (−/−) and wild-type (+/+) littermate control mice (n=10–15 per genotype) were subjected to the modified hole-board test 30 min after quinpirole injection (0 or 0.01 mg/kg i.p.) as described in the Supplementary data. (C) Total distance travelled after a low dose of haloperidol; 14–36-week-old CIN85Δex2 (−/−) and wild-type (+/+) littermate control mice (n=10–13 per genotype) were subjected to the modified hole-board test 30 min after haloperidol injection (0 or 0.05 mg/kg i.p.) as described in the Supplementary data. *P<0.05, vehicle versus haloperidol, Bonferroni's post-tests. (D) Total distance travelled after a low dose SCH23390; 10–11-week-old CIN85Δex2 (−/−) and wild-type (+/+) littermate control mice (n=10–12 per genotype) were subjected to the modified hole-board test 30 min after SCH23390 injection (0 or 0.03 mg/kg i.p.) as described in the Supplementary data. *P<0.05, **P<0.01, vehicle versus SCH23390, Bonferroni's post-tests. (E) Synaptosome fractions were prepared from wild-type and CIN85Δex2 knockout mouse striata by sucrose density-gradient centrifugation. In total, 25 μg of fractionated protein was analysed by immunoprecipitation (IP) followed by western blotting (WB) using the indicated antibodies. CIN85 co-immunoprecipitates with D2DRs, the endocytic proteins endophilin and dynamin, as well as the post-synaptic density protein PSD-95, in wild type, but not in CIN85Δex2 knockout samples. The upper band evident in the dynamin IP may be CIN85-xl. (F) The level of endophilin in complex with the D2DR is decreased in the striatal synaptosome fractions in CIN85Δex2 knockout mice. Synaptosomes of wild-type (+/+) and CIN85Δex2 knockout (−/−) mouse striata were prepared as in (E) and subjected to IP with anti-D2DR antibodies and subsequent immunoblot analysis with antibodies against endophilin, dynamin, PSD-95 and the D2DR (left and middle panels). Western blots from four independent experiments are shown in the middle panel. Relative intensities of the endophilin levels in CIN85Δex2 knock outs compared with wild type for each of the four experiments are indicated. A graph depicting the mean value of the relative endophilin intensities in CIN85Δex2 knockout (−/−) compared with wild-type (+/+) mice from the four experiments is shown in the right panel. The value is presented as mean±s.e. Differences between wild-type (+/+) and CIN85Δex2 knockout mice (−/−) were analysed using a Student's t-test, with the level of significance set at *P<0.05. +/+: n=4; −/−: n=4.
To further address the physiological relevance of CIN85 in dopaminergic neurotransmission, we investigated the effect of quinpirole and of haloperidol, a pharmacological inhibitor of dopamine receptors, on the behaviour of CIN85Δex2 knockout mice. At low doses, haloperidol is a selective antagonist for D2DR, whereas higher doses have been reported to act more promiscuously and target also D1DRs for inhibition (Leysen et al, 1988). Interestingly, we found that CIN85Δex2 animals were significantly less sensitive to low doses of quinpirole or haloperidol, compared with wild-type littermates (Figures 5B and C). Whereas wild-type mice exposed to a threshold dose of quinpirole (0.01 mg/kg) showed increased (approximately 34%) locomotor activity as indicated by total distance travelled (Figure 5B), CIN85Δex2 mutants seemed to be unaffected (genotype × treatment interaction: F(1,7)=4.17, P<0.05). Correspondingly, wild-type control animals displayed significantly reduced locomotor activity (approximately 40%) when treated with a low dose of haloperidol (0.05 mg/kg) (treatment effect: F(1,1)=8.51, P<0.01, Bonferroni's post-test vehicle versus haloperidol for wild type: t=2.847, P<0.05), whereas in contrast, CIN85Δex2 mutants did not (Bonferroni's post-test vehicle versus haloperidol for CIN85Δex2 mutants: t=1.331, NS) (Figure 5C). Higher doses of haloperidol (0.25 and 0.5 mg/kg) suppressed locomotor activity to a similar extent in both genotypes (data not shown). In contrast, treatment of wild-type and CIN85Δex2 mutants with a low dose of the D1-selective dopamine receptor antagonist SCH23390 (0.03 mg/kg; Figure 5D) yielded comparable suppression of locomotor activity in both genotypes (treatment effect: F(1,1)=16.89, P<0.001, Bonferroni's post-tests vehicle versus SCH23390 for wild type: t=2.365, P<0.05, and for CIN85Δex2 mutants: t=3.459, P<0.01; genotype × treatment interaction: F(1,43)=0.47, NS). Importantly, the effect produced by the chosen dose of the D1-ligand SCH23390 was in the same range as that of the two D2-ligands, that is 0.03 mg/kg SCH23390 reduced locomotor activity in wild-type mice by 28% (Figure 5D). Taken together, these findings reinforce the notion that CIN85 has a specific function in D2-mediated, but not in D1-mediated processes in vivo.
