Abstract
Background. Low nephron number is determined in utero and is a proposed risk for essential hypertension. Glomerular volume is inversely correlated with nephron number, and genetic and environmental factors that determine nephron number are thought to determine glomerular volume. This study compared total glomerular (nephron) number (Nglom), mean glomerular volume (Vglom) and kidney weight in two geographically separated black populations with significant common genetic ancestry.
Methods. Unbiased stereology was used to determine Nglom and Vglom in kidneys collected at coronial autopsy in an age- and sex-matched sample of 39 adult Africans from Dakar in Senegal, West Africa and 39 African Americans from Mississippi in the USA.
Results. African Americans were taller and heavier than their Senegalese counterparts. Nglom was remarkably similar—with a geometric mean of 937 967 in Senegalese and 904 412 in African Americans (P = 0.62). Vglom was correlated inversely with Nglom and directly with body surface area in both groups, but Vglom was 54% greater in African Americans than in Senegalese Africans [8.30 ± 2.92 (SD) and 5.38 ± 1.25 μm3 × 106, respectively] and remained significantly larger (38%) after adjustment for body size. Vglom increased with age in African Americans, but not in the Senegalese. Kidney weight was larger in African Americans (P < 0.0001), but kidney-to-body weight ratio was not different between groups.
Conclusions. Despite similar nephron numbers, a common genetic constitution, and even in relation to current body size, African Americans have larger Vglom than Senegalese subjects. This may mark exposure to environmental stressors or hereditary traits concentrated in the population's relocation to North America.
Keywords: African Americans, glomerular volume, kidney weight, nephron number, Senegalese Africans
Introduction
Recent autopsy studies have shown dramatic variation in total glomerular number (Nglom) in the human kidney. We have described a 9.6-fold range in Nglom (from 210 332 to 2 026 541) in the kidneys of 105 African Americans [1] and a 2.6–7.3-fold range in smaller studies of Caucasian Americans (n = 84) [1], Aboriginal (n = 19) [2] and Caucasian Australians (n = 24) [2] and Senegalese Africans (n = 28) [3]. Considerable differences in Nglom have also been observed between populations. Nyengaard and Bendtsen found a relatively low mean nephron number (617 000 nephrons) in an older sample of 37 Danes [4]. The mean number of nephrons in Senegalese Africans, African and Caucasian Americans varied from 843 106 to 925 485 nephrons with no significant difference between groups [1]. Only Australian Aborigines have been found to have a significantly lower mean nephron number than any other population with an Nglom of 713 209 ± 214 591 when compared to 861 541 ± 321 689 for Caucasian Australians [2].
Nephron endowment in humans is determined before term birth [5]. Following birth, the number of nephrons at any point in time is determined by the initial nephron endowment and by subsequent nephron loss. An increasing number of experimental and human studies have shown that single nucleotide polymorphisms [6–8] and aspects of the fetal environment [9–14] can influence nephron endowment. Several studies have shown age-associated nephron loss after birth [4,15].
Low nephron endowment has been proposed to increase the risk of hypertension in adult life, via a decrease in total filtration surface area and subsequent resetting of the pressure natriuresis curve [16]. Exacerbating lifestyle factors including obesity or a high-salt diet may further increase this risk in susceptible individuals [17]. Reduced nephron number has been related to hypertension in European and American Caucasians [18,19]. But nevertheless, despite their higher risk of hypertension and hypertensive renal disease compared to Caucasians, no significant differences in nephron number have been found between hypertensive and non-hypertensive African Americans [19,20].
Extensive variation in mean glomerular volume has also been observed in human kidneys collected at autopsy (up to 5.6-fold in adults) [15]. Increased glomerular volume is associated with higher body surface area (BSA) [3,4,15], lower nephron number [1,3,15] and with the presence of hypertension [2,18,19]. Large glomeruli are observed in populations at high risk of hypertension (e.g., African Americans and Aboriginal Australians) and renal disease (e.g., Pima Indians, African Americans and Aboriginal Australians) [2,21,22].
