Abstract
Background. Anaemia worsens as kidney function declines. Both conditions are associated with increased mortality. Serum cystatin C is purportedly a more sensitive marker of kidney disease and a better predictor of mortality than serum creatinine. However, studies suggest that extrarenal factors also influence cystatin C levels.
Methods. We determined whether estimates of glomerular filtration rate [estimated glomerular filtration rate (eGFR)] based on serum cystatin C alone or in combination with serum creatinine were superior to those based on serum creatinine in recognizing impaired kidney function in the setting of anaemia in a sub-sample of the Third National Health and Nutrition Examination Survey of the USA consisting of 6734 participants, 20 years or older.
Results. The prevalence of moderate to severe kidney disease (eGFR 15–59 mL/min/1.73 m2) among anaemic persons was 15–16% when based on serum creatinine alone (eGFRSCR) or combined with cystatin C (eGFRSCR + CYSC); this estimate increased to nearly 25% when kidney function was estimated by cystatin C (eGFRCYSC). The adjusted odds ratios of kidney disease in anaemic versus non-anaemic persons were slightly higher with eGFRCYSC than eGFRSCR and eGFRSCR + CYSC in younger adults [odds ratio (OR) = 5.22, 95% confidence interval (CI): 2.23, 12.17], women (OR = 5.34, 95% CI: 2.36, 12.06) and those with elevated C-reactive protein (CRP) (OR = 7.36, 95% CI: 1.98–27.36).
Conclusions. Impaired kidney function was common in individuals with anaemia. Among anaemic individuals, the prevalence estimate for kidney disease was notably higher when kidney function was estimated by cystatin C alone compared with the estimations by serum creatinine alone or in combination with serum cystatin C. eGFRCYSC may be particularly helpful in identifying kidney disease in the setting of anaemia among younger persons, women and those with elevated CRP. Regardless of which renal biomarker is used, our study suggests that an evaluation for underlying kidney disease should be considered in the standard workup of anaemia.
Keywords: anaemia, chronic kidney failure, creatinine, cystatin C, glomerular filtration rate
Introduction
Anaemia is associated with cognitive impairment [1,2], cardiovascular disease [3,4] and increased mortality [3,5,6]. It is also suspected of playing a role in chronic kidney disease progression [7]. Anaemia commonly occurs among the elderly and individuals with chronic illnesses, with a reported prevalence of ∼11% in persons 65 years and older [8,9]. Anaemia in these populations is often termed ‘anaemia of chronic disease’ or ‘anaemia of chronic inflammation’. It results from a cytokine-mediated disruption of iron metabolism and relative erythropoietin deficiency [10,11].
Anaemia associated with chronic kidney disease develops from similar pathological processes. In addition to decreased erythropoietin production, inflammation, which often accompanies kidney disease, leads to abnormal iron handling and blunted response to erythropoietin [12–14]. Some cases diagnosed as anaemia of chronic disease may result from occult kidney dysfunction, as anaemia often develops well before overt kidney disease is recognized [9,15,16]. This may be due, in part, to the insensitivity of serum creatinine to an early kidney function decline, and its dependence on age, gender, ethnicity, nutritional status and lean muscle body mass [17–19]. This insensitivity is particularly pertinent in individuals at high risk for both anaemia and kidney disease, in whom serum creatinine could be misleadingly low due to muscle loss or hyperfiltration. A more sensitive marker of kidney dysfunction may afford an earlier diagnosis of kidney disease among anaemic individuals.
Cystatin C is purportedly less affected by extrarenal factors [20] and has been more predictive of mortality than serum creatinine [21,22]. Therefore, it has been proposed as a better indicator of kidney function, particularly at milder levels of kidney dysfunction [23]. However, recent studies demonstrate positive correlations between serum cystatin C and increasing age as well as inflammatory markers [24,25]. Given serum cystatin C's positive relationship with these factors which also play a role in anaemia [26], we hypothesized that the estimates of kidney function based on serum cystatin C alone or in combination with serum creatinine would be superior to the estimates based on serum creatinine in identifying impaired kidney function in the anaemic individuals. Using a sub-sample of participants in the Third National Health and Nutrition Examination Survey (NHANES III), we determined whether the association between kidney disease and anaemia differed based on the method utilized to estimate kidney function, and examined factors which may impact this association.
Materials and methods
Study population
NHANES III was a cross-sectional survey conducted in the USA from 1988 to 1994 by the National Center for Health Statistics. In-person interviews and physical examinations were performed. Serum samples were obtained from non-institutionalized individuals. The survey employed a complex, multistage, clustered sampling design with oversampling of non-Hispanic blacks, Mexican Americans and elderly individuals.