Reduced complex formation between endophilins and D2DRs in CIN85Δex2 knockout mice
During receptor tyrosine kinase (RTK)-mediated endocytosis, CIN85 is targeted to activated receptors through the E3 ubiquitin ligase Cbl. Here, CIN85 functions to recruit components of the endocytic machinery that ultimately induces receptor internalization (Petrelli et al, 2002; Soubeyran et al, 2002). To investigate whether CIN85 has a similar function during D2DR endocytosis, we analysed whether CIN85 is associated with endocytic protein complexes in synaptosome fractions prepared from mouse striata, focusing on endocytic proteins earlier implicated either to interact with CIN85 or to have a function in dopamine receptor endocytosis. Similar to other cell surface receptors, we found that endogenous CIN85 co-immunoprecipitates with the D2DR receptor itself, as well as with the endocytic proteins endophilin and dynamin in samples from wild-type animals (Figure 5E). Interestingly, we also found CIN85 to associate with PSD-95, a prototypical scaffolding protein localized at post-synaptic densities, which has been shown to facilitate constitutive endocytosis of dopamine receptors (Figure 5E) (Zhang et al, 2007). As expected, neither of these interactions was observed in CIN85Δex2 knockout striata (Figure 5E).
Knowing that CIN85 is associated with several endocytic regulators, we next examined how loss of CIN85 affects the complex formation between these components and D2DRs. Again, we performed co-immunoprecipitation experiments in synaptosomal fractions from mouse striata. When concurrently analysing wild-type and CIN85Δex2 knockout preparations, it was clear that in the absence of CIN85, the complex formation of endophilin with D2DRs was strongly reduced, whereas the association of D2DRs with dynamin and PSD-95 was unaltered (Figure 5F, left panel). Quantification of the binding of endophilin to D2DRs in wild-type and CIN85Δex2 striatal synaptosome fractions from four independent experiments showed that the interaction between D2DR and endophilin in CIN85Δex2 knockout striata is significantly decreased to about 65% of the interaction observed in wild-type samples (Student's t-test, P<0.05) (Figure 5F, middle and right panels). We thus conclude that CIN85 has a function in engaging endophilins in D2DR complexes, thereby promoting receptor endocytosis.
Finally, we attempted to uncover the mechanism by which CIN85 may be recruited to dopamine receptor complexes. We could not detect a direct binding between dopamine receptors and CIN85 in GST overlay assays (data not shown), suggesting that the interaction is likely to be mediated through an intermediate mechanism, possibly by recruitment through one or several of the many dopamine receptor-interacting proteins (DRIPs) (Kabbani and Levenson, 2007; Yao et al, 2008). Whereas we were unable to detect an interaction between D2DRs and c-Cbl/Cbl-b (data not shown), we have found CIN85 to interact with p62 in GST pull-down and co-immunoprecipitation assays (Supplementary Figure S8). This is highly interesting in the light of a recent report describing p62 as a novel DRIP, specifically involved in the trafficking of D2DRs (Kim et al, 2008).