The objective of this study was to compare total nephron number (Nglom), mean glomerular volume (Vglom) and kidney weight in Senegalese Africans and African Americans. These two geographically separate black populations share significant common genetic ancestries, but have subsequently undergone different ancestral mixing over several centuries and have different current diets and lifestyles.
Materials and methods
Kidney collection
Kidneys were collected and weighed from Senegalese Africans coming to coronial autopsy at Hôpital Aristide Le Dantec in Dakar, Senegal from 2003 to 2005 and from African Americans coming to autopsy at the University of Mississippi Medical Center, Jackson, Mississippi, USA from 1998 to 2005. The right kidney was perfusion fixed with 10% formalin and systematically sampled (USA) or sent whole (Senegal) to Monash University, Melbourne, Australia for stereological analysis.
Kidney collection and analysis was carried out with permission of next-of-kin and in accordance with the standards of the Standing Committee on Ethics in Research Involving Humans at Monash University (project approval numbers 2002/204 and 2006/753).
Subject selection
The first 39 kidneys of Senegalese African adults to be analysed were compared to those of age- and sex-matched African Americans. African American subjects were selected from a cohort of 97 subjects whose kidneys had been previously examined [1] and were matched to the Senegalese Africans according to sex and age (Table 1). A random number generator was used for selection of African American subjects when more than one suitable subject was available. Findings from analysis of kidney pathology and heterogeneity of glomerular volume within the kidneys of some of these Senegalese and African Americans have been previously reported [3,23,24].
Table 1.
Physical characteristics of the 39 Senegalese Africans and the 39 adult African Americans. Values are mean (SD)
| Senegalese Africans | African Americans | P-value | |
|---|---|---|---|
| Age (years) | 42.3 (14.9) | 41.6 (13.8) | 0.83 |
| Male:Female | 29:10 | 29:10 | |
| Height (cm) | 168.2 (7.0) | 176.2 (12.6) | 0.0008 |
| Body weight (kg) | 71.0 (10.9) | 88.4 (26.1) | 0.0002 |
| BSA (m2) | 1.83 (0.16) | 2.08 (0.35) | 0.0001 |
| BMI (kg/m2) | 25.2 (4.2) | 29.2 (8.3) | 0.008 |
| Kidney weight (g) | 142.1 (32.6) | 180.1 (49.0) | 0.0001 |
| Kidney-to-body weight ratio (× 10−3) | 2.03 (0.51) | 2.18 (1.47) | 0.38 |
Cause of death
The main cause of death in the Senegalese Africans was death by misadventure (n = 28, 71.8%; accidents, homicides, suicide). Two deaths (5.1%) were cardiac related. The remaining deaths were due to infection, cancer or pulmonary related (n = 9, 23.1%). In the 39 African Americans, the proportion of deaths due to cardiac or cerebrovascular causes was much greater (n = 20, 51.3%). Accidental and violent deaths accounted for 17.9% of deaths (n = 7). The remainder were due to pulmonary embolism and other non-renal illnesses (n = 12, 30.8%).
Estimation of total nephron number and mean glomerular volume
Following systematic macroscopic sampling for stereology, selected blocks were embedded in glycol methacrylate and exhaustively sectioned at 20 μm. The physical disector/fractionator combination was used for unbiased stereological estimation of total glomerular (nephron) number. The disector/fractionator technique uses systematically sampled section pairs to count glomeruli at a unique point (when they first appear in the serial sections) in a known fraction of the kidney. Total glomerular number is calculated using basic algebra. Mean glomerular volume (Vglom) estimates were obtained by dividing the volume density of glomeruli in the kidney (VVglom,kid) (derived from point counting with a stereological test grid) by the numerical density of glomeruli in the kidney (NVglom,kid). These stereological methods have been described in detail in our previous publications [15,25].
Classification of hypertensive status
African American were categorized into hypertensive and non-hypertensive on the basis of a history of hypertension, consistently elevated blood pressures (≥140/90 mm Hg), mean arterial pressure (MAP) ≥ 107 mm Hg, the presence of cardiomegaly and severity of renal arteriosclerosis, as previously described [19]. One subject was classed as ‘probably hypertensive’ on the basis of a history of hypertension and confirmation by two other marks, but had no available blood pressure record. This subject was analysed with confirmed hypertensives. Hypertensive status was available for 38 African Americans. No information was available on the hypertensive status of the Senegalese autopsy subjects.
Statistical analysis
Analysis of data was performed using STATA statistical package, Version 8 (College Station, Texas, USA). Two-tailed Student t-tests were used to compare the mean values of the two groups. Variables with a skewed distribution were transformed using the natural logarithm, and geometric means were used to compare the two groups. Pearson's product moment correlation and linear regression were used for univariate analysis of parametric variables, while Spearman's rho correlation (r) was used for non-parametric data. One-way ANOVA with Bonferroni post hoc test was used to test differences in Vglom by hypertensive status and to compare to Senegalese. In all tests, P < 0.05 was considered significant.
Results
Physical characteristics of the sample populations
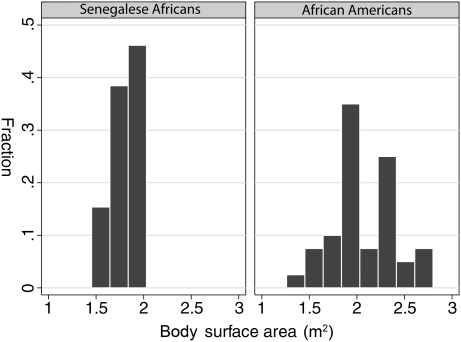
Characteristics of the two groups are presented in Table 1. As dictated by the study design, subjects were matched for gender and well matched for age. African Americans had significantly greater height, body weight, BSA and body mass index (BMI) than the Senegalese Africans. Mean height in the African Americans was 8 cm greater than Senegalese adults (P < 0.0008), and African Americans were on average 17 kg heavier than the Senegalese Africans (P = 0.0002). The striking difference in the range of BSA in the two groups is shown in Figure 1. All 39 Senegalese had a BSA <2.1 m2, whereas 44% of the African Americans had a BSA greater or equal to 2.1 m2.
Fig. 1.
Distribution of BSA in Senegalese Africans and age- and sex-matched African Americans.
Kidney weight
Kidney weight was 27% greater in African Americans than in Senegalese Africans (P < 0.0001). The difference in kidney weight between the races was reduced to 16% following adjustment for BSA (P = 0.02). The kidney-to-body weight ratio was similar in the two groups (P = 0.38), but variation was greater in African Americans (Table 1).
Total nephron number and mean glomerular volume
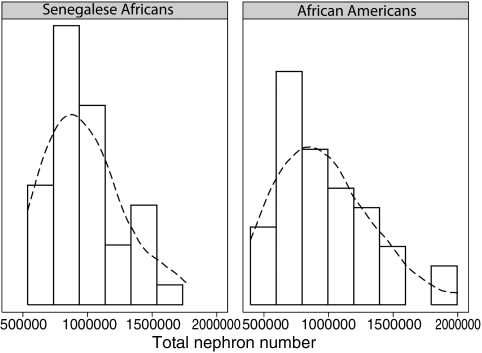
Total nephron number (Nglom) was remarkably similar in Senegalese Africans {geometric mean 937 967 [859 432–1 023 686 95% confidence interval (95% CI)]} and African Americans [geometric mean 904 412 (801 466–1 020 571 95% CI)], with the upper limit of the range being higher in African Americans (up to 2 026 541) (Table 2, Figure 2).
Table 2.
Total nephron number (Nglom) and mean glomerular volume (Vglom) in Senegalese Africans and African Americans
| Senegalese Africans | African Americans | P-value | |
|---|---|---|---|
| Nglom, gmean (95% CI) | 937 967 (859 432 – 1 023 686) | 904 412 (801 466 – 1 020 571) | 0.62 |
| Nglom range | 536 171 – 1 764 421 | 395 054 – 2 026 541 | |
| Vglom (μm3 × 106), mean (SD) | 5.38 (1.25) | 8.30 (2.92) | <0.0001 |
| Vglom range | 2.52 – 7.54 | 3.48 – 15.61 | |
| Vglom (BSA-adjusted) (μm3 × 106) | 5.74 | 7.94 | <0.0001 |
Gmean, geometric mean; 95% CI, 95% confidence interval.
Fig. 2.
Distribution of total nephron number (Nglom) in kidneys of Senegalese Africans and age- and sex-matched African Americans. Curves correlated using kernel density.
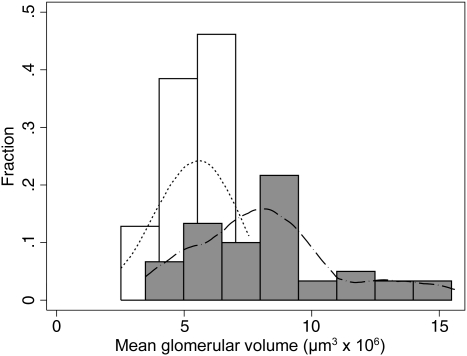
In contrast, mean glomerular volume (Vglom) differed greatly between the two populations (Table 2, Figure 3). Vglom was 54% greater in African Americans who also displayed a 4.5-fold range in Vglom compared to the 3.0-fold range in Senegalese. Furthermore, following adjustment for BSA, Vglom in African Americans remained 38% greater than in the Senegalese (P < 0.0001).
Fig. 3.
Distribution of mean glomerular volume (Vglom) in kidneys of Senegalese Africans (white columns) and age- and sex-matched African Americans (dark columns). Curves correlated using kernel density.
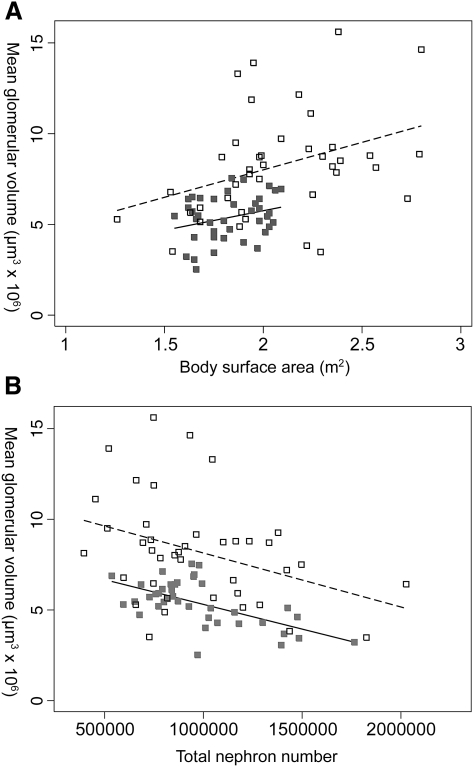
The significant difference in Vglom was also observed when the comparison was restricted to subjects with BSA within the range of 1.4–2.1 m2 (all 39 Senegalese, 21 African Americans). Vglom was found to be 48% larger in African Americans, with a mean of 7.95 μm3 × 106 in African Americans and 5.38 μm3 × 106 in Senegalese Africans (P < 0.0001), despite similar mean BSA (Senegalese: 1.83 m2 and African American: 1.85 m2, P = 0.60). Linear regression showed that predicted Vglom was greater in African Americans than in Senegalese Africans for all BSA (Figure 4A). The relationship between Vglom and BSA was similar between the groups with no significant interactions (P = 0.74).
Fig. 4.
