Abstract
Prostaglandin E2 (PGE2) is a key mediator of inflammation and contributes to pain hypersensitivity by promoting sensory neurons hyperexcitability. PGE2 synthesis results from activation of a multi-step enzymatic cascade that includes cyclooxygenases (COXs), the main targets of non-steroidal anti-inflammatory drugs (NSAIDs). Although NSAIDs are widely prescribed to reduce inflammatory symptoms such as swelling and pain, associated harmful side effects restrict their long-term use. Therefore, finding new drugs that limit PG production represents an important therapeutic issue. In response to peripheral inflammatory challenges, mice lacking the ATP-gated P2X4 channel (P2X4R) do not develop pain hypersensitivity and show a complete absence of inflammatory PGE2 in tissue exudates. In resting conditions, tissue-resident macrophages constitutively express P2X4R. Stimulating P2X4R in macrophages triggers calcium influx and p38 MAPK phosphorylation, resulting in cytosolic PLA2 (cPLA2) activation and COX-dependent release of PGE2. In naive animals, pain hypersensitivity was elicited by transfer into the paw of ATP-primed macrophages from wild type, but not P2X4R-deficient mice. Thus, P2X4Rs are specifically involved in inflammatory-mediated PGE2 production and might therefore represent useful therapeutic targets.
Keywords: inflammation, macrophages, pain, PGE2, P2X4R purinergic receptors
Introduction
Prostaglandins (PGs) are well-established mediators of inflammation. Prostaglandin E2 (PGE2), the main PG produced during inflammatory response, triggers pain hypersensitivity by promoting nociceptors sensitization and hyperexcitability (Portanova et al, 1996; Samad et al, 2002). Non-steroidal anti-inflammatory drugs (NSAIDs) are powerful analgesic molecules that inhibit cyclooxygenases (COXs), key enzymes of PG biosynthetic pathways. However, the beneficial effects of these drugs are counter balanced by severe long-term side effects such as gastric ulcerations or cardiovascular dysfunctions due to concomitant inhibition of homeostatic functions of prostanoids (Samad et al, 2002). Hence, it is important to get a better understanding of the specific signalling pathways leading to PG synthesis during inflammation. The first enzymatic step of PG biosynthesis is release of arachidonic acid (AA) from membrane phospholipids by the action of phospholipases A2 (Kudo and Murakami, 2002). Among different PLA2s, the cytosolic PLA2 α (cPLA2) has been directly linked to PGE2 release associated with inflammation and pain (Bonventre and Sapirstein, 2002; Lucas et al, 2005). The enzymatic activity of cPLA2 is highly controlled by intracellular calcium and phosphorylation (Gijon et al, 2000), and among the different extracellular signalling molecules capable of triggering a calcium rise, ATP is an efficient activator of cPLA2 in vitro (Balboa et al, 1999).
Extracellular ATP mediates its effect through two main families of membrane proteins, P2Y receptors that are G-protein-coupled receptors and P2X receptors (P2XRs) that are ATP-gated channels with high calcium permeability (Burnstock, 2007). Seven P2XRs have been characterized (P2X1–7); they have a broad expression profile that encompasses a variety of functions. In non-neuronal cells, these receptors initiate critical functions such as muscle contractility, vasodilation or secretion, whereas in neurons they regulate cell excitability and synaptic transmission (North, 2002; Burnstock, 2007). In sensory neurons, P2X3R and P2X2/3R have a well-established implication in pain processing (Wirkner et al, 2007). However, in chronic pain, their functions are supplemented by the action of other P2XRs, especially those expressed by immune cells such as microglia (Trang et al, 2006). P2X7R has been recognized as a key player of inflammation and pain (Chessell et al, 2005; McGaraughty et al, 2007); P2X7Rs are mainly expressed by myeloid cells and their activation provides a key physiological stimulus for the rapid activation of caspase-1 and subsequent release of the pro-inflammatory cytokine, IL-1β (Pelegrin and Surprenant, 2006). Similarly, after nerve injury, P2X4Rs are expressed de novo in the spinal cord by activated microglia, brain-resident macrophages, where they mediate neuropathic pain and allodynia (Tsuda et al, 2003; Ulmann et al, 2008). Recently, P2X4Rs have also been implicated in chronic inflammatory pain processing (Tsuda et al, 2009). Peripheral myeloid cells express different subtypes of purinergic receptors, including high levels of P2X4R (Wang et al, 2004); however, in the absence of subtype-specific antagonists, their functions in these cells are poorly documented. Yet, ATP has the ability to activate tissue-resident myeloid cells and is a powerful trigger of inflammation (Idzko et al, 2007). Activation of P2XRs in immune cells, by eliciting sustained calcium influx, is therefore likely to have a crucial function in peripheral inflammatory responses (Bours et al, 2006). In this study, using P2X4R-deficient mice, we examined whether P2X4Rs, which have a broad expression profile and high calcium permeability, might be involved in the initiation of peripheral inflammation.
Results
Impaired inflammatory pain behaviours in P2X4R-deficient mice
A cardinal feature of peripheral inflammation is pain. We thus analysed pain responses elicited by various inflammatory molecules in P2X4R-deficient mice, in particular formalin and carrageenan were used to induce acute chemical and inflammatory pain and complete Freund's adjuvant (CFA) to elicit long lasting inflammation. Injection of diluted formalin in the hindpaw elicits a stereotypic two-phase behavioural response, an early phase that depends on direct nociceptor stimulation and a delayed phase that is triggered by peripheral mechanisms (Le Bars et al, 2001). Injection of 20 μl of 5% formalin in the hindpaw induced the typical biphasic behavioural response with no difference between wild-type (WT) and P2X4-deficient mice. However, when a lower amount of formalin was injected (15 μl), we observed an attenuation of both phases in WT mice, whereas in P2X4R-deficient mice, the second phase was essentially absent, while the acute phase was unaffected (Figure 1A). We next measured sensitivity to light mechanical stimuli (allodynia) induced by injection of carrageenan in the hindpaw. Two hours after injection, the threshold of paw withdrawal in response to mechanical stimuli was significantly higher in P2X4R-deficient mice compared with WT animals, whereas basal mechanical thresholds were the same between genotypes (Figure 1B). Similarly, long lasting mechanical hypersensitivity assessed 24 h, 3 and 4 days after CFA injection in the paw was totally absent in P2X4R-deficient mice (Figure 1C).
Figure 1.