Discussion
CIN85 regulates locomotor and exploratory behaviour
In this study, we lay down an important piece of the puzzle regarding the regulation of dopamine receptor signalling circuits in the CNS. By conventional methods, we have generated mice deficient of CIN85 expression in the brain and thus concluded that loss of CIN85 gives rise to hyperactive phenotypes. In wild-type neurons, CIN85 is enriched in dendritic spines and it clearly has a crucial function in stabilizing endophilin binding to D2DRs in the striatum. In consequence, absence of CIN85 gives rise to insufficient endocytic internalization of D2DRs after dopamine stimulation, causing increased striatal dopamine receptor levels, which can, at least in part, explain the enhanced locomotor and exploratory behaviour we observe in the CIN85Δex2 mice.
The involvement of dopaminergic signalling in the regulation of movement, emotion and reward feelings is well established (Arias-Carrion and Poppel, 2007). In agreement, aberrations in these pathways have been strongly linked to various neurological disorders, including Parkinson's disease, schizophrenia, ADHD and Huntington's disease (Gainetdinov et al, 2004; Arias-Carrion and Poppel, 2007). The molecular defects underlying these pathologies have not been fully characterized, but may include alterations in the expression levels of dopamine ligands and/or receptors, as well as defects in downstream signalling events (Bellgrove and Mattingley, 2008; O'Connell et al, 2008). Interestingly, the hyperactive behaviour evoked by alterations in other pathways (such as glutamatergic and serotonergic cascades) or by the action of chemical substances (such as haloperidol and clozapine), in many cases, also indirectly affects dopaminergic signalling. Specifically, in several such cases, the described defect or compound has been reported to stimulate increased levels of high-affinity state D2DRs (D2High) in the striatum and nucleus accumbens (Seeman et al, 2006).
CIN85 regulates dopamine receptor endocytosis in the striatum
This report provides the first study of a mammal in which loss of CIN85 is analysed in vivo. Specifically, we have generated mice in which the two CIN85 isoforms expressed in the CNS, CIN85-xl and -l, have been removed. Most probably because of the presence of CD2AP, together with multiple alternative CIN85 isoforms expressed in other tissues, CIN85Δex2 mice are viable and fertile. This feature has thus enabled us to identify tissue-specific functions of CIN85 in the brain, in the regulation of proper neurotransmission.
Under physiological conditions, endocytic internalization of dopamine receptors is a crucial means by which neurons, in response to dopamine stimulation, can adjust their excitability potential, by either degrading or recycling receptors back to the plasma membrane (von Zastrow, 2003). A stringent control of this dynamic trafficking of dopamine receptors, as well as receptors for other neurotransmitter substances, is essential to ensure accurate transmission of neuronal information and proper regulation of synaptic plasticity (Gainetdinov et al, 2004). We have discovered that CIN85 affects the endocytosis of D2DR, but not D1DR, in primary striatal neurons. The resulting increase of surface-associated D2DRs in CIN85Δex2 knockout mouse striatal neurons and ensuing hyperactivity phenotype are in line with earlier findings, showing that activation of post-synaptic D2DRs results in increased locomotor activity and that D2DR knockout mice display reduced spontaneous movement (Jackson and Westlind-Danielsson, 1994; Baik et al, 1995). Importantly, activation of D1DRs, on the other hand, has little or no effect on locomotor activity (Jackson and Westlind-Danielsson, 1994). The specificity of CIN85 involvement in D2DR endocytosis could have multiple explanations. Either it may be a result of the restricted expression of D1DR and D2DR in distinct striatal neuron populations (Missale et al, 1998) causing a cell-specific positioning of CIN85 preferentially in D2DR-expressing neurons, or simply that CIN85 performs diverse functions during the internalization of different receptor types. Indeed, separate endocytic machineries and routes have been reported for D1DR versus D2DR (Vickery and von Zastrow, 1999; Iizuka et al, 2007; Kabbani and Levenson, 2007). Interestingly, we find CIN85 co-clustered with D2DRs in synapse-like structures in primary striatal neurons. Taken together, our data imply a specific function for CIN85 in the regulation of D2DR endocytosis in striatal neurons.