Relationships between mean glomerular volume and (A) BSA (r2 = 0.08, P = 0.09; r2 = 0.13, P = 0.03) and (B) total nephron number (r2 = 0.37, P < 0.0001; r2 = 0.14, P = 0.02) in Senegalese Africans (closed grey squares) and African Americans (open squares).
Relationships between Vglom, Nglom and age
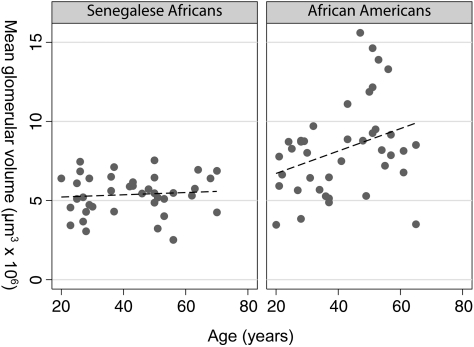
Vglom was inversely correlated with Nglom in both groups (Figure 4B). Similar relationships between Vglom and Nglom were observed for both groups: for every one million increase in nephron number, glomerular volume was found to be 2.94 μm3 × 106 smaller in African Americans and 2.73 μm3 × 106 smaller in Senegalese Africans. There was no difference between the beta coefficients of each group (P = 0.89). However, glomeruli of African Americans were significantly larger than those of Senegalese at all nephron numbers (P = 0.049); the difference in volume by race was 2.90 μm3 × 106 (P < 0.001). Vglom increased with increasing age in African Americans (r = 0.32, P = 0.04), but not in Senegalese Africans (r = 0.08, P = 0.61) (Figure 5).
Fig. 5.
Mean glomerular volume by age in Senegalese Africans (r2 = 0.01, P = 0.61) and African Americans (r2 = 0.10, P = 0.04).
Vglom and hypertension in African Americans
Twenty-four of the 38 African Americans (63%) were hypertensive. The hypertensive status of the Senegalese Africans is not known. However, there were no significant differences in Vglom among African Americans who were and were not hypertensive (P = 0.12), and the Vglom of those who were not hypertensive was still larger than in the Senegalese (P = 0.02) (Table 3). Nglom did not differ between hypertensive and non-hypertensive African Americans (P = 0.94) or compared to Senegalese Africans (P = 1.00, P = 1.00).
Table 3.
Physical characteristics and kidney data for Senegalese Africans compared to African Americans by hypertensive (HT) status
| Senegalese | African Americans |
P-value |
||||
|---|---|---|---|---|---|---|
| A. (n = 39) | B. Non-HT (n = 14) | C. HT (n = 24) | (A vs B) | (A vs C) | (B vs C) | |
| Age (years) | 42.3 (14.9) | 32.0 (11.5) | 46.7 (12.2) | 0.05 | 0.65 | 0.006 |
| Height (cm) | 168.2 (7.0) | 178.7 (16.2) | 175.1 (10.3) | 0.005 | 0.03 | 0.91 |
| Weight (kg) | 71.0 (10.9) | 83.3 (23.3) | 90.5 (27.9) | 0.15 | 0.001 | 0.88 |
| BSA (m2) | 1.83 (0.16) | 2.03 (0.33) | 2.10 (0.37) | 0.07 | 0.001 | 1.00 |
| BMI (kg/m2) | 25.2 (4.2) | 26.4 (6.8) | 30.5 (8.8) | 1.00 | 0.006 | 0.18 |
| Nglom | 972 825 (277 237) | 899 343 (334 835) | 1 011 123 (392 696) | 1.00 | 1.00 | 0.94 |
| Vglom (μm3 × 106) | 5.38 (1.25) | 7.31 (2.02) | 8.88 (3.30) | 0.02 | <0.0001 | 0.12 |
Cause of death analysis
A significantly greater proportion of African Americans died of coronary artery disease/cerebrovascular disease (CAD/CVD)-related causes (51.3%, 20 cases) compared to the Senegalese Africans (5.1%, two cases). Cause of death due to CAD/CVD did not appear to be associated with consistent variations in Nglom or Vglom, there being no difference in Vglom (P = 0.78), Nglom (P = 0.25), age (P = 0.74) or BSA (P = 0.58) between African Americans who died of CAD/CVD-related causes and the remaining African American subjects. Only kidney weight was significantly greater in those with cardiac-related deaths (P = 0.033), but this was predominantly accounted for by body weight, as the kidney-to-body weight ratio was not significantly different (P = 0.197). Cardiac-related deaths in the Senegalese sample (n = 2) were too few to allow meaningful comparison with the remaining subjects (n = 37).
