Abstract
Inhibition of cyclooxygenase-2 (COX-2) pathways may have significant implications for the prevention and treatment of head and neck squamous cell carcinoma (HNSCC). COX-2 is overexpressed in both premalignant lesions and invasive HNSCC. We examined COX-2 expression by immunohistochemistry in normal tissues, different stages of premalignant lesions, and carcinoma in-situ (CIS). We also evaluated the correlation between COX-2 expression and clinical characteristics of HNSCC patients. Tissue specimens were obtained from: premalignant lesions from 25 subjects enrolled in a biochemoprevention trial; tumor samples collected at diagnosis from 38 HNSCC patients enrolled in an induction chemotherapy trial; and normal control tissues from 10 non-cancer, non-smoking subjects. COX-2 was expressed in early and intermediate stages of premalignant lesions, increasing first in the basal and parabasal layers, then lower spinous, and upper spinous layers. This correlation was noted in normal epithelium (p<0.0001), histologically normal in-field samples (p<0.0001), low-grade dysplasia (p=0.024), and moderate-grade dysplasia (p=0.009), but was lost in the majority of high-grade dysplasia/CIS (p=0.896). COX-2 expression was also noted to increase progressively through the early stages of premalignancy, and to decrease in severe/CIS stage and invasive carcinoma. COX-2 expression in tumors from patients treated with induction chemotherapy was correlated with overall survival after controlling for clinical variables. These findings elucidate the differential expression pattern of COX-2 in stages of head and neck premalignant lesions and invasive carcinoma, supporting the rationale for COX-2 inhibition as an important strategy for cancer chemoprevention. Further validation of COX-2 expression is needed in prospective ongoing chemoprevention trials.
Keywords: COX-2, Head and neck cancer, premalignancy, chemoprevention, cyclooxygenase 2
Introduction
Head and neck squamous cell carcinoma (HNSCC) is an aggressive epithelial malignancy that is the sixth most common neoplasm worldwide, with 47,560 new cases diagnosed and more than 11,260 deaths in the United States estimated in 2008[1]. HNSCC is a phenotypically and biologically heterogeneous disease with widely variable patterns of behavior that represents a paradigm for cancer chemoprevention. Advances in the molecular biology of HNSCC[1] as well as the development of novel targeted therapies have led to increased interest in the study of chemoprevention in HNSCC.
Several laboratory investigations including animal models and epidemiological studies have provided evidence that inhibition of cyclooxygenase-2 (COX-2) pathways may have significant implications for cancer treatment or prevention in general[2–4], and in HNSCC in particular[5, 6].There are at least two forms of the cyclooxygenase (COX) isoenzyme, COX-1 and COX-2. COX-1 is constitutively expressed in most epithelial tissues and is thought to catalyze the formation of prostaglandins (PGs), which in turn promote tumorigenesis[7, 8]. COX-2, in contrast, is not constitutively expressed in most tissues but is induced by a wide spectrum of growth factors and cytokines in pathophysiological states[9]. Increased expression of COX-2 is commonly found in both premalignant and malignant tissues[9–11] [12]. Overexpression of COX-2 in epithelial cells has been shown to inhibit apoptosis and increase the invasiveness of tumor cells.
Evidence suggests that COX inhibitors, including non-steroidal anti-inflammatory drugs (NSAIDs), protect against a variety of tumors[13–15]. Selective inhibition of COX-2 avoids the side effects of NSAIDs that inhibit both COX-1 and COX-2. In patients with familial adenomatous polyposis, Celecoxib, a selective COX-2 inhibitor, caused a 28% reduction in the number of colorectal polyps compared with a 4.5% reduction for placebo[16].
Although the mechanism(s) by which NSAIDs inhibit tumor growth are not clearly understood, it could involve blockage of COXs, which suppress eicosanoid production, especially of PGs, and might affect cell proliferation, apoptosis and immune response[5, 17]. It has recently been shown that inhibition of COX pathways may also exert a negative effect on tumor angiogenesis[18, 19].
In HNSCC, COX-2 is expressed in both tumor tissue and the adjacent epithelium, with a higher expression level in invasive carcinoma compared to normal epithelium[20]. Western blot analysis of tissue from subjects at different stages of carcinogenesis and of normal mucosa, showed expression of COX-2 in all premalignant lesions lesions but not in normal mucosa, suggestive of a possible role for COX-2 inhibition in chemoprevention of HNSCC[21]. COX-2 inhibition has been shown to result in inhibition of cell growth in HNSCC [22–24] [25].Based on these findings, a number of chemoprevention and therapeutic trials in HNSCC using COX-2 inhibitors are underway[26].
We sought to examine the expression of COX-2 in normal and dysplastic tissues of different stages of premalignancy, including low-grade dysplasia (LD), moderate-grade dysplasia (MD), and high-grade dysplasia/carcinoma in-situ (CIS), to better understand the role it plays in tumorigenesis. We also evaluated the correlation between COX-2 expression and clinical outcomes of patients with HNSCC who were treated on an induction chemotherapy trial with TIC (paclitaxel, ifosfamide and carboplatin)[27].
Material and Methods
Tissue specimens
Specimens for this study were obtained from two separate trials, due to their availability in the University Of Texas M. D. Anderson Cancer Center. The biopsy tissues were obtained from consented patients following IRB protocol approval.
