Abstract
Background. Dialysis adequacy is currently judged by measures of urea clearance. However, urea is relatively non-toxic and has properties distinct from large classes of other retained solutes. In particular, intracellularly sequestered solutes are likely to behave differently than urea.
Methods. We studied an example of this class, the aliphatic amine monomethylamine (MMA), in stable haemodialysis outpatients (n = 10) using an HPLC-based assay.
Results. Mean MMA levels pre-dialysis in end-stage renal disease subjects were 76 ± 15 μg/L compared to 32 ± 4 μg/L in normal subjects (n = 10) (P < 0.001). Mean urea reduction was 62% while the reduction ratio for MMA was 43% (P < 0.01). MMA levels rebounded in the 1 hour post-dialytic period to 85% of baseline, whereas urea levels rebounded only to 47% of baseline. MMA had a much larger calculated volume of distribution compared to urea, consistent with intracellular sequestration. Measures of intra-red blood cell (RBC) MMA concentrations confirmed greater levels in RBCs than in plasma with a ratio of 4.9:1. Because of the intracellular sequestration of MMA, we calculated its clearance using that amount removed from whole blood. Clearances for urea averaged 222 ± 41 ml/min and for MMA 121 ± 14 ml/min, while plasma clearance for creatinine was 162 ± 20 ml/min (P < 0.01, for all differences). Using in vitro dialysis, in the absence of RBCs, solute clearance rates were similar: 333 ± 6, 313 ± 8 and 326 ± 4 ml/min for urea, creatinine and MMA, respectively. These findings suggest that the lower MMA clearance relative to creatinine in vivo is a result of MMA movement into RBCs within the dialyser blood path diminishing its removal by dialysis.
Conclusion. In conclusion, we find that, in conventional haemodialysis, MMA is not cleared as efficiently as urea or creatinine and raise the possibility that RBCs may limit its dialysis not merely by failing to discharge it, but by further sequestering it as blood passes through the dialyser.
Keywords: haemodialysis, uraemia
Introduction
Solute retention is a central component of the pathophysiology of end-stage renal disease (ESRD) [1,2]. Retained solutes are often labeled as ‘uraemic toxins’ and the levels of some solutes, such as inorganic phosphate, asymmetric dimethylarginine (ADMA) and p-cresol, have been correlated with mortality [3–5]. In isolation, however, many retained solutes do not exhibit significant toxicity, including urea [6]. Still, current measures of dialysis adequacy, such as Kt/V and urea reduction ratio (URR), focus exclusively on urea kinetics even though the physical and chemical properties of urea are not shared by all retained solutes.
Intracellularly sequestered solutes are molecules that accumulate in renal failure and are predicted to have haemodialysis kinetics different from urea [7,8]. Members of this class include not only phosphate but also the aliphatic amines, such as monomethylamine (MMA), which derives from dietary sources such as choline as well as endogenous protein metabolism [9]. Aliphatic amines may have both direct and indirect toxicities in uraemia [10–12]. MMA reduces appetite in mice [10]. Also, it can be oxidized to several toxic substances including formaldehyde and hydrogen peroxide [12]. Because MMA and other aliphatic amines are small and have high pKas, they can be sequestered in the relatively acidic environment within cells [13,14].
To gain better insight into the kinetics of the aliphatic amines as intracellularly sequestered solutes, we studied the levels of MMA in stable haemodialysis patients treated at an outpatient unit.
Materials and methods
Study participants
Subjects with ESRD (n = 10) were recruited from a university-affiliated outpatient haemodialysis unit. Exclusion criteria included age <18 years, time on haemodialysis <6 months, hospitalization or acute illness within 1 month, changes in dialysis prescription, dialysate composition or extra dialysis treatments within 1 month of sample collection. Subjects were dialysed with Fresenius 2008 machines. All patients were treated three times per week with single-use, high flux dialysers (F180NR, Fresenius). Ten normal subjects with normal renal function provided plasma. All subjects provided written informed consent. The study protocol was approved by the University's Committee on Clinical Investigations and by Fresenius Medical Care. Five of the subjects with ESRD were restudied under similar conditions.