A comparison of African Americans and Senegalese Africans dying of non-CAD/CVD-related deaths (n = 19 and n = 37) found the same relationships that were observed in the comparison of the whole population samples.
Discussion
The main objective of this study was to compare total nephron number and mean glomerular volume in age- and sex-matched African Americans and native West Africans from Dakar in Senegal. Three principal findings emerged from the comparison: (i) total nephron number per kidney (Nglom) was remarkably similar in the two races; (ii) mean glomerular volume (Vglom) was significantly greater in African Americans than in Senegalese and was not completely accounted for by differences in body size; and (iii) kidney weight was greater in African Americans than in Senegalese but was largely accounted for by the difference in body size.
The similarity in Nglom in African Americans and Senegalese Africans was remarkable, differing by only 3.6%, despite weakening of genetic ties by racial admixing and differences in prenatal nutrition and prenatal care in the different countries. Nglom measured at autopsy reflects both nephron endowment (the number of nephrons formed during fetal development) and subsequent nephron loss throughout postnatal life. However, regardless of endowment or possible nephron loss, the similarity in nephron number in these two groups, as determined at autopsy, means that in African Americans a similar complement of nephrons is required to provide for the demands of a larger body size (larger in both height and weight). In larger African Americans, particularly those with low numbers of nephrons, single nephron filtration rates are likely to be increased and greater strain may be placed on individual nephrons compared to their smaller African counterparts.
The difference in mean glomerular volume in these two populations of West African origin was striking. Although differences in Vglom could result from differences in tissue deformation during tissue processing, the strict histology protocols conducted within the same laboratory for all kidneys and the magnitude of the difference between the races make this an unlikely explanation. Vglom in African Americans was more than 1.5-times that in the Senegalese Africans from Dakar. Furthermore, a significant amount of glomerular enlargement in African Americans was independent of body size—larger body size in African Americans only accounted for 16% of the difference in the overall sample. Larger glomerular size was clearly observed in African Americans within the same range of body sizes as the Senegalese. A similar Vglom to the Senegalese has been observed in non-hypertensive Danes (5.98 μm3 × 106), who also had a low mean BSA (1.70 m2) [4]. Vglom in the African Americans in this study was not dissimilar to that in Caucasian Americans (7.1 μm3 × 106) and in Aboriginal Australians (7.7 μm3 × 106) simultaneously studied by our group [26]. Substantial proportions of those populations had hypertension documented prior to death, and Vglom tended to be higher in those who were hypertensive than those who were not [26].
Both populations of African origin demonstrated an inverse relationship between Vglom and Nglom, so that kidneys with more glomeruli tended to have smaller glomeruli than those with fewer nephrons. This fundamental relationship has been observed in many autopsy study populations [1], and here we demonstrated that the relationship was similar for both groups, but with greater variation in African Americans and increased glomerular volume in African Americans at all levels of nephron number compared to Senegalese.
The increased Vglom in African American subjects appeared to be related to age. This apparent age-related enlargement was not directly related to larger body size, as BSA did not change significantly with age. However, it may reflect accumulated exposure to environmental stressors with increasing age in African Americans that are not present to the same extent in Senegalese Africans.