The first group of tissue specimens included patients with premalignant lesions enrolled on the chemoprevention trial with 13-cis-retinoic acid, interferon-α2a and α-tocopherol[28] [29, 30]. A total of 36 participants were enrolled on this study, and 25 tissue specimens were obtained for our analysis at the time of diagnosis. In addition, 10 tissue samples from non-cancer patients who were non-smokers were collected and used as control. These biopsy samples were classified as: normal control (n = 10), histologically normal in-field (n = 25), low grade or mild dysplasia (LD) (n = 14), moderate grade dysplasia (MD) (n = 29), and severe dysplasia/carcinoma in-situ (CIS) (n = 14). For each of the samples, basal/parabasal (B/PB), lower spinous (LS), and upper spinous/granular (US/GR) were available for our analysis.
The second cohort of HNSCC tissue samples were collected at the time of diagnosis from 38 patients with HNSCC who enrolled on a phase II trial of induction chemotherapy with paclitaxel, ifosfamide, and carboplatin (TIC) followed by local therapy[27]. A total of 54 patients were enrolled on the trial and tissue was available for analysis from 38 patients. All eligible patients on this trial had locally advanced SCCHN and received two courses of induction chemotherapy with TIC. They subsequently underwent repeated clinical and radiologic evaluation. If they achieved complete or partial responses, they received two more courses of chemotherapy before undergoing definitive local treatment.
Clinical information for these patients was obtained from the pathology files in the University of Texas M. D. Anderson Cancer Center following the regulations of the Health Insurance Portability and Accountability Act (HIPAA).
Immunohistochemistry (IHC)
Unstained sections from tissue blocks were prepared using appropriately coated slides and stained with anti-COX-2 specific antibody (Cayman Chemical, Ann Arbor, Michigan) at a dilution of 1/100 following a standard IHC procedure. The slides were incubated with the primary antibody at 4°C overnight followed by incubation with a secondary antibody conjugated with horseradish peroxidase (R&D Systems, Minneapolis, MN) for 1 hour at room temperature. Antibody staining was visualized using DAB peroxidase substrate solution (Vector Labs, Burlingame, CA). Cell nuclei were counterstained using hematoxylin QS (Vector Labs). Mouse IgG without the primary antibody was used as a negative control.
COX-2 expression in the cell cytoplasm was scored by a head and neck pathologist blinded to the response or the outcome of patients using a semi-quantitative scale ranging from 0 (no expression) to 3+ (highest level of expression) on a cell-by-cell basis in 10 consecutive microscopic fields (x200) in a defined area. The weighted index of COX-2 expression was calculated as the sum of the number of counted cells multiplied each by the degree of intensity of each cell (0–3+) and divided by the total number of cells counted, as follows: weighted index of COX-2 expression = (number of cells counted × degree of intensity)/total number of cells counted)× 100[31]. COX-2 expression was determined in each of the epithelial layers of the different premalignant lesions and its correlation with clinical parameters was assessed.
Statistical analysis
For the analysis of premalignant lesions, Kruskal-Wallis test was carried out to compare the median expression level of COX-2 within each premalignant lesion and between the different degrees of premalignancies.
For the analysis of the 38 patients with invasive carcinomas, COX-2 expression was dichotomized at the mean (0: COX-2 expression below the mean, 1: COX-2 expression above the mean) and the Fisher exact test for comparing two population proportions was used to compare the proportion of men and women with COX-2 expression equal to 1. Similarly, Fisher exact test was used to compare COX-2 expression with respect to the binary variables listed in Table 1. When the variable had more than 2 categories, such as differentiation, the Chi-square exact test for comparing more than two population proportions was carried out. Kaplan-Meier estimator was used to estimate the survival distribution of all patients and to compare the overall survival of patients with COX-2 expression 0 relative to 1. The Cox proportional hazards model was used to compare the overall survival distributions between these two groups adjusting for relevant confounding variables.
Table 1.
High COX-2 expression in patients with HNSCC in correlation with clinical variables
| Characteristics | COX-2 expression above mean | P |
|---|---|---|
| Sex | 0.152 | |
| M | 13/33 | |
| F | 4/5 | |
| Response | 0.677 | |
| CR/PR | 13/31 | |
| NC/PD | 4/7 | |
| T stage | 0.033 | |
| T1/T2 | 2/12 | |
| T3/T4 | 15/26 | |
| N stage | 0.727 | |
| N0/N1 | 5/10 | |
| N2/N3 | 12/28 | |
| AJCC stage | 1.000 | |
| III | 2/5 | |
| IV | 15/33 | |
| Site | 1.000 | |
| HP/LX | 5/11 | |
| OC/OP | 11/25 | |
| Differentiation | 0.913 | |
| G1 | 3/6 | |
| G2 | 6/15 | |
| G3 | 8/17 | |
Results
Premalignant Lesions
A total of 36 patients were enrolled on the chemoprevention trial[29, 30]. Tissue samples were available from 25 subjects. Squamous mucosal samples were comprised of: 10 normal mucosa from healthy samples, 25 histologically normal in field of cancer, 14 with low grade or mild dysplasia (LD), 29 with moderate grade dysplasia (MD), and 14 with severe dysplasia or carcinoma in-situ (CIS). For each of the samples, basal/parabasal (B/PB), lower spinous (LS), and upper spinous/granular (US/GR) were available for our analysis, as shown in Figure 1.
Figure 1. COX-2 staining in premalignant and HNSCC samples.