Pain behaviours in P2X4R-deficient mice. (A) Behavioural response to different amounts of 5% formalin injection in the hindpaw. Left panel, no significant difference in nocifensive behaviour was observed between the two genotypes when 20 μl of formalin was injected. Right panel, behavioural response to 15 μl injection was attenuated in WT mice, and P2X4R-deficient mice show a significant reduction of phase 2 behaviour. N=6 mice per group. (B) Mechanical hypersensitivity induced by injection of carrageenan (15 μl, 1% in saline) was measured 2 h after injection. WT mice develop a strong hyperalgesic response compared with baseline (P=0.0004), which is significantly reduced in P2X4−/− mice. N=6 animals per group. (C) Lack of long lasting inflammatory pain in P2X4R-deficient mice. Withdrawal threshold to mechanical stimulation was measured on injected side before and 24 h, 3 and 7 days after subcutaneous CFA injection in the paw. N=12 animals per group. (D) In the hot-plate test, there was no difference in latency between WT and P2X4−/− mice at 50°C. Note a slight hypersensitivity in P2X4−/− mice at higher temperatures, N=10 animals per group. (E) Direct injection of PGE2 in the paw induces mechanical hypersensitivity in P2X4−/− mice. Paw withdrawal threshold to mechanical stimulation was measured before, 30 and 60 min after PGE2 (100 ng, 10 μl) injection. N=6 mice per group. For all panels, results are expressed as mean±s.e.m., *P<0.05, **P<0.01, ***P<0.005, one-way ANOVA.
mRNA expressing all P2XR subtypes, including P2X4R are expressed by DRG neurons (Collo et al, 1996). We therefore analysed the ability of sensory neurons to convey nociceptive information to the spinal cord in P2X4R-deficient mice. Sensitivity to a nociceptive 50°C heat stimulus measured using a hot-plate test was not different between WT and P2X4R-deficient mice (Figure 1D), although a slight, but significant higher sensitivity was observed in P2X4-deficient mice at temperatures above 52°C. Next, we analysed sensitization of nociceptive neurons by pro-inflammatory molecules. Mechanical hypersensitivity elicited by injection of PGE2 (100 ng, 10 μl) in the paw was measured. At 30 and 60 min after PGE2 injection, the reduction of withdrawal threshold values was identical in WT and P2X4R-deficient mice (Figure 1E). Altogether, these results show that in P2X4R-deficient mice, impaired inflammatory pain behaviours do not result from deficit of nociceptors function.
Impaired peripheral production of PGE2 in P2X4R-deficient mice
We reasoned that if sensitivity to pro-inflammatory molecules is unaltered in P2X4R-deficient mice, pro-inflammatory molecules production by peripheral tissues during inflammation might be affected. Among the different pro-inflammatory agents produced during an acute inflammation, PGE2 was a likely candidate. Indeed, neutralizing anti-PGE2 antibodies prevent development of carrageenan-induced hyperalgesia (Portanova et al, 1996). We thus analysed tissue-specific production of PGE2 after inflammatory challenges. Inflammation was induced by carrageenan injection and PGE2 levels were measured in paw exudates 2 h later. As expected, carrageenan injection induced a strong increase of PGE2 concentration in exudates of WT mice, which was completely abolished in P2X4R-deficient mice (Figure 2A). Interestingly, PGE2 levels in non-challenged animals were the same between genotypes, indicating that homeostatic production of PGE2 is not altered in P2X4R-deficient mice. Similar experiments were performed at different time points after CFA injection (Figure 2B). Here also, PGE2 levels in paw exudates from P2X4R-deficient mice were significantly reduced 2 and 4 h post-CFA injection. Reduced CFA-mediated PGE2 production in P2X4-deficient mice correlated with a reduction of sensitivity to light mechanical stimuli. These results show that in inflammatory conditions, PGE2 release directly relies on P2X4R activity.
Figure 2.
P2X4R-deficient mice show impaired peripheral PGE2 production in response to inflammatory challenge. PGE2 concentrations were measured in paw exudates in saline-injected animals, 2 h post-carrageenan injection (A) or 2 and 4 h after CFA injection (B). In both conditions, PGE2 concentrations were significantly reduced in paw exudates of P2X4R-deficient mice when compared with WT mice. Results were normalized to the mean concentration of PGE2 in WT saline group. N=6 mice per group, *P<0.05, ***P<0.005, Student t-test. (C) Mechanical allodynia was significantly reduced in P2X4R-deficient mice 2 and 4 h post-CFA injection. N=6 mice per group, *P<0.05, one-way ANOVA.
P2X7R promotes inflammatory pain behaviours that are very similar to those described in this study (Chessell et al, 2005; McGaraughty et al, 2007). Recent studies have shown that P2X4R and P2X7R physically interact in myeloid cells (Nicke, 2008; Boumechache et al, 2009), suggesting that altered pain phenotypes observed in P2X4R-deficient mice might result from an indirect downregulation of P2X7R. We thus analysed P2X7R expression in P2X4R-deficient mice. Western blot analysis of paw tissue and peritoneal macrophages shows that P2X7R expression was not reduced in WT and P2X4R-deficient mice. On the contrary, a small but consistent increase of P2X7 expression was observed (Supplementary Figure S1A and C). Similarly, there was no obvious change of P2X7R expression in paw tissue 2 h after injection of carrageenan (Supplementary Figure S1C). Furthermore, in P2X4R-deficient mice, CFA-induced increases in IL-1β and IL-6 in paw tissue were not different from WT animals, whereas it has been reported earlier that CFA-induced IL-1β or IL-6 production is severely impaired in P2X7R-deficient mice (Chessell et al, 2005) (Supplementary Figure S1D). These results show that expression and functions of P2X7R are not reduced in P2X4-deficient mice.
Functional P2X4R are constitutively expressed in tissue-resident macrophages
We next explored cell types that might be responsible for P2X4R-dependent PGE2 release. We focused our attention on tissue-resident macrophages that represent a well-characterized source of inflammatory PGE2. As shown in Figure 3A, in the CX3CR1+/eGFP mice in which enhanced green fluorescent protein (eGFP) is expressed in all myeloid cells (Jung et al, 2000), eGFP expressing cells were clearly present in paw tissue. These cells coexpressed the specific macrophage marker F4/80 and were positive for P2X4R immunostaining. In paw tissue, P2X4R immunostaining was almost exclusively restricted to eGFP-positive cells, demonstrating that in tissue, macrophages are the main cell type constitutively expressing P2X4R. Local recruitment of myeloid cells, such as inflammatory monocytes or neutrophils contributes to inflammatory pain hypersensitivity (Marchand et al, 2005). One possibility is that recruitment of these inflammatory cells is impaired in P2X4R-deficient mice. To test this hypothesis, cellular phenotyping of paw tissue 2 h post-carrageenan injection was performed in CX3CR1+/eGFP mice. No substantial change in the number of monocytes expressing eGFP or F4/80 was observed in paw tissue, as compared with control (Figure 3B). Similarly, western blot analysis indicated that P2X4R expression in paw was unaffected at this time (Figure 3C). Recruitment of neutrophils was assessed by GR1 staining or by measuring myeloperoxidase (MPO) activity. Here again, no recruitment of neutrophils could be detected 2 h after injection of carrageenan, whereas clearly present 24 h after CFA injection (Figure 3D and E). Thus, paw-resident macrophages are likely to be the main cell type expressing P2X4R, contributing to carrageenan-evoked PGE2 release.