Sample selection
A total of 15 488 NHANES III participants who had stored serum available and serum creatinine measured were eligible for serum cystatin C measurement. All eligible participants aged 60 years or older, those with elevated serum creatinine (>1.2 mg/dL in men and >1.0 mg/dL in women) aged 12 to 59 years and a random 25% sample of those aged 12 to 59 years underwent the serum cystatin C measurement [20]. Of 7596 participants who had serum cystatin C measured, 6951 were 20 years old or older. Among these, 6886 had haemoglobin previously measured. We excluded pregnant women (n = 46) and individuals with estimated glomerular filtration rates (eGFRs) <15mL/min/1.73 m2 based on either serum creatinine, cystatin C or both (n=106 125). The estimates provided in this study are therefore based on 6734 NHANES III participants 20 years or older.
Outcome
We defined anaemia using the World Health Organization (WHO) criteria of a haemoglobin level <13 g/dL in men and <12 g/dL in women [27]. Haemoglobin was determined using an automated haematology analyser (Coulter S-Plus; Beckman Coulter; Fullerton, CA). Serum ferritin, iron and total iron-binding capacity were measured, and transferrin saturation was calculated as previously described [28,29]. We categorized iron status into three groups according to serum ferritin and transferrin saturation. Absolute iron deficiency was defined by serum ferritin ≤40 ng/mL and transferrin saturation <20% while functional iron deficiency was defined by serum ferritin >40 ng/mL and transferrin saturation <20%. The remaining participants were classified as having a normal iron status [30].
Determination of kidney function
Kidney function was determined by GFR using serum creatinine, serum cystatin C or both. Serum creatinine was initially determined by the modified kinetic Jaffe reaction (Hitachi 737; Boehringer Mannheim; Indianapolis, IN) and subsequently calibrated to an enzymatic method (Roche; Basel, Switzerland) [31]. We estimated the serum creatinine-based GFR using the re-expressed four-variable Modification of Diet in Renal Disease equation: eGFRSCR = (175 × standardized serum creatinine−1.154 × age−0.203) × [0.742 (if female) × 1.212 (if black)] [32].
Serum cystatin C was measured using an automated particle-enhanced nephelometric assay (N Latex Cystatin C, Dade Behring, Deerfield, IL), as recently described [24]. We estimated the serum cystatin C-based GFR using: eGFRCYSC = (127.7 × cystatin C−1.17 × age−0.13) × [0.91 (if female) × 1.06 (if black)] [33]. We also estimated the GFR based on both serum creatinine and cystatin C using: eGFRSCR + CYSC = (177.6 × standardized serum creatinine−0.65 × cystatin C−0.57 × age−0.20) × [0.82 (if female) × 1.11 (if black)] [33]. The development and validation of these equations were recently detailed [33]. We reset the eGFR values which were improbably elevated to a maximum of 200 mL/min/1.73 m2 (n = 9). We defined moderate to severe kidney disease as an eGFR of 15–59 mL/min/1.73 m2.
Other independent variable measurements
The participants self-selected ethnicity and self-reported age and gender. The participants who reported other ethnicity were classified as non-Hispanic white. The self-reported diabetic history, a fasting blood glucose ≥126 mg/dL or a non-fasting blood glucose of ≥200 mg/dL defined diabetes. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or self-reported use of antihypertensive medications. Hypercholesterolaemia was defined as total serum cholesterol ≥200 mg/dL. We categorized participants as non-smokers (no prior smoking history and cotinine levels ≤15 ng/mL), former smokers (smoked >100 cigarettes in their lifetime, quit smoking and cotinine levels ≤15 ng/mL) or current smokers (self-reported smoking or cotinine levels >15 ng/mL). We calculated body mass index (BMI) from the measured height and weight and analysed it in increments of 10 kg/m2 to account for its non-linear relationship with haemoglobin. Urinary albumin-to-creatinine ratio was calculated as described previously [34]. We defined albuminuria as a urinary albumin-to-creatinine ratio >30 mg/g. Measurements of serum albumin, C-reactive protein (CRP), thyroid-stimulating hormone (TSH) and other data are described in detail elsewhere [35].
Statistical analysis
To obtain national estimates, we modified the sampling weights to account for the participants excluded from the sub-sample and serum cystatin C measurement [24,36]. We weighted all prevalence estimates to represent the civilian, non-institutionalized US population and derived standard errors (SEs) for the estimates using the Taylor series linearization method. To compare the general characteristics of individuals differentially categorized as having moderate to severe kidney disease by eGFRSCR and eGFRCYSC, we used survey design-corrected t-tests for continuous variables and chi-square tests for categorical variables. Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using logistic regression models. We conducted the analyses using Stata 9 ‘svy’ commands (StataCorp, College Station, TX).
The variables with skewed distributions were log-transformed for statistical analyses. We determined the prevalence of anaemia by eGFR as a continuous and as a binary variable (≥60 versus 15–59mL/min/1.73 m2). We used multivariable logistic regression models to assess the association between moderate to severe kidney disease and anaemia. Independent variables were introduced into the model based on their potential confounding of the relationship between kidney function and anaemia. Model building consisted of sequential addition of demographic factors, followed by comorbidities, smoking status, BMI, serum albumin, log-CRP and log-TSH. These variables were evaluated for interaction with kidney function. We performed stratified analyses when significant interactions were observed and based on age (<60 and ≥60 years old), ethnicity and CRP level (<1 and ≥1 mg/dL). The final models were based on a priori hypotheses, overall model fit and parsimony. This method was utilized for eGFRSCR, eGFRCYSC and eGFRSCR + CYSC. To predict the prevalence of kidney disease across the range of haemoglobin, we performed logistic regressions. This study was exempt from the Institutional Review Board.