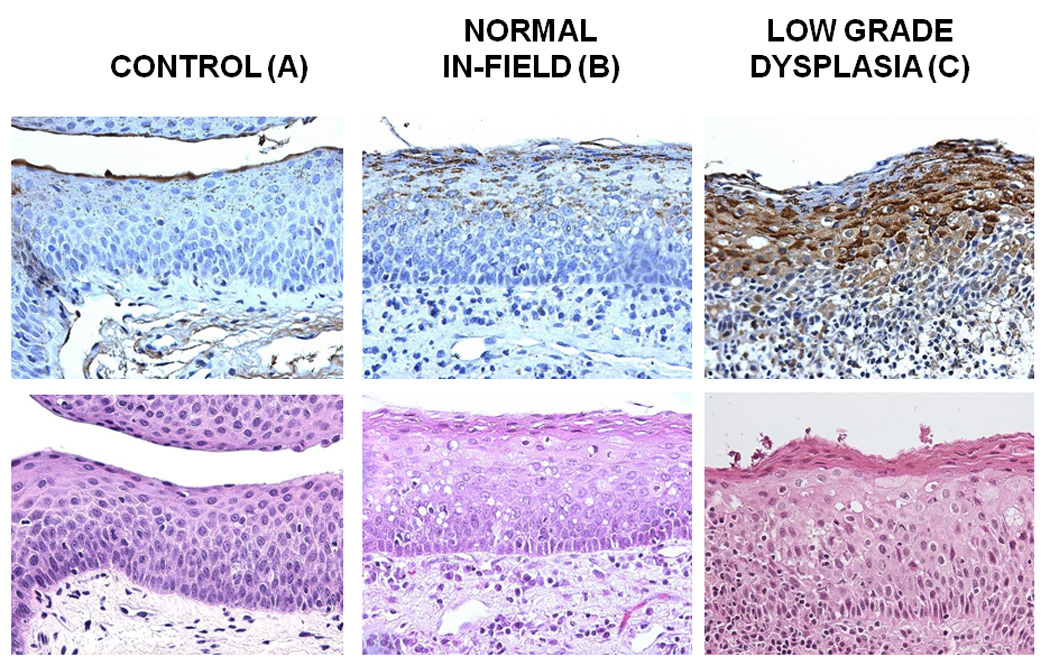
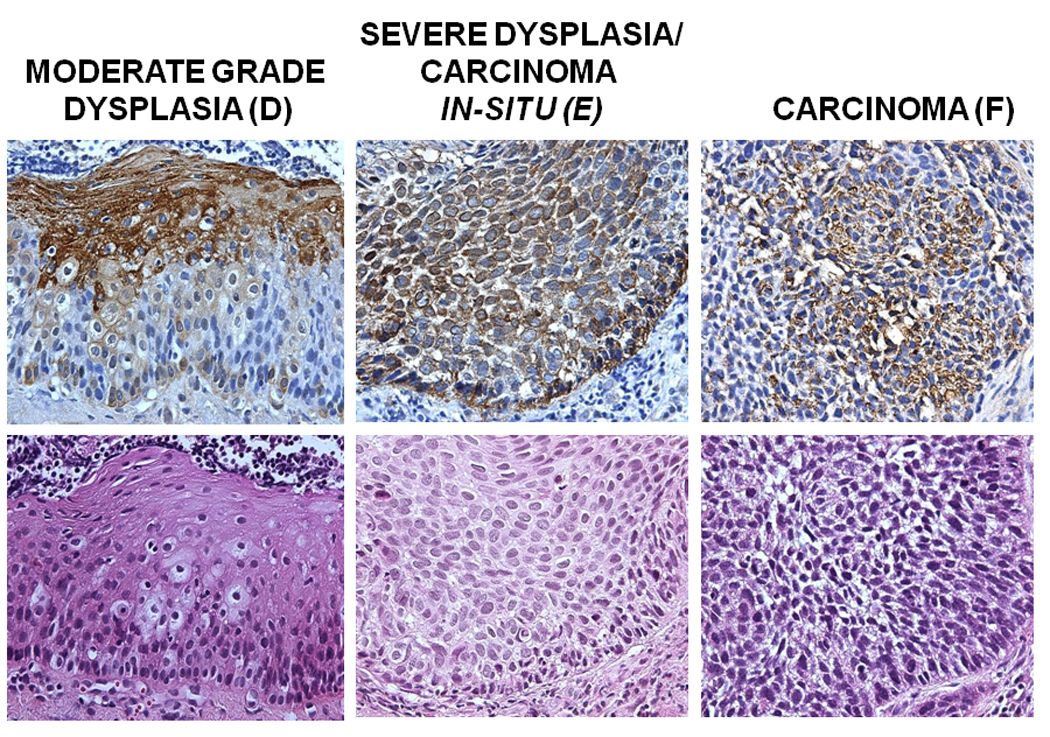
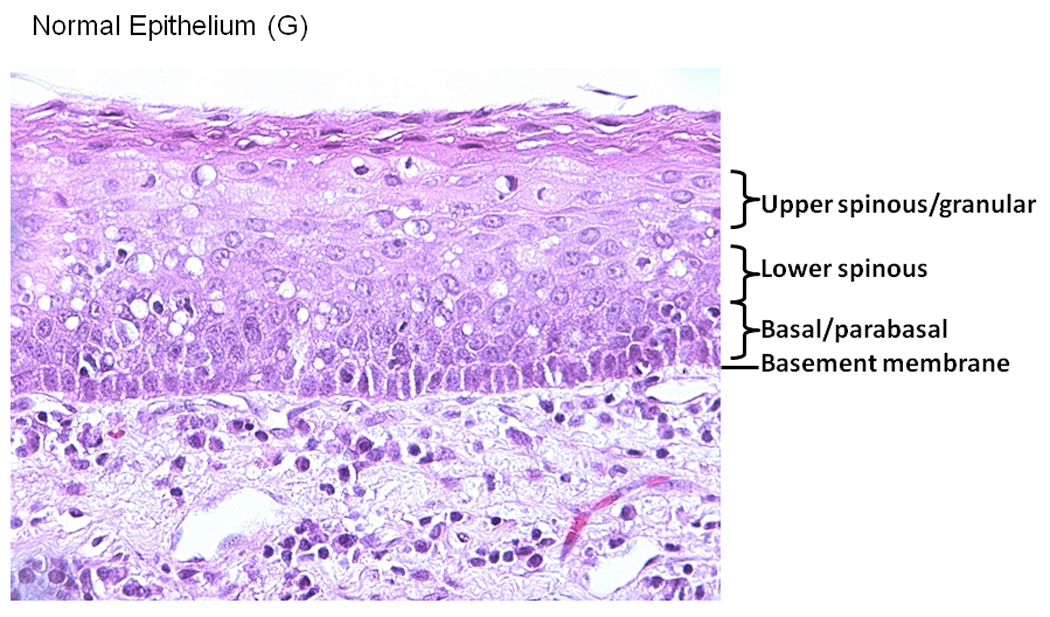
Tissue blocks were stained with anti-COX-2 specific antibody at a dilution of 1/100 following a standard IHC procedure. Antibody staining was visualized using DAB peroxidase substrate solution and cell nuclei were counterstained using hematoxylin QS. Mouse IgG without the primary antibody was used as a negative control. Representative samples of COX-2 staining in Control tissue (A), Normal in field (B), Low Grade Dysplasia (C), Moderate Grade Dysplasia (D), Carcinoma in Situ (E) is shown. A Representative image is showing the epithelial layers (basement membrane, basal/parabasal layer, lower spinous layer, and upper spinous layer) in a normal epithelial tissue sample, stained with hematoxylin (F).
COX-2 was found to be expressed in early and intermediate stages of HNSCC carcinogenesis, and increased through the different epithelial layers, its lowest expression being in the B/PB, and the highest in the US layers (Figures 1 and 2 and Table 