Figure 3.
Paw-resident macrophages express P2X4R. (A) In paw sections of CX3CR1+/eGFP mice, P2X4R immunostaining colocalizes with eGFP (top row, scale bar=50 μm). Bottom row shows the colocalization of eGFP with the specific macrophage marker F4/80 at lower magnification (scale bar=100 μm). (B) Absence of recruitment of inflammatory cells by carrageenan in paw of CX3CR1+/eGFP mice. Macrophages were identified in control conditions and 2 h post-carrageenan injection by F4/80-positive staining (top row, scale bar=100 μm) and by the expression of eGFP (bottom row, scale bar=50 μm). (C) Western blot analysis shows that P2X4R expression in paw tissue is not different between control and treated mice. Each lane represents two animals. Experiment was repeated twice. (D, E) Carrageenan did not induce neutrophil recruitment. Neutrophils were identified in WT mice using GR1 immunostaining (D), or myeloperoxidase (MPO) assay (E), 2 h post-carrageenan or 24 h post-CFA. No recruitment was observed 2 h post-carrageenan, whereas it was clearly present 24 h after CFA injection. Scale bar=100 μm. ***P<0.005, Student t-test.
One of the hallmarks of P2X4 channels is their high calcium permeability close to that of NMDA receptors (Egan and Khakh, 2004). We therefore investigated whether P2X4R mediates calcium influx in resident macrophages of the peritoneal cavity. We first examined expression of P2X4R in these cells. As in paw-resident macrophages, peritoneal cells coexpress both P2X4R and the macrophage-specific marker F4/80 (Figure 4A), whereas in P2X4R-deficient cells, F4/80 colocalized with β-galactosidase, which was knocked-in in the first coding exon of p2x4 gene (Sim et al, 2006). Western blot of peritoneal exudates or spleen revealed a strong expression of P2X4R, ruling out a potential induction of P2X4R expression during culture (Figure 4B). Next, we analysed ATP-induced intracellular calcium signals in peritoneal macrophage using Fura-2 calcium probe and videomicroscopy. Calcium signals were induced by three applications of 20 μM ATP (10 s) every 2 min, followed by a forth application in the presence of 3 μM ivermectin (IVM), a specific positive allosteric modulator of P2X4R (Khakh et al, 1999). Using this paradigm, repetitive ATP stimulations of WT macrophages induced calcium signals with a slight rundown over time (88.64±3.6% of the signal recorded on the first ATP application), which can be fully reversed by a subsequent ATP stimulation in the presence of IVM (109±10% compared with the first application) (Figure 4C and D). In P2X4R-deficient macrophages, repetitive application of ATP induced a significant rundown of calcium signals (65.5±6.3% compared with the first application) that could not be reversed by IVM (62.5±8% compared with the first application). ATP-induced calcium signals were not affected by coapplication with 10 μM A-740003 a specific P2X7R antagonist (Honore et al, 2006). At the same concentration, A-740003 completely abolished P2X7-mediated YO-PRO-1 uptake in macrophages (Supplementary Figure S1E). These results show that in macrophages, calcium influx through P2X4 channels contributes significantly to ATP-induced calcium signals. In addition, these data establish the specificity of IVM as a pharmacological tool to assess native P2X4R functions.
Figure 4.
Peritoneal macrophages express functional P2X4R. (A) In WT peritoneal macrophages, P2X4R immunostaining (green) colocalized with that of F4/80 (red), whereas in P2X4R-deficient cell β-galactosidase (green) substitutes for the P2X4R immunostaining. Scale bar=10 μm. (B) Western blot analysis of P2X4R expression in peritoneal exudates or spleen tissue. Representative experiment out of three. (C, D) Characterization of P2X4R-induced calcium signals in peritoneal macrophages. (C) Representative recording of ATP-induced calcium signals in single WT and P2X4R-deficient peritoneal macrophages. ATP (20 μM) was applied for 10 s three times every 2 min and then a fourth application was made in the presence of ivermectin (IVM, 3 μM). The rundown of ATP-induced calcium signals was much more pronounced in P2X4R-deficient macrophages, and it was not reversed by IVM. (D) Population analysis of ATP-induced calcium signals as described in (C) and performed in the presence of A-740003 (10 μM) a specific P2X7 antagonist (hatched bars). Fluorescence ratios were normalized to the mean of F340/F380 ratio measured for the first ATP application. N>50 cells. *P<0.05, **P<0.01, ***P<0.005, one-way ANOVA.
P2X4R activation evokes AA and PGE2 release from macrophages
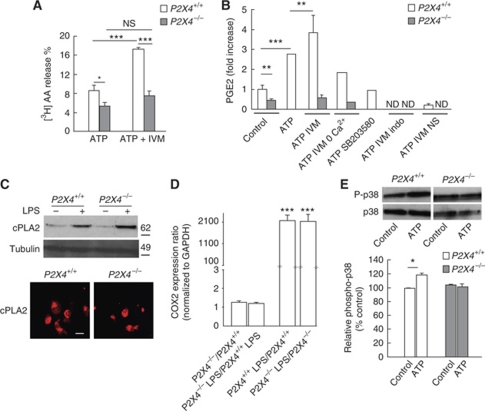
Recent studies have provided evidence that P2X4R-evoked calcium influx mediates the phosphorylation and subsequent activation of p38-MAPK (Trang et al, 2009). Both intracellular calcium and p38-MAPK are critical activators of cPLA2, one of the main AA releasing enzymes in macrophages (Gijon et al, 2000). We thus tested whether P2X4R activation could activate cPLA2 and trigger AA release. ATP-induced [3H]AA production was measured from WT and P2X4R-deficient LPS-primed cultured macrophages. In WT macrophages, ATP (50 μM) induced [3H]AA release that was two-fold larger in the presence of IVM (3 μM) (Figure 5A). In P2X4R-deficient macrophages, ATP-induced [3H]AA release was significantly reduced and not enhanced by IVM. We next analysed whether P2X4R-induced AA synthesis could lead to PGE2 release. In WT macrophage cultures, ATP stimulation induced a three-fold increase of PGE2 release over basal levels, and ATP-induced PGE2 release was potentiated by IVM (Figure 5B). In cultures from P2X4R-deficient mice, basal release of PGE2 was significantly reduced compared with WT and ATP/IVM stimulation failed to induce PGE2 release. Furthermore, ATP/IVM-induced PGE2 release was reduced by omission of extracellular calcium or after pretreatment with the p38 MAPK inhibitor, SB203580 (10 μM). Finally, ATP-induced PGE2 release was completely abolished by the non-selective COX inhibitor indomethacin (20 μM) or by the selective COX2 inhibitor NS-398 (10 μM) (Figure 5B).