Sample collection
Patients underwent their standard dialysis treatment as prescribed by their primary nephrologists on a Monday or a Tuesday. Blood samples were collected immediately prior to the start of haemodialysis and then every 20–45 minutes during the haemodialysis treatment and again at the end of treatment. Simultaneous samples were taken from the arterial and venous limbs of the haemodialysis circuit. Blood samples were also collected 30 and 60 minutes after the end of haemodialysis and then again immediately prior to the patients' next haemodialysis session (~44 hours later). Blood samples were collected in Benton–Dickinson plasma separator tubes, kept on ice and then centrifuged at 3800 rpm for 15 minutes after the end of the haemodialysis treatment. Plasma aliquots were stored at −80oC until analysed. For whole-blood level determinations, 1 ml of uncentrifuged blood was mixed with 9 ml of double distilled H2O (ddH2O). The resultant lysates were kept on ice for the duration of the dialysis treatment, then aliquoted and stored at −80°C until analysed. Blood and dialysate flow rates were recorded throughout the dialysis treatment. Patients were monitored for any adverse effects. For the five restudied subjects, blood samples were collected from the arterial and venous limbs of the dialysis circuit into heparinized blood gas syringes at 50 and 185 minutes into the dialysis treatment and immediately analysed to determine PCO2, PO2 and pH.
In an attempt to determine post-dialyser plasma MMA levels prior to any transcellular equilibration, we attempted a rapid isolation of post-dialyser plasma as follows: ~1 ml of blood drawn from the venous limb of the dialysis circuit was immediately aliquoted into a 1.5-ml Eppendorf tube and spun for 1 minute using a mini microcentrifuge (rcf ~2000 g). Plasma was removed by micropipette, placed on ice for the duration of the dialysis treatment and then stored at −80°C until analysed.
In another effort to test the possibility that rapid separation of red blood cell (RBC) and plasma might disclose an effect of high bicarbonate solutions to accentuate the RBC to plasma MMA ratios, we added to whole blood equal volumes of either 140 mM NaCl with 10 mM sodium bicarbonate or 110 mM NaCl with 40 mM sodium bicarbonate and 2 mM acetic acid. The latter solution approximates that of standard bicarbonate dialysate. After the additions under mineral oil, the mixtures were centrifuged rapidly with an estimated separation within 15 to 30 seconds.
MMA measurements
MMA levels were measured for each time point sampled by chromatography using a Shimadzu Prominence HPLC system which consisted of two LC-20AD pumps, a DGU-20A3 degasser, a SIL-20A autosampler and an RF-10AXL fluorescence detector. Separation was accomplished on a Phenomenex Onyx monolithic column (C-8, 150 × 4.6 mm) using an isocratic elution. The mobile phase consisted of 30% methanol (v/v), 30% acetonitrile (AcCN) (v/v) and 20 mM phosphate, and the pH was adjusted to 6.80. The flow rate was 1.0 mL/min. The wavelengths of the fluorescence detector for excitation and emission were λex = 420 and λem = 480 nm, respectively. Determinations were duplicated. Peak area was used for quantification.
The MMA was almost entirely free and not protein bound as 97 ± 1.3% (n = 3) passed through a filter with a nominal cutoff of 10 kD.
Urea and creatinine measurements
Urea was measured in duplicate for each time point using a commercially available assay based on the colorimetric Jung method (Quantichrom Urea Assay Kit, BioAssay Systems Hayward, CA) [15]. We attempted to measure urea levels in the whole-blood haemolysate but found these to be unreliable. Creatinine was measured in duplicate for each time point sampled using a commercially available assay based on the colorimetric Jaffe reaction (Quantichrom Creatinine Assay Kit, BioAssay Systems Hayward, CA). We attempted to measure creatinine levels in the whole-blood haemolysate but found these to be unreliable.