2). This correlation was statistically significant in histologically normal, normal in-field, LD, and MD epithelium, but did not reach statistical significance in the CIS cohorts (Table 2). In addition, COX-2 expression was found to increase in premalignant stages of HNSCC carcinogenesis from normal in-field to moderate grade dysplasia and carcinoma in-situ. (Table 2).
Figure 2. COX-2 expression level by IHC in different premalignant tissues.
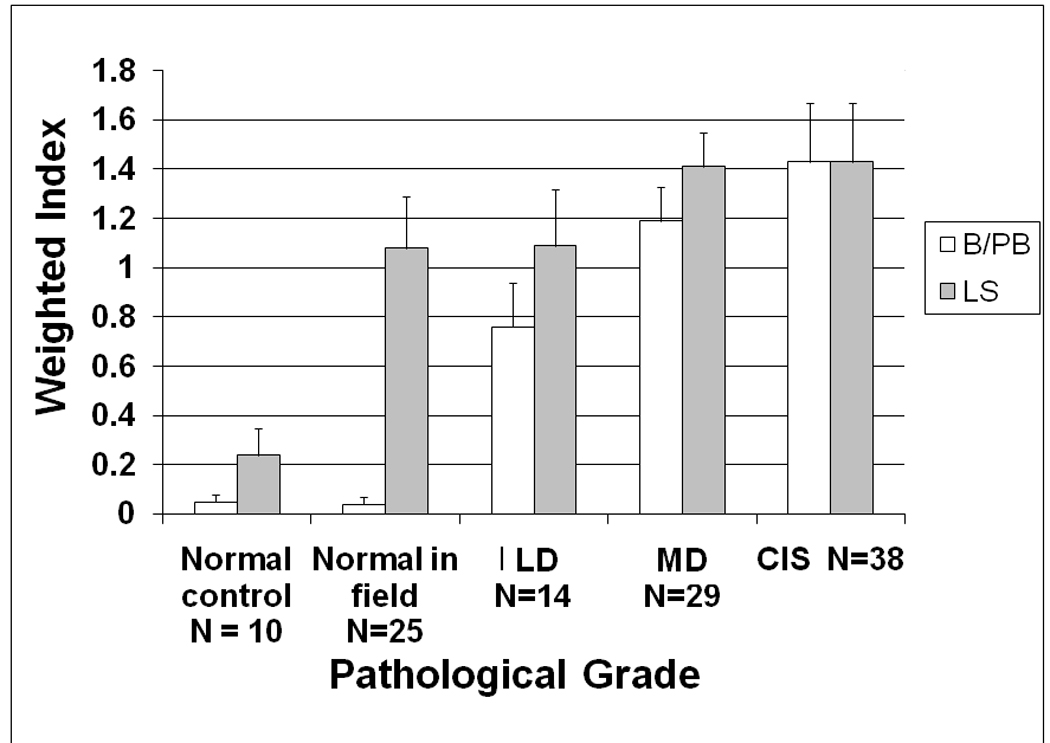
COX-2 expression in the cell cytoplasm was scored using a semi-quantitative scale ranging from 0 (no expression) to 3+ (highest level of expression) on a cell-by-cell basis in 10 consecutive microscopic fields (x200) in a defined area. The weighted index of COX-2 expression was calculated as follows: Weighted index of COX-2 expression = (number of cells counted × degree of intensity)/total number of cells counted)× 100. COX-2 expression is shown for the basal/parabasal (B/PB) and lower spinous (LS) layers of the different premalignant lesions. N=total number of samples for each category.