Figure 5.
P2X4R activation evokes arachidonic acid (AA) and PGE2 release from macrophages. (A) In WT macrophages, ATP (50 μM) induces [3H]AA release that is enhanced in the presence of IVM (3 μM), whereas in P2X4−/− macrophages ATP-evoked [3H]AA is reduced and IVM has no effect. N=5 independent experiments, one-way ANOVA, *P<0.05, ***P<0.005. (B) Stimulation of WT macrophages with ATP (50 μM) induced a three-fold increase of PGE2 release over baseline, which was further increased by IVM (3 μM). ATP-evoked PGE2 release was reduced in the absence of extracellular calcium and totally inhibited by pretreatment with p38 MAPK inhibitor SB203580 (10 μM), or with the COX inhibitors indomethacin (indo, 20 μM) or NS-398 (NS, 10 μM). Note that in P2X4−/− cells, basal PGE2 levels are reduced and that ATP/IVM do not trigger PGE2 release. To allow for inter-experiment comparisons, results were normalized to the mean concentration of PGE2 in WT control conditions, for each given experiment. N=3 independent experiments, one-way ANOVA, **P<0.01, ***P<0.005. ND, not detectable. (C) Western blot and immunocytochemistry analysis show that cPLA2 expression or its induction by LPS is not altered in P2X4−/− macrophages (scale bar=20 μm). N=2 experiments. (D) Transcriptional regulation of COX2 by LPS is not altered in P2X4−/− bone marrow-derived macrophages. Expression of COX2 mRNA was quantified in bone marrow-derived macrophages culture treated or not with LPS (1 μM, 6 h) from WT and P2X4R-deficient mice. Results are mean±s.e.m. of three independent experiments. One-way ANOVA, ***P<0.001. (E) Top panel, representative western blot analysis indicating that ATP (20 μM) induces an increase of P-p38 MAPK in WT but not in P2X4−/− macrophages. Bottom panel, quantification of N=3 independent experiments, one-way ANOVA, *P<0.05.
Western blot analysis and immunostaining indicated that neither the expression of cPLA2 nor its induction by LPS treatment was affected in P2X4R-deficient macrophages (Figure 5C). Similarly, LPS-dependent transcriptional upregulation of COX2 was not altered in P2X4R-deficient macrophages (Figure 5D). In microglia, P2X4R stimulation induced activation of p38 MAPK (Trang et al, 2009); we tested whether this regulation also occurred in macrophages. As shown in Figure 5E, in WT macrophages, ATP stimulation increased the phosphorylation of p38 MAPK, whereas such stimulatory effect was essentially absent from P2X4R-deficient cells. ATP-mediated p38 stimulation is not mediated by P2Y12 since, as reported earlier (Haynes et al, 2006), macrophages do not express this receptor (Supplementary Figure S2).
Altogether, these results show that in macrophages, P2X4R activation leads to COX-dependent PGE2 release by stimulating cPLA2 activity in a calcium- and p38 MAPK-dependent manner.
Acute adoptive transfer of P2X4R-stimulated macrophages induces COX-dependent mechanical hypersensitivity
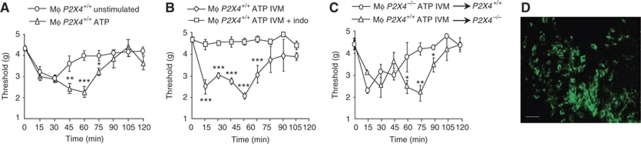
The above results show that reduced inflammatory pain behaviours in P2X4R-deficient mice are due to impaired production of PGE2, and that in vitro P2X4R activation elicits PGE2 release from macrophages. To establish a direct link between these two observations, we used acute adoptive macrophage transfer experiments. Cultured peritoneal macrophages from CX3CR1+/eGFP or (CX3CR1+/eGFP X P2X4−/−) mice were stimulated with ATP and injected in the hindpaw of WT mice; withdrawal thresholds to light mechanical stimulation were then measured every 15 min during a 2 h period. Transfer of ATP-stimulated CX3CR1+/eGFP macrophages induced the development of a biphasic mechanical hypersensitivity that peaked 15 and 60 min after injection, whereas that of unstimulated macrophages only induced the early phase and withdrawal thresholds returned to baseline value within 60 min (Figure 6A). Transfer of CX3CR1+/eGFP macrophages pretreated with indomethacin (20 μM) before ATP/IVM stimulation totally prevented development of pain hypersensitivity in recipient mice (Figure 6B). These later experiments also demonstrate that ATP and IVM coinjected with the cells do not contribute to mechanical hypersensitivity. When similar experiments were performed with P2X4R-deficient macrophages stimulated with ATP/IVM, the second phase was abolished and baseline threshold was reached within 60 min after injection (Figure 6C); Furthermore, transfer of ATP/IVM-stimulated CX3CR1+/eGFP macrophages in P2X4R-deficient mice induced both phases of hypersensitivity. Although hypersensitivity disappeared 2 h after injection, transferred CX3CR1+/eGFP macrophages were still present in paw tissue 6 h later (Figure 6D). As in these experiments macrophages were not LPS treated, this suggests that in the absence of inflammatory challenge, there is no endogenous release of ATP. Furthermore, it is likely that exogenous administered ATP is rapidly degraded by ectonucleotidases (Picher et al, 2004), thus only provoking transient P2X4R-dependent PGE2 release. Altogether, our data show that in macrophages, P2X4R stimulation triggers PGE2 release that is directly related to mechanical hyperalgesia.
Figure 6.
