Abstract
Background and Objectives
Associations have been reported between the serum uric acid (SUA) level, metabolic syndrome (MS), and atherosclerosis. We have determined the relationship between the SUA level, MS, and arterial stiffness in Korean.
Subjects and Methods
Cross-sectional data from 1,276 adults who underwent routine laboratory tests and pulse wave velocity (PWV) measurements during a health check-up were analyzed in a gender-specific manner. None of the participants had atherosclerotic cardiovascular disease, diabetes, renal disease, or systemic disease, or were under treatment which would affect SUA levels, or taking medications for hypertension or dyslipidemia.
Results
After adjustment for age, smoking status, total cholesterol (TC), and creatinine, the odds ratios (ORs, 95% confidence interval) of gender-specific quartiles of SUA for MS were 1.0, 1.28 (0.66-2.47), 1.46 (0.76-2.82), and 2.21 (1.15-4.26) in females, and 1.0, 1.33 (0.82-2.17), 1.60 (0.96-2.66), and 2.03 (1.21-3.40) in males. However, after adjustment for waist circumference, there were no significant differences in the ORs among the SUA quartile groups in females and males (both, p=NS). The Pearson's correlation coefficients for the relationship between SUA levels and heart-femoral (hf) PWVs or brachial-ankle (ba) PWVs were not significant in females and males (r=0.054 and r=0.015, respectively, in females; r=-0.036 and r=-0.015, respectively, in males; all, p=NS).
Conclusion
An elevated SUA level is associated with abdominal obesity among the MS components, but the SUA level is not associated with PWV in females or males.
Keywords: Uric acid, Metabolic syndrome
Introduction
Elevated serum uric acid (SUA) levels often exist in patients with metabolic syndrome (MS), and cross-sectional studies among various ethnic groups have shown that the prevalence of MS increases with increasing SUA levels.1-4) Epidemiologic studies have also shown associations between SUA levels and the components of MS,5-7) suggesting that hyperuricemia could be an additional component of MS. Moreover, the SUA level is increasingly elevated as the number of components of MS increases.1),6) However, strong intercorrelations among SUA levels and the variables of MS included in the diagnostic criteria8) make it difficult to determine whether or not an elevated SUA level is an additional active component of MS or just an associative link to MS or its components. Furthermore, the relationship between SUA levels, MS, and its variables still needs to be delineated in Korean subjects because clustering of risk factors is not the same among different ethnic groups.9)
Large epidemiologic studies have suggested that hyperuricemia may be a risk factor for cardiovascular disease.10),11) The association between SUA levels and cardiovascular disease may in part be attributable to cardiovascular risk factors clustering in subjects with MS. Moreover, a few studies have shown that the SUA level is independently associated with cardiovascular mortality,12) and atherosclerosis, including coronary artery calcifications13) and carotid atherosclerosis.3),14) Regardless of the role of the SUA level as an independent risk factor or marker for atherosclerosis, few studies have examined the relationship between SUA levels and arterial stiffness, which is also a surrogate marker of atherosclerosis.15),16) In particular, the available data are very limited on the relationship between SUA levels and pulse wave velocity (PWV), an index of arterial stiffness, even in Japanese individuals, despite the wide use of the automated measure of PWV in Japan.17) Moreover, it is unknown whether or not the SUA level is associated with PWV in Korean.
Therefore, in the present study we first determined the relationship between SUA levels, MS, and its variables. Then, we determined whether or not the SUA level is associated with PWV in Korean adults.
Subjects and Methods
Subjects
The present study included 1,276 adults, 20-80 years of age, who attended the Health Promotion Center and Outpatient Clinic of the Cardiovascular Center of Dongguk University Ilsan Hospital in Goyang, Korea between July 2005 and August 2007. The medical history, symptoms, and cigarette smoking were confirmed by the consulting physicians. All of the participants underwent the following laboratory tests as part of their health check-ups: routine blood tests, urinalysis, electrocardiography (ECG), chest radiography, and PWV measurement.
Subjects with atherosclerotic cardiovascular disease, diabetes, established renal disease (serum creatinine >1.4 mg/dL in females and >1.5 mg/dL in males), and systemic diseases, such as hyper- or hypo-thyroidism and gout, were excluded. Participants currently taking antihypertensives, lipid-lowering drugs, uricosuric agents, and hormone replacement for menopause were also excluded.
