EMBO J 29 14, 2407–2420 (2010); published online June042010
Microtubule poisons induce mitotic arrest that leads to apoptotic cell death if not resolved in a timely manner, but the mechanisms that directly link this cell cycle arrest to apoptosis have been elusive. In this issue of The EMBO Journal, Clarke and colleagues show that Mcl-1, an anti-apoptotic Bcl-2 family protein, is phosphorylated by the mitotic kinase CDK1/cyclin B1. This targets the protein for degradation by anaphase-promoting complex/cyclosome (APC/C)-mediated ubiquitination, in a manner such that only prolonged arrest allows sufficient Mcl-1 phosphorylation and degradation to trigger apoptosis. Thus, the APC/C, a major effector of the spindle assembly checkpoint (SAC), not only ensures cell cycle arrest upon spindle disruption, but promotes cell death when the duration of mitotic arrest is too long.
Apoptotic cell death proceeds through a series of signalling events that bring about the dismantling of cellular components by a family of cysteine proteases, the caspases. In particular, genotoxic and/or cytotoxic stress promotes the release of cytochrome c, a component of the electron transport chain in the mitochondria, to the cytoplasm, which in turn triggers activation of the initiator and executioner caspases, caspase-9 and caspase-3, respectively. It is well recognized that a failure in cell cycle progression, such as prolonged mitotic arrest, can induce apoptosis by triggering mitochondrial cytochrome c release. When a cell encounters a defect in mitotic spindle assembly (as when cells are treated with microtubule poisons), entry into anaphase is inhibited by the SAC that suppresses APC/C-mediated cyclin B1 degradation until metaphase can be completed; if the spindle defect is unrepairable, apoptotic cell death ensues. Cyclin B1 degradation is slowed, but not halted, during prolonged mitotic arrest because of residual APC/CCdc20 activity. As a result, when CDK1 activity drops below the threshold required for maintenance of mitosis, cells eventually exit M phase with a defect in chromosome segregation. Thus, mitotic cell death seems to be a preventive mechanism that avoids the creation of carcinogenic aneuploids.
Harley et al (2010) now show that the degradation of the anti-apoptotic Bcl-2 family member Mcl-1 has an important function in such mitotic cell death. Cytochrome c release is regulated by the balance of pro- and anti-apoptotic Bcl-2 family proteins. Owing to its very short half-life (<0.5 h), Mcl-1 protein levels, and its consequent contribution to cell survival, can be rapidly tuned by changes in protein stability. Polyubiquitination and proteasomal degradation of Mcl-1 in interphase has been attributed to the action of two E3 ligases, Mule and SCFβ-TrCP; this can be counter-acted by the deubiquitinase USP9X (Zhong et al, 2005; Ding et al, 2007; Schwickart et al, 2010). Harley et al (2010) add another layer of Mcl-1 stability control, showing that APC/CCdc20 is a mitosis-specific E3 ligase for Mcl-1, which prompts the destruction of Mcl-1 during prolonged mitotic arrest. As is true for multiple APC/C substrates, the interaction between Mcl-1 and the APC/C activator Cdc20 is mediated through a D-box motif. Unlike cyclin B, but similar to the situation for cyclin A, APC/C-mediated Mcl-1 ubiquitination is not inhibited by the SAC. Further, APC/CCdc20-mediated ubiquitination of Mcl-1 is unusual in that it requires earlier phosphorylation of Mcl-1 by CDK1/cyclin B1. Thus, Mcl-1 carrying either a phospho-site or a D-box mutation exhibits marked stability during prolonged mitosis. The authors also show that expression of the mutant Mcl-1, but not wild-type protein, renders cells resistant to apoptotic cell death induced by mitotic arrest, indicating that the APC/CCdc20-mediated degradation of Mcl-1 is critical for mitotic cell death. As Cdc20-Mcl-1 interactions do not seem to be modulated by this phosphorylation, Harley et al speculate that the ability of the APC/C to act on this complex may be phospho-dependent or that a deubiquitinase countering Mcl-1 polyubiqutiylation may be antagonized by Mcl-1 phosphorylation. A further important facet of the proposed model is a timing mechanism. During a normal mitosis, the transient phosphorylation of Mcl-1 by CDK1/cyclin B1 is insufficient to drive degradation of the majority of Mcl-1 before cyclin B1 degradation and loss of CDK1 activity. Thus, cells are not unduly susceptible to apoptosis if mitosis proceeds normally. However, on prolonged SAC arrest with sustained CDK1/cyclin B1 activity, the accrued phosphorylation promotes sufficient degradation to drop Mcl-1 below the threshold necessary to sustain cell viability. This ensures that only prolonged arrest induces apoptosis.