Transfer of ATP-primed macrophages induces P2X4-dependent pain hypersensitivity. (A) Peritoneal macrophages from CX3CR1+/eGFP mice were stimulated with ATP (50 μM) or left untreated and injected in the paw of WT mice; withdrawal thresholds to mechanical stimulation were measured every 15 min. Administration ATP-primed macrophages induced biphasic hypersensitivity, whereas that of unstimulated macrophages only induced a transient response. *P<0.05, **P<0.01, ***P<0.005, one-way ANOVA. (B) Similar experiments were performed with macrophages pretreated (or not) with indomethacin (20 μM) before wash and further stimulation with ATP (50 μM) and IVM (3 μM). Indomethacin completely abolished both phases of hypersensitivity. (C) ATP/IVM-primed macrophages from (CX3CR1+/eGFP X P2X4−/−) and CX3CR1+/eGFP mice were transferred to P2X4+/+ or P2X4−/− mice, respectively. Transfer of P2X4-deficient macrophages induced only transient hypersensitivity that returned to basal threshold values within 60 min. Transfer of CX3CR1+/eGFP macrophages in P2X4-deficient mice induced biphasic hypersensitivity, as observed in P2X4+/+ recipient mice N=4–8 animals per point. *P<0.05, **P<0.01, ***P<0.005, one-way ANOVA. All results are expressed as mean±s.e.m. (D) Transferred CX3CR1+/eGFP macrophages were still present in paw 6 h after injection. Scale bar=20 μm.
Discussion
Our findings show that P2X4R-deficient mice display reduced inflammatory pain behaviours and an impaired production of PGE2 in peripheral tissue in response to inflammatory challenges. We identified paw-resident macrophages as the main cell type responsible for P2X4R-evoked PGE2 production, and we demonstrate that in macrophages, P2X4R activation triggers the necessary intracellular signals leading to the activation of cPLA2/COX pathway and PGE2 synthesis. P2X4R deletion did not affect basal tissue levels of PGE2, while strongly reducing carrageenan- or CFA-induced PGE2 release. These observations identify P2X4Rs as key receptors mediating inflammatory PGE2 release in vivo and support their specific involvement in inflammation.
Our behavioural analysis of P2X4-deficient mice show that P2X4Rs mediate both acute and long lasting inflammatory pain but are not involved in acute nociception. A recent study also performed in P2X4-deficient mice provided essentially similar results (Tsuda et al, 2009). However, we observed reduced nocifensive behaviours to formalin in P2X4-deficient mice when a lower amount of formalin was injected in the paw. Intensities of formalin-induced responses are dose dependent (Le Bars et al, 2001). It is therefore possible that higher formalin concentrations trigger additional cellular mechanisms that are independent of P2X4Rs, such as direct activation of TRPA1 channels (McNamara et al, 2007). Peripheral EP2 PG receptors are likely to mediate formalin-induced nocifensive behaviours (Hosl et al, 2006). This is in good agreement with our present data showing that peripheral inflammatory challenges lead to local P2X4R-dependent PGE2 release.
Other P2XRs have been reported to mediate inflammatory pain responses. Particularly, genetic or pharmacological inhibitions of non-neuronal P2X7Rs result in similar alteration of pain behaviours as seen in P2X4-deficient mice. Recent studies have suggested that P2X4 and P2X7 subunits are likely to physically interact, although they do not appear to form heteromeric channels (Nicke, 2008; Boumechache et al, 2009). One interpretation could be that p2x4 gene deletion might affect P2X7R expression and functions, thus indirectly altering inflammatory pain processing. Our results clearly show that expression of P2X7R is not reduced in tissue or macrophages from P2X4-deficient mice. On the contrary, a small increase of P2X7R expression was repeatedly observed in tissue of P2X4R-deficient mice; similar results have been described in microglial cells (Ulmann et al, 2008). In addition, we show that CFA-induced elevations of IL-1β and IL-6, that are impaired in P2X7R-deficient mice (Chessell et al, 2005), are identical in WT and P2X4R-deficient mice. P2X4R and P2X7R are thus likely to contribute to peripheral inflammatory pain through the activation of independent cellular signalling pathways.
Tissue-resident macrophages express other purinergic receptors. In macrophage cell lines, ATP is a known activator of cPLA2, producing AA and a variety of pro-inflammatory lipids including PGE2 (Balboa et al, 1999; Buczynski et al, 2007). We cannot totally exclude that other purinergic receptors contribute to ATP-mediated PGE2 release, particularly through intracellular calcium increase. Indeed, in P2X4R-deficient cells, ATP triggers residual intracellular calcium increase, likely mediated by P2Y2 receptors (Bowler et al, 2003; Wang et al, 2004). Our data show that reducing extracellular calcium is sufficient to abrogate ATP-evoked PGE2 release, suggesting that calcium influx through P2X4 channels is necessary, if not sufficient, to activate cPLA2. Microglia, brain-resident macrophages, express high levels of the P2Y12R (Haynes et al, 2006; Avignone et al, 2008), which is known to mediate neuropathic pain processing through activation of the p38-MAPK pathway (Kobayashi et al, 2008; Tozaki-Saitoh et al, 2008). However, we observed that paw-resident macrophages do not express P2Y12R receptors, as suggested earlier (Haynes et al, 2006). Altogether, these findings support that PGE2 release from tissue-resident macrophages in response to acute inflammatory challenge is likely to be mediated by P2X4R. Yet, the mechanisms by which inflammatory agents such as carrageenan or CFA lead to P2X4R activation still remain to be elucidated. Studies have proposed that activation of Toll-like receptors (TLR) triggers ATP release from monocytes (Ferrari et al, 1997; Piccini et al, 2008). Carrageenan or CFA are known to activate TLR4 (Bhattacharyya et al, 2008) and can conceivably induce TLR-dependent ATP release and autocrine activation of P2X4Rs.
In peritoneal or alveolar macrophages, P2X4R-mediated currents displayed very small amplitude, raising questions about the function of this receptor in these cells (Bowler et al, 2003; Sim et al, 2007). One of the hallmarks of P2X4R is the very high calcium permeability (Egan and Khakh, 2004). Using calcium imaging, we show that, in peritoneal macrophages, activation of P2X4R contributes significantly to ATP-induced calcium signals. We also defined a specific calcium signature for P2X4R characterized by a slow run down that can be reversed by IVM. These data are consistent with studies suggesting that once activated, P2X4R internalizes through clathrin-dependent endocytosis (Royle et al, 2005). IVM, by reducing receptor internalization, enhances P2X4R-evoked responses (Toulme et al, 2006). Despite the alleged poor specificity of IVM (Dawson et al, 2000), our data using P2X4R-deficient macrophages provide convincing evidence for the specificity of IVM positive allosteric modulation of P2X4R. Thus, IVM represents a useful tool to address P2X4R functions while awaiting the development of specific antagonists.
Calcium influx through P2X channels can bind to specific proteins and activate intracellular calcium-dependent pathways (Chaumont et al, 2008). During inflammation, cytosolic PLA2 has a central function in AA release and PGE2 synthesis from macrophages (Bonventre and Sapirstein, 2002), and consequently its activity is highly regulated. Increase in intracellular calcium is an absolute requirement for cPLA2 activity, which is enhanced by p38 MAPK phosphorylation (Gijon et al, 2000). Our data show that, in macrophages, P2X4R activation triggers calcium influx and phosphorylation of p38 MAPK. In addition, we provide a direct demonstration that ATP-induced PGE2 release depends on P2X4R-mediated calcium influx and p38 MAPK activity, consistent with a direct activation of cPLA2 by P2X4R. Similar synergies between P2X4R-evoked calcium influx and p38 MAPK activation have been involved in the regulation of BDNF secretion from microglia as well as in the facilitation of synaptic transmission in the spinal cord (Gong et al, 2009; Trang et al, 2009). Thus, intracellular calcium and p38 MAPK are common intracellular messengers downstream of P2X4Rs that can trigger specific cellular functions, depending on the cell type in which the receptors are expressed.
AA is metabolized by two main enzymatic pathways, lipoxygenase (LOX) and COX, resulting in the synthesis of pro-inflammatory leukotrienes and prostanoids, respectively (Samad et al, 2002). P2X4R-evoked AA release could also be metabolized though the LOX pathway producing the pro-inflammatory leukotriene LTB4, which is known to promote inflammation though its neutrophil chemoattractant properties (Guerrero et al, 2008). However, we found no evidence for the presence of neutrophils after injection of carrageenan, whereas pain hypersensitivity had already developed and neutrophil recruitment was not affected in P2X4R-deficient mice in long lasting inflammatory conditions. Furthermore, in the macrophages transfer experiments, COX inhibition is sufficient to abolish pain hypersensitivity, suggesting a preferential coupling between cPLA2 and COX enzymes (Takano et al, 2000). Altogether, our results suggest that P2X4R preferentially activates the cPLA2-COX pathway at least during the acute phase of inflammation, although we cannot exclude the involvement of the LOX pathway, which depends on sustained intracellular calcium increase (Buczynski et al, 2007), at later time points of inflammation.
One question is whether P2X4R can also mediate PGE2 release in other pathologies, particularly within the CNS. In neuropathic pain or neurodegenerative diseases, both neurons and microglia support PGE2 biosynthesis (Block and Hong, 2005; Vardeh et al, 2009). There are now strong data for an upregulation of P2X4Rs in activated microglia in chronic brain disease (Avignone et al, 2008) as well as for its expression in different neuronal population. Therefore, dual expression of P2X4R and COX2 in neurons or microglia could conceivably promote PGE2 release and inflammation associated with chronic brain diseases or sustained neuronal activity.
Here, we identify P2X4R as the main trigger of inflammation-evoked PGE2 synthesis in vivo. We provide evidence that the restricted expression of P2X4Rs in tissue-resident macrophages accounts for the specific link between P2X4R and inflammatory-dependent PGE2 release. Beyond inflammatory pain, P2X4Rs expressed by resident myeloid cells, by virtue of their high calcium permeability may also contribute significantly to other types of inflammation such as airway inflammation in which purinergic receptors have clearly been involved (Idzko et al, 2007). Hence, P2X4Rs represent a potential therapeutical target alternative to traditional NSAID that could specifically limit PGE2 production during inflammatory events.
Materials and methods
Targeting of the P2X4 gene and generation of mutant mice
Mice carrying a targeted null mutation of the P2X4 gene have been described elsewhere (Sim et al, 2006). Briefly, a β-galactosidase-neomycin cassette was inserted in place of the coding sequence of the first exon of the p2x4 gene. In the resulting allele, the P2X4 promoter drives β-galactosidase expression. Mice were backcrossed on C57Bl6/J for at least 20 generations and were housed in groups of 5–12 under a standard 12-h light/dark cycle with food and water available ad libitum. All testing began when the mice were between 6 and 12 weeks of age and were sex matched. All procedures fully complied with French legislation (décret 87–848, 19 October 1987, and order, 19 April 1988), which implement the European directive (86/609/EEC) on research involving laboratory animals, and were performed according to the requirements of CNRS ethical standards.
Generation of CX3CR1+/−X P2X4−/− mice
CX3CR1−/− mice obtained from the Jackson Laboratory were crossed with P2X4−/− mice to obtain double heterozygote animals, which were further intercrossed. All mice were genotyped for each allele.
Macrophage culture
Macrophages were obtained from peritoneal cavity lavage. After euthanasia, the peritoneal cavity was injected with 10 ml of DMEM containing antibiotics and the abdomen was gently rubbed. Peritoneal lavage was collected through a 21-gauge needle. After washes, cells were plated in 24-well dishes and non-adherent cells were flushed 2 h later. Where indicated, macrophages were activated for 6 h with 1 μg/ml LPS. Cytometric analysis of cultured macrophages from CX3CR1+/− mice indicated that 85–95% of cells expressed GFP.
[3H]AA release assay
Cultures of P2X4+/+ and P2X4−/− peritoneal macrophages were incubated with [3H]AA (6.6 μCi/ml) overnight, unincorporated radioactivity was discarded. Cells were then activated with 1 μg/ml LPS for 6 h and the medium was collected for counting. After washes, macrophages were stimulated with ATP (50 μM) alone or in combination with IVM (3 μM) for 10 min and media collected. Cells were collected from tissue dishes to measure residual radioactivity. Radioactivity present in all fractions was measured by scintillation counting.
PGE2 assay
Macrophages were cultured and stimulated as described for [3H]AA assay. Where indicated, cultures were pretreated with indomethacin (20 μM, Sigma) or NS398 (10 μM, Cayman Chemical) before stimulation. PGE2 concentration in macrophage culture supernatant was measured in duplicate using PGE2 metabolite EIA system (Cayman Chemical) according to the manufacturer's instructions.
To determine PGE2 levels in inflamed tissue, 2 to 4 h after inflammation, mice were euthanized and subsequently indomethacin (30 μl, 20 μM) was injected in the paw to avoid further activation of COX. After dissection, paw exudates were collected by centrifugation (10 000 g, 10 min). After appropriate dilution, PGE2 concentrations in individual paw exudates were measured in duplicate as described for cell culture supernatant. Results are means±s.e.m. of three independent animals. All experiments were replicated three times.
