Abstract
Background. Type of health insurance is an important mediator of medical outcomes in the United States. Medicaid, a jointly sponsored Federal/State programme, is designed to serve medically needy individuals. How these patients differ from non-Medicaid-enrolled incident dialysis patients and how these differences have changed over time have not been systematically examined.
Methods. Using data from the United States Renal Data System, we identified individuals initiating dialysis from 1995 to 2004 and categorized their health insurance status. Longitudinal trends in demographic, risk behaviour, functional, comorbidity, laboratory and dialysis modality factors, as reported on the Medical Evidence Form (CMS-2728), were examined in all insurance groups. Polychotomous logistic regression was used to estimate adjusted generalized ratios (AGRs) for these factors by insurance status, with Medicaid as the referent insurance group.
Results. Overall, males constitute a growing percentage of both Medicaid and non-Medicaid patients, but in contrast to other insurance groups, Medicaid has a higher proportion of females. Non-Caucasians also constitute a higher proportion of Medicaid patients than non-Medicaid patients. Body mass index increased in all groups over time, and all groups witnessed a significant decrease in initiation on peritoneal dialysis. Polychotomous regression showed generally lower AGRs for minorities, risk behaviours and functional status, and higher AGRs for males, employment and self-care dialysis, for non-Medicaid insurance relative to Medicaid.
Conclusions. While many broad parallel trends are evident in both Medicaid and non-Medicaid incident dialysis patients, many important differences between these groups exist. These findings could have important implications for policy planners, providers and payers.
Keywords: demographics, dialysis, end-stage renal disease, insurance, Medicaid
Introduction
Health insurance has been shown to be an important mediator of medical outcomes across a broad range of disease states and clinical scenarios [1–4]. In the setting of end-stage renal disease (ESRD), type of health insurance has been associated with timing of [5] and initial laboratory values at [6] dialysis initiation, self-reported medication adherence [7] and accessibility to kidney transplantation [8]. Type of health insurance and extent of insurance coverage may, therefore, be important mediators of outcomes in the ESRD population.
Since 1972, nearly all adults with ESRD have been entitled to Medicare coverage regardless of age [9]. While not all individuals receiving chronic dialysis are Medicare enrollees, the majority are. In addition to Medicare, typical sources of coverage for healthcare expenses include private insurance carriers, the Department of Veterans Administration (DVA), Medicaid, or out-of-pocket expenditures by the patient. Of these, Medicaid is the second largest payer group for ESRD. Medicaid, a public insurer funded jointly by federal and state governments, provides broad medical care benefits including prescription drugs for low-income or medically needy patients, although the specifics of plan benefits vary by state. As such, Medicaid stands apart from most other health insurance programmes with higher enrolments of minorities and women.
In ESRD, ∼22% of incident patients have Medicaid coverage, although many dialysis patients later attain Medicaid coverage, and prevalence rates rise to ∼32% as dialysis patients become impoverished due to high medical care costs [10]. Since Medicaid-enrolled incident dialysis patients are likely to be more financially and medically needy relative to non-Medicaid-enrolled incident dialysis patients, demographic characteristics, comorbidities and type of dialysis modality initiated could be hypothesized to be dissimilar between these groups. However, no one, to our knowledge, has systematically explored differences between groups of dialysis patients based on insurance status or how such differences have changed over time.
Knowledge of trends in Medicaid-enrolled incident dialysis patients and of the factors associated with Medicaid enrolment at dialysis initiation could have important implications for policy planners, providers and payers. Accordingly, we designed a study to investigate whether there are distinctions in demographic characteristics, medical characteristics and incident dialysis modalities differences between individuals with various types of insurance. The insurance category was classified as one of six core groups: Medicaid (with or without Medicare), Medicare only, Medicare plus another type of (non-Medicaid) insurance, DVA, private insurance or ‘other’ (a group consisting of uninsured individuals, those without a specified insurance carrier or those with multiple payer sources not already classified). Our overall goal was to determine whether and how individuals with Medicaid, potentially the most medically needy subgroup of patients, are dissimilar to the incident dialysis population without Medicaid, and how such differences have changed longitudinally from 1995 to 2004.
Subjects and methods
Data sources for analysis
To examine factors associated with health insurance payer status access for newly initiating chronic dialysis patients over time, we used data obtained from the United States Renal Data System (USRDS) [11]. The USRDS, established in 1988 under a contract with the Health Care Financing Administration (now known as the Centers for Medicare and Medicaid Services, or CMS), tracks nearly all dialysis patients from initiation of dialysis through transplantation or death.
We used the USRDS core compact disc (CD) data to capture patients who initiated dialysis during each year from 1995 to 2004 (10 years total). Data contained in the core CD data are generated upon initiation of dialysis, when providers are required to submit to CMS a Medical Evidence Form (CMS-2728) documenting patient demographic characteristics, comorbid conditions, laboratory values prior to the first dialysis treatment, date of dialysis initiation and dialysis modality and setting. CMS forwards the information to the USRDS Coordinating Center, currently located in Minneapolis, MN, USA. Over time, the changes in patient residence, payer status, treatment history (including dialysis modality), transplant information and death were submitted to CMS and subsequently incorporated into the core USRDS CD.
