Abstract
Background
Although there is a statistically significant association between modestly raised baseline plasma C-reactive protein (CRP) values and future cardiovascular events, the debate is still unsettled as to whether CRP plays a causal role in the pathogenesis of atherosclerosis.
Methods and Results
We generated two lines of transgenic (Tg) rabbits expressing human CRP (hCRP). The plasma levels of hCRP in hCRP-Tg-1 and hCRP-Tg-2 rabbits were 0.4 ± 0.13 (n=14) and 57.8 ± 20.6 mg/L (n=12), respectively. Also, hCRP isolated from Tg rabbit plasma exhibited the ability to activate the rabbit complement. To define the role of hCRP in atherosclerosis, we compared the susceptibility of hCRP-Tg rabbits to cholesterol-rich diet-induced aortic and coronary atherosclerosis with that of non-Tg rabbits. After being fed with a cholesterol-rich diet for 16 weeks, Tg and non-Tg rabbits developed similar hypercholesterolemia and lesion sizes in both aortic and coronary arteries. Immunohistochemical staining and Western blotting revealed that hCRP was indeed present in the lesions but did not affect macrophage accumulation and smooth muscle cell proliferation of the lesions.
Conclusions
Neither high nor low plasma concentrations of human CRP affected aortic or coronary atherosclerosis lesion formation in hCRP-Tg rabbits.
Keywords: atherosclerosis, inflammation, acute phase protein, risk factors
There have been many controversial and contradictory results published on the effects of C-reactive protein (CRP), and a very active debate continues regarding its role in the pathogenesis of cardiovascular disease (CVD)1–5. Despite the clinical importance of CRP as a potential marker of increased risk of CVD6, the lack of an appropriate animal model has made it difficult to determine whether CRP is merely a marker or an active mediator in the progression of CVD1. Several lines of evidence have revealed that CRP may modulate vascular function, thereby directly participating in the pathogenesis of atherosclerosis7, 8. This notion has been suggested by the pathological demonstration of CRP in atherosclerotic lesions9 and the finding that CRP causes a number of biological changes in endothelial cells, smooth muscle cells, and macrophages in vitro which are considered to promote lesion progression7. In addition, the recent JUPITER trial (Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin) showed that a lipid-lowering drug, rosuvastatin (Crestor) can significantly reduce the incidence of major cardiovascular events even in apparently healthy subjects not exhibiting established risk factors such as hyperlipidemia but with relatively high baseline plasma CRP levels (2 mg/L or greater)10. These studies so far have raised concerns as to whether we should develop CRP-lowering therapies for reducing CVD or whether we should aggressively treat those CVD patients with high levels of CRP in both primary and secondary prevention stages in the same way used to treat hyperlipidemia11, 12.
Unfortunately, the critical issue of whether high levels of CRP are indeed atherogenic remains unresolved3. Many researchers have attempted to address this issue using transgenic (Tg) mice expressing either human or rabbit CRP, but the results so far are quite controversial and contradictory: CRP is either pro-atherogenic13, 14, has no effect on atherosclerosis15–19, or is even athero-protective20. Although the cause of these discrepancies is unclear, it appears that the mouse is not an appropriate model for evaluation of CRP because plasma levels of CRP in mice, even in the presence of inflammatory stimuli, are extremely low compared with humans and rabbits21. Furthermore, human CRP and rabbit CRP cannot activate complement in the mouse17. Given the limitations of the CRP Tg mouse models, it is imperative to develop CRP Tg rabbits as an alternative model for the study of CRP in vivo. Rabbits have been used as an excellent model for human atherosclerosis because their lipoprotein metabolism and cardiovascular system are similar to those of humans22. In addition, the acute-phase reactant CRP response of rabbits resembles humans more than the mice23 and rabbit and human CRP have similar characteristics in structure and function24. Furthermore, we have demonstrated that the severity of atherosclerosis is also closely associated with plasma CRP levels in cholesterol-fed and Watanabe heritable hyperlipidemic rabbits9. In the current study, we have successfully generated two lines of human CRP Tg rabbits and compared the susceptibility of Tg rabbits to cholesterol-rich diet-induced aortic and coronary atherosclerosis with that of non-Tg rabbits.
Methods
Generation and characterization of transgenic (Tg) rabbits
Tg rabbits were generated by the methods described previously22. In this study, Japanese white rabbits (std:JW/CSK) were purchased from SLC, Inc. (Shizuoka, Japan) and zygotes were microinjected with a DNA construct consisting of 1.13 kb human C-reactive protein (hCRP) cDNA under the control of liver-specific expression elements from the human apolipoprotein E gene25 with 4 copies of the chicken beta-globin insulator (kindly provided by Dr. Gary Felsenfeld, NIH) (Fig. 1A). Insulators can prevent the position effect of transgenes26. All animal experiments were performed with the approval of the Animal Care Committee of the Universities of Yamanashi and Saga, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Two Tg founders were identified by Southern blotting25 and mated with non-Tg rabbits to produce F1 progeny. To examine the mRNA expression of hCRP, total RNA was isolated from various tissues of the Tg rabbits using Trizol reagent (Invitrogen, Life Technologies, Inc., Carlsbad, CA) and Northern blotting was performed as described previously27.