Calculating amounts removed and clearances
Amounts of urea and MMA removed were calculated by plotting the differences between arterial and venous limb plasma solute concentrations vs. time (plasma based) and whole-blood lysates vs. time (whole blood based). The equations for the best-fit curves were then integrated over the length of the dialysis treatments to determine the total amount of solute removed. Dialysate and blood flow rates, haematocrits and amounts of ultrafiltration were incorporated into the calculations. Urea was treated as removed from plasma water and RBC water. RBC water was estimated as 70% of the haematocrit volume. Intra-RBC urea concentrations were assumed to be equivalent to plasma urea concentrations. For MMA removal, total blood flow rate was used to calculate amounts removed. Clearances were calculated as the volume of plasma cleared per minute.
Calculating volumes of distribution
Volumes of distribution for each solute were calculated as follows:
Where the level at initial is the initial, pre-dialysis concentration and the level at 60 minutes after haemodialysis is the equilibrium concentration. For the five restudied subjects, equilibrium concentrations were estimated based on the measured rebound from the initial 10 subjects. Equilibrium concentrations for MMA were assumed to be 85% of initial values.
Transcellular distribution ratios were calculated first by determining intra-erythrocytic solute levels ([RBC]):
The transcellular ratio was then calculated as: [RBC]/[plasma]
In vitro dialysis
Urea, creatinine and MMA solutions at concentrations of 200, 20 and 486 μg/L, respectively, were run through an F180 dialyser (Fresenius) with a flow rate of 360 ml/min against a countercurrent dialysate flow rate of 800 ml/min. Samples pre- and post-dialyzer were analysed for each solute after 5 and 10 minutes of dialysis, and clearance rates were calculated as volume of solution cleared per minute. Data presented represents the average of four independent experiments.
PCO2, PO2 and pH
PCO2, PO2 and pH measurements were made using the clinical lab of Weiler Hospital, the teaching hospital of Albert Einstein College of Medicine. Measurements were performed using a radiometre ABL 700 blood gas analyser.
In vitro RBC distribution
Freshly drawn blood samples from normal subjects were separated into 0.5-ml aliquots. The mean haematocrit value was 45%. MMA levels were measured by HPLC as above. Some aliquots were used to determine plasma and whole-blood lysate MMA levels. MMA (46.5 ng) was added to the remaining aliquots to increase the whole-blood level by 93 µg/L. The mixtures were incubated at 37°C for 15 minutes, and plasma and whole-blood MMA concentrations were then measured again. Recovery amounts were calculated as the proportion of MMA measured in plasma and whole-blood lysate after subtraction of baseline levels compared to that added.
For the RBC addition studies, 0.5-ml aliquots of whole blood were spun at 10 000 rpm for 3 minutes, and the plasma was discarded. The precipitated RBCs were then mixed with 0.5-ml aliquots of whole blood pre-treated with MMA to raise the whole-blood MMA concentration by 93 μg/ml. The final haematocrit was 62%. The mixtures were incubated at 37°C for 15 minutes. Plasma and whole-blood lysate MMA concentrations were measured as above. Recovery amounts were calculated as above.
Statistics
Correlations between baseline variables and solute levels were assessed by calculating Pearson correlation coefficients. Comparisons for degree of solute rebound, clearance rates, amounts removed, extraction ratios and volumes of distribution were determined by using two-sided paired Student's t-tests. Comparisons between recovery amounts for in vitro studies were determined by using the two-sided Student's t-test.
Results
MMA as a retained solute
The baseline data for 10 subjects, of whom five were restudied, are summarized in Table 1. We found no correlation between pre-dialysis plasma MMA and urea levels (r2 = .003, P = 0.99). At the beginning of dialysis, plasma levels were consistently elevated in these subjects with a mean MMA concentration of 76 ± 15 μg/L. These values were more than double the average random level in subjects with normal renal function, 32 ± 4 μg/L (P = <0.001).