Study cohort and rationale for analytic approach
We identified unique individuals over the age of 18 years who were initiating dialysis without a prior transplant from 1 January 1995 through 31 December 2004. Health insurer coverage was determined for each subject at dialysis initiation. Coverage was categorized into one of six groups: Medicaid (including dually-eligible individuals, or persons also eligible for Medicare), Medicare only, Medicare combined with another (non-Medicaid) insurance group, DVA, private coverage and ‘other’; the latter group included uninsured persons, persons with combinations of payers not otherwise categorized as well as persons without a specified carrier. We compared patients across their demographic, risk behaviour, functional, comorbidity, laboratory and dialysis modality status, as recorded on the baseline CMS-2728 form, stratified by their baseline health insurer.
Demographic variables considered were age, sex and race by ethnicity (four mutually exclusive groups consisting of non-Hispanic Caucasian, non-Hispanic African American, Hispanic and other) and employment (unemployed versus part or fully employed). Risk behaviour factors examined were smoking and substance abuse (alcohol or illicit drugs), and functional status markers were inability to ambulate and inability to transfer. The causes of ESRD were grouped into four mutually exclusive categories (diabetes, hypertension, glomerulonephritis or other). Major comorbidities, recorded on the CMS-2728 form at the time of dialysis initiation, were considered to be diabetes (types I and II combined), hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease and cardiac dysrhythmia. Since the CMS-2728 form is structured such that diseases like diabetes or hypertension may be classified as a cause of ESRD and/or a comorbidity, diabetes and hypertension were considered present in an individual if they were listed as either the cause of ESRD or as a comorbidity. The BMI was classified into four categories; >25–29.99 kg/m2 was considered overweight and ≥30 kg/m2 obese. The sole laboratory value analysed was haemoglobin, with a cut-off score of 11 g/dL; there were too many missing values to analyse serum albumin. Dialysis modality consisted of in-centre haemodialysis (HD), home HD or peritoneal dialysis (PD).
Statistical analyses: overall and specific approaches
To simplify the presentation of large amounts of data, descriptive data for the first and last biennial periods (1995–96 and 2003–04, respectively) were presented. The descriptive statistics were designed to illustrate how ESRD patients who had Medicaid coverage at dialysis initiation differed from each of their non-Medicaid counterparts and how such differences may have changed over time. The temporal trends within each insurance group over the 10-year observation period were estimated using unconditional logistic regression models for dichotomous response variables and ordinary least squares regression for continuous measures. These models included explanatory variables, insurance status, time and their interaction. Linear contrasts of the parameter estimates from these models were performed to test the within payer status slopes over time using Wald chi-square tests and F-tests for the logistic and linear regression models, respectively.
We modelled the nominal response variable, type of insurance coverage, with polychotomous logistic regression with a generalized logit function [12]. This modelled the natural log of the ratio of the likelihoods (or probabilities) of each of the various coverage types to the likelihood of Medicaid coverage, which served as the referent category. The explanatory variables used to model these ratios of likelihoods were the baseline demographic and clinical characteristics outlined above. We present the relative comparisons between levels of the explanatory variables with respect to type of coverage (using Medicaid as the referent category) by presenting the ratios of these ratios of likelihoods. Though these comparisons were similar to comparisons of the odds produced by dichotomous logistic regression (i.e., similar to the odds ratios), the relative comparison was of these likelihood or probability ratios rather than of odds; hence, we referred to these comparisons as adjusted generalized ratios (AGRs) to distinguish our findings from adjusted odds ratios produced by dichotomous logistic regression models.
Due to the large size of the study population, we attempt to distinguish between the many findings that were only statistically significant from those that were more likely to be clinically meaningful. All statistical analyses were performed with SAS 9.1 (Cary, NC, USA).
Data for this project were obtained from the USRDS under a data use agreement for research-identifiable files, and the study protocol was approved by the University of Kansas Medical Center Human Subjects Committee.
Results
Over the 10 years, there were 883 380 persons who initiated dialysis, after excluding 62 persons who had missing demographic data. The largest proportion was covered by Medicare (42.2%): those with Medicare and some secondary insurance, n = 250 106 (28.3%) and Medicare only, n = 123 164 (13.9%). Medicaid was the next largest payer accounting for 213 388 (24.2%) persons. The other group included 166 895 persons (18.9%), and private coverage accounted for 123 985 (14.0%) persons. Finally, the DVA was the sole payer for 5842 (0.7%) persons.
We analysed both longitudinal trends in incident dialysis patients by payer status as well as factors associated with payer enrolment for the entire cohort, relative to Medicaid.
Longitudinal trends in incident dialysis patients by the payer group
First, we summarized within-insurance group longitudinal trends in patients from each of the six insurance categories according to demographic, risk behaviour, functional, comorbidity, treatment modality and laboratory status. Tables 1 (demographics, risk behaviours and functional limitations) and 2 (cause of ESRD, comorbidities, BMI, laboratory values and dialysis modality) illustrate these trends and the associated level of within-group statistical significance over the course of the observation period. Given the large size of dataset, many within-group trends were found to be highly statistically significant (P < 0.001) despite very modest changes in raw percentages. Overall, each of the six payer groups expanded in size over the 10 years. Medicaid constituted 25.1% of the 2003–04 cohort, and Medicare coverage (solo and combined) accounted for 44.0% of the 2003–04 cohort. As of 2003–04, ∼13.5% had private coverage and 16.8% were classified as ‘other’; as stated, these latter individuals may or may not have had insurance coverage. The DVA provided coverage to <1% of the cohort.