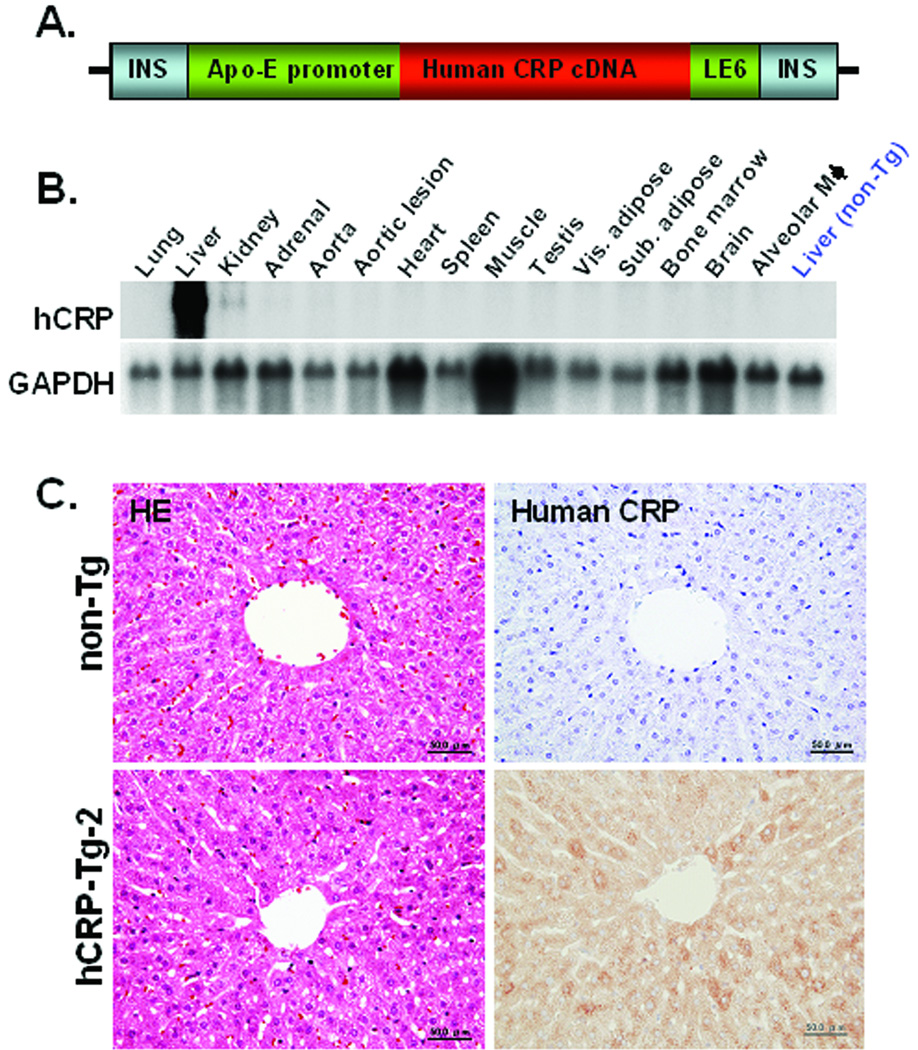
Figure 1.
Transgenic construct for the generation of transgenic rabbits (A). The 10.9 kb transgenic construct contains the human apoE promoter, human CRP cDNA and apoE liver element sequences (LE6) with 4 copies of chicken beta-globin insulator (INS). Northern blotting was performed to examine the hCRP transgene expression in hCRP-Tg-2 rabbits (B). H&E staining (on the left) and immunohistochemical staining of the liver using mAb against hCRP (on the right) are shown in (C).
Western blotting and complement consumption assay
Human CRP concentrations in the plasma of Tg rabbits were measured by latex agglutination using an automatic analyzer (JCA-BM2250, JEOL, Tokyo, Japan). Two founder Tg rabbits expressed hCRP in the plasma at levels of 0.8 mg/L (designated as Tg-1) and 50 mg/L (designated as Tg-2), respectively. To analyze hCRP proteins in Tg rabbit plasma, we subjected the plasma to electrophoresis on a 4–12% non-denaturing polyacrylamide gradient gel without sodium dodecyl sulfate (SDS)28 and also on 10% SDS-polyacrylamide gels (SDS-PAGE), followed by immunoblotting with hCRP mAb. To investigate whether hCRP produced by Tg rabbits was physiologically functional, we isolated hCRP from Tg-2 rabbit plasma using an affinity column with rabbit mAb against hCRP (Epitomics, Inc., Burlingame, CA) and 0.1M glycine-HCl (pH 2.5) as elution buffer. As described previously17, a complement consumption assay was conducted using enzymatically-modified human LDL (E-LDL) as a CRP-ligand. E-LDL concentrations (400 to 800 µg/ml) were adjusted in accordance with the rabbit sera so that a background consumption of approximately 50% was achieved without CRP. The complement consumption induced by E-LDL alone was used as a control and expressed as 100%. Total consumption of CRP from different sources (normal rabbit, human, and Tg-2 rabbit) was compared with that of the controls.
Analysis of blood and plasma biochemistry
To exclude the possibility that expression of hCRP may have any adverse effects on rabbit health, blood cells were analyzed using an automated hematology analyzer Sysmex XE-2100 (Sysmex Co., Kobe, Japan), and plasma biochemistry was measured using an autoanalyzer (JCA-BM2250, JEOL, Tokyo, Japan).
Cholesterol-rich diet experiments
To investigate the effect of hCRP on the development of atherosclerosis, male Tg rabbits (4–5 months) and sex- and age-matched non-Tg littermates were fed a diet containing 0.3% cholesterol and 3% soybean oil for 16 weeks. To minimize the variations of plasma cholesterol concentrations in cholesterol-fed rabbits, we measured plasma lipids biweekly and adjusted the cholesterol content of the diet according to the changes in plasma cholesterol of each animal.
Hypercholesterolemia of both Tg and non-Tg rabbits was induced and maintained at “atherogenic levels” (600~1200 mg/dl) throughout the experiment (see below). The animals were fed ad libitum, and plasma levels of total cholesterol, triglycerides, and HDL-cholesterol were measured using Wako assay kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Plasma levels of hCRP were measured before and after cholesterol diet feeding for 16 weeks. For the analysis of lipoprotein profiles and apolipoproteins, plasma lipoproteins from rabbits at 8 and 16 weeks of cholesterol diet feeding were isolated by sequential ultracentrifugation and analyzed as described previously29.