Table 1.
Baseline patient characteristics
| Age (years) | Sex (M/F) | BMI (kg/m2) | Hct (%) | Time on HD (months) | Qb (ml/min) | Qd (ml/min) | |
|---|---|---|---|---|---|---|---|
| Subjects (n = 10) | |||||||
| Mean (SD) | 60 (13) | 4/6 | 33.9 (10.0) | 37.4 (2.2) | 45 (34) | 379 (36) | 569 (54) |
| Subjects (n = 5) | |||||||
| Mean (SD) | 60 (13) | 2/3 | 31.8 (7.4) | 37.2 (5.4) | 63 (47) | 380 (27) | 570 (41) |
Intradialytic solute levels and rebound
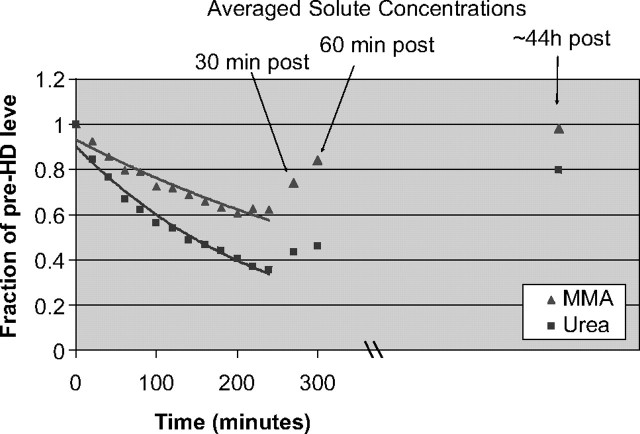
Urea levels declined during haemodialysis therapy in the expected exponential fashion (Figure 1). The mean URR was 62%, whereas the mean reduction ratio for MMA was significantly lower at 43% (P < 0.01). At 1 hour after the termination of haemodialysis, mean urea levels were still 53% lower than initial values. In contrast, MMA levels displayed considerable rebound, and by 1 hour post-dialysis, were at 85% of mean initial levels. This large degree of rebound supports the notion that there are large pools of sequestered MMA outside of the plasma space.
Fig. 1.
The mean solute levels of MMA and urea throughout the course of haemodialysis, 30 and 60 minutes post-dialysis and prior to the next haemodialysis treatment (values are expressed as a fraction of the pre-dialysis, initial plasma level).
At the beginning of the next dialysis session, urea levels had returned to 80% of prior pre-dialysis values. This is consistent with an expected rise over 2 days where the initial value followed a 3-day interdialytic interval. MMA levels essentially returned to initial values, 98% of initial.
Solute removal and volume of distribution
We measured the relative MMA concentrations in RBCs and plasma. For MMA concentration, the erythrocyte to plasma ratio was found to be 4.9:1 for arterial samples at the initiation of dialysis. Thus, MMA is sequestered in RBCs.
Because of its intracellular sequestration, we measured its removal by the A–V difference in whole blood during a separate set of five dialysis treatments in five of the same subjects. MMA removal averaged 1696 ± 230 μg per treatment. Using A–V differences for urea, the mean VdUREA was 56 L. Using whole blood based removal amounts for MMA the mean VdMMA was greater at 133L (P < 0.001). Thus, as for the direct measurements of RBC to plasma ratio of MMA, its whole body Vd suggests a major degree of sequestration in the cellular space. These results fit the prediction that aliphatic amines would be sequestered in cells, and this sequestration likely explains in part the large post-dialysis rebound of MMA.