Table 1.
Ten-year trends in demographic, risk-behaviour and functional limitation status characteristics by the insurance group
| Payer status | Medicaid | Medicare alone | Medicare comb.a | DVAb | Private | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 |
| Number | 32 238 | 50 722 | 16 881 | 29 503 | 35 364 | 59 386 | 1128 | 1444 | 19 054 | 27 323 | 28 435 | 33 945 |
| Percentage of total | 24.2 | 25.1 | 12.7 | 14.6 | 26.6 | 29.4 | 0.8 | 0.7 | 14.3 | 13.5 | 21.4 | 16.8 |
| Demographics | ||||||||||||
| Age (mean years) | 57.6 | 59.7† | 67.9 | 69.7† | 70.6 | 73.7† | 57.8 | 59.7† | 51.1 | 51.7† | 55.6 | 54.3† |
| Male gender | 39.3% | 42.8%† | 56.2% | 57.9%* | 57.3% | 58.1%* | 82.7% | 95.6%† | 57.6% | 58.1% | 56.9% | 59.4%† |
| Race | ||||||||||||
| Caucasian | 31.4% | 33.1%† | 55.9% | 55.1% | 75.7% | 77.0%* | 37.9% | 50.8%† | 54.0% | 52.5%† | 46.1% | 41.3%† |
| African American | 41.9% | 38.5%† | 30.6% | 30.5% | 14.7% | 14.2% | 39.1% | 36.4% | 29.4% | 30.9%† | 29.7% | 32.0%† |
| Hispanic | 18.0% | 20.0%† | 8.5% | 11.0%† | 4.1% | 5.8%† | 16.6% | 9.6%† | 9.0% | 10.4%† | 16.4% | 19.5%† |
| Other | 8.7% | 8.4%* | 5.1% | 3.4%† | 5.5% | 3.1%† | 6.5% | 3.3%† | 7.6% | 6.3%† | 7.9% | 7.3%† |
| Unemployed | 35.7% | 32.3%† | 15.1% | 12.9%† | 6.5% | 5.4%† | 32.5% | 21.2%† | 12.6% | 11.1%† | 30.4% | 37.7%† |
| Risk behaviours | ||||||||||||
| Smoking | 7.0% | 6.5% | 5.9% | 4.8%† | 4.8% | 3.5%† | 11.1% | 14.1% | 5.9% | 5.1%† | 5.6% | 5.9% |
| Substance abuse | 4.5% | 3.3%† | 2.1% | 1.4%† | 1.1% | 0.7%† | 6.5% | 7.4% | 1.7% | 1.4% | 3.4% | 3.7% |
| Functional limitations | ||||||||||||
| Unable to ambulate | 7.0% | 6.5% | 5.7% | 5.0% | 5.2% | 4.5%* | 7.4% | 4.6%† | 2.2% | 1.5%† | 2.8% | 2.3%† |
| Unable to transfer | 2.5% | 2.7% | 2.1% | 1.9% | 1.8% | 1.5% | 2.6% | 1.7% | 0.7% | 0.4%† | 0.9% | 0.8% |
aMedicare combined group consists of Medicare plus any additional non-Medicaid insurance.
bDVA, Department of Veterans Affairs.
*10-year trend from 1995 to 2004 significant within the payer group, P < 0.01.
†10-year trend from 1995 to 2004 significant within the payer group, P < 0.001.
Characteristics shown as percentage, unless otherwise noted.
Table 2.