Quantification of aortic and coronary atherosclerosis
At the end of the cholesterol diet feeding, all rabbits were sacrificed by injection of an overdose of sodium pentobarbital solution. The aortas were en face stained with Sudan IV for evaluation of gross atherosclerotic lesions as described previously30. For microscopic quantification of lesion areas, each portion of the aorta was dehydrated in ethanol and embedded in paraffin (10 segments for the aortic arch and abdominal aorta, and 20 for the thoracic aorta). All specimens were cut into 3-µm-thick sections and stained with hematoxylin and eosin (H&E), and Elastica van Gieson (EVG). For microscopic evaluation of the cellular components of the lesions, serial paraffin-embedded sections of the aorta were immunohistochemically stained with mAbs against macrophages (RAM11) and α-smooth muscle actin (HHF35)30 as shown in S-Table 1 and visualized with Histofine® Simple Stain MAX-PO(M) kits (Nichirei Biosciences Inc., Tokyo, Japan). To assess coronary atherosclerosis, rabbit hearts were sectioned into 7 blocks and the lesions of the left and right coronary arteries were quantified under a light microscope and expressed as the stenosis (%) of the lumen area [lesion area/(total lumen area) × 100%] by the method described previously30, 31 . All measurements were performed blindly and independently by 2 separate researchers. To detect CRPs in lesions, immunohistochemical staining was performed using Abs against human and rabbit CRP. We first evaluated the reactivity of two Abs against denatured proteins by SDS-PAGE followed by Western blotting and found that human CRP mAb showed slight cross-reactivity with rabbit plasma CRP whereas rabbit CRP polyclonal Ab cross-reacted with human CRP (S-Fig.1). For native CRP (though fixed) in the lesions, human CRP mAb showed slight cross-reactivity with rabbit CRP (see below). Because this cross-reactivity was faint and not often present compared to the reactivity of rabbit CRP Ab in the same section, we could evaluate the human CRP deposition in the lesions by immunohistochemical staining. For negative controls, primary Abs were replaced by either nonspecific mouse IgG or chicken IgY. In addition, the lesions of aortas were homogenized and proteins (10 µg) were run on SDS-PAGE followed by immunoblotting with hCRP mAb.
Statistical analysis