Urea, creatinine and MMA clearances
Solute clearances were calculated for MMA, creatinine and urea (Table 2). For the five sessions during which whole-blood measurements of MMA were determined, the average blood flow rates, dialysate flow rates and haematocrits were 380 ± 27 ml/min, 570 ± 41 ml/min and 37.2 ± 5.4%, respectively. The mean clearances of urea, creatinine and MMA were 222 ± 41, 161 ± 20 and 121 ± 14 ml/min, respectively (P < 0.01 for urea and creatinine vs. MMA; P < 0.02 for urea vs. creatinine). These clearance values are presented using the conventional units of millilitre per minute of plasma. The urea clearance was calculated from the A–V values for plasma urea concentration and dialyser blood flow assuming urea equilibration between plasma and RBC [16]. The creatinine clearance was calculated from A–V values for plasma creatinine concentration and dialyser plasma flow. The MMA clearance was calculated from A–V values for whole-blood MMA and dialyser blood flow using arterial plasma MMA to express the clearance in units of millilitre per minute plasma. Consistent with its lower clearance rates, MMA also had significantly lower trans-dialyser plasma extraction ratios as compared to urea and creatinine (19 ± 2% for MMA vs. 62 ± 8% and 64 ± 4% for urea and creatinine, respectively; P < 0.001 for both MMA vs. urea and MMA vs. creatinine; Table 3). Of note, the fall in measured plasma MMA concentration across the dialyser was less than the fall in plasma urea and creatinine concentrations. Thus, a calculation of MMA clearance based on A–V differences for plasma MMA concentration yielded value of 53 ± 6 ml/min which was even lower than the MMA clearance calculated based on whole-blood MMA concentrations.
Table 2.
Solute clearance rates
| Urea (ml/min) | Creatinine (ml/min) | MMA (ml/min) | |
|---|---|---|---|
| Subject | |||
| 1′ | 195 | 149 | 131 |
| 2′ | 164 | 143 | 100 |
| 3′ | 263 | 178 | 129 |
| 4′ | 250 | 148 | 111 |
| 5′ | 237 | 186 | 132 |
| Mean (SD) | 222 (41) | 161 (20) | 121 (14) |
Table 3.
Average in vivo trans-dialyser extraction ratios
| Urea | Creatinine | MMA | |
|---|---|---|---|
| Subject | |||
| 1′ | 59% | 61% | 20% |
| 2′ | 50% | 66% | 18% |
| 3′ | 71% | 70% | 21% |
| 4′ | 69% | 61% | 18% |
| 5′ | 62% | 64% | 16% |
| Mean | 62% | 64% | 19% |
| SD | 8% | 4% | 2% |
The apparent failure of the dialyser to remove MMA from the plasma was surprising as MMA is smaller at 31D than either urea (60D) or creatinine (113D), and we have shown that it is not protein bound. In vitro experiments showed that all three small solutes were dialysed effectively from buffered saline in the absence of RBCs. In this setting, MMA clearance was 327 ± 6 ml/min compared to 333 ± 8 and 313 ± 4 ml/min for urea and creatinine.
The finding of lower in vivo than in vitro clearance for MMA suggested that RBC impeded MMA clearance. Because we suspected that the acute changes in pH, bicarbonate and pCO2 experienced by blood during the transit through the dialyser (see below and Discussion) would lead to a movement of MMA into the RBCs, we attempted to separate RBC and plasma rapidly (~30 seconds from sample withdrawal from the lines) but found no difference from standard preparation times with a persistent ~5:1 RBC: plasma ratio. We also examined in vitro whether reproducing the acute pH effects by adding low (10 mM) or high (40 mM) sodium bicarbonate solutions to whole blood would yield a detectable accentuation of the RBC sequestration of MMA. This procedure took ~15 to 30 seconds from mixing to the beginning of the centrifugation step but again, no greater RBC content of the MMA could be detected with the higher bicarbonate solution.
Blood pH, PCO2 and PO2
We observed the following trans-dialyser changes: a decrease in pH from 7.44 ± 0.05 to 7.34 ± 0.04, an increase in PCO2 from 52 ± 5 to 72 ± 6 mmHg and an increase in PO2 from 61 ± 24 to 73 ± 30 mmHg (P < 0.001 for all changes).
In vitro MMA distribution
To test whether RBCs sequester MMA in vitro, we added exogenous MMA (93 μg/L) to whole blood. With a mean haematocrit of 45%, if MMA were equally distributed between plasma and RBC water, we would expect to find an increase of plasma MMA levels of 93 μg/L. However, after exogenous addition of MMA, plasma levels only rose by an average of 54 μg/L. Thus, we calculated a mean recovery of exogenous MMA of 58% based on plasma levels. However, based on whole-blood lysate levels, recovery of exogenous MMA was 100%, suggesting that MMA was taken up by RBCs.
As a further test of the ability of RBC to take up MMA, we added fresh normal RBCs to whole blood spiked with exogenous MMA (93 μg/L). We calculated a mean recovery of exogenous MMA from plasma of 30%. Based on whole-blood lysate levels, though, recovery of exogenous MMA was 99%. The lower recovery (30% vs. 58%; P < 0.001) based on plasma levels with equivalent recovery based on whole-blood lysates strongly suggest that MMA is sequestered by RBCs.
Discussion
We studied the behaviour of MMA in stable outpatient haemodialysis subjects to better understand how a sequestered solute is handled by conventional haemodialysis. Our study shows that MMA is a retained solute in haemodialysis subjects, is intracellularly sequestered and rebounds to high plasma levels post dialysis, and that its clearance is significantly lower than that of urea and creatinine.
RBCs readily liberate intracellular urea as they transit the dialyser, probably via their membrane urea transporters [16]. Creatinine also permeates RBCs but its removal from them is slower than urea leading to disequilibrium between RBC and plasma creatinine in the venous effluent of the dialyser and contributing to its lower clearance than urea [17–21]. However, the finding that MMA has a lower clearance than creatinine despite being smaller (31D and 113D, respectively) and not protein bound suggests that MMA's plasma levels actually fall within the dialyser, due to its movement into the RBCs as they flow through the dialyser. That RBCs hamper MMA removal is further supported by our in vitro dialysis experiments, wherein MMA clearance rates were essentially equivalent to urea and creatinine clearances in the absence of RBCs.
Molecules with high pKa such as MMA or ammonia distribute between the cell and the extracellular space based at least in part on the diffusion of their neutral form. The generally more acidic intracellular space traps their cationic, protonated form accounting for higher intracellular total concentrations. Since RBCs are acidic compared to plasma, this non-ionic diffusion likely explains at least a part of the baseline high RBC to plasma ratios of MMA that we measured in arterial pre-dialyser blood. Recently, the Rh glycoproteins expressed on the RBC membrane, and in other tissues, have been recognized as transporters for ammonia and small aliphatic amines including MMA [22]. These may, in yet unclear ways, further contribute to RBC sequestration of amines.
This intracellular accumulation may be accentuated within the dialyser with current bicarbonate dialysis. Bicarbonate dialysate has a relatively high PCO2 compared to venous or arterial blood. Several studies have shown that as blood courses through the dialyser, PCO2 rises disproportionately to bicarbonate, and as a consequence, blood pH falls. Also PO2 rises due to the near atmospheric level of O2 in the dialysate. Our measurements confirmed these changes identified by prior studies [23,24]. These events should lower the pH within the RBC at least as much as, and perhaps transiently more than, in the adjacent plasma as CO2 rapidly diffuses into the RBC, and carbonic anhydrase generates carbonic acid. The slight elevation in PO2 would exacerbate the intracellular acidosis as oxyhaemoglobin functions as a weaker buffer than the deoxygenated form. Such an intracellular acidification would be predicted to shift MMA into the RBC and thereby reduce its plasma level and in turn diminish its removal by dialysis. The Rh transporter of the RBC would be expected to at least facilitate such an intracellular shift. This acute change in pH would not be expected to affect urea or creatinine distribution.