CIN85 facilitates complex formation between endophilins and D2DRs
Molecularly, we have observed that CIN85 displays important scaffolding functions in the complex formation between endophilins and D2DRs in striatal neurons, a finding that is in agreement with earlier discoveries showing a direct interaction between endophilins and CIN85 during the initiation of receptor internalization (Petrelli et al, 2002; Soubeyran et al, 2002). The endophilins constitute a family of proteins with well-established functions in the process of endocytosis, during which they are important both for the recruitment of endocytic machinery components to sites of activated receptors, as well as for the induction of membrane curvature, a function mediated through their N-BAR domains (Gallop et al, 2006; Masuda et al, 2006). Insufficient targeting of endophilins to dopamine receptor complexes could, therefore, result in accumulation of activated receptors at the plasma membrane of neurons, thereby enhancing dopaminergic signalling activities, and in extension cause aberrations in behaviour.
Importantly, this study provides the first evidence that CIN85/endophilin complexes are involved in the endocytosis of a member of the GPCR family. In the light of this finding, it is interesting to remark that the arrestins, another family of adaptor proteins with well-established functions in GPCR endocytosis and trafficking, also have a function in internalization of RTKs (Lefkowitz et al, 2006), thus exposing both CIN85 and arrestins as adaptors with the capacity to regulate the endocytosis and trafficking of a broad range of receptor types, including both RTKs and GPCRs. Despite extensive efforts, we have not been successful in clearly establishing the mechanism of CIN85 recruitment to dopamine receptor complexes. The interaction is likely to be mediated through an intermediate mechanism, possibly by recruitment through one or several of the many DRIPs, such as p62, which we indeed have found in complex with CIN85 (Kabbani and Levenson, 2007; Yao et al, 2008). The multiplicity of possible interacting partners and the redundancy between distinct important pathways could explain why the loss of CIN85 function can have deleterious effects on a selection of receptor types in specific tissues, whereas other classes of receptors in contrast seem to be unaffected (Petrelli et al, 2002; Soubeyran et al, 2002; Kobayashi et al, 2004). Indeed, a function of CIN85 specifically in the regulation of D2DR activity would be in agreement with the reported hypoactivity (i.e. reduced locomotor activity) displayed by D2DR knockout mice, which is in contrast to the hyperactivity, which has been observed in mice deficient of other D2-like dopamine receptor sub-types (Holmes et al, 2004).
Increased D2DR levels in the absence of CIN85 correlate with hyperactive behaviour and altered metabolism
During our extensive physiological examination of the CIN85Δex2 mice, we were interested to find that mice lacking striatal expression of CIN85 display a lean body composition, even though consuming more food than wild-type animals. However, in contrast to c-Cbl knockouts in which the anomalous lean body composition is accompanied by alterations in insulin metabolism, CIN85Δex2 mice did not show any significant improvements in conventional glucose tolerance tests (data not shown). The explanation for this phenotypical trait may instead be directly linked to the observed alterations in dopaminergic activity, given the earlier reported involvement of D2DR-mediated signalling in the regulation of appetite, energy intake and obesity. Multiple studies have shown a correlation between striatal D2DR expression levels and body composition and have associated low D2DR with obesity (Epstein et al, 2007). A recent report has furthermore showed a strong link between certain D2DR allelic variations and obesity, suggesting that individuals with certain genotypes resulting in dopaminergic hypofunction are prone to obesity (Stice et al, 2008). Increased dopaminergic signalling is, therefore, in agreement with the slim appearance of CIN85Δex2 knockouts.
Concluding remarks
Notably, a function of CIN85 at neuronal synapses is well in line with the reported importance of CD2AP/CIN85 at immunological synapses in T cells, as well as in the slit diaphragm in kidney glomeruli, given the structural as well as functional similarities between neuronal synapses and these specialized cell surface contacts (Shih et al, 1999; Dustin and Colman, 2002). In conclusion, CIN85 is a novel regulator of D2DR endocytosis in striatal neurons, involved in controlling locomotor and exploratory behaviour in mice.