ANOVA was used to assess differences between three groups of gross aortic lesions and plasma biochemistry. Two-factor repeated-measures ANOVA was used for the time-course data of plasma lipids after cholesterol-rich diet. One-way ANOVA with Scheffe’s F test or Kruskal-Wallis test was used for parametric and non-parametric analysis. Microscopic analysis of aortic lesions, coronary arterial lesions and plasma lipoproteins between two groups were compared by Student’s t-test or Mann-Whitney’s U test depending on the data distribution. In all cases, statistical significance was set at P< 0.05.
Results
Generation and characterization of transgenic rabbits
We generated two lines of Tg rabbits expressing different levels of plasma hCRP. Average plasma levels of hCRP in F1 Tg-1 and Tg-2 rabbits at 3 to 4 months were 0.4 ± 0.13 (n=14) and 57.8 ± 20.6 mg/L (n=12), respectively (S-Fig.2). The hCRP transcripts were almost exclusively expressed in the liver of Tg rabbits (Fig. 1B). Histological examination revealed no abnormalities in the liver of Tg rabbits and hCRP immunoreactive proteins were immunohistochemically detected only in hepatocytes but not in blood vessels or bile ducts (Fig. 1C).
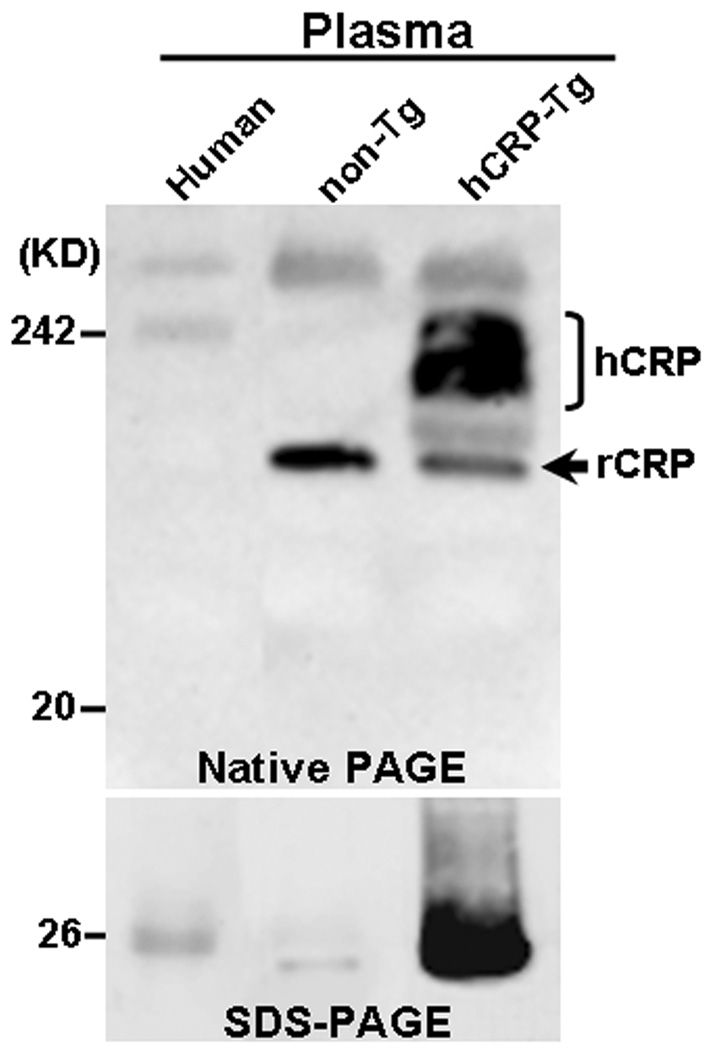
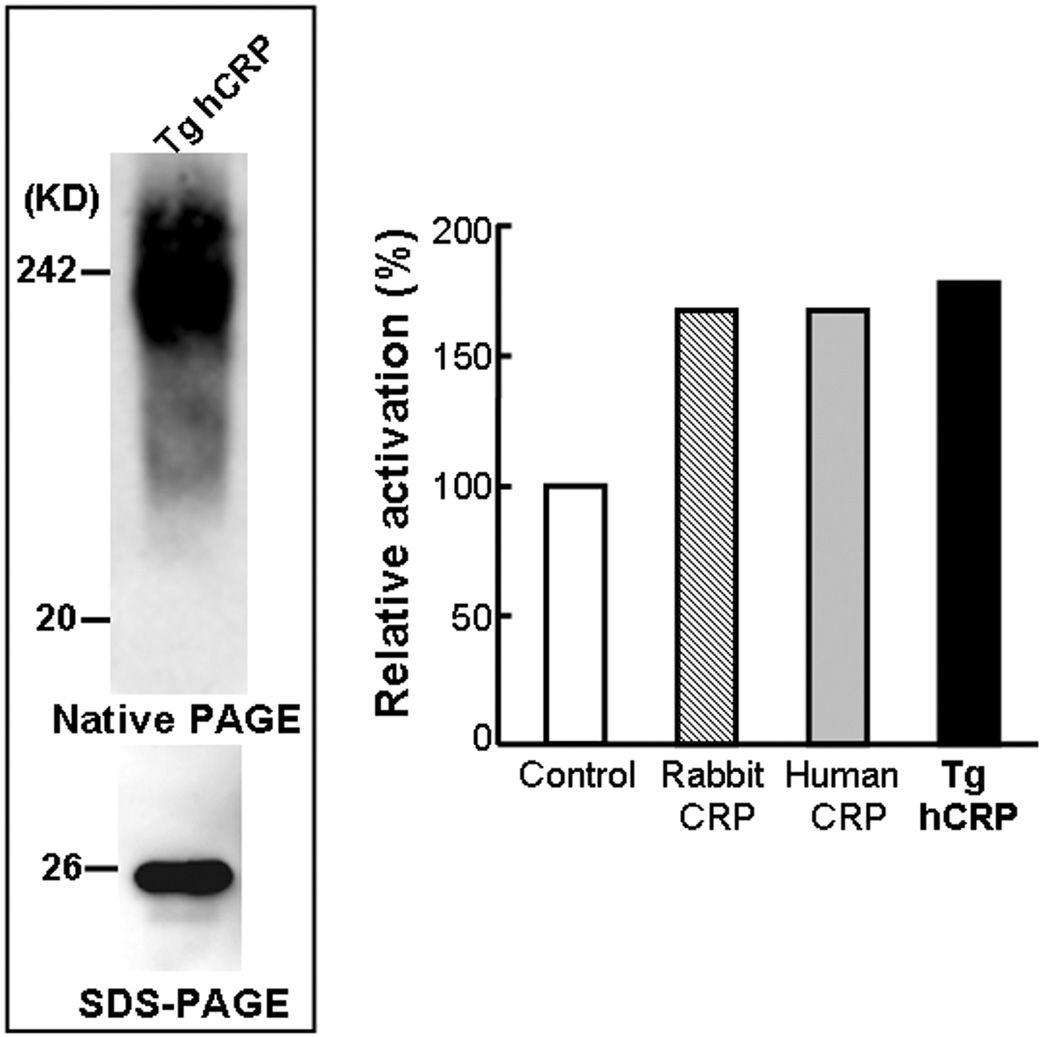
Western blotting analysis revealed that hCRP in the plasma of Tg rabbits was present as a complex with a high molecular weight (pentamer) on non-denaturing gels and a monomer on SDS-PAGE (with an approximate molecular weight of 26 kD) (Fig.2). To investigate whether hCRP produced by Tg rabbits was physiologically functional, we conducted a complement consumption assay using E-LDL as a CRP-ligand. We found that isolated hCRP from Tg-2 rabbis exhibited the same ability to augment activation of rabbit serum complement in the presence of E-LDL as native rabbit and human CRP (Fig.3).
Figure 2.
Western blotting analysis of plasma CRP of Tg-2 and non-Tg rabbits by either non-denaturing gel (top) or SDS-PAGE (bottom) and immunoblotted with hCRP mAb as described in Methods. Human plasma obtained from a volunteer was used as a positive control.
Figure 3.
Western blotting analysis and complement activation assay. Human transgenic CRP was isolated from Tg-2 rabbit plasma as described in Methods and analyzed by either non-denaturing gel (top) or SDS-PAGE (bottom) and immunoblotted with hCRP mAb (left panel). Isolated human transgenic CRP from Tg rabbits exhibited the same ability to augment activation of rabbit serum complement by E-LDL as native rabbit and human CRP (right panel).