Results
Table 1 presents the number of survey participants and the estimated distribution of characteristics among the non-institutionalized US population 20 years or older with and without anaemia. The anaemic group was older and had a greater proportion of women and non-Hispanic blacks compared to the non-anaemic group. While the anaemic persons were more likely to have hypertension and had higher CRP levels, they were less likely to have hypercholesterolaemia than the non-anaemic group. Although the average serum creatinine and the eGFRSCR levels were similar between the two groups, the anaemic persons had slightly higher levels of serum cystatin C and lower estimates of kidney function based on cystatin C alone or combined with serum creatinine.
Table 1.
Characteristics of individuals aged 20 and older with and without anaemia in NHANES III (1988–94)
| Characteristic | Unweighted n | Participants without anaemia (n = 5992) | Participants with anaemiaa (n = 742) | P-value | |
|---|---|---|---|---|---|
| Age, years | 6734 | 44.4 (0.8) | 48.8(1.1) | <0.01 | |
| Men, % (SE) | 3227 | 49.2 (1.5) | 22.6 (2.9) | <0.01 | |
| Ethnicity, % (SE) | |||||
| Non-Hispanic white | 3487 | 85.4(1.0) | 61.0 (0.8) | <0.01 | |
| Non-Hispanic black | 1657 | 9.5(0.8) | 33.5 (3.6) | <0.01 | |
| Mexican American | 1590 | 5.0 (0.5) | 5.5 (0.7) | 0.51 | |
| Haemoglobin, g/dL | 6734 | 14.3 (0.1) | 11.4(0.1) | <0.01 | |
| Transferrin saturation <20%, % (SE) | 2223 | 28.0 (1.2) | 59.7 (3.6) | <0.01 | |
| Serum ferritin <40 μg/L, % (SE) | 1271 | 22.4(1.2) | 54.8 (3.5) | <0.01 | |
| Serum creatinine, mg/dL | 6734 | 0.82 (1.01) | 0.81 (1.02) | 0.32 | |
| Serum cystatin C, mg/L | 6734 | 0.87 (1.01) | 0.93 (1.02) | <0.01 | |
| eGFR, mL/min/1.73 m2b | 6734 | ||||
| SCr-based | 90.3 (1.01) | 87.7 (1.02) | 0.19 | ||
| CysC-based | 88.5(1.01) | 79.8 (1.03) | <0.01 | ||
| SCr and CysC-based | 94.7 (1.01) | 88.1(1.03) | <0.01 | ||
| Microalbuminuria, % (SE) | 6526 | 1.0 (0.2) | 3.5 (0.8) | <0.01 | |
| Smoking status, % (SE) | |||||
| Non-smoker | 3001 | 42.5 (1.2) | 55.9 (3.0) | <0.01 | |
| Former smoker | 1767 | 22.9 (1.3) | 21.1 (3.4) | <0.01 | |
| Current smoker | 1900 | 34.6 (1.5) | 22.9 (2.7) | 0.61 | |
| Comorbid conditions, % (SE) | |||||
| Diabetes mellitus | 1313 | 10.0 (0.8) | 11.5 (1.9) | 0.42 | |
| Hypertension | 3087 | 24.3 (1.3) | 36.0 (3.7) | <0.01 | |
| Hypercholesterolaemia | 3964 | 51.6 (1.3) | 38.6 (3.7) | <0.01 | |
| BMI, kg/m2 | 6721 | 26.7 (0.1) | 26.7 (0.3) | 0.88 | |
| Serum albumin, g/dL | 6733 | 4.2 (0.1) | 3.9 (0.1) | <0.01 | |
| C-reactive protein, mg/dLb | 6727 | 0.29 (1.02) | 0.37 (1.06) | <0.01 | |
| Thyroid-stimulating hormone, mU/Lb | 6616 | 1.70 (1.04) | 1.56 (1.07) | 0.28 | |
Data presented as mean (SE) unless otherwise specified.
BMI, body mass index; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; CysC, serum cystatin C.
Anaemia defined as haemoglobin <13 g/dL in men; <12 g/dL in women.
Geometrical mean (SE) presented.
While 838 participants were classified as having moderate to severe kidney disease by eGFRCYSC but not eGFRSCR, far fewer individuals (n = 181) were classified as having moderate to severe kidney disease by eGFRSCR but not eGFRCYSC. Table 2 displays the characteristics of individuals according to the agreement or disagreement in kidney disease classification by eGFRSCR and eGFRCYSC. Compared to the persons who were classified as not having kidney disease by both GFR-estimating methods, those who were classified as having kidney disease by both methods were older, more likely to be women and anaemic. They also had a greater prevalence of kidney disease risk factors, microalbuminuria and elevated CRP levels. In general, those who were differentially classified as having kidney disease by eGFRCYSC and eGFRSCR were older and more likely to be non-Hispanic white and former smokers than those who were classified as not having kidney disease by both eGFR methods. They were also more likely to have kidney disease risk factors. Compared with the individuals classified as not having kidney disease by both eGFR methods, the individuals who were classified as having kidney disease by eGFRCYSC but not eGFRSCR were more likely to be anaemic, with a greater proportion of individuals having functional iron-deficiency anaemia. They were also more likely to have microalbuminuria, higher BMIs and elevated CRP levels.
Table 2.
Characteristics of individuals by differing methods of estimating GFR
| Characteristic | eGFRSCR and eGFRCYSC ≥ 60 | eGFRSCR and eGFRCYSC 15–59 | P-valuea | eGFRSCR ≥60 eGFRCYSC 15–59 | P-valuea | eGFRSCR 15–59 eGFRCYSC ≥ 60 | P-valuea |
|---|---|---|---|---|---|---|---|
| (n = 4996) | (n = 719) | (n = 838) | (n = 181) | ||||
| Age, mean years (SE) | 41.6 (0.6) | 73.1 (0.7) | <0.01 | 69.8 (1.2) | <0.01 | 60.8 (1.6) | <0.01 |
| Men, % (SE) | 48.9 (1.5) | 34.1 (2.9) | <0.01 | 37.6 (3.2) | <0.01 | 42.4 (5.2) | 0.28 |
| Ethnicity, % (SE) | |||||||
| Non-Hispanic white | 83.1 (1.2) | 91.4 (1.0) | 90.3 (1.1) | 92.2 (1.6) | |||
| Non-Hispanic black | 11.4 (1.0) | 7.1 (1.0) | 6.3 (1.0) | 6.9 (1.5) | |||
| Mexican American | 5.4 (0.6) | 1.5 (0.2) | <0.01 | 3.4 (1.0) | <0.01 | 0.9 (0.3) | <0.01 |
| Anaemia, % (SE)b | 4.9 (0.5) | 19.4 (1.7) | <0.01 | 13.2 (2.8) | <0.01 | 8.2 (3.0) | 0.27 |
| Iron-deficiency anaemia | 12.2 (0.9) | 9.1 (1.1) | 10.0 (3.0) | 5.7 (1.9) | |||
| Functional iron-deficiency anaemia | 16.8 (1.0) | 33.5 (2.6) | 24.0 (2.5) | 27.7 (3.7) | |||
| Other anaemia | 70.9 (1.3) | 57.4 (3.0) | 0.01 | 65.9 (3.2) | 0.65 | 66.6 (4.0) | 0.70 |
| Microalbuminuria, % (SE)b | 0.7 (0.2) | 9.1 (1.5) | <0.01 | 3.4 (0.7) | <0.01 | <0.01 | 0.16 |
| Smoking status, % (SE) | |||||||
| Non-smoker | 43.7 (1.2) | 43.5 (2.8) | 38.9 (2.3) | 36.3 (7.1) | |||
| Former smoker | 21.4 (1.4) | 38.0 (2.3) | 30.3 (2.3) | 38.5 (6.4) | |||
| Current smoker | 34.9 (1.5) | 18.5 (2.1) | <0.01 | 30.8 (2.6) | <0.01 | 25.2 (5.5) | 0.06 |
| Comorbid conditions, % (SE) | |||||||
| Diabetes mellitus | 8.3 (0.8) | 0.29 (1.9) | <0.01 | 23.5 (1.9) | <0.01 | 21.9 (3.7) | <0.01 |
| Hypertension | 19.8 (1.2) | 81.7 (1.9) | <0.01 | 65.3 (3.1) | <0.01 | 46.5 (4.5) | <0.01 |
| Hypercholesterolaemia | 48.7 (1.4) | 75.0 (2.5) | <0.01 | 62.4 (3.1) | <0.01 | 75.9 (4.2) | <0.01 |
| BMI, mean kg/m2 (SE) | 26.5 (0.2) | 27.7 (0.3) | <0.01 | 28.5 (0.4) | <0.01 | 27.1 (0.3) | 0.13 |
| Serum albumin, mean g/dL (SE) | 4.2 (0.0) | 4.0 (0.0) | <0.01 | 4.0 (0.0) | <0.01 | 4.1 (0.0) | 0.26 |
| CRP ≥1 mg/dL, % (SE) | 6.3 (0.7) | 19.3 (1.9) | <0.01 | 19.3 (3.1) | <0.01 | 7.2 (2.0) | 0.06 |
| TSH, mean mU/L (SE) | 1.62 (1.04) | 2.73 (1.10) | <0.01 | 2.25 (1.10) | <0.01 | 2.16 (1.09) | <0.01 |
eGFRSCR, serum creatinine-based estimated GFR; eGFRCYSC, cystatin C-based estimated GFR (units in ml/min/1.73 m2); BMI, body mass index; CRP, C-reactive protein; TSH, thyroid-stimulating hormone.
Compared to individuals with eGFRSCR and eGFRCYSC ≥60mL/min/1.73 m2.
Definitions provided in the Materials and Methods section of text.
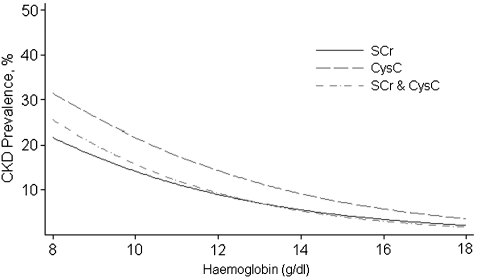
Figure 1 shows the predicted prevalence of moderate to severe kidney disease by eGFRSCR, eGFRCYSC and eGFRSCR + CYSC throughout the range of haemoglobin. In general, eGFRCYSC provided increasingly higher prevalence estimates of kidney disease than eGFRSCR and eGFRSCR + CYSC at lower haemoglobin levels. Among the persons with anaemia, the prevalence estimates of kidney disease yielded by eGFRCYSC were significantly higher [24.5% (95% CI: 18.7, 30.3%)] than those provided by either eGFRSCR [15.3% (95% CI: 12.4, 18.2%)] and eGFRSCR + CYSC [16.3% (95% CI: 13.5, 19%)]. These differences were consistent throughout the different forms of anaemia; however, the 95% confidence intervals for the prevalence estimates overlapped (Table 3).
Fig. 1.
Predicted prevalence of kidney disease (defined as eGFR 15–59 mL/min/1.73 m2) using different GFR-estimating methods in US adults aged 20 years or older by haemoglobin levels. Estimated GFR are based separately on serum creatinine (SCr), serum cystatin C (CysC) and combined serum creatinine and cystatin C (SCr & CysC). Prevalence curves are truncated when the number of relevant participants is <30.
Table 3.
Prevalence of moderate to severe kidney disease by GFR-estimating equation in US adults with anaemia
| Type of anaemia |
||||
|---|---|---|---|---|
| Anaemia | Absolute iron deficiency | Functional iron deficiency | Other | |
| (n = 742) | (n = 227) | (n = 193) | (n = 320) | |
| eGFRSCR | 15.3 (12.4, 18.2) | 6.0 (3.5, 8.6) | 38.2 (27.4, 49.0) | 15.9 (10.9, 20.9) |
| eGFRCYSC | 24.5 (18.7, 30.3) | 15.2 (3.0, 27.3) | 57.0 (45.9, 68.2) | 21.3 (15.8, 26.7) |
| eGFRSCR+CYSC | 16.3 (13.5, 19.0) | 6.6 (3.8, 9.4) | 41.9 (31.3, 52.5) | 16.2 (11.9, 20.5) |
Data presented as prevalence (95% CI).
Table 4 displays the unadjusted and multivariable-adjusted odds ratios of kidney disease associated with anaemia. Overall, anaemia was associated with more than 3-fold higher odds of moderate to severe kidney disease. In the overall multivariable models for kidney disease based on GFR estimated by serum creatinine, cystatin C or both, older age (OR = 1.09–1.13 per 1 year increase in age), hypertension (OR = 1.80–2.17), and elevated CRP (OR = 1.37–1.49 per log 1-mg/dL increase) and TSH (OR = 1.13–1.40 per log 1-mU/L increase) levels were also consistently associated with higher odds of kidney disease (all P-values <0.01). Hypercholesterolaemia was also associated with higher odds of kidney disease in the overall adjusted models using eGFRSCR (OR = 1.66, P < 0.01) and eGFRSCR + CYSC (OR = 1.49, P < 0.01), while diabetes history was associated with 1.4-fold higher odds of kidney disease (P<0.01) only in the overall adjusted model using eGFRSCR. In addition, higher BMI was also associated with slightly higher odds of kidney disease in the overall models utilizing eGFRCYSC (OR = 1.06, P < 0.01) and eGFRSCR + CYSC (OR = 1.04, P = 0.02). In younger individuals, women and those with elevated CRP levels, there was a trend of higher odds of kidney disease associated with anaemia when GFR was estimated by serum cystatin C rather than by serum creatinine alone or in combination with serum creatinine. In contrast, there was a trend for the higher odds of kidney disease associated with anaemia when kidney function was based on eGFRSCR and eGFRSCR + CYSC rather than eGFRCYSC in non-Hispanic whites and Mexican Americans.
Table 4.