Ten-year trends in the cause of ESRD and comorbidity, body mass index, laboratory and initial dialysis modality status characteristics by insurance group
| Payer status | Medicaid | Medicare alone | Medicare comb.a | DVAb | Private | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 | 1995–96 | 2003–04 |
| Cause of ESRD | ||||||||||||
| Diabetes | 50.4% | 52.2%† | 43.6% | 46.6%† | 38.8% | 41.7%† | 41.4% | 52.5%† | 40.1% | 41.6% | 39.5% | 41.7%† |
| Hypertension | 22.2% | 24.5%† | 26.7% | 28.6%† | 25.5% | 28.6%† | 23.9% | 19.8%* | 17.9% | 19.0%† | 24.1% | 25.9%† |
| Immune | 11.4% | 8.2%† | 10.0% | 6.5%† | 11.7% | 7.5%† | 14.6% | 7.5%† | 21.5% | 18.5%† | 15.7% | 13.2%† |
| Other | 16.0% | 15.2%* | 19.7% | 18.3%† | 24.1% | 22.2%† | 20.1% | 20.2% | 20.6% | 20.9% | 20.7% | 19.3%† |
| Comorbidities | ||||||||||||
| Diabetes | 51.6% | 54.4%† | 44.7% | 49.0%† | 40.4% | 44.3%† | 42.4% | 55.5%† | 41.0% | 43.3%† | 40.4% | 43.1%† |
| Hypertension | 75.3% | 84.2%† | 72.9% | 84.4%† | 74.7% | 84.2%† | 75.6% | 85.9%† | 73.2% | 83.0%† | 68.7% | 82.8%† |
| Heart failure | 33.0% | 33.1% | 36.1% | 37.2% | 40.3% | 40.3% | 24.5% | 25.8% | 21.6% | 19.0%† | 22.9% | 24.1% |
| CAD | 21.6% | 24.1%† | 29.2% | 33.2%† | 35.8% | 39.9%† | 24.3% | 27.4% | 16.0% | 15.7% | 16.7% | 17.4% |
| PVD | 13.8% | 14.0% | 16.9% | 17.3% | 19.2% | 19.1% | 15.2% | 13.6% | 8.6% | 7.7%† | 9.7% | 8.7%† |
| CVA | 9.6% | 10.5%† | 10.0% | 11.5%† | 10.6% | 11.4%† | 11.1% | 8.9% | 4.8% | 4.7% | 5.5% | 5.6% |
| Dysrhythmia | 4.5% | 4.8%* | 7.1% | 7.8%† | 8.9% | 10.7%† | 4.8% | 4.6% | 2.8% | 3.0% | 3.2% | 3.6% |
| Body mass index | ||||||||||||
| <20 kg/m2 | 17.6% | 10.8%† | 16.1% | 10.6%† | 15.0% | 9.5%† | 17.5% | 8.8%† | 13.7% | 6.3%† | 14.9% | 9.1%† |
| 20–24.99 kg/m2 | 29.8% | 29.8% | 32.6% | 32.2% | 34.6% | 34.0% | 31.0% | 30.1% | 29.7% | 26.2%† | 27.6% | 31.7%† |
| 25–29.99 kg/m2 | 20.6% | 25.9%† | 21.6% | 28.8%† | 23.1% | 30.0%† | 20.0% | 32.3%† | 23.2% | 29.0%† | 19.3% | 28.1%† |
| +30 kg/m2 | 19.5% | 32.3%† | 14.3% | 27.6%† | 13.7% | 25.8%† | 14.1% | 27.7%† | 19.8% | 37.6%† | 15.3% | 30.1%† |
| Haemoglobin | ||||||||||||
| <11.0 g/dL | 70.5% | 68.6%† | 64.2% | 67.1%† | 62.1% | 62.9% | 66.2% | 63.0% | 66.9% | 64.1%† | 65.5% | 69.7%† |
| ≥11.0 g/dL | 12.3% | 24.0%† | 13.4% | 25.7%† | 15.9% | 29.6%† | 13.0% | 27.6%† | 15.3% | 27.3%† | 13.2% | 23.5%† |
| Dialysis modality | ||||||||||||
| In-centre HD | 90.9% | 95.5%† | 88.9% | 94.7%† | 86.0% | 93.2%† | 87.2% | 95.1%† | 76.6% | 85.0%† | 80.1% | 92.3%† |
| Home HD | 0.4% | 0.3% | 0.8% | 0.7% | 0.6% | 0.4%† | 0.7% | ‡ | ‡ | 0.3%† | 2.6% | 0.3%† |
| PD | 8.8% | 4.2%† | 10.4% | 4.7%† | 13.4% | 6.4%† | 12.1% | ‡ | ‡ | 14.7%† | 17.3% | 7.3%† |
aMedicare combined group consists of Medicare plus any additional non-Medicaid insurance.
bDVA, Department of Veterans Affairs.
*10-year trend from 1995 to 2004 significant within payer group, P < 0.01.
†10-year trend from 1995 to 2004 significant within payer group, P < 0.001.
‡Not reportable due to Centres for Medicare & Medicaid restrictions on cell sizes.
CAD, coronary artery disease; PVD, peripheral vascular disease; CVA, cerebrovascular accident; HD, haemodialysis; PD, peritoneal dialysis.
Characteristics shown as a percentage, unless otherwise noted.
Because of missing values for body mass index and haemoglobin, totals do not add to 100% in these categories.
Notable findings are that the mean age at dialysis initiation increased overall by ∼2 years over the observation period for all payer groups except for those with private or ‘other’ coverage. Medicaid enrollees were consistently 10 or more years younger throughout the decade than those with Medicare-only or Medicare-combined coverage. The mean age for Medicaid enrollees was comparable to that of the DVA and ‘other’ groups and was actually higher than those with private healthcare coverage. Males constituted a growing percentage of all groups, though the increases were typically modest; the proportion of males on Medicaid increased by 3.5%, and the increase was larger than all groups except for the DVA. However, while a majority of Medicaid enrollees were females, the reverse was true for individuals in all other payer groups. The proportion of individuals who were African American in 2003–04 was highest among Medicaid enrollees (38.5%), although this was a decline from 1995 to 1996. Nearly one-third of the cohort was African American in each of the other payer groups, except for the Medicare-combined group, in which African Americans numbered roughly one in seven. Hispanics were most prevalent in Medicaid and other groups. Unemployment in 2003–04 was higher in the Medicaid group than in all others save the ‘other’ group.
Smoking and substance abuse rates were highest for DVA followed by Medicaid and other; most groups, including Medicaid, showed a decline over time. Substance abuse rates demonstrated a trend generally similar to that of smoking. Physical function limitations were most prevalent in DVA and Medicaid.
Diabetes as the primary cause of ESRD was increasingly prevalent for all payer groups except the privately insured; it was the cause of ESRD for >50% of the Medicaid group in 2003–04, a rate exceeded only by the DVA group. Rates of diabetes and hypertension as comorbidities upon dialysis initiation increased across the observation period for the Medicaid and Medicare groups. Diabetes was present in nearly half of the overall cohort by 2003–04 and hypertension in over three-quarters. Heart failure and coronary artery disease were the next most prevalent comorbidities; coronary artery disease increased for Medicaid and Medicare groups over time. The proportion of each payer group that was overweight or obese increased dramatically, with 40.1% of the individuals in the Medicaid cohort falling into one of these two groups in 1995–96 and 58.2% in 2003–04; these rates of increase were exceeded by the DVA and privately insured groups. Haemoglobin demonstrated the most variable findings; while roughly two-third of each payer group initiated dialysis with a haemoglobin <11 g/dL, some groups saw an increase and others a decrease.
Rates of initiation of in-centre HD increased globally for all groups, approaching or even exceeding 95% by 2003–04 in all groups except those with private insurance. Rates of self-care dialysis (PD or home HD) fell correspondingly in all groups.
Factors associated with Medicaid status at dialysis initiation
After eliminating records missing at least one data element (∼17% of total), we had adequate data to analyse 730 560 individuals in the multivariable model. The number (percentage of payer group) with missing data excluded across the entire 10-year period was comparable across the payer groups: Medicaid, n = 34 478 (16.2%); Medicare only, n = 20 945 (17.0%); Medicare combined, n = 43 021 (17.2%); DVA, n = 1225 (21.0%); Private, n = 21 855 (17.6%) and other, n = 31 296 (18.8%).
We assessed which factors were associated with payer status using polychotomous logistic regression. The AGRs are presented in Table 3; Medicaid was established as the referent payer group. AGRs, which differ from classic adjusted odds ratios, are properly interpreted as follows, taking gender as an example. Among ESRD patients, the ratio of DVA coverage to Medicaid coverage was 14.6 times higher among males than females, controlling for other factors. The ratios for males receiving healthcare coverage from each of the remaining payer groups, compared to Medicaid, ranged from 1.83 to 2.15 times higher. Of note, while this range of AGRs does not necessarily mean that there were fewer males within Medicaid, it can be seen from the bivariate analyses presented in Table 1 that Medicaid had the highest proportion of females.
Table 3.
Associations between baseline characteristics and payer status for the 10-year cohort of dialysis initiators, shown as adjusted generalized ratios (AGRs)
| Payer status | Medicare | Medicare comb.a | DVAb | Private | Other |
|---|---|---|---|---|---|
| Number | 102 219 | 207 085 | 4617 | 102 130 | 135 599 |
| AGR | AGR | AGR | AGR | AGR | |
| Demographics | |||||
| Age (years) | 1.06* | 1.08* | 1.01* | 0.96* | 0.99* |
| Male gender | 2.15* | 2.07* | 14.6* | 1.83* | 1.87* |
| Race (Caucasian = referent) | |||||
| African American | 0.66* | 0.26* | 0.78* | 0.47* | 0.53* |
| Hispanic | 0.38* | 0.15* | 0.36* | 0.29* | 0.65* |
| Other | 0.28* | 0.20* | 0.44* | 0.55* | 0.67* |
| Employed | 1.76* | 3.22* | 1.45* | 5.12* | 1.06* |
| Risk behaviours | |||||
| Smoking | 0.87* | 0.73* | 1.45* | 0.71* | 0.76* |
| Substance abuse | 0.73* | 0.55* | 1.49* | 0.43* | 0.83* |
| Functional limitations | |||||
| Inability to ambulate | 0.66* | 0.57* | 0.91 | 0.39* | 0.53* |
| Inability to transfer | 0.84* | 0.71* | 0.82 | 0.76* | 0.80* |
| Cause of ESRD (Diabetes = referent) | |||||
| Hypertension | 0.98 | 1.07 | 0.89 | 0.81* | 0.92 |
| Glomerulonephritis | 1.13* | 1.54* | 1.26 | 1.23* | 0.98 |
| Other | 1.08 | 1.31* | 1.21 | 1.05 | 0.89 |
| Comorbidities | |||||
| Diabetes | 0.94 | 1.08* | 1.19 | 0.79* | 0.68* |
| Hypertension | 1.01 | 1.10* | 1.28* | 1.15* | 0.99 |
| Heart failure | 0.91* | 0.90* | 0.69* | 0.73* | 0.83* |
| CAD | 1.07* | 1.17* | 1.14* | 0.91* | 0.92* |
| PVD | 1.05* | 1.05* | 0.94 | 0.79* | 0.86* |
| CVA | 0.88* | 0.85* | 0.87* | 0.61* | 0.70* |
| Dysrhythmia | 1.05* | 1.09* | 1.02 | 0.91* | 1.00 |
| BMI (20–24.99 kg/m2 = referent) | |||||
| Low | 0.91* | 0.81* | 1.00 | 0.79* | 0.92* |
| Overweight | 1.07* | 1.11* | 1.16* | 1.29* | 1.06* |
| Obese | 1.00 | 1.03* | 0.99 | 1.29* | 0.96* |
| Laboratory | |||||
| Haemoglobin (≥11 g/dL = referent) | 1.02 | 0.94* | 0.95 | 0.91* | 1.04* |
| Modality (in-centre HD = referent) | |||||
| PD | 1.40* | 1.85* | 1.20* | 2.34* | 1.49* |
| Home HD | 1.94* | 1.57* | 4.35* | 1.47* | 5.21* |
aMedicare combined group consists of Medicare plus any additional non-Medicaid insurance.
bDepartment of Veterans Affairs.
*Statistically significant at P < 0.01 (99% Wald confidence limits).
Referent group for payer status is Medicaid (n = 178 910).
Number with missing data and therefore excluded: Medicaid n = 34 478 (16.2%); Medicare n = 20 945 (17.0%); Medicare combined.
n = 43 021 (17.2%); DVA n = 1225 (21.0%); Private n = 21 855 (17.6%); other n = 31 296 (18.8%).
ESRD, end-stage renal disease; CAD, coronary artery disease; PVD, peripheral vascular disease; CVA, cerebrovascular accident; BMI, body mass index; HD, haemodialysis; PD, peritoneal dialysis.
Statistically significant findings from the polychotomous regression results were noted for nearly every demographic and clinical variable. In addition to the above-mentioned AGR >1 for males, AGRs >1 were found for employment and initiation on self-care dialysis, while AGRs <1 were found for Africans Americans, Hispanics and other non-whites, for smoking and substance abuse (<1.0 for all but the DVA) and for inability to ambulate and inability to transfer.
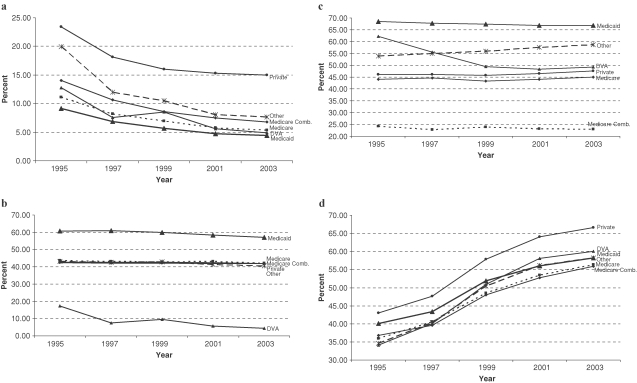
Several longitudinal trends of particular clinical importance, previously highlighted, are illustrated in Figure 1. Figure 1(a) illustrates both the overall decline in initiation in rates of self-care dialysis as well as the lowest absolute rate in Medicaid patients. Figure 1(b) not only shows how the percentage of females is decreasing among all payer groups but also demonstrates the discordance in the female-to-male sex ratio by insurance status; Medicaid patients consist of more females than males, while the reverse is true for non-Medicaid patients. Figure 1(c) demonstrates how non-Caucasians constitute a higher proportion of Medicaid-enrolled incident dialysis patients than of non-Medicaid patients. Finally, Figure 1(d) documents the considerable increase in obesity among dialysis initiates.
Fig. 1.
Trends in longitudinal differences between Medicaid and non-Medicaid incident dialysis patients, 1995–2004. (a) Self-care dialysis as initial dialysis modality; (b) females; (c) minorities; (d) overweight or obese, by body mass index.
Discussion
The 10-year trends mentioned above and the analyses of differences between incident dialysis patients enrolled in Medicaid and other health insurance programmes presented herein illustrate important distinctions in demographic and medical characteristics. While observers familiar with the characteristics of the broader Medicaid population could anticipate many of the differences that we report in dialysis patients, our findings firmly establish their details.
Several major trends over time are notable across insurance groups. Hispanics, who represent a rapidly growing segment of the US society [13], constituted an increasing proportion of new dialysis patients, as has been predicted [14]; this trend was evident in all insurance groups except for the DVA. For Medicaid, the decline in African Americans was offset by increases in both Hispanics and Caucasians. There was also an overall increase in the proportion of ESRD patients who were males, a finding previously recognized by the nephrology community [15]. However, Medicaid continued to provide coverage more often to females as compared to males.
An increase in BMI, a trend that grew alarmingly in the general population during our period of observation [16], has been previously noted in dialysis patients [17] and was also observed in our study sample. Our analysis makes clear that, despite important differences in Medicaid and non-Medicaid patients, the magnitude and time course of the increase in BMI are similar across all groups. Indeed, all groups’ BMIs averaged ∼28 kg/m2 in 2004, values that are classified as overweight [18]. In addition, diabetes and hypertension as comorbidities were prevalent among an increasing proportion of incident dialysis patients, irrespective of insurance status. These entities are manifestations of the metabolic syndrome, which accompanies increases in BMI in both the general and CKD [19,20] populations.
The percentage of individuals with haemoglobin >11 g/dL, the current lower threshold recommend by the KDOQI guidelines, has increased significantly in all insurance groups. These trends probably reflect the traditional belief in the deleterious consequences of low haemoglobin in kidney disease patients [21,22] which gave rise to recent randomized controlled trials addressing this issue [23,24] and, therefore, are likely to reflect changes in care rendered by providers in the pre-ESRD period. Nevertheless, less than one-third of all individuals, irrespective of insurance status, had haemoglobin levels exceeding this target, indicating a substantial need for improvement.