Cholesterol-rich diet experiments
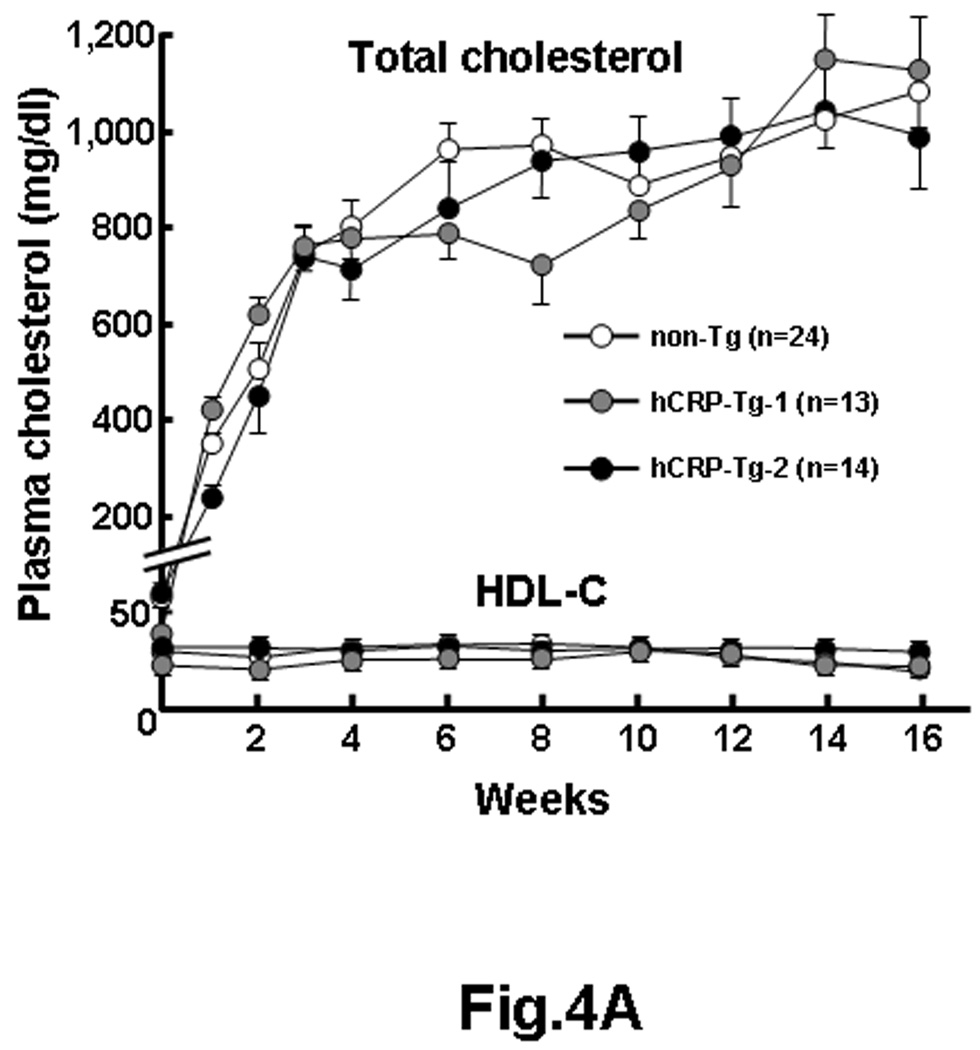
To investigate the effect of hCRP on the development of atherosclerosis, male Tg rabbits and non-Tg littermates were fed a cholesterol-rich diet for 16 weeks. Both Tg and non-Tg rabbits developed similar hypercholesterolemia during the experimental period and lipoprotein profiles were identical (Fig.4A, left and S-Fig. 3). Plasma hCRP levels of Tg-2 rabbits remained as “high” as those of a normal chow diet-fed rabbits during the cholesterol diet whereas plasma hCRP levels of Tg-1 rabbits were constantly “low” (0.4~5 mg/L) (S-Fig.2). Expression of hCRP did not lead to obvious changes in the variables of blood and plasma in both Tg rabbits compared to non-Tg rabbits during the experiment (S-Table 2).
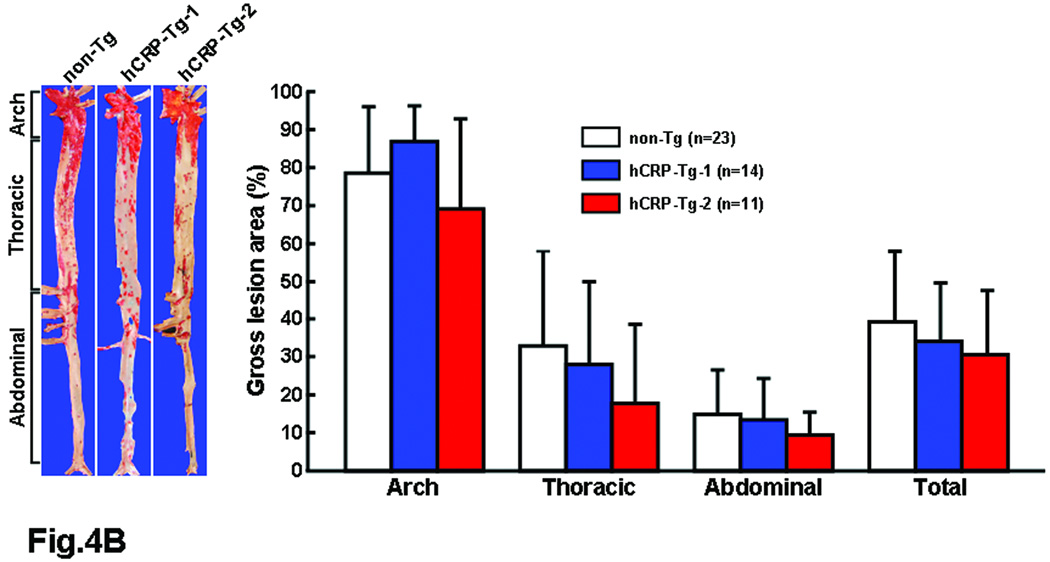
Figure 4.
hCRP-Tg rabbits developed similar hypercholesterolemia to non-Tg rabbits during cholesterol diet feeding (A, left). The values are expressed as mean ± SE.
Representative photographs of pinned-out aortic trees stained with Sudan IV from each group are shown in (B, left) and aortic atherosclerotic lesions (defined by Sudanophilic area) on the surface were quantified by an image analysis system (B, right). The values are expressed as mean ± SD. P<0.05, =0.36, =0.52, and =0.49 in arch, thoracic, abdominal and whole aorta by ANOVA. Since p<0.05 by ANOVA was noted in arch, we further analyzed these data by Scheffe’s F test and found that p=0.38 (non-Tg vs. Tg-1) and 0.35 (non-Tg vs. Tg-2). Therefore, there was no statistical difference between Tg and non-Tg rabbits in all parts of the aorta.