Rates of hypertension are known to increase with age in both populations [27,28], with considerable prevalence reported for both populations. Direct comparisons of the prevalence in each population are difficult due to differences in collection methods and definitions. In 1990, 18.3% of urban Senegalese males and 33.9% of urban Senegalese females aged 45–55 years were hypertensive (defined as blood pressure (BP) ≥ 160/95 mm Hg) [27]. The National Health and Nutrition Examination Survey (NHANES) survey found that the prevalence of hypertension (defined as ≥ 140/90 mm Hg) in African Americans was 40% in those aged 40–59 years [28]. Comparing standardized and adjusted rates using the World Health Organization Global Comparable Estimates Tool [29], mean BP in Senegal (133.8 mm Hg) was higher than the national mean for the USA (123.3 mm Hg).
Analysis of the effect of hypertension on glomerular volume was not the main aim of this study as individual blood pressure data were not available for the Senegalese African cohort. Despite this, our study demonstrated that Vglom in African Americans was significantly greater compared to Senegalese regardless of their hypertensive status in this small sample. This finding suggests that other factors, or at least factors acting prior to a diagnosis of hypertension, may be driving greater glomerular hypertrophy in the African Americans.
African Americans had a larger kidney weight than the Senegalese Africans, but kidney-to-body weight ratio was not significantly different. Increased BSA is associated with increased kidney weight [3,4,15]. Recorded kidney weights of previously studied autopsy populations vary widely, from 161.9 to 217.6 g in Australians and Americans. In the older Danish population, mean kidney weight was 131 g [4], and a study of kidney weight in autopsy cases from black tribes in Southern Africa in 1983–1985 found mean combined kidney weight varied from 213 to 245 g [30], therefore ∼107 to 123 g for single kidneys. In the current study, while kidneys of African Americans were heavier compared to those of Senegalese Africans, glomerular volume per gramme of kidney weight was significantly greater in African Americans than in Senegalese. When considered with the similarity of total nephron number in the two groups, this highlights the greater degree of nephron and/or glomerular enlargement occurring in the African Americans.
The increased glomerular volume of African Americans compared to their African counterparts may reflect the genetic and environmental factors that have changed with the relocation of Africans to North America. Enlarged glomeruli are thought to be more susceptible to sclerosis as a consequence of hyperfiltration and podocyte dysfunction [31,32], and larger glomerular size has been demonstrated in populations, including African Americans, who are at high risk of chronic kidney disease [22,33]. The inheritance of several MYH9 polymorphisms has been linked to the increased risk of African Americans for non-diabetic chronic kidney disease. The increased risk is strongest for focal segmental glomerulosclerosis but also includes hypertension. MYH9 encodes non-muscle β-actin which is a major cytoskeletal component of podocytes. Individuals who have inherited African rather than protective Caucasian alleles may be more susceptible to podoycte injury for any of several causes of glomerular stress that may themselves be environmental [34].
In summary, this study demonstrated similar numbers of nephrons in the kidneys of African Americans and Senegalese Africans from an urban community, but much greater glomerular volumes in the kidneys of African Americans. Glomerular enlargement in African Americans was largely independent of body size and was proportionately greater than the overall kidney enlargement. The findings in these two black populations with common genetic constitution suggest that environmental factors or gene–environment interactions are likely to underlie the glomerular enlargement in African Americans.
Acknowledgments
The authors would like to acknowledge Sue Connell and Julie Hickey for their assistance in sectioning the tissue samples. PhD scholarship funding for B.J.M. was provided by an Australian Postgraduate Award and a Faculty of Medicine Dean's Excellence Award. Partial support for this work was provided by grants from the National Institutes of Health NIH 1 RO1 DK065970-01, NIH Center of Excellence in Minority Health 5P20M00534-02 and National Health and Medical Research Council (NHMRC) Program grant 502009. Glomerular counts for African American kidneys were performed by R.N.D.-D. for previous publications [1,15,19].
Conflict of interest statement. None declared.