Odds ratio of kidney disease (defined as eGFR 15–59 mL/min/1.73 m2) in NHANES III (1988–94) participants aged 20 years or older with versus without anaemia by each GFR-estimating method
| Unadjusted model (n = 6734) |
Multivariable model (n = 6621) |
|||||
|---|---|---|---|---|---|---|
| ORSCR | ORCYSC | ORSCR+CYSC | ORSCR | ORCYSC | ORSCR+CYSC | |
| Overalla | 3.47 (2.77, 4.36) | 3.65 (2.65, 5.03) | 3.93 (3.04, 5.09) | 3.31 (2.44, 4.49) | 3.58 (2.01, 6.38) | 3.45 (2.70, 4.15) |
| Ageb | ||||||
| <60 years | 1.91 (0.98, 3.71) | 8.43 (2.68–26.51) | 4.03 (1.94, 8.38) | 2.61 (1.07, 6.33) | 5.22 (2.23, 12.17) | 2.92 (1.14, 7.52) |
| ≥60 years | 3.12 (2.36, 4.13) | 2.59 (1.87, 3.58) | 3.27 (2.44, 4.38) | 3.82 (2.89, 5.06) | 2.85 (2.08, 3.91) | 3.78 (2.89, 4.95) |
| Ethnicityc | ||||||
| Non-Hispanic white | 4.47 (3.30, 6.07) | 4.96 (3.21, 7.66) | 4.96 (3.54, 6.94) | 3.49 (2.42, 5.04) | 4.60 (2.15, 9.82) | 3.62 (2.58, 5.07) |
| Non-Hispanic black | 2.99 (2.02, 4.42) | 2.86 (1.89, 4.32) | 3.21 (2.15, 4.79) | 2.57 (1.72, 3.82) | 2.20 (1.33, 3.65) | 2.61 (1.60, 4.25) |
| Mexican American | 6.85 (3.69, 12.71) | 4.74 (2.13, 10.56) | 7.29 (4.13, 12.87) | 7.94 (3.68, 17.12) | 3.52 (1.24, 10.02) | 10.41 (4.66, 23.25) |
| Genderd | ||||||
| Male | 10.65 (7.14, 15.88) | 9.18 (6.09, 13.85) | 11.43 (7.97, 16.38) | 3.57 (1.86, 6.84) | 2.22 (1.44, 3.43) | 5.91 (3.86, 9.05) |
| Female | 1.97 (1.43, 2.71) | 2.31 (1.53, 3.49) | 2.28 (1.65, 3.13) | 3.34 (2.24, 4.97) | 5.34 (2.36, 12.06) | 3.16 (2.08, 4.79) |
| C-reactive proteine | ||||||
| <1 mg/dL | 3.54 (2.69, 4.67) | 2.97 (2.28, 3.88) | 3.98 (2.94, 5.37) | 3.76 (2.71, 5.21) | 2.75 (1.98, 3.81) | 3.92 (2.87, 5.35) |
| ≥1 mg/dL | 2.30 (1.04, 5.10) | 5.47 (2.24, 13.35) | 2.57 (1.16, 5.68) | 2.01 (0.85, 4.74) | 7.36 (1.98, 27.36) | 2.16 (0.95, 4.94) |
Data presented as OR (95% CI).
SCR, serum creatinine; CYSC, serum cystatin C; SCR + CYSC, serum creatinine and cystatin C.
Multivariable model adjusted for age, ethnicity, gender, diabetes mellitus, hypertension, hypercholesterolaemia, smoking status, BMI, serum albumin, log-CRP and log-TSH.
Multivariable model adjusted for all variables included in overall adjusted model except for age.
Multivariable model adjusted for all variables included in overall adjusted model except for ethnicity.
Multivariable model adjusted for all variables included in overall adjusted model except for gender.
Multivariable model adjusted for all variables in overall adjusted model except for C-reactive protein.
Of the covariates, age was found to modify the association of anaemia with kidney disease (P interaction = 0.02) in the model utilizing eGFRCYSC. In the adjusted, age-stratified model using eGFRCYSC, there was a trend for higher odds of kidney disease associated with anaemia in individuals <60 years of age compared to older individuals. Adjustment for age and ethnicity attenuated the odds ratio in men while it strengthened the odds ratio in women; however, we did not observe an interaction between age and gender. The addition of albuminuria to the multivariable model did not appreciably alter the estimates (data not shown).
Discussion
Our study shows that moderate to severe kidney disease occurs commonly among individuals with anaemia. The prevalence of kidney disease among anaemic persons was greater with eGFRCYSC compared with eGFRSCR and eGFRSCR + CYSC. Overall, persons who were differentially classified as having moderate to severe kidney disease by eGFRSCR and eGFRCYSC had similar prevalence of kidney disease risk factors; however, those who were classified as having moderate to severe kidney disease by eGFRCYSC alone were more likely to have microalbuminuria and elevated CRP, two conditions which have been linked to anaemia, compared to individuals who were classified as having kidney disease by eGFRSCR only.
Prior studies have demonstrated the inverse association between kidney function and anaemia [9,37]. Anaemia develops early in the course of chronic kidney disease, with nearly one-third of the individuals with a GFR of 60–89 mL/min/1.73 m2 meeting WHO criteria for anaemia [37]. However, these prior studies either utilized serum creatinine-based estimates of kidney function or were not representative of the general population [9,37]. In contrast, our study used a large, nationally representative population to examine the differences in the association of anaemia with kidney dysfunction based on serum creatinine, cystatin C or both.