Quantification of aortic and coronary atherosclerosis
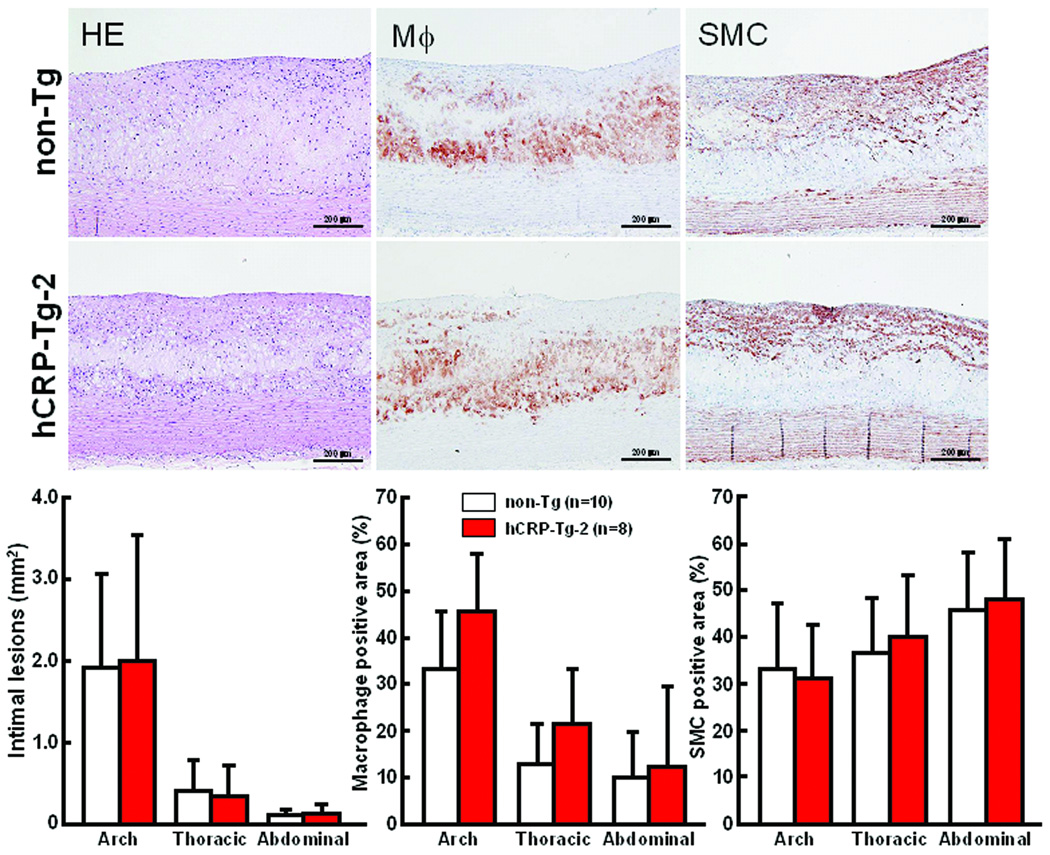
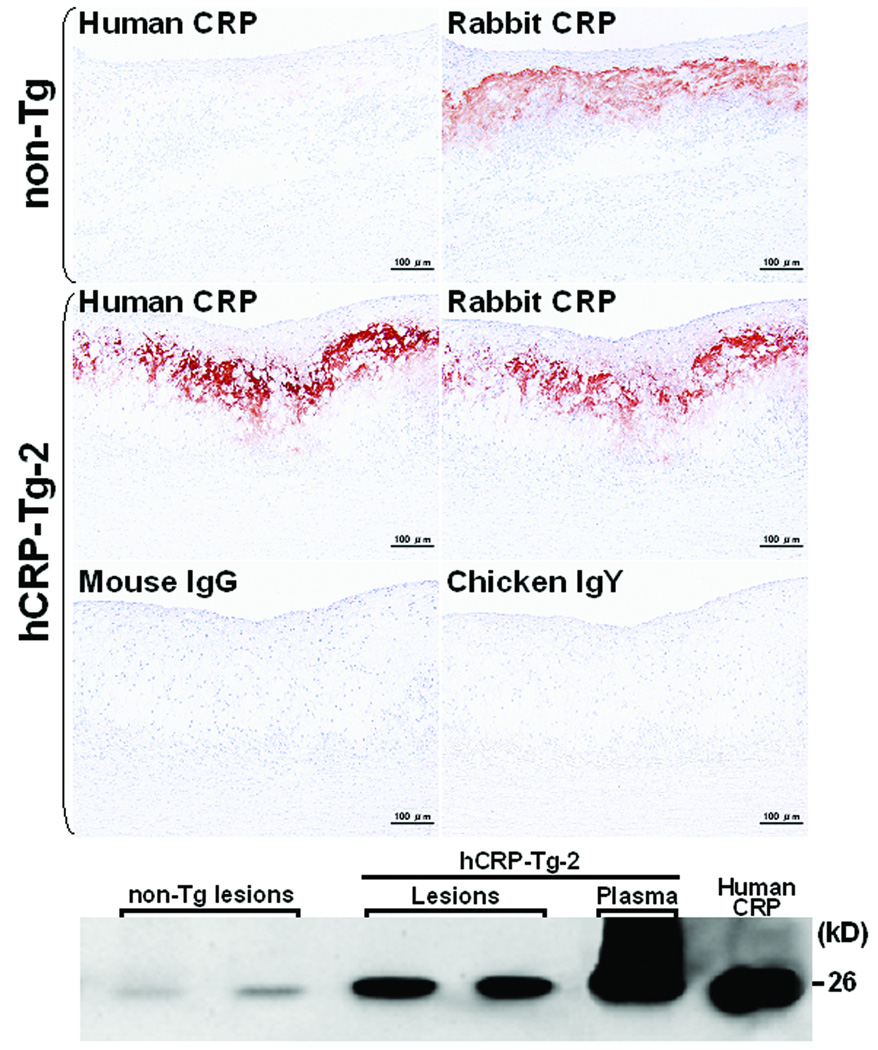
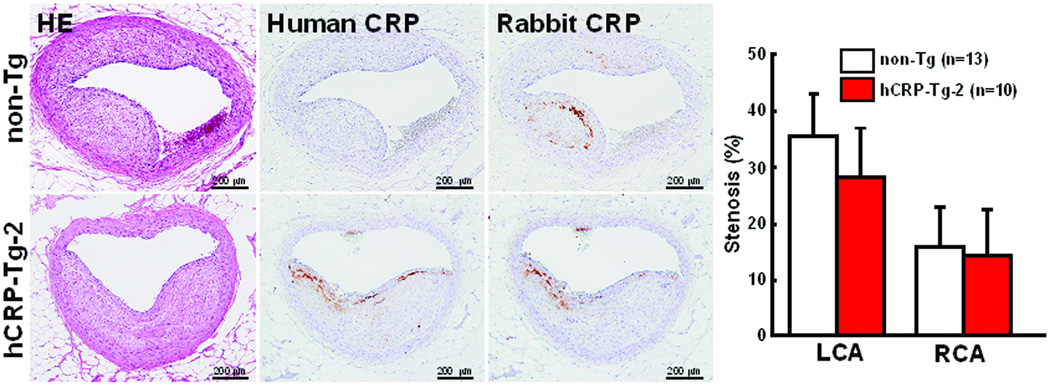
At the end of the experiment, all rabbits were sacrificed and the severity of aortic and coronary atherosclerosis was examined and quantified using an image analysis system. Compared to non-Tg control rabbits, neither of the Tg rabbit lines showed any statistical differences (p=0.5 vs. non-Tg by ANOVA) in aortic atherosclerotic lesions defined by Sudan IV staining (Fig.4B). Since plasma levels of hCRP in both lines of Tg rabbits were quite variable at 16 weeks (S-Fig. 2), we also evaluated the correlation between plasma hCRP and the extent of aortic lesions of each Tg rabbit. However, we did not find any correlations between plasma hCRP and aortic lesions in all Tg rabbits (data not shown). We further examined sections of the lesions under a light microscope and quantified the microscopic lesion areas. However, we did not find any differences in lesion sizes or cellular components (macrophages and smooth muscle cells) between Tg-2 and non-Tg rabbits (Fig.5). To confirm the presence of CRP in lesions, we performed immunohistochemical staining using Abs against either human or rabbit CRP and showed that hCRP immunoreactive proteins were regularly detected in atherosclerotic lesions of Tg rabbits whereas rabbit CRP was present in both Tg and non-Tg rabbit lesions (Fig.6, top panel and S-Fig.4). Western blotting analysis of the aortic lesions confirmed that the CRP contents were markedly increased in the lesions of Tg rabbits compared to non-Tg rabbits (Fig.6, bottom panel). Finally, we examined the effect of hCRP on coronary arterial lesions. As shown in Fig.7, the coronary stenosis of Tg-2 rabbits was not statistically different from that of non-Tg rabbits (p=0.33 in left and p=0.64 in right coronary artery vs. non-Tg) even though CRP immunoreactive proteins were detected in the lesions. Taken together, human CRP does not affect the development of atherosclerosis in Tg rabbits, which is supported by two recent human genetic studies32, 33.
Figure 5.
Representative micrographs of the aortic lesions from Tg and non-Tg rabbits (top). Serial paraffin cross-sections of aortic lesions were stained with H&E or immunohistochemically stained with mAbs against either macrophages (Mϕ) or α-smooth muscle actin for SMCs. Microscopic analysis of the intimal lesion size and cellular components by immunohistochemical staining is shown (bottom). Intimal lesions on EVG-stained sections were quantified with an image analysis system (left). Positive-stained areas of immunostained macrophages and SMCs are quantified (middle and right). The values are expressed as mean ± SD. Intimal lesions: p=0.90, 0.66 and 0.54 in arch, thoracic and abdominal aorta. Mϕ positive area: p=0.06, 0.10 and 0.76 in arch, thoracic and abdominal aorta. SMC positive area: p=0.74, 0.56 and 0.76, in arch, thoracic and abdominal aorta. All comparisons were made by Student’s t-test.