Materials and methods
Generation of the CIN85Δex2 knockout mice
The removal of exon 2 from the mouse CIN85 locus by homologous recombination and the generation of the CIN85Δex2 knockout mice are described in the Supplementary data.
Phenotypic analyses of behaviour, metabolism and other parameters
See the Supplementary data.
Immunofluorescence of primary neurons
See the Supplementary data.
Isolation of synaptosomes and post-synaptic density fractions
See the Supplementary data.
Dopamine receptor biotinylation and endocytosis assay
See the Supplementary data.
Supplementary Material
Acknowledgments
We are grateful to Amparo Acker-Palmer, Harald Stenmark and members of our laboratories for discussions, constructive comments and critical reading of the paper. We thank the members of the German Mouse Clinic for the primary analysis in the other screens and the fruitful discussion, Ingrid Konrad and Masuda Sader for help with breeding and genotyping and Dr Yoshikatsu Kanai (Osaka University) and Dr Makoto Kinoshita (Kyoto University) for their technical advice. We thank Inger Drescher, Claudia Meyer and Astrid Rhyner for expert help with behavioural testing and data analysis. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (18059005) and (18590216) from MEXT of Japan to NS, and by grants from Deutsche Forschungsgemeinschaft (DI 931/1-1 to ID; DE 551/8-1 to TD; JE 528/1-1 to PJ; SCHU 1412/2-1 to CS), as well as National Cancer Institute of the National Institutes of Health, USA (R01-CA108500 to OB and ID) and SNF NCCR Neural Plasticity and Repair (to DPW). KH and CG acknowledge support from The International Human Frontier Science Program Organization. This work has also been funded by a grant from the Research Council of Norway (to KH) and the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN), Förderkennzeichen 01GR0430 and by an EU grant (LSHG-2006-037188) (to MHA, GMC).
Footnotes
The authors declare that they have no conflict of interest.
References
- Arias-Carrion O, Poppel E (2007) Dopamine, learning, and reward-seeking behavior. Acta Neurobiol Exp (Wars) 67: 481–488 [DOI] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424–428 [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Mattingley JB (2008) Molecular genetics of attention. Ann N Y Acad Sci 1129: 200–212 [DOI] [PubMed] [Google Scholar]
- Bian M, Yu M, Yang S, Gao H, Huang Y, Deng C, Gao Y, Sun F, Huang F (2008) Expression of Cbl-interacting protein of 85 kDa in MPTP mouse model of Parkinson's disease and 1-methyl-4-phenyl-pyridinium ion-treated dopaminergic SH-SY5Y cells. Acta Biochim Biophys Sin (Shanghai) 40: 505–512 [DOI] [PubMed] [Google Scholar]
- Booth RF, Clark JB (1978) A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J 176: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman VL, Luke C, Borthwick EB, Gout I, Ninkina N (2002) Organization of the mouse Ruk locus and expression of isoforms in mouse tissues. Gene 295: 13–17 [DOI] [PubMed] [Google Scholar]
- Chen B, Borinstein SC, Gillis J, Sykes VW, Bogler O (2000) The glioma-associated protein SETA interacts with AIP1/Alix and ALG-2 and modulates apoptosis in astrocytes. J Biol Chem 275: 19275–19281 [DOI] [PubMed] [Google Scholar]
- Cormont M, Meton I, Mari M, Monzo P, Keslair F, Gaskin C, McGraw TE, Le Marchand-Brustel Y (2003) CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic 4: 97–112 [DOI] [PubMed] [Google Scholar]
- Culver KE, Szechtman H, Levant B (2008) Altered dopamine D2-like receptor binding in rats with behavioral sensitization to quinpirole: effects of pre-treatment with Ro 41-1049. Eur J Pharmacol 592: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I (2002) CIN85/CMS family of adaptor molecules. FEBS Lett 529: 110–115 [DOI] [PubMed] [Google Scholar]
- Dustin ML, Colman DR (2002) Neural and immunological synaptic relations. Science 298: 785–789 [DOI] [PubMed] [Google Scholar]
- Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS (1998) A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94: 667–677 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS (2007) Food reinforcement and eating: a multilevel analysis. Psychol Bull 133: 884–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finniss S, Movsisyan A, Billecke C, Schmidt M, Randazzo L, Chen B, Bogler O (2004) Studying protein isoforms of the adaptor SETA/CIN85/Ruk with monoclonal antibodies. Biochem Biophys Res Commun 325: 174–182 [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Romano A, Routtenberg A (2004) Transcription factor NF-kappaB activation after in vivo perforant path LTP in mouse hippocampus. Hippocampus 14: 677–683 [DOI] [PubMed] [Google Scholar]
- Gaidos G, Soni S, Oswald DJ, Toselli PA, Kirsch KH (2007) Structure and function analysis of the CMS/CIN85 protein family identifies actin-bundling properties and heterotypic-complex formation. J Cell Sci 120: 2366–2377 [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27: 107–144 [DOI] [PubMed] [Google Scholar]
- Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT (2006) Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 25: 2898–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunkemeyer JA, Kwoh C, Huber TB, Shaw AS (2005) CD2-associated protein (CD2AP) expression in podocytes rescues lethality of CD2AP deficiency. J Biol Chem 280: 29677–29681 [DOI] [PubMed] [Google Scholar]
- Holmes A, Lachowicz JE, Sibley DR (2004) Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology 47: 1117–1134 [DOI] [PubMed] [Google Scholar]
- Hutchings NJ, Clarkson N, Chalkley R, Barclay AN, Brown MH (2003) Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J Biol Chem 278: 22396–22403 [DOI] [PubMed] [Google Scholar]
- Iizuka Y, Sei Y, Weinberger DR, Straub RE (2007) Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci 27: 12390–12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DM, Westlind-Danielsson A (1994) Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther 64: 291–370 [DOI] [PubMed] [Google Scholar]
- Jozic D, Cardenes N, Deribe YL, Moncalian G, Hoeller D, Groemping Y, Dikic I, Rittinger K, Bravo J (2005) Cbl promotes clustering of endocytic adaptor proteins. Nat Struct Mol Biol 12: 972–979 [DOI] [PubMed] [Google Scholar]
- Kabbani N, Levenson R (2007) A proteomic approach to receptor signaling: molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur J Pharmacol 572: 83–93 [DOI] [PubMed] [Google Scholar]
- Kawata A, Iida J, Ikeda M, Sato Y, Mori H, Kansaku A, Sumita K, Fujiwara N, Rokukawa C, Hamano M, Hirabayashi S, Hata Y (2006) CIN85 is localized at synapses and forms a complex with S-SCAM via dendrin. J Biochem (Tokyo) 139: 931–939 [DOI] [PubMed] [Google Scholar]
- Kim OJ, Ariano MA, Namkung Y, Marinec P, Kim E, Han J, Sibley DR (2008) D2 dopamine receptor expression and trafficking is regulated through direct interactions with ZIP. J Neurochem 106: 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch KH, Georgescu MM, Shishido T, Langdon WY, Birge RB, Hanafusa H (2001) The adapter type protein CMS/CD2AP binds to the proto-oncogenic protein c-Cbl through a tyrosine phosphorylation-regulated Src homology 3 domain interaction. J Biol Chem 276: 4957–4963 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sawano A, Nojima Y, Shibuya M, Maru Y (2004) The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1). FASEB J 18: 929–931 [DOI] [PubMed] [Google Scholar]
- Kowanetz K, Husnjak K, Holler D, Kowanetz M, Soubeyran P, Hirsch D, Schmidt MH, Pavelic K, De Camilli P, Randazzo PA, Dikic I (2004) CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol Biol Cell 15: 3155–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakin AV, Wu S, Bredesen DE (2003) Atypical recognition consensus of CIN85/SETA/Ruk SH3 domains revealed by target-assisted iterative screening. J Biol Chem 278: 34102–34109 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ (2006) New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24: 643–652 [DOI] [PubMed] [Google Scholar]
- Leysen JE, Gommeren W, Janssen PF, Van Gompel P, Janssen PA (1988) Receptor interactions of dopamine and serotonin antagonists: binding in vitro and in vivo and receptor regulation. Psychopharmacol Ser 5: 12–26 [DOI] [PubMed] [Google Scholar]
- Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH (2000) CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol 279: F785–F792 [DOI] [PubMed] [Google Scholar]
- Lynch DK, Winata SC, Lyons RJ, Hughes WE, Lehrbach GM, Wasinger V, Corthals G, Cordwell S, Daly RJ (2003) A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J Biol Chem 278: 21805–21813 [DOI] [PubMed] [Google Scholar]
- Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N (2006) Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J 25: 2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189–225 [DOI] [PubMed] [Google Scholar]
- Molero JC, Jensen TE, Withers PC, Couzens M, Herzog H, Thien CB, Langdon WY, Walder K, Murphy MA, Bowtell DD, James DE, Cooney GJ (2004) c-Cbl-deficient mice have reduced adiposity, higher energy expenditure, and improved peripheral insulin action. J Clin Invest 114: 1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfetta R, Belleudi F, Peruzzi G, Morrone S, Leone L, Dikic I, Piccoli M, Frati L, Torrisi MR, Santoni A, Paolini R (2005) CIN85 regulates the ligand-dependent endocytosis of the IgE receptor: a new molecular mechanism to dampen mast cell function. J Immunol 175: 4208–4216 [DOI] [PubMed] [Google Scholar]
- Moncalian G, Cardenes N, Deribe YL, Spinola-Amilibia M, Dikic I, Bravo J (2006) Atypical polyproline recognition by the CMS N-terminal Src homology 3 domain. J Biol Chem 281: 38845–38853 [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Bellgrove MA, Dockree PM, Lau A, Fitzgerald M, Robertson IH (2008) Self-alert training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 46: 1379–1390 [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29: 192–199 [DOI] [PubMed] [Google Scholar]
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S (2002) The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416: 187–190 [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T (2006) Psychosis pathways converge via D2high dopamine receptors. Synapse 60: 319–346 [DOI] [PubMed] [Google Scholar]
- Segal M (2005) Dendritic spines and long-term plasticity. Nat Rev Neurosci 6: 277–284 [DOI] [PubMed] [Google Scholar]
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315 [DOI] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I (2002) Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416: 183–187 [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM (2008) Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322: 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DP, Liu QY, Koshiya N, Gu H, Alkon D (2006) Enhancement of long-term memory retention and short-term synaptic plasticity in cbl-b null mice. Proc Natl Acad Sci USA 103: 5125–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E, Cossart P (2005) Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 7: 894–900 [DOI] [PubMed] [Google Scholar]
- Vickery RG, von Zastrow M (1999) Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol 144: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Vallone D, Sadile A (2004) Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast 11: 97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M (2003) Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci 74: 217–224 [DOI] [PubMed] [Google Scholar]
- Welsch T, Endlich N, Kriz W, Endlich K (2001) CD2AP and p130Cas localize to different F-actin structures in podocytes. Am J Physiol Renal Physiol 281: F769–F777 [DOI] [PubMed] [Google Scholar]
- Yao WD, Spealman RD, Zhang J (2008) Dopaminergic signaling in dendritic spines. Biochem Pharmacol 75: 2055–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, Isacson O, Caron MG, Yao WD (2007) Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem 282: 15778–15789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Okada M, Yoshida S, Hirose S, Kaneko S (2004) Both 3,4-dihydroxyphenylalanine and dopamine releases are regulated by Ca2+-induced Ca2+ releasing system in rat striatum. Neurosci Lett 362: 244–248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.