The mechanism of mitotic cell death has long been unclear. A recent study showed that Bcl-xL and Bcl-2 are phosphorylated by CDK1/cyclin B1 and inhibited in mitosis (Terrano et al, 2010). However, it has also been shown that CDK1 activity is required to protect cells from apoptosis in mitosis (O'Connor et al, 2002). How can we reconcile these apparently paradoxical results? In contrast to prolonged mitotic arrest, cells in normal mitosis exhibit resistance to apoptosis. For instance, during M phase, caspase-2 and caspase-9 are inhibited by CDK1/cyclin B1-mediated phosphorylation, thus preventing apoptosis (Allan and Clarke, 2007; Andersen et al, 2009).
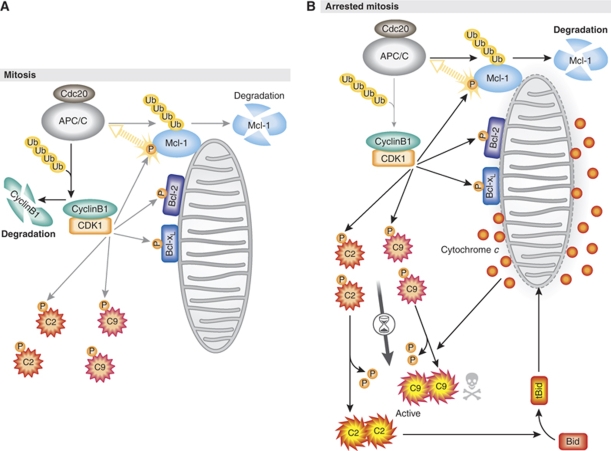
The findings by Harley et al (2010) provide new insight into the CDK1/cyclin B1-mediated regulation of pro- and anti-apoptotic proteins in mitosis. It is suggested that during mitosis in which high CDK1/cyclin B1 activity is maintained, the anti-apoptotic function of Bcl-xL and Bcl-2 is suppressed, whereas Mcl-1 is gradually degraded (Figure 1A). Concomitantly, CDK1/cyclin B1 inhibits the activation of the initiator caspase-2 and caspase-9, thereby preventing the full initiation of the apoptotic cascade (Figure 1A). When mitotic arrest is protracted, the loss of Mcl-1 ultimately elicits the release of cytochrome c from the mitochondria (Figure 1B). Interestingly, the authors show that during mitotic arrest, Mcl-1 degradation precedes the dephosphorylation of caspase-9. In that a non-phosphorylatable mutant of caspase-2 or caspase-9 can enhance sensitivity to apoptosis in mitotic cells (Allan and Clarke, 2007; Andersen et al, 2009), it is likely that a signal distinct from Mcl-1 degradation also takes part in mitotic cell death through the dephosphorylation of the caspases. Lastly, it is known that some cancer cells overexpress Mcl-1, whereas others do not (Warr and Shore, 2008; Schwickart et al, 2010). It will be interesting to compare the efficacy of microtubule-directed chemotherapeutics in cancers expressing varying levels of Mcl-1 in that this may serve as a novel biomarker to predict patient responsiveness to these agents.
Figure 1.
Model for mitosis-induced apoptosis. (A) In normal mitosis, CDK1/cyclin B1-mediated phosphorylation (P) inhibits pro- and anti-apoptotic proteins, including Bcl-xL, Bcl-2, caspase-2 (C2) and caspase-9 (C9), whereas it causes the APC/CCdc20-mediated ubiquitination (Ub) and subsequent degradation of Mcl-1. (B) When mitotic arrest is prolonged, constant degradation of Mcl-1 ultimately triggers mitochondrial outer membrane permeabilization and the release of cytochrome c. Presumably, prolonged mitotic arrest also causes dephosphorylation of caspase-2 and caspase-9, which can induce activation of the apoptotic pathways upstream and downstream of the mitochondria, respectively. See the text for details.
Acknowledgments
MK is supported by a grant from the NIH (K99 CA140948). SK is supported by NIH RO1 GM080333.
Footnotes
The authors declare that they have no conflict of interest.
References
- Allan LA, Clarke PR (2007) Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26: 301–310 [DOI] [PubMed] [Google Scholar]
- Andersen JL, Johnson CE, Freel CD, Parrish AB, Day JL, Buchakjian MR, Nutt LK, Thompson JW, Moseley MA, Kornbluth S (2009) Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2. EMBO J 28: 3216–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, Lee DF, Liu JC, Zhong Q, Wang X, Hung MC (2007) Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol 27: 4006–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley ME, Allan LA, Sanderson HS, Clarke PR (2010) Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J 29: 2407–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Wall NR, Porter AC, Altieri DC (2002) A p34cdc2 survival checkpoint in cancer. Cancer Cell 2: 43–54 [DOI] [PubMed] [Google Scholar]
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM (2010) Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463: 103–107 [DOI] [PubMed] [Google Scholar]
- Terrano DT, Upreti M, Chambers TC (2010) Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol 30: 640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Shore GC (2008) Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med 8: 138–147 [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X (2005) Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121: 1085–1095 [DOI] [PubMed] [Google Scholar]