Figure 6.
Demonstration of human and rabbit CRPs in aortic lesions by immunohistochemical staining (top) and Western blotting analysis (bottom). Human and rabbit CRP immunoreactive proteins were stained by mouse mAb against human CRP and chicken polyclonal Ab, respectively. The negative control staining was performed by using mouse non-specific IgG and chicken IgY. Human CRP mAb shows slight cross-reaction with rabbit endogenous CRP (top, left). Purified human CRP from Sigma-Aldrich was used as a positive control in Western blotting.
Figure 7.
Histological analysis of coronary arterial atherosclerosis and immuno-detection of human and rabbit CRP in lesions (left) and the quantitatively measured lesion size and expressed as the stenosis (%) of the lumen area [lesion area/(total lumen area) × 100%] (right). The values are expressed as mean ± SE. P=0.33 in LCA and p=0.64 in RCA vs. non-Tg, analyzed by Mann-Whitney’s U test.
Discussion
For the first time, we have successfully generated two lines of hCRP Tg rabbits to define the role of CRP in atherosclerosis. CRP is a highly controversial maker of cardiovascular disease. The rabbit model was selected for this undertaking because of its usefulness in studying both the development of atherosclerosis and CRP pathophysiological functions22, 34.
Human CRP isolated from Tg rabbit plasma exhibited the ability to activate the rabbit complement in the presence of E-LDL, confirming that hCRP of Tg rabbits is functional in vivo. Expression of hCRP in Tg rabbits did not lead to any health problems since we did not find any pathological abnormalities and hematological and biochemical data of blood were unchanged compared to non-Tg rabbits. Spontaneously atherosclerotic lesions were not detected in both lines of hCRP-Tg rabbits on a chow diet for up to one year (data not shown). Therefore, we administered a cholesterol-rich diet for 16 weeks, a method that has been used in many studies for investigating the interactions between different genes and the development of atherosclerosis in rabbits22. Plasma total cholesterol levels and lipoprotein profiles of Tg rabbits were basically similar to non-Tg rabbits. Taken together, we have established hCRP-Tg rabbits that allow us to investigate the direct effects of plasma hCRP on the development of atherosclerosis.