Blood chemistry
Blood samples were obtained after the participants had fasted overnight. Serum levels of total cholesterol (TC), triglycerides, high density lipoprotein-cholesterol (HDL-C), fasting plasma glucose, creatinine, and uric acid were determined by standard automated bioassays (Modular DPE; Roche Diagnostics, Mannheim, Germany).
The SUA level was measured by the uricase-peroxidase method. The mean SUA level was significantly lower in females (4.3±0.9 mg/dL) than males (6.0 ±1.3 mg/dL, p<0.001). Therefore, gender-specific quartiles of the SUA levels were used. Gender-specific SUA quartiles were as follows: 1.9-3.6, 3.7-4.2, 4.3-4.9, and 5.0-8.6 mg/dL in females; and 1.9-5.1, 5.2-6.0, 6.1-6.8, and 6.9-10.1 mg/dL in males.
Measurement of pulse wave velocity
Arterial stiffness was assessed by measuring the heart-femoral (hf) and brachial-ankle (ba) PWVs using an automatic waveform analyzer (VP-2000; Colin Co., Komaki, Japan).15),16),18) The VP-2000 simultaneously records pulse waves, blood pressure (BP; both arms and ankles), ankle-brachial pressure index (ABI), ECG, and heart sounds, as described elsewhere.15),16) Pearson's correlation coefficients of intra- and inter-observer reproducibility of hfPWV and baPWV were as follows, as described elsewhere:15) hfPWV (r=0.912 and r=0.802, respectively); and baPWV (r=0.976 and 0.972, respectively). All participants included in the present study had a normal ABI (>0.9). A high hfPWV or baPWV was defined as the gender-specific highest quartile of the values among the study subjects {hfPWV ≥900 cm/s in females and ≥968 cm/s in males; baPWV (the mean of the right and left values) ≥1,490 cm/s in females and ≥1,509 cm/s in males}.
Definition of metabolic syndrome
Using the modified National Cholesterol Education Program Adult Treatment Panel Guideline III8) and central obesity criteria for Korean,19) participants with ≥3 of the following criteria were diagnosed with MS: 1) central obesity (waist circumference ≥85 cm in females; and ≥90 cm in males); 2) elevated fasting plasma glucose (≥100 mg/dL); 3) elevated triglycerides (≥150 mg/dL); 4) low HDL-C (<50 mg/dL in females; and <40 mg/dL in males); and 5) elevated BP (≥130/85 mmHg). The BP recorded was the higher BP measured in both arms by oscillomety in a supine position during PWV measurement. Smoking was defined as any cigarette use in the past month.
Statistical analysis
The data are expressed as the mean±SD, percentage, and the mean with 95% confidence interval. Before statistical analysis, the levels of triglycerides and creatinine were log-transformed because of the skewed distribution. Differences between groups were tested by Student's t-test, χ2 test, one-way analysis of variance, and analysis of covariance, when appropriate. The relationships between SUA levels, the variables of MS, and the PWV values were assessed by Pearson's correlation coefficients. The relationship between the SUA quartiles and MS was evaluated by logistic regression analysis. We calculated the unadjusted odds ratios (ORs) and multivariate ORs of gender-specific SUA quartiles for MS using the lowest quartile as a reference.
In model 1, we adjusted for age, smoking, TC, and creatinine. In model 2, we added waist circumferences among the variables of MS. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 11.5 (SPSS, Chicago, IL, USA). A p<0.05 was considered statistically significant.
Results
Characteristics of the subjects
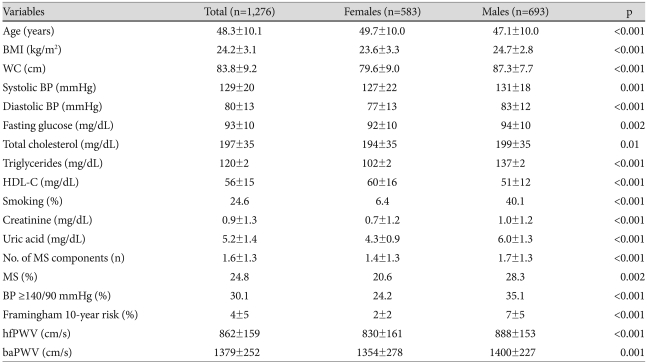
Among a total of 1,276 subjects, 583 were females and 693 were males. The mean age was 48.3±10.1 years. The mean body mass index was 24.2±3.1 kg/m2. The Framingham 10-year coronary heart disease (CHD) risk was 4±5%. Among 1,276 subjects, 30.1% had a systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg, and 24.8% had MS. Current smokers comprised 24.6% of the participants. The SUA level and other variables significantly differed between females and males (Table 1).
Table 1.
Characteristics of the study participants according to gender
Values are the mean±SD or percentage. For levels of triglycerides and creatinine, the mean values are expressed as the geometric mean because of the skewed distribution. baPWV: brachial-ankle pulse wave velocity, BMI: body mass index, BP: blood pressure, HDL-C: high density lipoprotein-cholesterol, hfPWV: heart-femoral pulse wave velocity, MS: metabolic syndrome, WC: waist circumference
Relationship between serum uric acid levels and the variables of metabolic syndrome
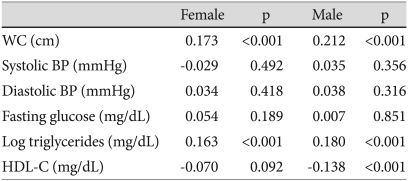
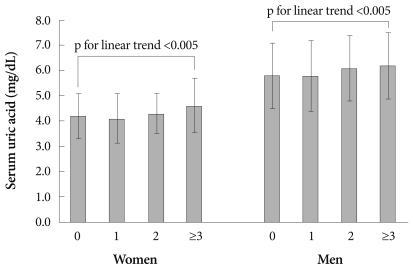
In females and males, the SUA levels were significantly and positively correlated with waist circumference and log triglycerides, but not with BP (systolic or diastolic) and fasting glucose. SUA levels were significantly and negatively correlated with HDL-C in males, but not in females (Table 2). SUA levels increased significantly with an increasing number of MS components (0, 1, 2, and ≥3) in females and males (both, p for linear trend <0.005) (Fig. 1).
Table 2.
Pearson's correlation coefficients between serum uric acid level and metabolic syndrome variables
BP: blood pressure, HDL-C: high density lipoprotein-cholesterol, WC: waist circumference
Fig. 1.
Serum uric acid levels according to the number of metabolic syndrome components and gender. Error bars indicate SDs.
Relationship between serum uric acid levels and metabolic syndrome status
Females with MS (n=120) had higher SUA levels than females without MS (n=463; 4.6±1.1 vs. 4.2±0.9 mg/dL, p<0.001). Males with MS (n=196) also had higher SUA levels than males without MS (n=497; 6.2±1.3 vs. 5.9±1.3 mg/dL, p=0.006). The differences retained significance after adjustment for age, smoking, TC, and creatinine in females (p=0.014) and males (p=0.011).
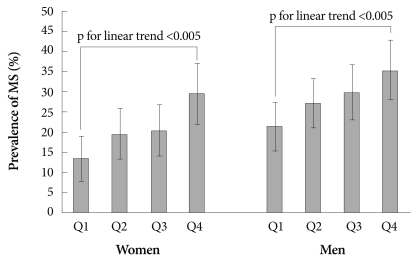
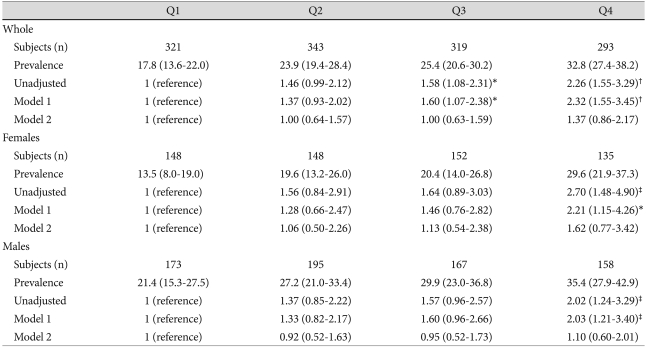
The prevalence of MS according to the gender-specific SUA quartile is shown in Fig. 2. In females and males, the prevalence of MS increased gradually with increasing SUA quartiles (both, p for linear trend <0.005). There were graded increases in the unadjusted ORs for the association between increasing SUA levels and MS. Similarly, increasing trends of the multivariate ORs persisted in model 1. However, after additional adjustment for waist circumference (model 2), there were no significant differences in the multivariate ORs among the SUA quartile groups in both genders (both, p=NS) (Table 3).
Fig. 2.
Prevalence of metabolic syndrome according to the serum uric acid quartile and gender. Error bars indicate 95% confidence intervals. MS: metabolic syndrome.
Table 3.
Prevalence of metabolic syndrome and odds ratio for metabolic syndrome according to the serum uric acid quartile
Values are the number of subjects, % of prevalence, and odds ratio (95% CI). Model 1: adjusted for age, smoking, total cholesterol, creatinine (females or males), and gender (whole). Model 2: adjusted for age, smoking, total cholesterol, creatinine, waist circumference (females or males), and gender (whole). *p<0.05, †p<0.001, ‡p<0.01 vs. the lowest quartile (reference)
Relationship between serum uric acid levels and pulse wave velocity
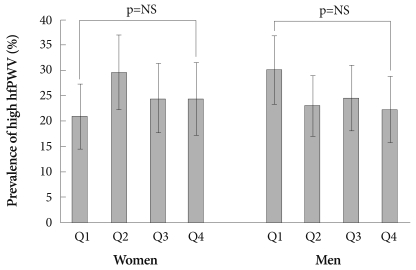
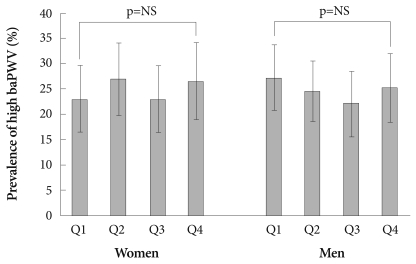
The prevalence of high hfPWV or high baPWV was neither different nor increasing among participants in the first, second, third, or fourth SUA quartiles of both genders (Figs. 3 and 4). Furthermore, Pearson's correlation coefficients for the relationship between SUA levels and hfPWV or baPWV were not significant in both genders (r=0.054 and r=0.015, respectively, in females; and r=-0.036 and r=-0.015, respectively, in males; all, p=NS).
Fig. 3.
Prevalence of high heart-femoral pulse wave velocity (hfPWV) according to the serum uric acid quartile and gender. Error bars indicate 95% confidence intervals.
Fig. 4.
Prevalence of high brachial-ankle pulse wave velocity (baPWV) according to the serum uric acid quartile and gender. Error bars indicates 95% confidence intervals.
Discussion
In the present study, we showed that an elevated SUA level is associated with MS, but the association is dependent on waist circumference. Further, SUA is not associated with PWV in Korean adults.
In our study population, SUA levels were significantly and positively associated with waist circumference and triglycerides in both genders, and significantly and negatively associated with HDL-C in males. These relationships are generally similar to those reported in previous cross-sectional studies.1),3),4),6),7) The Pearson's correlation coefficient between the SUA level and waist circumference was the highest value in females and males, which is compatible with the finding that abdominal obesity is the main determinant of elevated SUA levels.4),20) The lack of a significant association between the SUA level, BP, and fasting glucose in our study population was consistent with the significant associations reported by other investigators.1),3),4),6),7) SUA levels were significantly correlated with these variables when the data of females and males were combined (data not shown).
As the number of subjects with four or five components of the MS was small, participants with three or more components were combined in the present study. We found a significant positive and linear relationship between SUA levels and the number of MS components in both genders. This result has also been demonstrated in other studies among Koreans1) and Chinese.6) However, gender-specific results were not reported in these studies.
Regarding the relationship between SUA levels and MS status, the present study clearly showed that a graded increase in the prevalence of MS with increasing SUA levels. This finding is in accordance with other cross-sectional studies among various ethnic groups.1-4) However, cross-sectional studies1-3) have not demonstrated whether the positive association between SUA and MS exists independently of the variables of MS. The multivariate ORs of model 2 in the present study indicate that the association between SUA and MS status is not independent, but dependent on waist circumference among the variables of MS. When we added triglycerides instead of waist circumference in model 2, the OR of the highest SUA quartile for MS was decreased, but the statistical significance was maintained in the whole participants (data not shown). Thus, this result suggests that hyperuricemia is closely related to abdominal obesity among the components of MS. In a study involving a Turkish population, Onat et al.4) showed that the ORs of the upper SUA tertile for MS were markedly decreased in females and males, but lost significance in males when waist circumference was added in multivariate logistic regression models. The presence of hyperuricemia should trigger a high level of clinical suspicion and investigation for the potential co-existence of MS as a more potentially life-threatening factor than hyperuricemia per se.2) The present and previous cross-sectional studies1-4) have limitations in elucidating of the significance of hyperuricemia in relation to MS. Two recent prospective studies21),22) are noteworthy. Hyperuricemia was shown to be an independent predictor of incident MS in MS-free Korean young male workers21) and US middle-aged females and males.22)
If an elevated SUA level is a risk factor for cardiovascular disease, a positive relationship between SUA levels and surrogate markers of atherosclerosis is expected. Among the surrogate markers of atherosclerosis, coronary artery calcifications,13),23) carotid plaque,3) carotid intima-media thickness14),24),25) have been shown to be associated with SUA levels. However, the associations have not always been significant after adjustment for other risk factors or according to MS status. Thus, it is still inconclusive whether SUA is an independent risk factor of atherosclerosis.