While there are many similarities between Medicaid and non-Medicaid dialysis patients, important differences exist. These are illustrated in the patterns of the AGRs. The other insurance groups, relative to Medicaid, had AGRs <1 for minorities, risk behaviours (with the exception of the DVA) and diminished functional status, while simultaneously demonstrating AGRs >1 for employment and initiation on self-care dialysis modalities. These findings were expected given the needs that Medicaid is designed to meet within the US healthcare system. [25]. However, changes in Medicaid in the latter 1990s, which were designed to broaden its reach [26], are also in evidence in our results; males, for example, made up an increasingly larger proportion of individuals in the Medicaid group. That the proportion of unemployed individuals fell in the Medicaid group, a finding which has previously been noted in ESRD patients [17], may also be the result of the concurrent expansions of Medicaid eligibility in a number of states.
Of great importance to patients, healthcare providers and payers is the decline in self-care dialysis modalities worldwide [27], even in areas with strong traditions of PD [28]. Recent analysis of this unfortunate trend has concluded that demographic and comorbidity factors do not adequately account for this finding and that provider beliefs and attitudes, or other not-readily-observable healthcare system factors, may be responsible [15,17]. This trend appears not to be supported by high-grade medical evidence such as clinical trials or observational outcome data free of residual confounding [15] and, as such, has caused much consternation and debate within the nephrology community [27,29,30]. As expected, we observed a broad decline in self-care dialysis (the majority of which is in the form of PD) across all categories of insurance. The percentage of individuals on self-care dialysis by 2004 was lowest in the Medicaid group (4.5%), a finding not unexpected given work by previous investigators who reported that minority status [31,32], unemployment [32], poor functional status [32] and overall lower socioeconomic status [31] were inversely associated with use of PD. Given that these factors are disproportionately represented in individuals with Medicaid, this is not surprising. How the overall structure of the US healthcare system may contribute to the worldwide decline in self-care dialysis is less certain. It has been suggested by several authors that the structure of national healthcare systems influences use of PD, and that private insurance-based healthcare systems like that of the USA disincentivize use of self-care therapies [27,33]. We found that the rates of PD were highest among the privately insured and that these individuals as a whole experienced less of a proportional decline in PD use over time relative to the other groups. This does not necessarily imply, of course, that a wholesale shift to private insurance would increase rates of self-care dialysis, or would arrest the historical decline in PD presently being witnessed. It may be that the most affluent individuals with generally higher levels of functional status, and perhaps more education and social support, are able to procure and maintain private insurance; these same individuals are also the most likely to be placed on PD. In distinction, individuals with lower socioeconomic and functional status may be both less likely to have private insurance and be placed on PD or home HD. Our descriptive study does not allow us to determine whether causality exists between insurance and initial dialysis modality, and, if so, in what direction it may be operating.
Our study is subject to several important limitations. The completion of the CMS-2728 form relies, at least in part, on some degree of patient self-reporting, and this is likely to be a source of inaccuracy. For example, the smoking rates reported above seem unintuitively low. Additionally, information on individual patients’ comorbidities is likely to be imperfect [34], as the provider who completes the form may have an incomplete access to aspects of the patient's medical history. However, the CMS-2728 form is a foundational source of information about characteristics of incident dialysis patients and is also a basis for policy planning, so its use in an analysis of this type is likely to yield important information of broad public health relevance. Finally, many of the longitudinal trends in incident dialysis patients that we highlight, such as the increase in BMI, are not specific to dialysis patients or to renal disease and are likely to reflect broad societal changes. Nonetheless, an understanding of these trends provides important insight into the changing face of the incident dialysis population.
In conclusion, our examination of longitudinal trends in demographic, risk behaviour, functional, comorbidity, laboratory and treatment modality characteristics in incident dialysis patients and their respective associations with insurance status reveals findings of clinical and public health importance. Our observations appear to reflect the interplay between transformations in demography, evolution in health policy and alteration in provider behaviour. In this descriptive analysis, we are unable to dissect all aspects of this complex interplay, but the differences noted highlight the need for more in-depth analyses.
Medicaid coverage has been extended to a significant proportion of the incident ESRD population. These dialysis initiates are more likely to be younger, minority and female. They are also more likely to have significant risk factors for poor outcomes, including certain lifestyle factors and functional limitations. Research on how insurance status affects dialysis morbidity and mortality is urgently needed.
Acknowledgments
The authors thank Galen Cook-Weins, MS, for his assistance with the analysis and Connie Wang, MD, for her assistance with manuscript preparation. This study was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (1R01DK080111-01, Shireman PI), from a KUMC Lied Center Clinical Pilot Grant, and from funds provided by the Kidney Institute and the Landon Center on Aging at KUMC. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Conflict of interest statement. None declared.