As illustrated by our analysis, average plasma levels of hCRP-Tg-2 rabbits are above the risk levels (3~10 mg/L) generally proposed in humans35, 36. Plasma hCRP levels of Tg-1 rabbits were initially less than 1 mg/L but increased to 4.97 ± 4.63 mg/L at 16 weeks of cholesterol diet. Regardless of hCRP expression in Tg rabbits, both lines of Tg rabbits did not show any enhancement of either lesion size or any changes in the cellular components (macrophages and smooth muscle cells) of lesions. Immunohistochemical staining coupled with Western blotting revealed that hCRP immunoreactive proteins were indeed present in lesions. Because hCRP, like endogenous rabbit CRP is exclusively expressed by the liver but not by aorta or macrophages in Tg rabbits, we considered that CRP in the lesions was essentially derived from the circulation rather than synthesized de novo by vascular cells34. Despite this observation, both aortic and coronary atherosclerotic lesions were not significantly changed in both Tg rabbits compared to non-Tg rabbits, suggesting that hCRP at these levels is not pro-atherogenic in Tg rabbits. In the past years, many studies attempted to demonstrate the atherogenic effect of CRP in genetically-modified mice but the results so far are controversial13, 15–17, 19, 20. It is apparent that our results obtained from two lines of Tg rabbits expressing different plasma levels of human CRP rebut the notion that CRP is pro-atherogenic. Our data are also in support of the recent study showing that genetically-elevated CRP does not play a causal role in ischemic vascular disease32, 33. Nevertheless, we cannot exclude the possibility that human CRP may have a pathophysiological role in aspects of cardiovascular disease that are not modeled in the present study, such as myocardial infarction37 and thrombosis38. It also remains to be determined whether local CRP present in the arterial wall is involved in plaque rupture. Cross-breeding hCRP Tg rabbits with Watanabe heritable hyperlipidemic MI rabbits that develop spontaneous atherosclerosis in both aorta and coronary arteries as well as myocardial infarction39 will certainly provide a powerful model to examine these hypotheses in future.
In summary, the present study does not support a direct role of human CRP in the pathogenesis of atherosclerosis in hCRP-Tg rabbits, suggesting that CRP is a marker rather than a maker in the development of atherosclerosis.
Clinical perspectives
Despite the clinical importance of c-reactive protein (CRP) as a potential marker for cardiovascular diseases, the lack of an appropriate animal model has made it difficult to determine whether CRP is merely a marker or an active mediator in the pathogenesis of atherosclerosis. In the past years, studies using transgenic (Tg) mice expressing either human or rabbit CRP have generated quite controversial and contradictory results. In fact, mice are not appropriate for evaluation of CRP pathophysiology because CRP in mice is not functional in terms of complement activation. In the current study, we have generated novel Tg rabbits expressing human CRP (hCRP) and documented that hCRP does not affect aortic or coronary atherosclerosis lesion formation in hCRP-Tg rabbits. Taken together, our data suggest that CRP may be not a maker of human atherosclerosis.
Supplementary Material
Acknowledgements
We thank our students Maeda T, Aoki T, and Jin Y for their help with animal experiments. We also thank Sato K and Ohta M, Department of Clinical and Laboratory Medicine, University of Yamanashi Hospital, for analysis of rabbit plasma and blood.
Funding Sources
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports and Technology, Japan, a research grant for cardiovascular disease from the Ministry of Health, Labor, and Welfare of Japan, NIH R01HL088391 (YEC), an American Heart Association National Career Development Grant (0835237N to JZ), the Takeda Science Foundation, the Ono Medical Research Foundation, the Naito Foundation for the Promotion of Science, the Uehara Memorial Foundation, the Japan Heart Foundation and a research grant from AstraZeneca. YEC is an established investigator of the American Heart Association (0840025N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject code: [130] Animal models of human disease [134] Pathophysiology [135] Risk Factors
[96] Mechanism of atherosclerosis/growth factors
Disclosures
None
References
- 1.Nilsson J. CRP--marker or maker of cardiovascular disease? Arterioscler Thromb Vasc Biol. 2005;25:1527–1528. doi: 10.1161/01.ATV.0000174796.81443.3f. [DOI] [PubMed] [Google Scholar]
- 2.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schunkert H, Samani N. Elevated C-reactive protein in atherosclerosis - chicken or egg? N Engl J Med. 2008;359:1953–1955. doi: 10.1056/NEJMe0807235. [DOI] [PubMed] [Google Scholar]
- 4.Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, Rumley A, Fowkes FG, Humphries SE, Hingorani AD. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med. 2008;264:295–314. doi: 10.1111/j.1365-2796.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–2150. [PubMed] [Google Scholar]
- 8.Devaraj S, Singh U, Jialal I. The evolving role of C-reactive protein in atherothrombosis. Clin Chem. 2009;55:229–238. doi: 10.1373/clinchem.2008.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun H, Koike T, Ichikawa T, Hatakeyama K, Shiomi M, Zhang B, Kitajima S, Morimoto M, Watanabe T, Asada Y, Chen YE, Fan J. C-reactive protein in atherosclerotic lesions: its origin and pathophysiological significance. Am J Pathol. 2005;167:1139–1148. doi: 10.1016/S0002-9440(10)61202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Danielson E, Fonseca F, Genest J, Gotto AJ, Kastelein J, Koenig W, Libby P, Lorenzatti A, MacFadyen J, Nordestgaard B, Shepherd J, Willerson J, Glynn R, Group JS. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 11.Jialal I, Devaraj S. Jupiter to Earth: CRP Promotes Atherothrombosis. Metab Syndr Relat Disord. 2009;7:1–3. doi: 10.1089/met.2009.EDI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuster V, Bansilal S. JUPITER strikes earth. Nat Clin Pract Cardiovasc Med. 2009;6:159. doi: 10.1038/ncpcardio1454. [DOI] [PubMed] [Google Scholar]
- 13.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 14.Xing D, Hage FG, Chen YF, McCrory MA, Feng W, Skibinski GA, Majid-Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (Fc gamma RI)-dependent. Am J Pathol. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trion A, de Maat MP, Jukema JW, van der Laarse A, Maas MC, Offerman EH, Havekes LM, Szalai AJ, Princen HM, Emeis JJ. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 16.Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, Dhillon AP, Tennent GA, Pepys MB. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reifenberg K, Lehr HA, Baskal D, Wiese E, Schaefer SC, Black S, Samols D, Torzewski M, Lackner KJ, Husmann M, Blettner M, Bhakdi S. Role of C-reactive protein in atherogenesis: can the apolipoprotein E knockout mouse provide the answer? