Abstract
Aging is associated with a decreased capacity for dentate gyrus (DG) granule cell depolarization as well as reduced perforant path activation. Although it is well established that the maintenance of DG long-term potentiation (LTP) over days is impaired in aged, as compared to young animals, the threshold for inducing this LTP has never been investigated in aged, awake animals. In addition, although exposure to novelty prior to θ-burst stimulation (TBS) increases both the induction and longevity of DG LTP in adult rats, the effects of exposure to novelty on LTP in aged rats have never been investigated. Here, we report that although TBS delivered in the home cage induces robust and long-lasting DG LTP in young rats, TBS fails to induce DG LTP in aged rats. Interestingly, delivery of TBS to aged rats exploring novel environments induces robust and long-lasting LTP, with the induction, but not the longevity, of this LTP being similar in magnitude to that observed in young rats delivered TBS in the home cage. These results indicate that although TBS-induced DG LTP is impaired in aged, as compared to young rats, TBS during exploration of novel environments is sufficient to rescue age-related deficits in DG LTP. We discuss these observations in the context of previous findings suggesting that the facilitation of LTP by exposure to novel environments results as a consequence of reduced network inhibition in the DG and we suggest that, in spite of age-related changes in the DG, this capacity persists in aged rats and represents a nondietary and nonpharmacological way to facilitate DG LTP during aging.
Keywords: aging, hippocampus, novel environment, synaptic plasticity, in vivo
Introduction
LTP is a persistent increase in synaptic strength observed after high-frequency stimulation (HFS) of afferent fibers (Bliss and Lomo, 1973). Much evidence indicates that LTP is one mechanism underlying memory processes (Morris et al., 1986; Barnes, 1995; Moser et al., 1998), and thus LTP is the most intensely studied cellular model of synaptic plasticity (Bliss and Collingridge, 1993). An interesting aspect of LTP is that, like memory, it is subject to modification by environmental influences (Izquierdo et al., 2001; Straube et al., 2003a; Davis et al., 2004).
The hippocampus may function as a novelty detection network (Lisman and Otmakhova, 2001) and numerous hippocampal models suggest that environmental novelty facilitates a state of hippocampal function thought ideal for the encoding of new information (Hasselmo et al., 1995, 2002; Lisman and Otmakhova, 2001; Meeter et al., 2004). Given that LTP is thought important for memory processes (Bliss and Collingridge, 1993; Barnes, 1995), the observation of increased LTP in the DG of adult rats as a consequence of exploration of novel environments (Davis et al., 2004; Sanberg et al., 2006) parallels previous observations that the DG is essential for new encoding (McNaughton and Morris, 1987; McNaughton et al., 1989). Indeed, the DG displays plastic changes in response to novel stimuli (Montag-Sallaz et al., 1999) and shows increased activation with new learning (Zeineh et al., 2003). Thus, exposure to novel environments may facilitate processes favoring hippocampal synaptic plasticity and new learning (Paulsen and Moser, 1998; Guzowski et al., 2004; Sanberg et al., 2006).
Recent studies indicate that, in adult rats, brief exposure to novelty can facilitate DG LTP (Straube et al., 2003a,b; Sanberg et al., 2006). In the DG, LTP can be either facilitated by brief novelty exploration around the time of tetanization (Straube et al., 2003a,b; Sanberg et al., 2006) or impaired by exploration of novelty subsequent to tetanization (Xu et al., 1998). Therefore, brief novelty exploration around the time of weak tetanization creates conditions that favor novelty-mediated facilitation of LTP (Davis et al., 2004; Sanberg et al., 2006).
Aging is associated with an increased threshold for LTP induction in the DG such that greater postsynaptic depolarization is required to induce LTP (Barnes et al., 2000). Thus far, the novelty-mediated enhancement of LTP has only been reported in adult animals and whether exposure to novelty can facilitate LTP in aged animals is currently unknown. In adult rats, novelty exploration causes a decrease in GABAergic inhibition in the DG (Wilson and McNaughton, 1993; Moser, 1995, 1996), a condition thought to favor LTP by increasing postsynaptic depolarization (Wigstrom and Gustaffson, 1983, 1985; Sanberg et al., 2006) and facilitates DG population spikes (Kitchigina et al., 1997). In addition, it is suggested that, in adult rats, novelty may facilitate DG LTP by inhibition of DG interneurons, thus creating a net effect of increased network excitability, which may serve to increase LTP (Sanberg et al., 2006). Aged rats display reduced cellular excitability in the DG as reflected by an increased threshold for LTP induction (Barnes et al., 2000) and thus experimental manipulations that reduce interneuron-mediated inhibition in the DG may be especially effective in facilitating DG LTP in aged rats. Therefore, we studied the effect of brief novelty exploration on DG LTP induction and maintenance in chronically implanted aged rats. To create optimal conditions for novelty-mediated facilitation of LTP, we used weak tetanization parameters to induce LTP around the time of novelty exploration. Some of these data were presented in abstract form (Sierra et al., 2003).
Materials and Methods
Subjects
The subjects were young (6 months) and aged (22–24 months) male Fischer 344 rats (NIA/Harlan, Indianapolis, IN). Rats were maintained on a 12:12 h light–dark cycle, housed individually, and had access to food and water ad libitum. All animals were allowed to acclimate for a 1-week period after arrival and handled daily after surgery until completion of recordings.
Surgical Procedure
For surgery, rats were anesthetized with sodium pentobarbital (Nembutal) (50 mg/kg intraperitoneally) and maintained at a surgical level of anesthesia with supplemental injections as needed. For each rat, body temperature was maintained at 37°C with a heating pad. Each rat's head was mounted into the frame of a stereotaxic apparatus, and a sterile stainless steel blade was used to make a vertical incision to expose the skull. Throughout the surgical procedures, gauze moistened with sterile saline was used to keep the tissue moist. After surgery, rats were removed from the stereotaxic apparatus, kept on a heating pad until recovery from the anesthesia, and given a subcutaneous injection of 150,000 U of penicillin G to prevent infections. Rats were given a solution of Tylenol and water (1%) ad libitum for 3 days after surgery. All experiments were conducted in accordance with National Institutes of Health guidelines for the care and use of animals in research and with approval from the UTSA Institutional Animal Care and Use Committee.
Electrophysiology
Bipolar electrodes were used to stimulate the medial perforant path in the angular bundle (AP -8.5, ML 4.4, DV 3.0 mm; Paxinos and Watson, 1994). We recorded extracellular excitatory postsynaptic potentials (EPSPs) referenced and grounded with screws mounted on the anterior and posterior portions of the skull, respectively. EPSPs were amplified (500×) with a Grass P511 A.C. preamplifier, filtered at 1 Hz to 3 kHz and stored for off-line analysis using commercially available software (Data-Wave Technologies, Thornton, CO). A digital stimulator (A-M Systems 2,100, Everett, WA) was used to deliver constant current stimulation. Stimulation (biphasic constant current pulses, 0.2 ms duration) was delivered with two twisted Teflon-coated stainless steel wires (0.005 inch diameter, A-M Systems). A single Teflon-coated stainless steel wire (0.005 inch diameter, A-M Systems) was used to record EPSPs from the DG (AP -3.5, ML 2.0, DV 3.0–3.5 mm). Final dorsal–ventral coordinates for stimulating and recording electrodes were determined by electrophysiological criteria that maximized the EPSP peak with the smallest amount of current delivery. We confirmed the accuracy of electrode placements by stereotaxic coordinates and electrophysiological criteria in all rats (McNaughton and Barnes, 1977). Electrodes were permanently implanted as previously described (Dieguez and Barea-Rodriguez, 2004). Briefly, electrodes were attached to gold Amphenol pins, mounted in 9-pin Malino/MacIntyre sockets (Ginder Scientific, Canada), and affixed to the skull with dental acrylic (Patterson Dental Supply, San Antonio, TX). All rats were allowed a minimum period of 1 week for recovery from surgery before electrophysiological recordings.
Experimental
Four groups of rats were used in this study: young rats delivered TBS in the home cage (n = 4), aged rats delivered TBS in the home cage (n = 6), aged rats delivered TBS in a novel environment (n = 5), and aged rats delivered low-frequency stimulation (LFS) in a novel environment (n = 3). At the beginning of each experiment, maximal responses for each rat were evoked by stimulating with up to 379 μA of current intensity (biphasic pulses, 0.2 ms duration). For each rat, two input–output curves were collected by averaging 10 responses at each of 10 current intensities evoked at 120% increments of the minimal intensity required to elicit a stable, recognizable EPSP. Baseline responses were evoked at 0.05 Hz with the average current intensity that elicited a response magnitude 50% of maximum slope as determined by the input–output curves. Measurements of DG responses were restricted to the initial slope (dV/dt) of field EPSPs measured between 1 and 3 ms subsequent to response onset. Ten responses were evoked at 20-s intervals at a fixed time of day for each rat (during the middle of the light cycle). Daily responses were collected for a minimum of 10 min on each of 3 consecutive days before TBS. TBS was delivered to experimental rats in the novel cage but was delivered to control rats while in their home cage.
The novel cage (47 × 25 × 20 cm) was identical to the home cage with the exception of various novel objects being permanently mounted to the cage floor. Novel objects were small metal or plastic toys. On the day of LTP induction, baseline responses were collected in the animal's home cage for 10 min before novelty exposure. After baseline recordings in the home cage, experimental rats were placed in the novel cage and allowed to explore freely for 5 min, at which point 3 θ-burst trains were delivered at 5-min intervals. After spending 15 min in the novel cage, experimental rats were returned to their home cage. After each use of the novel cage, the cage and toys were wiped clean with soapy water and bedding was replaced. To control for possible handling effects, control rats were handled briefly in the home cage immediately before and after TBS. TBS consisted of three sets of 10 pulses delivered at 400 Hz, with an interpulse interval of 2.5 ms and pulse duration of 0.2 ms. Each of the three trains had a duration of 25 ms, with a 200-ms intertrain interval and a 5-min interburst interval. None of the animals displayed afterdischarges subsequent to TBS.
Because stimulation with a current intensity of about 75% of maximum effectively induces perforant path-DG LTP (McNaughton et al., 1978), we stimulated with a current intensity of 50% during TBS to ascertain whether novelty could facilitate LTP that would otherwise be unlikely to occur. Using a 50% of maximum current intensity, low-frequency (0.05 Hz) responses were recorded for an additional hour. In addition, daily responses were then collected for each rat until LTP decayed to baseline. Changes in synaptic responses are presented as the percent change from the average of baseline responses collected over the 3-day period before TBS. Post-TBS slopes showing an increase of at least 20% above that of baseline slopes were considered LTP. LTP was considered decayed when daily responses did not differ significantly from the average baseline response.
LTP induction and maintenance data were analyzed by two-way repeated measure ANOVAs, followed by Tukey post hoc tests. To assess LTP longevity, the daily magnitude of responses was compared to baseline values. A significance level of 0.05 was chosen for all analyses.
Results
LTP Induction
With TBS in the home cage, young rats showed significant DG LTP induction whereas aged rats did not ([F (1,8)] = 59.067, P < 0.001). However, this failure of DG LTP induction in aged rats was rescued by delivery of TBS while aged rats explored novel environments, such that LTP in young rats acquired in the home cage was indistinguishable from that acquired by aged rats in the novel cage ([F (1,7)] = 0.700, P > 0.05, n.s.). In addition, TBS delivered to aged rats during exploration of the novel environment significantly induced DG LTP, whereas this stimulation failed to change synaptic efficacy in aged rats in the home cage ([F (1,9)] = 24.177, P < 0.001) (Fig. 1A). Young rats in the home cage showed a mean increase in responses of 123 ± 3%, aged rats in the novel environment showed a mean increase in responses of 121 ± 2%, and aged rats in the home cage showed a mean increase in responses of 99 ± 3%. These trends are evident in the depicted waveforms (Fig. 1B).
FIGURE 1.
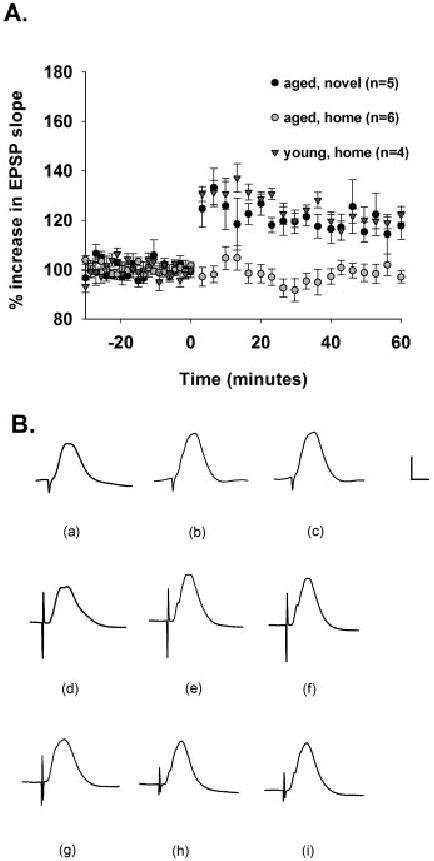
Aging impairs TBS-induced DG LTP in the home cage, but TBS delivery during exploration of novel environments facilitates DG LTP in aged rats. A: Medial perforant path-DG LTP induction as a consequence of TBS in the home cage in young rats (black triangles, n = 4), in the home cage in aged rats (gray circles, n = 6), and in a novel environment in aged rats (black circles, n = 5). After a 30-min baseline, TBS was delivered at time 0, and responses were recorded for an additional 1 h. TBS induced DG LTP to comparable levels in both young rats delivered TBS in the home cage and aged rats delivered TBS in the novel environment (P > 0.05, n.s.). In addition, young rats delivered TBS in the home cage showed DG LTP, whereas aged rats delivered TBS in the home cage failed to acquire LTP (P < 0.001). TBS delivered to aged rats in the home cage failed to induce DG LTP, but TBS delivered to aged rats exploring novel environments induced DG LTP (P < 0.001). B: Depicted are waveforms (a) during baseline, (b) 30 min into the post-TBS recording period, and (c) 60 min into the post-TBS recording period for a young rat delivered TBS in the home cage. Also shown are waveforms (d) during baseline, (e) 30 min into the post-TBS recording period, and (f) 60 min into the post-TBS recording period for an aged rat delivered TBS in a novel environment. Depicted are also waveforms (g) during baseline, (h) 30 min into the post-TBS recording period, and (i) 60 min into the post-TBS recording period for an aged rat delivered TBS in the home cage. Calibration bar: 2 mV, 5 ms.
LTP Maintenance
Over a 7-day recording period, young rats delivered TBS in the home cage showed long-lasting LTP while responses recorded in aged rats delivered TBS in the home cage failed to show potentiation over days ([F (1,8)] = 54.728, P < 0.01). Post hoc analyses showed that, on days 1–4 of recording, responses were significantly greater in young rats delivered TBS in the home cage as compared to that observed in aged rats delivered TBS in the home cage (*Tukey Test, P < 0.05). Responses recorded over a 7-day recording period were significantly different between young rats delivered TBS in the home cage and aged rats delivered TBS in the novel environment ([F (1,7)] = 7.264, P < 0.04). Post hoc analyses showed that, on days 1, 2, and 4 of recording, responses were significantly greater in young rats delivered TBS in the home cage as compared to those observed in aged rats delivered TBS in the novel environment (†Tukey Test, P < 0.05). In addition, responses recorded over a 7-day recording period were significantly different between aged rats delivered TBS in the home cage and those delivered TBS in the novel environment ([F (1,9)] = 42.646, P < 0.001). Post hoc analyses showed that, on days 1–3, responses were significantly greater in aged rats delivered TBS in the novel environment than in those delivered TBS in the home cage (§Tukey Test, P < 0.05) (Fig. 2A). These trends are evident in the depicted waveforms (Fig. 2B).
FIGURE 2.
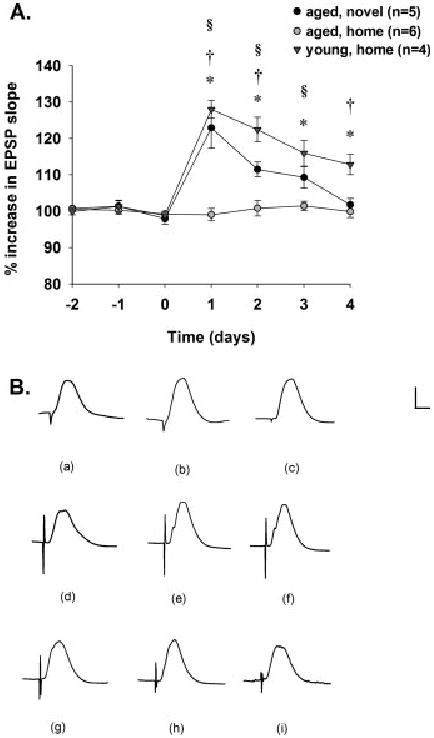
Delivery of TBS to aged rats induces long-lasting DG LTP in aged rats tetanized during exploration of novel, but not home, environments. A: Medial perforant path-DG LTP maintenance as a consequence of TBS in the home cage in young rats (black triangles, n = 4), in the home cage in aged rats (gray circles, n = 6), and in a novel environment in aged rats (black circles, n = 5). After a 30-min baseline, TBS was delivered at time 0, and responses were recorded for an additional 4 days. TBS-induced DG LTP was more enduring in young rats delivered TBS in the home cage as compared to that observed in aged rats delivered TBS in the novel environment (P < 0.04). Post hoc tests showed differences between these groups on days 1, 2, and 4 (†Tukey test, P < 0.05). In addition, young rats delivered TBS in the home cage showed long-lasting DG LTP, whereas aged rats delivered TBS in the home cage failed to show LTP over days (P < 0.01). Post hoc tests showed differences between these groups on days 1–4 (*Tukey test, P < 0.05). Furthermore, TBS delivered to aged rats in the novel environment induced DG LTP that was greater in magnitude than responses recorded from aged rats delivered TBS in the home cage (P < 0.01). Post hoc tests revealed differences between these groups on days 1–3 (§Tukey test, P < 0.05). B: Depicted are waveforms (a) during baseline, (b) 2 days into the post-TBS recording period, and (c) 4 days into the post-TBS recording period for a young rat delivered TBS in the home cage. Also shown are waveforms (d) during baseline, (e) 2 days into the post-TBS recording period, and (f) 4 days into the post-TBS recording period for an aged rat delivered TBS in a novel environment. Depicted are also waveforms (g) during baseline, (h) 2 days into the post-TBS recording period, and (i) 4 days into the post-TBS recording period for an aged rat delivered TBS in the home cage. Calibration bar: 2 mV, 5 ms.
Low-Frequency Stimulation
To address whether general changes in activity during exploration of novel environments contributed to the novelty-mediated enhancement of LTP in aged rats, we recorded DG responses utilizing low-frequency (0.05 Hz) stimulation during novelty exploration. LFS did not cause a change in DG EPSPs of aged rats, neither immediately after novelty exploration ([F (2, 38)] = 1.118, P > 0.05, n.s.) (Fig. 3A) nor on any of several days afterwards ([F (2,6)] = 0.472, P > 0.05, n.s.) (Fig. 3B).
FIGURE 3.
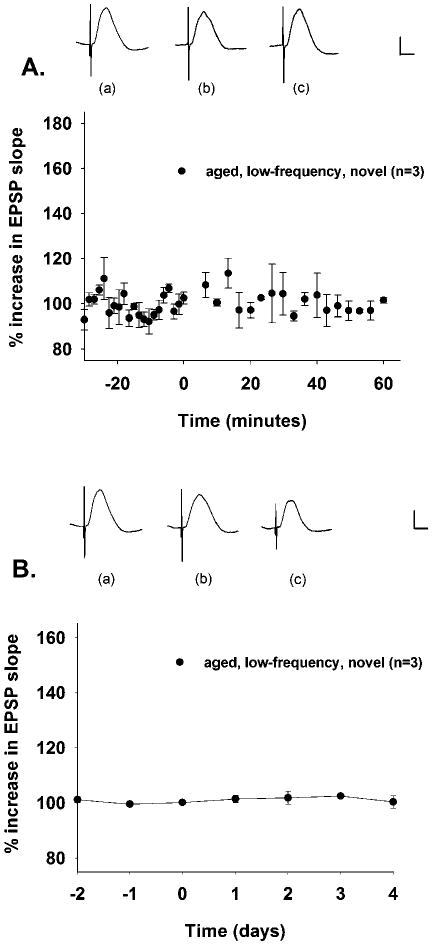
LFS delivered to aged rats briefly exploring novel environments fails to cause changes in DG responses over minutes or days. A: LFS delivered to aged rats briefly exploring novel environments does not cause changes in DG responses recorded for 60 min after the postbaseline recording period (P > 0.05, n.s.). Rats were transferred to novel environments at time 0 for 15 min and were subsequently returned to the home cage, where DG responses were recorded for 1 additional hour. Depicted are waveforms from an aged rat (a) during baseline, (b) 30 min into the postnovelty exposure home cage recording period, and (c) 60 min into the postnovelty exposure home cage recording period. B: LFS delivered to aged rats briefly exploring novel environments does not cause changes in DG responses recorded for 4 days after the postbaseline recording period (P > 0.05, n.s.). Rats were transferred to novel environments at time 0 for 15 min and were subsequently returned to the home cage, where DG responses were recorded for an additional 4 days. Depicted are waveforms from an aged rat (a) during baseline, (b) 2 days into the postnovelty exposure home cage recording period, and (c) 4 days into the postnovelty exposure home cage recording period. Calibration bar: 1 mV, 5 ms.
Input–Output Curves
Input–output EPSP slopes were not statistically different for the following groups: aged home, aged novel, and aged LFS (Tukey Test, P > 0.05, n.s.). However, the input–output EPSP slopes for the young home group were significantly increased as compared to those of the aged home, aged novel, and aged LFS groups ([F (3,9)] = 3.631, P < 0.001) (Fig. 4). Specifically, post hoc analyses revealed that EPSP slopes for the young home group were significantly elevated as compared to that of all other groups at input–output current intensity levels 4–10 (*P < 0.05, Tukey Test).
FIGURE 4.
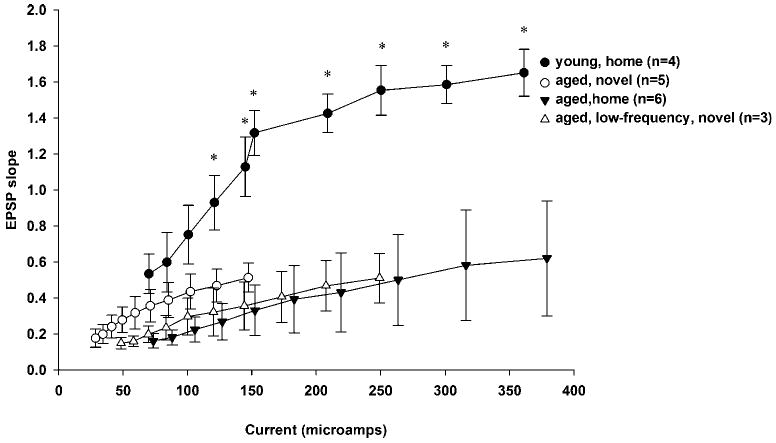
Input–output curves for all groups of rats utilized in this study. Input–output EPSP slopes were not statistically different for the following groups: aged home, aged novel, and aged LFS (Tukey Test, P > 0.05, n.s.). However, the input–output EPSP slopes for the adult home group were significantly increased as compared to those of the aged home, aged novel, and aged LFS groups ([F (3,9)] = 3.631, P < 0.001). Specifically, post hoc analyses revealed that EPSP slopes for the young home group were significantly elevated as compared to those observed in all other groups at input–output current intensity levels 4–10 (*P < 0.05, Tukey test).
Discussion
The current data demonstrates that aged, as compared to young, rats are impaired in their ability to acquire DG LTP as a consequence of perithreshold stimulation (i.e., TBS) in the home cage. Remarkably, brief exposure to novel environments facilitates TBS-induced DG LTP in aged rats as compared to that observed in aged rats delivered TBS in the home cage. Although delivery of TBS to aged rats exploring novel environments induces DG LTP to a degree that is similar to that observed in young rats delivered TBS in the home cage, the maintenance of this LTP over days is still impaired in aged (delivered TBS during novelty exploration), as compared to young (delivered TBS in the home cage), rats. This observation is consistent with previously reported age-related deficits in the maintenance of hippocampal LTP over days (Barnes, 1979; Dieguez and Barea-Rodriguez, 2004). Interestingly, DG LTP observed in aged rats delivered TBS during exploration of novel environments is still elevated for several days as compared to that observed in aged rats delivered TBS in the home cage. These observations suggest that TBS during exploration of novel environments can overcome the age-related decrease in DG excitability (Barnes et al., 2000) and/or the age-related deficit in perforant path activation (Foster et al., 1991) to facilitate LTP induction and can even augment LTP maintenance to some degree. Previous reports indicate that novelty facilitation of adult DG LTP lasts for about 14 days (Davis et al., 2004; Sanberg et al., 2006), whereas our current data demonstrate that novelty facilitates DG LTP for 4 days in aged rats. A comparison of the effect of novelty on DG LTP between young adult and aged rats is beyond the scope of this report. However, it may be inferred that novelty facilitation of DG LTP induced by TBS is greater in adult than in aged rats.