References
- 1.Douglas-Denton RN, McNamara BJ, Hoy WE, et al. Does nephron number matter in the development of kidney disease? Ethn Dis. 2006;16 S2-40–45. [PubMed] [Google Scholar]
- 2.Hoy WE, Hughson MD, Singh GR, et al. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–110. doi: 10.1038/sj.ki.5000397. [DOI] [PubMed] [Google Scholar]
- 3.McNamara BJ, Diouf B, Hughson MD, et al. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Tranplant. 2008;23:2576–2585. doi: 10.1093/ndt/gfn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 5.Hinchliffe SA, Sargent PH, Howard CV, et al. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–784. [PubMed] [Google Scholar]
- 6.Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17:1568–1575. doi: 10.1681/ASN.2005101074. [DOI] [PubMed] [Google Scholar]
- 7.Quinlan J, Lemire M, Hudson T, et al. A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol. 2007;18:1915–1921. doi: 10.1681/ASN.2006101107. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Quinlan J, Hoy W, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19:2027–2034. doi: 10.1681/ASN.2007101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 10.Lelievre-Pegorier M, Vilar J, Ferrier ML, et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54:1455–1462. doi: 10.1046/j.1523-1755.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 11.Merlet-Benichou C, Gilbert T, Muffat-Joly M, et al. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 12.Merlet-Bénichou C, Gilbert T, Vilar J, et al. Nephron number: variability is the rule. Causes and consequences. Lab Invest. 1999;79:515–527. [PubMed] [Google Scholar]
- 13.Ortiz LA, Quan A, Weinberg A, et al. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Cullen-McEwen L, Kett M, et al. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579:503–513. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. 2003;83:S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 16.Brenner B, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 17.Praga M. Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Tranplant. 2005;20:2594–2597. doi: 10.1093/ndt/gfi201. [DOI] [PubMed] [Google Scholar]
- 18.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 19.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 20.Hughson MD, Gobe GC, Hoy WE, et al. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt K, Pesce C, Liu Q, et al. Large glomerular size in Pima Indians: lack of change with diabetic nephropathy. J Am Soc Nephrol. 1992;3:229–235. doi: 10.1681/ASN.V32229. [DOI] [PubMed] [Google Scholar]
- 22.Young RJ, Hoy WE, Kincaid-Smith P, et al. Glomerular size and glomerulosclerosis in Australian aborigines. Am J Kidney Dis. 2000;36:481–489. doi: 10.1053/ajkd.2000.9788. [DOI] [PubMed] [Google Scholar]
- 23.Samuel T, Hoy WE, Douglas-Denton R, et al. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 24.McNamara B, Diouf B, Hughson M, et al. Associations between age, body size and nephron number with individual glomerular volumes in urban West African males. Nephrol Dial Tranplant. 2009;24:1500–1506. doi: 10.1093/ndt/gfn636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- 26.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 27.Astagneau P, Lang T, Delarocque E, et al. Arterial hypertension in urban Africa: an epidemiological study on a representative sample of Dakar inhabitants in Senegal. J Hypertens. 1992;10:1095–1101. [PubMed] [Google Scholar]
- 28.Hajjar I, Kotchen TA, Hajjar I, et al. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 29.WHO . World Health Organization Comparable Estimates. 2002. https://apps.who.int/infobase/comparestart.aspx. [Google Scholar]
- 30.Moar JJ, Reinach SG. Renal weights in the southern African black population. Am J Phys Anthropol. 1988;76:105–110. doi: 10.1002/ajpa.1330760109. [DOI] [PubMed] [Google Scholar]
- 31.Fogo AB. Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases. Kidney Int Suppl. 2000;75:S15–S21. [PubMed] [Google Scholar]
- 32.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 33.Hughson MD, Johnson K, Young RJ, et al. Glomerular size and glomerulosclerosis: relationships to disease categories, glomerular solidification, and ischemic obsolescence. Am J Kidney Dis. 2002;39:679–688. doi: 10.1053/ajkd.2002.31980. [DOI] [PubMed] [Google Scholar]
- 34.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]