Table 2.
COX-2 correlation with each stage of premalignancy and its corresponding epithelial layers
| Epithelial layer | Basal/ Parabasal layer |
Lower spinous layer |
Upper spinous layer |
Total | Correlation P value |
|
|---|---|---|---|---|---|---|
| Normal control | N | 11 | 11 | 11 | 33 | <0.0001 |
| COX-2 | 0.0 | 0.0 | 2.0 | |||
| Normal in-field | N | 25 | 25 | 25 | 75 | <0.0001 |
| COX-2 | 0.0 | 1.0 | 2.0 | |||
| Low-grade dysplasia |
N | 14 | 14 | 14 | 42 | 0.024 |
| COX-2 | 1.0 | 0.9 | 1.9 | |||
| Moderate-grade dysplasia |
N | 29 | 29 | 29 | 87 | 0.009 |
| COX-2 | 1.3 | 1.0 | 2.0 | |||
| Carcinoma in-situ | N | 14 | 14 | 14 | 42 | 0.896 |
| COX-2 | 1.3 | 1.2 | 1.4 | |||
| Correlation P value | <0.0001 | 0.002 | 0.1 | |||
N = Total number of samples per category
COX-2 = Mean COX-2 value obtained for each category
Invasive Carcinoma
A total of 38 of the 54 patients with invasive HNSCC had COX-2 expression analyzed in tissue samples. Their clinical characteristics are shown in Table 1. The male to female ratio was 7:1. Response to systemic therapy was reported as 81% (31/38), including complete and partial remissions. The majority of patients (87%) had stage IV disease (33/38). We used a cut-off point of COX-2 expression of 0.856 to differentiate between low versus high expressers in our survival analysis. Four patients had no expression of COX-2. Seventeen patients had COX-2 expression below the mean (<0.856) whereas 21 had expression above the mean (>0.856). Of note is the lack of association between COX-2 expression and gender, response to therapy, nodal stage, AJCC stage, site and degree of differentiation. There was, however, a correlation noted with primary tumor T stage (p=0.033).
The 5-year overall survival rate of the 38 patients was 63% (figure 3), which is comparable to that in more recent trials using induction chemotherapy in treatment of HNSCC[32]. Expression of COX-2 appeared to correlate with overall survival. Patients whose tumor had COX-2 expression of <0.856 (below the mean) had a 5-year survival rate of 78%, significantly greater than that in patients whose COX-2 level was >0.856 (p=0.026), who had a 5-year survival rate of 49% (HR=3.19, Figure 4). A multivariable analysis adjusting for clinical and demographic variables including smoking status showed that COX-2 expression was still a statistically significant predictor of survival (p = 0.033) with an adjusted hazards ratio of 3.64. When we stratify by smoking status, the test of hypothesis that Cox2 expression is the same in all strata had a p-value 0.1694.
Figure 3. TIC induction trial: Reported overall survival.
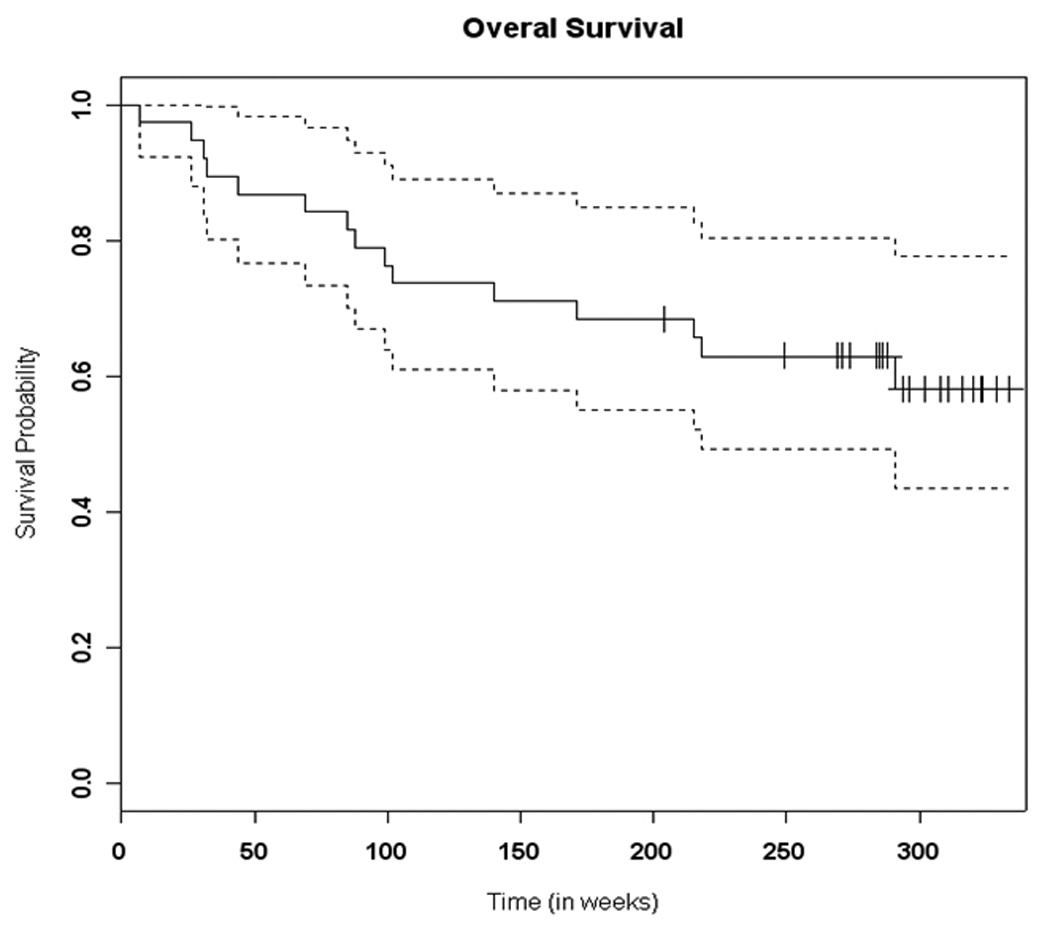
The 5-year overall survival rate of the 38 patients treated on the TIC induction trial.
Figure 4. COX-2 expression: Impact on survival in induction trial.
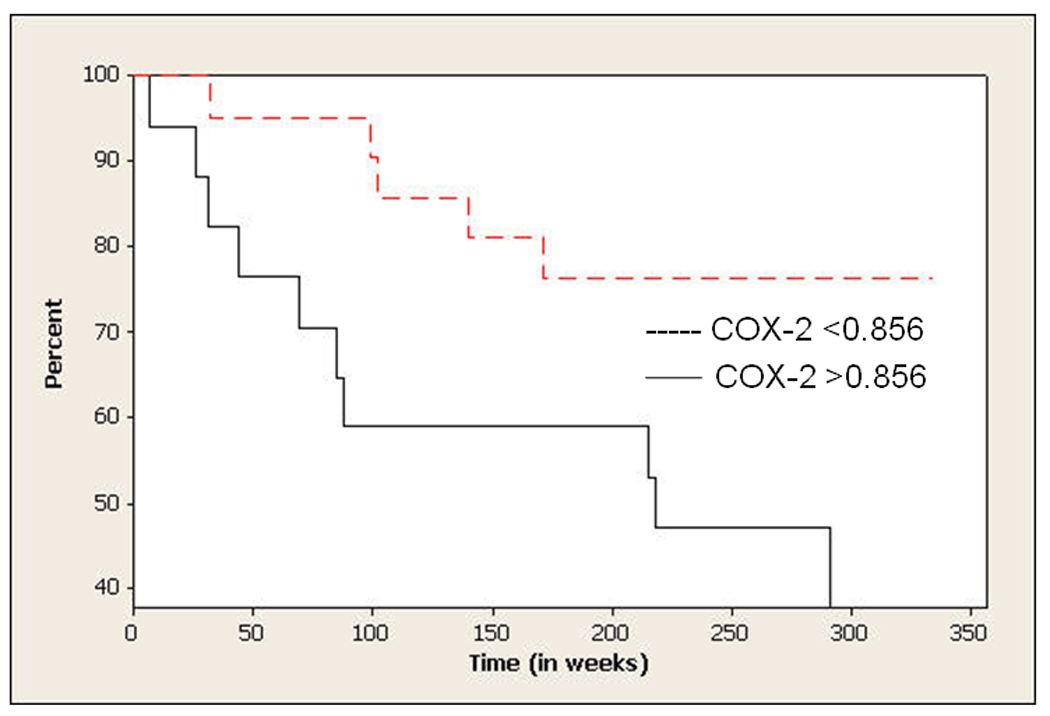
Kaplan-Meier estimator was used to estimate the survival distribution of all patients and to compare the overall survival of patients with high versus low COX-2 expression. A cut-off point of COX-2 expression of 0.856 was used to differentiate between low versus high expressers in the survival analysis.
Discussion
Our findings support those of other published reports in showing that COX-2 is involved in early and intermediate stages of carcinogenesis in HNSCC, although we found its expression to decrease in invasive carcinoma.
Studies looking at COX-2 expression in various stages of carcinogenesis are scarce. COX-2 expression has been investigated in other premalignant and malignant lesions besides HNSCC. In gastric lesions from Helicobacter Pylori-infected patients with chronic active gastritis, gastric atrophy, intestinal metaplasia and frank carcinoma [33]. Other studies have suggested a close relationship between hepatitis B infection and COX-2 expression in chronic hepatitis B and hepatocellular carcinoma [34]. In squamous cell carcinoma of the lung, there seems to be a clear correlation between the stage of the pre-neoplastic lesion and the degree of COX-2 expression, with severe dysplasia being a cut-off point and seemingly a key step in early lung carcinogenesis. In this and other similar studies, COX-2 expression seems to be weak in metaplasia and only increases in dysplasia and CIS, which is consistent with our findings in premalignant lesions of the head and neck[35].
Our findings indicate that COX-2 is involved in early and intermediate stages of carcinogenesis in HNSCC. As carcinogenesis progresses, COX-2 levels increase from the basal/parabasal layer to the lower spinous and upper spinous layers in all stages of carcinogenesis except in CIS. Even though we cannot explain the observed pattern of COX-2 expression in different epithelial layers from a biologic standpoint, it may reflect the role COX-2 plays in HNSCC carcinogenesis. It is worth noting that other markers such as EGFR and proliferating cell nuclear antigen expression have been noted to be overexpressed in the basal and parabasal layers compared to the superficial layers in premalignant head and neck tissues[36–38]. This, in addition to our findings, suggests that these distinct epithelial layers may play different roles in cellular differentiation and carcinogenesis. These patterns while only descriptive at the present, may have a bearing on future understanding of the role COX-2 plays in the different stages of cell differentiation and malignant transformation.
COX-2 expression in premalignant oral lesions has been studied by several groups. COX-2 has been shown to be extensively expressed in oral leukoplakia and premalignant lesions. Its expression seems to increase in high-grade premalignant lesions[39]. In a study looking at COX-2 gene expression in healthy persons, persons with oral dysplasia, and carcinomas, COX-2 was upregulated from healthy (3%) to premalignant lesions (41%) to oral carcinomas (88%)[40]. In contrast to our findings, there was no correlation found in this study between histologic grading of oral premalignant lesions and the level of COX-2 expression. In another study of similar design, the level of COX-2 expression in oral premalignancies including oral leukoplakia and leukoplakia with and without dysplasia was significantly higher than those in normal mucosa (p<0.05), but lower than those in oral invasive carcinoma (p<0.01). There was also no statistically significant difference in the expression of COX-2 among the oral premalignancy groups (p>0.05)[41]. Other studies reported a correlation between COX-2 level and grade of dysplasia, with the highest expression found in severe dysplasia[42]. Our findings are therefore consistent with others, showing that the level of COX-2 expression increases throughout the process of carcinogenesis. Possible biases in our study include the fact that not all tissues were available for IHC for both premalignant as well as carcinoma samples from both premalignant and carcinoma cohorts. Even though this could have potentially biased the results, no specific characteristics could be singled out that would predict the lack of tissue availability on either studies. Further investigation will be required to determine the exact role of COX-2 in the different premalignancy stages of head and neck carcinogenesis
For HNSCC, our results suggest a correlation between COX-2 expression and HNSCC tumor size, which duplicates findings from other studies,[43] showing a correlation between COX-2 mRNA level in peripheral blood and both tumor size and prognosis, although no correlation was found with nodal status, response to therapy, site, overall stage and grade of disease in our study. These and similar reported findings suggest the potential use of COX-2 expression as a reliable tumor marker. In contrast to other reports, however, we found a lower level of COX-2 expression in HNSCC compared to that in premalignant lesions. These findings however, do not necessarily reflect the actual pattern of COX-2 expression between premalignant and malignant lesions. The lack of progressive increase in COX-2 may be related to the heterogeneity as well as a possible modulation of expression when progressing through the different stages of premalignancies and ultimately to carcinoma. This may partially explain the decrease in COX-2 expression we observed in carcinoma compared to premalignant samples. It is also not inconceivable that a genetic alteration occurring at a later stage in the carcinogenenic process may interact with COX-2 and affect its expression in malignant tissues.
In looking at COX2 expression in malignant HNSCC samples there was no specific rationale for choosing patients treated with TIC chemotherapy beyond the fact that they were treated on the same study which promoted homogeneity. We felt that this would provide more reliable information compared to a retrospective analysis of patients outside of a clinical study. It is also worth noting that all analyzed tissues were from biopsies were obtained prior to any anticancer therapy. Validation of COX-2 expression and its prognostic significance in invasive carcinomas of the head and neck needs further confirmation in large sample-size clinical trials.
In conclusion, our findings elucidate the expression level of COX-2 in different stages of head and neck premalignant lesions, and correlate COX-2 expression with clinical outcome in patients with invasive HNSCC. It indicates that COX-2 seems to play a role in the process of transformation from lower to higher grades of premalignancies, and continues to play a role in the behavior of invasive carcinoma where its higher expression seems to correlate with worse clinical outcome. An expanding body of evidence indicates that downregulation of the cyclooxygenases will be an important strategy for preventing cancer. Whether this downregulation will translate into benefits to patients with invasive carcinomas as well, is the current topic of investigation on clinical studies.
Evaluation of COX-2 inhibition seems to be justified in trials focusing on both primary chemoprevention, second primary tumor prevention for HNSCC as well as possible therapy for invasive HNSCC. Evaluation of the role of COX-2 inhibitors as targeted therapies in combination with other potentially chemopreventive agents, such as EGFR inhibitors, in premalignant head and neck lesions as well as invasive carcinomas is the subject of currently ongoing studies.
Acknowledgements
This work has been supported by R01 CA112643, U01 CA101244, and P50 CA128613 (P.I., D.M. Shin) from the National Cancer Institute.