Our attempts at rapid separations may not have been fast enough to detect transient disequilibrium shifts of MMA into RBCs within the dialyser. Indeed, the transit time for blood through the dialyser was ~15 seconds and neither of our rapid separations was so short. Two findings do directly support the idea that MMA moves into the RBC for at least some fraction of the blood's flow through the dialyser and thereby diminishing its clearance. Firstly, MMA clearance from blood was markedly lower than that of urea and lower even than creatinine from plasma. Secondly and by contrast, all three compounds had similar clearances with RBC free in vitro dialysis.
Our studies were not designed to test the toxicity of MMA, although both direct and indirect toxicity have been ascribed to it and other small aliphatic amines [10–12]. However, these present results do emphasize that some uraemic retention solutes have behaviours that are not well predicted by the behaviour of urea and that RBCs may actively and adversely influence clearance of some solutes. Indeed, urea may be unique in the ease with which it exits the RBC during dialysis presumably due to the urea transporters in the RBC membrane. Eloot and colleagues have also noted that the guanidino compounds have kinetics different than urea [25,26]. They estimated large volumes of distribution for them but found dialyser clearances not much different than urea. The present studies differ not only in studying a compound of a different class but in finding a low clearance and documenting a major post-dialysis rebound.
Of note, longer dialysis is predicted to effect better removal of intracellularly sequestered solutes. Indeed, such seems to be the case with phosphate, another intracellular solute, as slow nocturnal dialysis is highly effective at controlling phosphate levels [27]. Thus, the epidemiologically noted benefits of longer dialysis times may reflect the better removal of many such solutes [28–33].
Acknowledgments
This study was supported by grants from the National Institutes of Health (R21 DK077326 to T.H.H., R33 DK71251 to T.W.M. and T32 DK007110 to M.P.P.).
Conflict of interest statement. None declared.
References
- 1.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 2.Dhondt A, Vanholder R, Van Biesen W, et al. The removal of uremic toxins. Kidney Int Suppl. 2000;76:S47–S59. doi: 10.1046/j.1523-1755.2000.07606.x. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 5.Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 6.Johnson WJ, Hagge WW, Wagoner RD, et al. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin Proc. 1972;47:21–29. [PubMed] [Google Scholar]
- 7.Kaufman AM, Schneditz D, Smye S, et al. Solute disequilibrium and multicompartment modeling. Adv Ren Replace Ther. 1995;2:319–329. doi: 10.1016/s1073-4449(12)80030-6. [DOI] [PubMed] [Google Scholar]
- 8.Schneditz D, Daugirdas JT. Compartment effects in hemodialysis. Semin Dial. 2001;14:271–277. doi: 10.1046/j.1525-139x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell SC, Zhang AQ. Methylamine in human urine. Clin Chim Acta. 2001;312:107–114. doi: 10.1016/s0009-8981(01)00608-8. [DOI] [PubMed] [Google Scholar]
- 10.Pirisino R, Ghelardini C, Banchelli G, et al. Methylamine and benzylamine induced hypophagia in mice: modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br J Pharmacol. 2001;134:880–886. doi: 10.1038/sj.bjp.0704316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simenhoff ML, Saukkonen JJ, Burke JF, et al. Importance of aliphatic amines in uremia. Kidney Int Suppl. 1978:S16–S19. [PubMed] [Google Scholar]
- 12.Yu PH, Wright S, Fan EH, et al. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim Biophys Acta. 2003;1647:193–199. doi: 10.1016/s1570-9639(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 13.Ihle BU, Cox RW, Dunn SR, et al. Determination of body burden of uremic toxins. Clin Nephrol. 1984;22:82–89. [PubMed] [Google Scholar]