Formalin test
Mice were placed in individual plexiglass cages and allowed to acclimatize to the testing environment for 15 min. Formalin (5%, 15 or 20 μl in PBS) was then injected into the left hindpaw. The length of nocifensive events (paw flinching, licking, bitting) for each animal was recorded during 5-min periods with a stopwatch.
Carrageenan-induced inflammatory mechanical hyperalgesia
Carrageenan (30 μl, 1% in PBS) was injected subcutaneously in the plantar hindpaw of two groups of six animals. Mechanical hyperalgesia was measured using an Ugo-Basile Dynamic Plantar test aesthesiometer 37450 (Bioseb), with a progressive pressure (cutoff 0.5 g/s for 10 s). Measurements were made in triplicate prior and 60 and 120 min after carrageenan injection.
PGE2 and CFA induced mechanical hypersensitivity
PGE2 (100 ng, 10 μl) or CFA (30 μl) were injected in paw and mechanical hyperalgesia was measured using an analgesimeter as previously described Ulmann et al (2008). After the establishment of baseline thresholds, withdrawal thresholds were measured at 30 and 60 min post-PGE2 injection or 24 h later for CFA. Acute mechanical hyperalgesia to CFA injection was measured 2 and 4 h after injection using the Ugo-Basile Dynamic plantar test aesthesiometer as described earlier.
Macrophage transfer and mechanical hypersensitivity measurements
Cultures of non-LPS-primed macrophages were prepared as described above, except that cells were seeded on RepCell temperature-responsive dishes (CellSeed), which allow for temperature-dependent cell detachment. After 24 h in culture, dishes were placed at 20°C for 15 min and cells were detached by gentle flushing. After washes, 5 × 105 cells were placed in collection tube in 800 μl DMEM and stimulated with 50 μM ATP±3 μM IVM for 10 min at 37°C; 30 μl of stimulated cell suspension were injected in the hindpaw without further wash. Pretreatment with indomethacin (20 μM) was performed for 30 min before cell detachment and stimulation. Mechanical hypersensitivity was measured using an Ugo-Basile Dynamic Plantar test aesthesiometer as described earlier. For each mouse, ipsilateral thresholds were measured in triplicate every 15 min for 2 h. Four to eight mice were used for each point.
Measurement of MPO activity
The neutrophil content in paw was evaluated using a MPO colorimetric assay. Paws were collected 2 h after carrageenan or 24 h after CFA injection and dissociated in phosphate buffer containing 0.5% hexadecyl trimethylammonium bromide. After three cycles of freezing and thawing, 20 μl of supernatant were mixed with phosphate buffer containing 0.167 mg/ml O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The MPO activity was assayed spectrophotometrically at 450 nm.
Cytokines profiling
Paw samples from the 24 h post-CFA treated and untreated P2X4−/− and P2X4+/+ mice were dissected and homogenized in PBS containing ‘complete' protease inhibitor cocktail (Roche) using a Mixer Mill MM300 (Qiagen). Homogenates were centrifuged and supernatants were harvested. Cytokine content was analysed by Luminex using a BioPlex cytokine kit (Bio-Rad Laboratories) following the manufacturer's instructions.
Immunohisto- and cytochemistry
The following antibodies were purchased from commercial sources and used at the indicated dilution: mouse anti-β-galactosidase (1/50 000, Promega), mouse anti-cPLA2 (1/300, Santa Cruz), rat anti-mouse F4/80 (1/1000, AbD Serotec), rat anti-mouse GR1 (1/1000, AbD Serotec) and rabbit anti-rat P2X7 (1/500, Alomone Labs). Rabbit anti-mouse P2X4 antibody (1/500) was produced in the laboratory. Rabbit anti-mouse P2Y12R antibody (1/1000) was a gift from Professor Julius (Haynes et al, 2006).
Mice were lethally anaesthetized with 5% pentobarbital and tissues were fixed through a trans-cardiac perfusion of 4% paraformaldehyde in PBS and post-fixed overnight. Vibratom paw sections (30 μm) were permeabilized in PBS containing, 0.1% Triton X-100 and 10% fetal calf serum for 30 min at room temperature and then incubated overnight at 4°C with primary antibody. After three washes in PBS, sections were incubated for 2 h with appropriate secondary antibody. Sections were mounted and observed on a Bio-Rad MRC 1024 laser scanning confocal.
For immunocytochemistry, macrophages plated on cover slips were fixed for 10 min with 4% paraformaldehyde in PBS and treated as described earlier. Image acquisitions were performed with DMRA2 microscope (Leica).
Western blot
Primary macrophages or paw tissues were homogenized in lysis buffer (20 mM Hepes, pH 7.4, 100 mM NaCl, 5 mM EDTA, 1% Triton X-100 and Complete Protease Inhibitor cocktail (Roche)). Lysates were clarified by centrifugation and protein concentration from tissues determined using protein assay kit (Bio-Rad). Proteins were separated on 12–4% NuPage precast gel (Invitrogen), and transferred on nitrocellulose membrane. The membrane was blocked with 5% non-fat dry milk/0.5% Tween 20 in Tris-buffered saline (TBST) overnight at 4°C. The membrane was then incubated overnight at 4°C with antibody diluted in TBST as indicated above or using rabbit anti-P2X4 (1/300, Alomone Labs), mouse anti-tubulin (1/2500, Sigma), mouse anti-p38 and anti-phospho-p38 (1/1000, Cell Signaling). After washes in TBST, the membrane was then treated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature and revealed with ECL+detection kit (Amersham). Western blots were analysed using Image J analysis software (NIH) to quantify band intensity and the data normalized to control conditions.
Quantitative PCR analysis
Total RNA from macrophage cultures was extracted with Trizol. A measure of 2 μg of total RNA were reverse transcribed using random hexamers and SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed by using SYBR Green dye detection according to the manufacturer's instruction (SYBR Green PCR Master Mix, Roche) on the LightCycler480 system. PCR reactions were performed in a 10 μl volume containing 2.5 μl of diluted RT product, 1 μl of forward and reverse primers (GAPDH: Mm_Gapdh_3_SG, COX2: Mm_Ptgs2_1__SG, QuantiTect Primer Assay; Qiagen) and 5 μl of PCR master mix. Negative controls using non-reverse-transcribed RNA were performed simultaneously. For each reaction, Cq was determined using the 2nd Derivative Max tool of LightCycler480 software. The relative ratios of specifically amplified cDNAs were calculated using the ΔCq method (Pfaffl, 2001). RNA from two independent cultures were used in this study. All four experimental conditions (P2X4+/+, P2X4+/+_LPS, P2X4−/− and P2X4−/−_LPS) were processed at the same time. For each RNA sample, two reverse transcriptions were performed and analysed.