In the present study we determined the relationship between the SUA level and PWV as an index of arterial stiffness, which is also a surrogate marker of atherosclerosis.15),16) In contrary to the positive association between the SUA level and baPWV in Japanese individuals by Ishizaka et al.26) and Saijo et al.27) we found no association between the SUA level and baPWV or hfPWV. There are possible reasons why our results differed from that of these two studies.26),27) First, differences in the baseline characteristics of a study population may explain the disparate result. Of note, are potential effects of morbidity and medications on PWV. For example, PWV was increased in patients with diabetes28) and decreased by antihypertensive drugs in hypertensive patients.18) We excluded subjects with diabetes and those taking medications, such as antihypertensive or lipid-lowering drugs, whereas these subjects were included in the study by Ishizaka et al.26) Moreover, it was not reported whether the study participants were taking medications potentially affecting PWV in the study of Saijo et al.,27) except for the current use of hormone therapy for menopause. Age and systolic BP are the primary determinants of PWV.17) However, it is unknown whether the relative impact of the SUA level on PWV is changed by confounders, such as diabetes and antihypertensive medications, which result in a positive association between the SUA level and PWV. Second, SUA levels may not affect arterial stiffness in a population at low risk for cardiovascular disease. In the present study, the Framingham 10-year CHD risk reflects a low-risk cardio vascular profile for our study population. In fact, 84% of our study participants had a Framingham CHD risk <10% (data not shown).
Tomiyama et al.29) showed no independent relationship between the SUA level and baPWV in a healthy Japanese population. Cuspidi et al.30) showed that the SUA level was not associated with organ damage, including left ventricular hypertrophy, carotid alteration, or microalbuminuria, in a population of recently diagnosed uncomplicated hypertensives with a low prevalence of hyperuricemia. Although arterial stiffness was not measured in this study, the result could suggest that the SUA level is not associated with atherosclerosis in a population at low-risk for cardiovascular disease. Third, the association between the SUA level and PWV may depend on gender, MS, or MS components.
In the study of Ishizaka et al.,26) Pearson's correlation coefficient between the SUA level and baPWV was significant in females, but not in males. The SUA levels were associated with carotid plaque in males without MS3) and with carotid intima-media thickness in males without MS14) and in hypertensive patients.24) Although MS was associated with increased hfPWV or baPWV after adjustment for age and gender in the present study (data not shown), we found no significant correlations between SUA levels and hfPWV or baPWV when we divided our study participants according to MS status or each component of MS in females and males (data not shown). Thus, the third reason is not likely to explain our negative results. However, we cannot completely exclude the possibility that our negative results were due to an insufficient number of participants when we were grouping our study participants. Thus, community-based, large population studies may be necessary to determine whether SUA levels are associated with PWV independently of other confounding risk factors.
The strength of the present study was the relative homogeneity of the study subjects enhancing the internal validity of our findings by reducing confounding factors potentially affecting SUA and PWV. The limitations of the current study include a single center study, the number of study subjects, the lack of control of alcohol intake, and the cross-sectional nature of the study, thereby reducing the statistical power and precluding temporality and causal inference.
In conclusion, based on our findings, an elevated SUA level is associated with abdominal obesity among the MS components and is not associated with increased PWV in Korean at low-risk for cardiovascular disease.
References
- 1.Yoo TW, Sung KC, Shin HS, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J. 2005;69:928–933. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 2.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–447. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaka N, Ishizaka Y, Toda E, Nagai R, Yamakodo M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–1044. doi: 10.1161/01.ATV.0000161274.87407.26. [DOI] [PubMed] [Google Scholar]
- 4.Onat A, Uyarel H, Hergenc G, et al. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am J Hypertens. 2006;19:1055–1062. doi: 10.1016/j.amjhyper.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25:1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 6.Lin SD, Tsai DH, Hsu SR. Association between serum uric acid levels and components of the metabolic syndrome. J Chin Med Assoc. 2006;69:512–516. doi: 10.1016/S1726-4901(09)70320-X. [DOI] [PubMed] [Google Scholar]
- 7.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Ann Epidemiol. 1998;8:250–261. doi: 10.1016/s1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 9.Reimann M, Schutte AE, Malan L, Huisman HW, Malan NT. Hyperuricema is an independent risk factor for the metabolic syndrome in a sub-Saharan African population: a factor analysis. Atherosclerosis. 2008;197:638–645. doi: 10.1016/j.atherosclerosis.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson C, Lapidus L, Stendahl C, Waldenström J. Hyperuricemia and risk of cardiovascular disease and overall death: a 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand. 1988;224:549–555. [PubMed] [Google Scholar]
- 11.Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 12.Niskanen LK, Laaksonen DE, Nyyssonen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middles-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 13.Santos RD, Dasir K, Orakzai R, Meneghelo RS, Carvalho JA, Blumenthal RS. Relation of uric acid levels to presence of coronary artery calcium detected by electron beam tomography in men free of symptomatic myocardial ischemia with versus without the metabolic syndrome. Am J Cardiol. 2007;99:42–45. doi: 10.1016/j.amjcard.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto R, Tomita H, Oka Y, Ohtsuka N. Relationship between serum uric acid concentration, metabolic syndrome and carotid atherosclerosis. Intern Med. 2006;45:605–614. doi: 10.2169/internalmedicine.45.1661. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Kim D. The relation of pulse wave velocity with Framingham risk score and SCORE risk score. Korean Circ J. 2005;35:22–29. [Google Scholar]
- 16.Na SH, Kim YS, Bae JH, et al. Effects of physical activity and aerobic exercise capacity on aortic stiffness in patients with untreated hypertension. Korean Circ J. 2009;39:52–56. [Google Scholar]
- 17.Tanaka H, Munakawa M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- 18.Jung AD, Kim W, Park SH, et al. The effect of telmisartan on endothelial function and arterial stiffness in patients with essential hypertension. Korean Circ J. 2009;39:180–184. doi: 10.4070/kcj.2009.39.5.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Yamamoto T, Tsutsuni Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46:1162–1165. doi: 10.1016/s0026-0495(97)90210-9. [DOI] [PubMed] [Google Scholar]
- 21.Ryu S, Song J, Choi BY, et al. Incidence and risk factors for metabolic syndrome in Korean male workers, ages 30 to 39. Ann Epidemiol. 2007;17:245–252. doi: 10.1016/j.annepidem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–852. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF, 2nd, Kullo IJ. Associations of serum uric acid with markers of inflammation, metabolic syndrome and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Tavil Y, Kaya MG, Oktar SÖ, et al. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197:159–163. doi: 10.1016/j.atherosclerosis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Iribarren C, Folsom AR, Eckfeldt JH, McGovern PG, Nieto FJ. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC study. Ann Epidemiol. 1996;6:331–340. doi: 10.1016/s1047-2797(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 26.Ishizaka N, Ishizaka Y, Toda EI, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192:131–137. doi: 10.1016/j.atherosclerosis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Saijo Y, Utsugi M, Yoshioka E, et al. Relationships of C-reactive protein, uric acid, and glomerular filtration rate to arterial stiffness in Japanese subjects. J Hum Hypertens. 2005;19:907–913. doi: 10.1038/sj.jhh.1001913. [DOI] [PubMed] [Google Scholar]
- 28.Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52:448–452. doi: 10.2337/diabetes.52.2.448. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Yamashina A, Arai T, et al. Influence of age and gender on the results of noninvasive brachial-ankle pulse wave velocity measurement-a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 30.Cuspidi C, Valero C, Sala C, et al. Lack of association between serum uric acid and organ damage in a never-treated essential hypertensive population at low prevalence of hyperuricemia. Am J Hypertens. 2007;20:678–685. doi: 10.1016/j.amjhyper.2007.01.013. [DOI] [PubMed] [Google Scholar]