Anaemia commonly occurs in individuals with chronic conditions such as diabetes [38,39] and hypertension [40], which are also known risk factors for chronic kidney disease [41]. Recommended clinical approaches to the workup of anaemia, however, only marginally refer to kidney disease as a possible cause for normocytic anaemia and neglect to recommend renal function assessment in anaemic individuals [42]. In our study, we found that >15% of anaemic individuals had impaired kidney function when based on eGFRSCR or eGFRSCR + CYSC. The use of eGFRCYSC to estimate kidney function led to a 9% higher prevalence estimate of kidney disease in anaemia. The mechanisms underlying these disparities among the different eGFR methods likely stem from differing effects of extrarenal factors on both biomarkers and haemoglobin. In the Multi-Ethnic Study of Atherosclerosis (MESA), eGFRSCR inversely correlated while serum cystatin C positively correlated with several inflammatory markers in persons with chronic kidney disease [25]. In individuals without impaired kidney function, serum cystatin C remained significantly correlated with several inflammatory markers while eGFRSCR only correlated with tumour necrosis factor-α receptor1 (TNF-αR1) [25]. This study did not evaluate the association between eGFRCYSC and inflammation, which would have partially accounted for the effects of age, race and gender on serum cystatin C [25]. A more recent study, however, of >3000 individuals supports the notion that serum cystatin C and creatinine are differentially affected by non-renal factors [44]. After adjustment for measured GFR, age and gender were found to have greater effects on serum creatinine than on serum cystatin C. Whereas serum creatinine was 9.2% lower with each 20-year increase in age and 31.7% lower in women, serum cystatin C was 4.3% and 9.2% lower, respectively. Moreover, a higher CRP was associated with a lower serum creatinine (−3.3%) while it was associated with a higher serum cystatin C (2.3%). The association of these factors with serum creatinine noticeably diminished but had minimal effect on these associations with serum cystatin C after further adjustment for proxies of muscle mass [44]. Our results showing an overall odd ratio for kidney disease based on eGFRSCR + CYSC, which was in between those by eGFRSCR and eGFRCYSC, imply that the combined equation may mediate some of the differential effects of extra-renal factors on serum creatinine and cystatin C. However, this hypothesis could not be tested given our study’s lack of direct GFR measurements. Nonetheless, the use of eGFRCYSC to assess kidney function in anaemic persons may be particularly helpful in persons aged <60 years, women and those with ongoing inflammation. Its stronger association with mortality compared with eGFRSCR and eGFRSCR + CYSC [43] may provide an added benefit of prognostication in using eGFRCYSC for kidney function examination in anaemia.
The limitations of our study to consider include its cross-sectional design and our choice of equation to calculate eGFRCYSC. Due to a lack of temporality inherent in cross-sectional studies, we are unable to determine if serum cystatin C predicts earlier declines in haemoglobin than serum creatinine. However, NHANES III provides a unique opportunity to examine the relationship between eGFRCYSC and haemoglobin in an ethnically diverse, nationally representative sample. Although the eGFRCYSC equation used in our study addressed some biases associated with age, race and gender, it may not fully account for bias as the equation was developed in a study population enriched with participants afflicted with chronic kidney disease [33]. Our study lacks direct GFR measurements; therefore, we cannot discern the true impact of extra-renal influences on serum creatinine, serum cystatin C and anaemia. No direct comparisons have been performed between the eGFRCYSC equation we used and those developed by other investigators; however, we believe that this equation, which was externally validated [33], currently provides the most reliable estimate of GFR based on serum cystatin C in adults. Our prevalence estimates for microalbuminuria differed from those previously reported by Coresh and colleagues [34]. A selection bias may have occurred in the process of selecting the NHANES III sub-sample included in our analysis. Alternatively, our prevalence estimates may have diminished accuracy compared with those by Coresh and colleagues given our smaller study sample size [34]. Despite these limitations, our study provides a thorough comparison of the association of anaemia with kidney disease based on the serum creatinine- and cystatin C-based estimates of kidney function.
In conclusion, the prevalence of kidney disease among anaemic persons was greater with eGFRCYSC than with eGFRSCR and eGFRSCR + CYSC. Anaemia may be more strongly associated with eGFRCYSC rather than eGFRSCR and eGFRSCR + CYSC in persons aged <60 years, women and those with ongoing inflammation. This observation may be due to disparities in the effect of non-renal factors on both renal biomarkers. Further studies with measured GFR are needed to examine the differential effects of non-renal factors on the three GFR-estimating equations. Our study suggests that an assessment of kidney function, regardless of which GFR-estimating method is used, may need to be incorporated in to the routine workup of anaemia given the high prevalence of renal disease among anaemic individuals.