- 14.Smith JL, Wishnok JS, Deen WM. Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol. 1994;125:296–308. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- 15.Jung D, Biggs H, Erikson J, et al. New colorimetric reaction for end-point, continuous-flow, and kinetic measurement of urea. Clin Chem. 1975;21:1136–1140. [PubMed] [Google Scholar]
- 16.Cheung AK, Alford MF, Wilson MM, et al. Urea movement across erythrocyte membrane during artificial kidney treatment. Kidney Int. 1983;23:866–869. doi: 10.1038/ki.1983.108. [DOI] [PubMed] [Google Scholar]
- 17.Descombes E, Perriard F, Fellay G. Diffusion kinetics of urea, creatinine and uric acid in blood during hemodialysis. Clinical implications. Clin Nephrol. 1993;40:286–295. [PubMed] [Google Scholar]
- 18.Gotch FA, Panlilio F, Sergeyeva O, et al. Effective diffusion volume flow rates (Qe) for urea, creatinine, and inorganic phosphorous (Qeu, Qecr, QeiP) during hemodialysis. Semin Dial. 2003;16:474–476. doi: 10.1046/j.1525-139x.2003.16102.x. [DOI] [PubMed] [Google Scholar]
- 19.Langsdorf LJ, Zydney AL. Effect of uremia on the membrane transport characteristics of red blood cells. Blood. 1993;81:820–827. [PubMed] [Google Scholar]
- 20.Skalsky M, Schindhelm K, Farrell PC. Creatinine transfer between red cells and plasma: a comparison between normal and uremic subjects. Nephron. 1978;22:514–521. doi: 10.1159/000181522. [DOI] [PubMed] [Google Scholar]
- 21.Schneditz D, Platzer D, Daugirdas JT. A diffusion-adjusted regional blood flow model to predict solute kinetics during haemodialysis. Nephrol Dial Transplant. 2009;24:2218–2224. doi: 10.1093/ndt/gfp023. [DOI] [PubMed] [Google Scholar]
- 22.Ripoche P, Bertrand O, Gane P, et al. Human Rhesus-associated glycoprotein mediates facilitated transport of NH(3) into red blood cells. Proc Natl Acad Sci U S A. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sombolos KI, Bamichas GI, Christidou FN, et al. pO2 and pCO2 increment in post-dialyzer blood: the role of dialysate. Artif Organs. 2005;29:892–898. doi: 10.1111/j.1525-1594.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 24.Symreng T, Flanigan MJ, Lim VS. Ventilatory and metabolic changes during high efficiency hemodialysis. Kidney Int. 1992;41:1064–1069. doi: 10.1038/ki.1992.162. [DOI] [PubMed] [Google Scholar]
- 25.Eloot S, Torremans A, De Smet R, et al. Complex compartmental behavior of small water-soluble uremic retention solutes: evaluation by direct measurements in plasma and erythrocytes. Am J Kidney Dis. 2007;50:279–288. doi: 10.1053/j.ajkd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Eloot S, Torremans A, De Smet R, et al. Kinetic behavior of urea is different from that of other water-soluble compounds: the case of the guanidino compounds. Kidney Int. 2005;67:1566–1575. doi: 10.1111/j.1523-1755.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 27.Mucsi I, Hercz G, Uldall R, et al. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53:1399–1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 28.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan CT, Notarius CF, Merlocco AC, et al. Improvement in exercise duration and capacity after conversion to nocturnal home haemodialysis. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm368. [DOI] [PubMed] [Google Scholar]
- 30.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. Jama. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 31.McPhatter LL, Lockridge RS, Jr, Albert J, et al. Nightly home hemodialysis: improvement in nutrition and quality of life. Adv Ren Replace Ther. 1999;6:358–365. doi: 10.1016/s1073-4449(99)70048-8. [DOI] [PubMed] [Google Scholar]
- 32.Nesrallah G, Suri R, Moist L, et al. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42:13–17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 33.Eloot S, Van Biesen W, Dhondt A, et al. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008;73:765–770. doi: 10.1038/sj.ki.5002750. [DOI] [PubMed] [Google Scholar]