References
- 1.Baker DW, Sudano JJ, Albert JM, et al. Lack of health insurance and decline in overall health in late middle age. N Engl J Med. 2001;345:1106–1112. doi: 10.1056/NEJMsa002887. [DOI] [PubMed] [Google Scholar]
- 2.Wharam JF, Landon BE, Galbraith AA, et al. Emergency department use and subsequent hospitalizations among members of a high-deductible health plan. JAMA. 2007;297:1093–1102. doi: 10.1001/jama.297.10.1093. [DOI] [PubMed] [Google Scholar]
- 3.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliams JM, Meara E, Zaslavsky AM, et al. Health of previously uninsured adults after acquiring Medicare coverage. JAMA. 2007;298:2886–2894. doi: 10.1001/jama.298.24.2886. [DOI] [PubMed] [Google Scholar]
- 5.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34:2024–2027. [PubMed] [Google Scholar]
- 6.Ward MM. Laboratory abnormalities at the onset of treatment of end-stage renal disease: are there racial or socioeconomic disparities in care? Arch Intern Med. 2007;167:1083–1091. doi: 10.1001/archinte.167.10.1083. [DOI] [PubMed] [Google Scholar]
- 7.Holley JL, DeVore CC. Why all prescribed medications are not taken: results from a survey of chronic dialysis patients. Adv Perit Dial. 2006;22:162–166. [PubMed] [Google Scholar]
- 8.Gill JS, Hussain S, Rose C, et al. Access to kidney transplantation among patients insured by the United States Department of Veterans Affairs. J Am Soc Nephrol. 2007;18:2592–2599. doi: 10.1681/ASN.2007010050. [DOI] [PubMed] [Google Scholar]
- 9.Lockridge RS., Jr The direction of end-stage renal disease reimbursement in the United States. Semin Dial. 2004;17:125–130. doi: 10.1111/j.0894-0959.2004.17209.x. [DOI] [PubMed] [Google Scholar]
- 10.United States Renal Data System., USRDS 2006 Annual Data Report. Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2006.
- 11.USRDS United States Renal Data System 2004 Annual Data Report. Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2004 In.
- 12.Agresti A. Categorical Data Analysis. 2nd edn. New York: John Wiley & Sons, Inc.; 990. 1. [Google Scholar]
- 13.Rutledge MS, McLaughlin CG. Hispanics and health insurance coverage: the rising disparity. Med Care. 2008;46:1086–1092. doi: 10.1097/MLR.0b013e31818828e3. [DOI] [PubMed] [Google Scholar]
- 14.Benabe JE, Rios EV. Kidney disease in the Hispanic population: facing the growing challenge. J Natl Med Assoc. 2004;96:789–798. [PMC free article] [PubMed] [Google Scholar]
- 15.Khawar O, Kalantar-Zadeh K, Lo WK, et al. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol. 2007;2:1317–1328. doi: 10.2215/CJN.02550607. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers L, Kaskel FJ, Bamgbola O. The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv Chronic Kidney Dis. 2006;13:352–364. doi: 10.1053/j.ackd.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Ching-Ha Kwan B, Beddhu S. Metabolic syndrome and chronic kidney disease. Minerva Urol Nefrol. 2006;58:1–12. [PubMed] [Google Scholar]
- 21.Eschbach JW, Kelly MR, Haley NR, et al. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med. 1989;321:158–163. doi: 10.1056/NEJM198907203210305. [DOI] [PubMed] [Google Scholar]
- 22.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 23.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 24.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 25.Iglehart JK. The American health care system—Medicaid. N Engl J Med. 1999;340:403–408. doi: 10.1056/NEJM199902043400525. [DOI] [PubMed] [Google Scholar]
- 26.Dubay L, Kenney G. Expanding public health insurance to parents: effects on children's coverage under Medicaid. Health Serv Res. 2003;38:1283–1301. doi: 10.1111/1475-6773.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lameire N, Peeters P, Vanholder R, et al. Peritoneal dialysis in Europe: an analysis of its rise and fall. Blood Purif. 2006;24:107–114. doi: 10.1159/000089446. [DOI] [PubMed] [Google Scholar]
- 28.Oreopoulos DG, Coleman S, Doyle E. Reversing the decreasing peritoneal dialysis (PD) trend in Ontario: a government initiative to increase PD use in Ontario to 30% by 2010. Perit Dial Int. 2007;27:489–495. [PubMed] [Google Scholar]
- 29.Mendelssohn DC. PD and the future: the role of PD in the overall management of ESRD. Blood Purif. 2003;21:24–28. doi: 10.1159/000067853. [DOI] [PubMed] [Google Scholar]
- 30.Jindal K. Revitalizing peritoneal dialysis: the Ontario approach. Perit Dial Int. 2007;27:526–528. [PubMed] [Google Scholar]
- 31.Winkelmayer WC, Glynn RJ, Levin R, et al. Late referral and modality choice in end-stage renal disease. Kidney Int. 2001;60:1547–1554. doi: 10.1046/j.1523-1755.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 32.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol. 2002;13:1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 33.Horl WH, de Alvaro F, Williams PF. Healthcare systems and end-stage renal disease (ESRD) therapies—an international review: access to ESRD treatments. Nephrol Dial Transplant. 1999;14(Suppl 6):10–15. doi: 10.1093/ndt/14.suppl_6.10. [DOI] [PubMed] [Google Scholar]
- 34.Longenecker JC, Coresh J, Klag MJ, et al. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]