Video microscopy
The functional expression of P2X4 receptors was evaluated by recording the changes in the cytoplasmic Ca2+ concentration using the ratiometric fluorescent probe Fura-2. Macrophages were loaded with 2 μM of Fura-2 acetoxymethyl ester (Fura-2/AM, Invitrogen), 0.5% BSA in the recording saline solution containing (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 Hepes, pH 7.4. After 1 h incubation at room temperature, cells were washed three times. The Metafluor Imaging system (Molecular Devices) was used for fluorescence acquisition and analysis of individual cells. Fluorescence was excited by illumination through × 20 water immersion objective with a light wavelength switch provided by a DG4 filter wheel and detected with a CCD camera under the Metafluor software control. Pairs of images were acquired every 2 s. Intracellular calcium is expressed throughout as the fluorescence ratio F340/F380, calculated after background subtraction. For YO-PRO uptake, cells were incubated for 10 min with YO-PRO (1 μM, Invitrogen) in a divalent-free solution containing (in mM): NaCl 145, KCl 3, CaCl2 0.1, Hepes 10, pH 7.3 and stimulated with 1 mM ATP±10 μM A-740003 in the low divalent solution for 1 min. Fluorescence was excited at 350 nm and emitted light was collected above 540 nm. Images were acquired every 2 s. Results are expressed as the mean fluorescence expressed in arbitrary units of all recorded cells (N>50). A-740003 was custom synthesized (ArtMolecule, Poitiers, France). Experiments were performed at room temperature (22–26°C). Drugs were applied locally through a second multi-channel gravity-fed perfusion system controlled by solenoids, whereas control saline was applied using an independent perfusion. ATP (20 μM) or ATP and IVM (3 μM) were applied alternatively for 10 s every 2 min.
Statistics
Difference between groups were assessed using Student's t-test, or for multiple comparisons between groups by a one-way ANOVA followed by a Bonferroni post hoc test (Prism 2; GraphPad Software).
Supplementary Material
Acknowledgments
This work was supported by the CNRS, the Agence Nationale de la Recherche (ANR-05-NEUR-037), the Fondation pour la Recherche Médicale and l'Institut UPSA de la Douleur. This work was made possible, thanks to the Animal facility of Institut Fédératif de Recherche 3 and the Montpellier Réseau Inter-Organisme Imaging facility. We thank Drs J Hatcher, IP Chessels and JP Hughes for their help during the initial phase of this study and Dr IA Lefevre for editing the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E (2008) Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci 28: 9133–9144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa MA, Balsinde J, Johnson CA, Dennis EA (1999) Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J Biol Chem 274: 36764–36768 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Gill R, Chen ML, Zhang F, Linhardt RJ, Dudeja PK, Tobacman JK (2008) Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem 283: 10550–10558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76: 77–98 [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Sapirstein A (2002) Group IV cytosolic phospholipase A2 (PLA2) function: insights from the knockout mouse. Adv Exp Med Biol 507: 25–31 [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R (2009) Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 284: 13446–13454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112: 358–404 [DOI] [PubMed] [Google Scholar]
- Bowler JW, Bailey RJ, North RA, Surprenant A (2003) P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol 140: 567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA (2007) TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem 282: 22834–22847 [DOI] [PubMed] [Google Scholar]
- Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797 [DOI] [PubMed] [Google Scholar]
- Chaumont S, Compan V, Toulme E, Richler E, Housley GD, Rassendren F, Khakh BS (2008) Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal 1: RA8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114: 386–396 [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G (1996) Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16: 2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM (2000) Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther 295: 1051–1060 [PubMed] [Google Scholar]
- Egan TM, Khakh BS (2004) Contribution of calcium ions to P2X channel responses. J Neurosci 24: 3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med 185: 579–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC (2000) Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that do and do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem 275: 20146–20156 [DOI] [PubMed] [Google Scholar]
- Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, Zhang T, Liu XG (2009) ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2X4 receptors and p38 MAPK in microglia. Glia 57: 583–591 [DOI] [PubMed] [Google Scholar]
- Guerrero AT, Verri WA Jr, Cunha TM, Silva TA, Schivo IR, Dal-Secco D, Canetti C, Rocha FA, Parada CA, Cunha FQ, Ferreira SH (2008) Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol 83: 122–130 [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9: 1512–1519 [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319: 1376–1385 [DOI] [PubMed] [Google Scholar]
- Hosl K, Reinold H, Harvey RJ, Muller U, Narumiya S, Zeilhofer HU (2006) Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor alpha3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain 126: 46–53 [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919 [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA (1999) Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci 19: 7289–7299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K (2008) P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28: 2892–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I, Murakami M (2002) Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat 68–69: 3–58 [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53: 597–652 [PubMed] [Google Scholar]
- Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA (2005) Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol 144: 940–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB (2005) Role of the immune system in chronic pain. Nat Rev Neurosci 6: 521–532 [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF (2007) P2X7-related modulation of pathological nociception in rats. Neuroscience 146: 1817–1828 [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104: 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A (2008) Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun 377: 803–808 [DOI] [PubMed] [Google Scholar]
- North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067 [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA 105: 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picher M, Burch LH, Boucher RC (2004) Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem 279: 20234–20241 [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC (1996) Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med 184: 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen DJ, Murrell-Lagnado RD (2005) Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci 118 (Part 14): 3073–3080 [DOI] [PubMed] [Google Scholar]
- Samad TA, Sapirstein A, Woolf CJ (2002) Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med 8: 390–396 [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F (2006) Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci 26: 9006–9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Park CK, Oh SB, Evans RJ, North RA (2007) P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol 152: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Panesar M, Papillon J, Cybulsky AV (2000) Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat 60: 15–26 [DOI] [PubMed] [Google Scholar]
- Toulme E, Soto F, Garret M, Boue-Grabot E (2006) Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol 69: 576–587 [DOI] [PubMed] [Google Scholar]
- Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K (2008) P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 28: 4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW (2006) Purinoceptors in microglia and neuropathic pain. Pflugers Arch 452: 645–652 [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW (2009) P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29: 3518–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–783 [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ, Fitzgerald GA, Samad TA (2009) COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest 119: 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jacobsen SE, Bengtsson A, Erlinge D (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Sperlagh B, Illes P (2007) P2X3 receptor involvement in pain states. Mol Neurobiol 36: 165–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.