To our knowledge, this is the first report of a nondietary, nonpharmacological environmental manipulation that facilitates hippocampal LTP in aged animals. A previous study reported normal TBS-mediated LTP in the DG of aged rats (Maroun and Richter-Levin, 2002), but the current intensity was increased to 70–90% of maximum during tetanization, which had the net effect of increasing the number of afferent fibers stimulated during tetanus and thereby increasing the likelihood of obtaining LTP (McNaughton et al., 1978). In our study, aged rats failed to show DG LTP when TBS was delivered with a 50% of maximum current intensity, but, under the same conditions, DG LTP was observed in aged rats exposed briefly to novelty during TBS. Thus, a TBS paradigm, which is normally ineffective in inducing DG LTP in aged rats, is able to induce DG LTP when aged rats explore novel environments during delivery of TBS. Thus, as previously reported in adult rats (Sanberg et al., 2006), novelty exploration may serve to lower the threshold for DG LTP in aged rats as well. In addition, these data are consistent with a recent report that θ-contingent training of aged animals on an eyeblink conditioning task improves performance of aged animals, suggesting that θ-patterned neuronal activity can be used to improve synaptic plasticity during aging (Asaka et al., 2005). Thus, it appears that θ-patterned stimulation during brief exposure to novel environments is sufficient to ameliorate age-related deficits in DG LTP.
Sanberg et al. (2006) recently reported that, in adult rats, novelty-mediated enhancement of DG LTP is independent of changes in general activity during novelty exploration. In addition, our data show that the novelty-mediated enhancement of DG LTP in aged rats is not merely a consequence of changes in exploration during novelty exposure because, in our studies, aged rats exposed to novelty but given only LFS show no changes in responses either immediately after novelty exploration or for several days afterward, a finding which is also suggestive of the stability of our recordings over time. In addition, aged Fischer 344 rats do not show changes in any of several parameters of activity between home and novel environments, nor do they show changes in rearing activity during exploration of novel environments as compared to that observed in young rats (Casadesus et al., 2001), which is a primary response to novelty exploration in adult rats (Rosenthal et al., 1989). Thus, the novelty-mediated enhancement of DG LTP in aged rats may occur as a consequence of novelty-mediated processes that may directly facilitate hippocampal synaptic plasticity. Together, these observations indicate that the magnitude of evoked hippocampal EPSPs in aged rats is unaffected by LFS in either home (Dieguez and Barea-Rodriguez, 2004) or novel cages (current data) and suggests, rather, that HFS during exploration of novel environments facilitates the induction and maintenance of synaptic potentiation.
It is suggested that the novelty-mediated enhancement of DG LTP in adult rats may occur as a consequence of novelty-mediated inhibition of DG interneurons (Sanberg et al., 2006), thereby creating the net effect of increased cellular excitability (Wilson and McNaughton, 1993). Consistent with this hypothesis, in adult rats, increases in DG population spikes can be elicited by novelty exploration (Kitchigina et al., 1997) or by local application of a GABA-A receptor antagonist in the DG (Maroun and Richter-Levin, 2002). Specifically, novelty may facilitate DG LTP in young adult rats via inhibition of hilar perforant path-associated (HIPP) interneurons, which terminate in the molecular layer of the DG where the perforant path synapses onto DG principal cell dendrites (Freund and Buzsaki, 1996). This mechanism may also be operating in the DG of aged rats to facilitate DG LTP during novelty exploration. Interestingly, aging is associated with a loss of HIPP interneurons (Vela et al., 2003), an effect which may facilitate DG LTP, especially during exploration of novel environments, because these cells are inhibited in the DG of adult rats during exploration of novel environments (Wilson and McNaughton, 1993) to facilitate DG LTP in novel contexts (Sanberg et al., 2006). In aged animals, enhancement of DG LTP by novelty exposure may be augmented, because there are fewer HIPP cells to begin with and thus inhibition of fewer HIPP cells is required to elicit a novelty-mediated facilitation of LTP. However, in response to the reduced GABAergic inputs to the molecular layer of the DG of aged rats, GABA-A receptors in this region show an upregulation of α1 GABA-A receptor subunits (Gutierrez et al., 1996) and display increased sensitivity (Ruano et al., 1995), both of which constitute an adaptive response of DG principal cells to maintain network properties (Vela et al., 2003). In spite of this compensation, which might serve to reduce novelty-mediated facilitation of DG LTP, brief exposure to novelty nonetheless appears sufficient to facilitate DG LTP in aged rats, presumably via novelty-mediated disinhibition in the DG as observed in adult rats (Wilson and McNaughton, 1993).
It should be noted, however, that we cannot rule out the possibility that the novelty-mediated enhancement of DG LTP in aged rats may occur via other additional mechanisms or by unique mechanisms as compared to those operating in adult rats. It is reported that, in young adult rats, the novelty-mediated enhancement of DG responsiveness or LTP depends on adrenergic (Kitchigina et al., 1997; Straube et al., 2003a) dopaminergic (Li et al., 2003), and serotonergic (Richter-Levin and Segal, 1990; Levkovitz and Segal, 1997; Sanberg et al., 2006), but not cholinergic (Li et al., 2003; Davis et al., 2004), neurotransmission. In addition, although corticosterone can facilitate primed burst potentiation (Diamond et al., 1992), exploration of a novel environment does not significantly increase blood corticosterone concentration as compared with exploration of a familiar environment (Xu et al., 1998; Straube et al., 2003b) and exposure to a novel environment reverses stress-related effects on hippocampal synaptic plasticity (Yang et al., 2006), suggesting that stress hormones and exploration of a novel environment do not share a common mechanism but rather have unique effects on LTP. It is possible that exploration of novel environments during aging may facilitate DG LTP by increasing dopaminergic, cholinergic, or adrenergic neurotransmission. However, all these systems are negatively affected during aging (Luine et al., 1990; Chouinard et al., 1995; Stemmelin et al., 2000) and the facilitation of LTP by dopaminergic (Bach et al., 1999), cholinergic (Frey et al., 2001; Bergado and Almaguer, 2002), and adrenergic (Seidenbecher et al., 1997; Bergado and Almaguer, 2002) inputs are impaired during aging and thus are unlikely to facilitate DG LTP during exploration of novel environments. Further studies will be required to definitively identify the neuromodulators involved in the novelty-mediated enhancement of DG LTP during aging.
Besides exposure to novelty, a number of studies report pharmacological enhancement of hippocampal synaptic plasticity during aging. Age-related deficits in hippocampal LTP can be attenuated by the application of (Watson et al., 2006) or dietary supplementation with (Murray and Lynch, 1998; McGahon et al., 1999) antioxidants. In addition, calcium chelation (Tonkikh et al., 2006) or pharmacological blockade of L-type calcium channels (Norris et al., 1998; Yu et al., 2003) improves hippocampal synaptic plasticity during aging. Lastly, pharmacological supplementation with nerve growth factor improves hippocampal LTP in aged, cognitively impaired rats (Bergado et al., 1997).
The age-related reduction in basal DG synaptic transmission, as revealed by deficits in input–output functions in the aged groups before our LTP experiments, is a manifestation of the age-related decrease in the number of perforant path afferents to the DG (Geinisman et al., 1992) and is supported by previous electrophysiological studies (Barnes and McNaughton, 1980). In our view, the observation of age-related deficits in basal DG synaptic transmission, as indicated by input–output functions, does not compromise our finding of enhanced DG LTP by novelty in aged rats. In fact, our view is that the finding of enhanced DG LTP by novelty in aged rats is made even more important by the presence of age-related deficits in basal DG synaptic transmission. Interestingly, our data suggest that although the aged DG shows deficits in basal synaptic transmission, it still possesses the ability to show enhanced synaptic plasticity, as revealed by LTP, in response to brief exposure to novel environments. It should be noted here that basal synaptic transmission and LTP are unique, dissociable phenomena, and a deficit in one does not necessarily imply a deficit in the other. For example, Foster et al. (1996) showed that enriched environments can increase basal CA1 synaptic transmission without increasing CA1 LTP (actually, CA1 LTP was decreased by enriched environments). In addition, Dieguez and Barea-Rodriguez (2004) showed that the age-related deficits in CA3 LTP occur in aged rats without an accompanying decrease in basal input–output functions (note, however, that this study did not include an exhaustive investigation of basal synaptic transmission).
Exploration of novel environments may represent an ideal condition under which to reveal increases in DG plasticity mediated by network disinhibition. We thus extend previous findings that novelty facilitates DG LTP in adult rats (Sanberg et al., 2006) and now indicate that aged animals are capable of showing novelty-mediated facilitation of DG LTP. Thus, brief novelty exposure appears sufficient to facilitate DG LTP in aged animals and represents a nondietary, nonpharmacological environmental manipulation that facilitates hippocampal synaptic plasticity during aging.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: GM07717, T32-MH65728 and G12RR1346-02-RCMI NCRR.