Acknowledgments
This work was supported by the National Institute of Diabetes, Digestive and Kidney Disease (grant number 1K23DK081317-01A1 to M.M.E.; UO1 DK 053869, UO1 DK 067651, UO1 DK 35073 and R01DK07677001 to B.C.A.; K01DK076595 to E.S.; U01DK057304 and 1R01DK072367 to R.S.P.) and the German Research Foundation Fellowship (to A.K.).
Conflict of interest statement. M.M.E. received an honorarium from Watson Pharmaceuticals, Inc in July 2007 for involvement in a CME monograph.
References
- 1.Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. BMJ. 1990;300:573–578. doi: 10.1136/bmj.300.6724.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Astor BC, Coresh J, Heiss G, et al. Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2006;151:492–500. doi: 10.1016/j.ahj.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovitz CT, Abramson JL, Vaccarino LV, et al. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2003;14:2919–2925. doi: 10.1097/01.asn.0000092138.65211.71. [DOI] [PubMed] [Google Scholar]
- 5.Culleton BF, Manns BJ, Zhang J, et al. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 6.Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J. 2005;149:391–401. doi: 10.1016/j.ahj.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Kohagura K. Kidney Int Suppl. [2007]. Anemia as a risk factor for chronic kidney disease; pp. S4–S9. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the united states: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 9.Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nangaku M, Eckardt KU. Pathogenesis of renal anemia. Semin Nephrol. 2006;26:261–268. doi: 10.1016/j.semnephrol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P. The role of inflammation in the anaemia of end-stage renal disease. Nephrol Dial Transplant. 2001;16:36–40. doi: 10.1093/ndt/16.suppl_7.36. [DOI] [PubMed] [Google Scholar]
- 14.Drueke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16:25–28. doi: 10.1093/ndt/16.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 15.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit. Nephrol Dial Transplant. 2004;19:1792–1797. doi: 10.1093/ndt/gfh248. [DOI] [PubMed] [Google Scholar]
- 16.Thomas MC, Tsalamandris C, Macisaac R, et al. Functional erythropoietin deficiency in patients with type 2 diabetes and anaemia. Diabet Med. 2006;23:502–509. doi: 10.1111/j.1464-5491.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS. Measurement of renal function in chronic renal disease (clinical conference) Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 19.Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function—a review. Clin Chem Lab Med. 1999;37:389–395. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 21.Ix JH, Shlipak MG, Chertow GM, et al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 23.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 24.Kottgen A, Selvin E, Stevens LA, et al. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Keller C, Katz R, Cushman M, et al. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) BMC Nephrol. 2008;9:9. doi: 10.1186/1471-2369-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivares M, Hertrampf E, Capurro MT, et al. Prevalence of anemia in elderly subjects living at home: role of micronutrient deficiency and inflammation. Eur J Clin Nutr. 2000;54:834–839. doi: 10.1038/sj.ejcn.1601099. [DOI] [PubMed] [Google Scholar]
- 27.World health organization . Nutritional Anemias: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1968. [PubMed] [Google Scholar]
- 28.Summary of a report on assessment of the iron nutritional status of the United States population. Expert Scientific Working Group. Am J Clin Nutr. 1985;42:1318–1330. doi: 10.1093/ajcn/42.6.1318. [DOI] [PubMed] [Google Scholar]
- 29.McLaren CE, Li KT, Gordeuk VR, et al. Relationship between transferrin saturation and iron stores in the African American and US Caucasian populations: analysis of data from the Third National Health and Nutrition Examination Survey. Blood. 2001;98:2345–2351. doi: 10.1182/blood.v98.8.2345. [DOI] [PubMed] [Google Scholar]
- 30.Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. 1974. Nutrition. 1992;8:443–447. discussion 448. [PubMed] [Google Scholar]
- 31.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 35. NHANES III Data Files, Documentation, and Codebooks. Accessed 30 May 2008. [Google Scholar]
- 36.Ezzati TM, Massey JT, Waksberg J, et al. Vital Health Stat 2. 1992. Sample design: Third National Health and Nutrition Examination Survey; pp. 1–35. [PubMed] [Google Scholar]
- 37.Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adetunji OR, Mani H, Olujohungbe A, et al. ‘Microalbuminuric anaemia’—the relationship between hemoglobin levels and albuminuria in diabetes. Diabetes Res Clin Pract. 2009;85:179–182. doi: 10.1016/j.diabres.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Goldhaber A, Ness-Abramof R, Ellis MH. The prevalence of anemia among unselected adults with diabetes mellitus and normal creatinine levels. Endocr Pract. 2009;15:1–20. doi: 10.4158/EP09119.ORR. [DOI] [PubMed] [Google Scholar]
- 40.Paul B, Wilfred NC, Woodman R, et al. Prevalence and correlates of anaemia in essential hypertension. Clin Exp Pharmacol Physiol. 2008;35:1461–1464. doi: 10.1111/j.1440-1681.2008.05031.x. [DOI] [PubMed] [Google Scholar]
- 41.McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89:419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80:923–936. doi: 10.4065/80.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astor BC, Levey AS, Stevens LA, et al. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]