References
- 1.American Cancer Society. Cancer statistics. 2009 http://www.cancer.org/docroot/STT/stt_0.asp. [Google Scholar]
- 2.Wang D, D RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannenberg AJ, S K. Targeting cyclooxygenase-2 in human neoplasia: Rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 4.Dannenberg AJ, et al. Cyclooxygenase-2 and Epidermal Growth Factor Receptor: Pharmacologic Targets for Chemoprevention. J Clin Oncol. 2005;23(2):254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 5.Chan G, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 6.Cohen EG, et al. Microsomal Prostaglandin E Synthase-1 Is Overexpressed in Head and Neck Squamous Cell Carcinoma. Clinic Cancer Res. 2003;9:3425–3430. [PubMed] [Google Scholar]
- 7.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Subbaramaiah K, Telang N, Ramonetti JT. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- 9.O'Mahony CA, et al. Cyclooxygenase-2 alters transforming growth factor-beta 1 response during intestinal tumorigenesis. Surgery. 1999;126(2):364–370. [PubMed] [Google Scholar]
- 10.Williams CS, et al. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenerology. 1996;111(4):1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 11.Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res. 2000;6:2424–2430. [PubMed] [Google Scholar]
- 12.Dannenberg AJ, et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 13.Backlund MG, Mann JR, D RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69 Suppl 1:28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, DuBois RN. Prostaglandins and cancer. Gut. 2005;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams CS, et al. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 16.Staeinbach G, Lynch PM, P RK. The effect of Celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 17.Sawaoka H, et al. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;247:G1061–G1067. doi: 10.1152/ajpgi.1998.274.6.G1061. [DOI] [PubMed] [Google Scholar]
- 18.Jones MK, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 19.Ams CS, et al. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan G, Boyle JO, Y EK. Cyclo-oxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 21.Nathan CA, et al. COX-2 expression in dysplasia of the head and neck: correlation with eIF4E. Cancer. 2001;92(7):1888–1895. doi: 10.1002/1097-0142(20011001)92:7<1888::aid-cncr1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZG, et al. Simultaneously Targeting Epidermal Growth Factor Receptor Tyrosine Kinase and Cyclooxygenase-2, an Efficient Approach to Inhibition of Squamous Cell Carcinoma of the Head and Neck. Clin Cancer res. 2004;10:5930–5939. doi: 10.1158/1078-0432.CCR-03-0677. [DOI] [PubMed] [Google Scholar]
- 23.Ondrey FG, Juhn SK, A GL. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope. 1996;106:129–134. doi: 10.1097/00005537-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 24.cia KA, et al. Role of arachidonic acid metabolites in tumor growth inhibition by nonsteroidal antiinflammatory drugs. Am J Otolaryngol. 1997;18:1–8. doi: 10.1016/s0196-0709(97)90041-7. [DOI] [PubMed] [Google Scholar]
- 25.Panje WR. Regression of head and neck carcinoma with a prostaglandin-synthesis inhibitor. Arch Otolaryngol. 1981;107:658–663. doi: 10.1001/archotol.1981.00790470006003. [DOI] [PubMed] [Google Scholar]
- 26.Papadimitrakopoulou VA, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer res. 2008;14(7):2095–2101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 27.Shin DM, et al. Phase II study of induction chemotherapy with paclitaxel, ifosfamide, and carboplatin (TIC) for patients with locally advanced squamous cell carcinoma of the head and neck. Cancer. 2003;95(2):322–330. doi: 10.1002/cncr.10661. [DOI] [PubMed] [Google Scholar]
- 28.Papadimitrakopoulou VA, et al. Biologic Correlates of a Biochemoprevention Trial in Advanced Upper Aerodigestive Tract Premalignant Lesions. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:1605–1610. [PubMed] [Google Scholar]
- 29.Papadimitrakopoulou VA, et al. Biochemoprevention for Dysplastic Lesions of the Upper Aerodigestive Tract. Arch Otolaryngol. 1999;125:1083–1089. doi: 10.1001/archotol.125.10.1083. [DOI] [PubMed] [Google Scholar]
- 30.Shin DM, et al. Biochemopreventive Therapy for Patients With Premalignant Lesions of the Head and Neck and p53 Gene Expression. J Natl Cancer Inst. 2000;92:69–73. doi: 10.1093/jnci/92.1.69. [DOI] [PubMed] [Google Scholar]
- 31.Tae K, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer res. 2000;6(7):2821–2828. [PubMed] [Google Scholar]
- 32.Posner MR, et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 33.Sung JY, et al. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. The American journal of pathology. 2000;157:729–735. doi: 10.1016/S0002-9440(10)64586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng AL, et al. Expression of HBx and COX-2 in chronic hepatitis B, cirrhosis and hepatocellular carcinoma: implication of HBx in upregulation of COX-2. Modern Pathology. 2004;17:1169–1179. doi: 10.1038/modpathol.3800196. [DOI] [PubMed] [Google Scholar]
- 35.Mascaux C, et al. COX-2 expression during early lung squamous cell carcinoma oncogenesis. Eur Respir J. 2005;26:198–203. doi: 10.1183/09031936.05.00001405. [DOI] [PubMed] [Google Scholar]
- 36.Shin DM, et al. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54(12):3153–3159. [PubMed] [Google Scholar]
- 37.Shin DM, et al. Sequential increases in proliferating cell nuclear antigen expression in head and neck tumorigenesis: a potential biomarker. J Natl Cancer Inst. 1993;85(12):971–978. doi: 10.1093/jnci/85.12.971. [DOI] [PubMed] [Google Scholar]
- 38.Shin DM, Hittelman WN, Hong WK. Biomarkers in upper aerodigestive tract tumorigenesis: a review. Cancer Epidemiology, Biomarkers & Prevention. 1994;3(8):697–709. [PubMed] [Google Scholar]
- 39.Banerjee AG, et al. Deregulated Cyclooxygenase-2 expression in oral premalignant tissue. Mol Cancer Ther. 2002;1:1265–1271. [PubMed] [Google Scholar]
- 40.Sudb J, et al. Cyclooxygenase-2 (COX-2) expression in high risk premalignant oral lesions. Oral Oncol. 2003;39:497–505. doi: 10.1016/s1368-8375(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 41.Musikasukont P, et al. Cyclooxygenase-2 expression in oral premalignancies. Mahidol Dent. 2006;26(3):171–178. [Google Scholar]
- 42.Shibata M, et al. Cyclooxygenase-1 and 2 expression in human oral mucosa, dysplasia and squamous cell carcinomas and their pathological significance. Oral Oncol. 2005;41:304–312. doi: 10.1016/j.oraloncology.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Grau JJ, et al. Expression of cyclooxygenase-2 mRNA (COX2-mRNA) in peripheral blood (PB) of head and neck cancer patients (HNC) and healthy controls (HC) Proceedings of J Clin Oncol. 2004;22(14S):#5521. [Google Scholar]