References
- Asaka Y, Mauldin KN, Griffin AL, Seager MA, Shurell E, Berry SD. Nonpharmacological amelioration of age-related learning deficits: The impact of hippocampal theta-triggered training. Proc Natl Acad Sci USA. 2005;102:13284–13288. doi: 10.1073/pnas.0506515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related deficits in spatial memory are correlated with deficits in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Involvement of LTP in memory: Are we “searching under the street light”? Neuron. 1995;15:751–754. doi: 10.1016/0896-6273(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal grannule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path–granule cell synapse. Neurobiol Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Almaguer W. Aging and synaptic plasticity: A review. Neural Plast. 2002;9:217–232. doi: 10.1155/NP.2002.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Fernandez CL, Gomez-Soria A, Gonzalez O. Chronic intraventricular infusion with NGF improves LTP in old cognitively-impaired rats. Brain Res. 1997;770:1–9. doi: 10.1016/s0006-8993(97)00610-0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus G, Shukitt-Hale B, Joseph JA. Automated measurement of age-related changes in the locomotor response to environmental novelty and home-cage activity. Mech Ageing Dev. 2001;122:1887–1897. doi: 10.1016/s0047-6374(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Chouinard ML, Gallagher M, Yasuda RP, Wolfe BB, McKinney M. Hippocampal muscarinic receptor function in spatial learning-impaired aged rats. Neurobiol Aging. 1995;16:955–963. doi: 10.1016/0197-4580(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose G. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Dieguez D, Jr, Barea-Rodriguez EJ. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52:53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: Relation to long-term potentiation. Brain Res. 1996;736:243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, De Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, Ruano D, Miralles CP, Victoria J, Deblas AL. Aging-related subunit expression changes of the GABAA receptor in the rat hippocampus. Neuroscience. 1996;74:341–348. doi: 10.1016/0306-4522(96)00137-6. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Viola H, Barros DM, Alonso M, Vianna MR, Furman M, Levi de Stein M, Szapiro G, Rodrigues C, Choi H, Medina JH, Izquierdo I. Novelty enhances retrieval: Molecular mechanisms involved in rat hippocampus. Eur J Neurosci. 2001;13:1464–1467. doi: 10.1046/j.0953-816x.2001.01530.x. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Segal M. Serotonin 5HT1A receptors modulate hippocampal reactivity to afferent stimulation. J Neurosci. 1997;17:5591–5598. doi: 10.1523/JNEUROSCI.17-14-05591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: Contributions of monoaminergic systems. Brain Res. 1990;537:271–278. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Local circuit plasticity in the rat dentate gyrus: characterization and aging-related impairment. Neuroscience. 2002;112:1001–1007. doi: 10.1016/s0306-4522(02)00045-3. [DOI] [PubMed] [Google Scholar]
- McGahon BM, Murray CA, Horrobin DF, Lynch MA. Age-related changes in oxidative mechanisms and LTP are reversed by dietary manipulation. Neurobiol Aging. 1999;20:643–653. doi: 10.1016/s0197-4580(99)00027-5. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: Cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- Meeter M, Murre JM, Talamini LM. Mode shifting between storage and recall based on novelty detection in oscillating hippocampal circuits. Hippocampus. 2004;14:722–741. doi: 10.1002/hipo.10214. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 1999;38:234–246. [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser EI. Learning-related changes in hippocampal field potentials. Behav Brain Res. 1995;71:11–18. doi: 10.1016/0166-4328(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Moser EI. Altered inhibition of dentate granule cells during spatial learning in an exploration task. J Neurosci. 1996;16:1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long-term potentiation in dentate gyrus. J Biol Chem. 1998;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca+2 channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O, Moser E. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Sterotaxic atlas of the rat brain, 2nd ed, plate 50. New York: Academic Press; 1994. [Google Scholar]
- Richter-Levin G, Segal M. Effects of serotonin releasers on dentate granule cell excitability in the rat. Exp Brain Res. 1990;82:199–207. doi: 10.1007/BF00230852. [DOI] [PubMed] [Google Scholar]
- Rosenthal MJ, Varela M, Garcia A, Britton DR. Age-related changes in the motor response to environmental novelty in the rat. Exp Gerontol. 1989;24:149–157. doi: 10.1016/0531-5565(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Ruano D, Benavides J, Machado A, Victoria J. Aging-associated changes in the pharmacological properties of the benzodiazepine (w) receptor isotypes in the rat hippocampus. J Neurochem. 1995;64:867–873. doi: 10.1046/j.1471-4159.1995.64020867.x. [DOI] [PubMed] [Google Scholar]
- Sanberg CD, Jones FL, Do VH, Dieguez D, Jr, Derrick BE. 5-HT1a receptor antagonists block perforant path-dentate LTP induced in novel, but not familiar, environments. Learn Mem. 2006;13:52–62. doi: 10.1101/lm.126306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci USA. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra D, Dieguez D, Jr, Barea-Rodriguez EJ. Brief novelty exposure facilitates dentate gyrus LTP in aged rats. Soc Neurosci Abst 56.4. 2003 doi: 10.1002/hipo.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Lazarus C, Cassel S, Kelche C, Cassel JC. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neuroscience. 2000;96:275–289. doi: 10.1016/s0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol (London) 2003a;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Korz V, Frey J. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 2003b;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Tonkikh A, Janus C, El-Beheiry H, Pennefather PS, Samoilova M, McDonald P, Ouanounou A, Carlen PL. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol. 2006;197:291–300. doi: 10.1016/j.expneurol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Victoria J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem. 2003;85:368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- Watson JB, Arnold MM, Ho YS, O'Dell TJ. Age-dependent modulation of hippocampal long-term potentiation by antioxidant enzymes. J Neurosci Res. 2006;84:1564–1574. doi: 10.1002/jnr.21040. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 1983;275:153–158. doi: 10.1016/0006-8993(83)90428-6. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustaffson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Novelty exploration elicits a reversal of acute stress-induced modulation of hippocampal synaptic plasticity in the rat. J Physiol. 2006;577:601–615. doi: 10.1113/jphysiol.2006.120386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IT, Kim JS, Lee SH, Lee YS, Son H. Chronic lithium treatment enhances hippocampal long-term potentiation, but not neurogenesis, in the aged rat dentate gyrus. Biochem Biophys Res Commun. 2003;303:1193–1198. doi: 10.1016/s0006-291x(03)00494-7. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]


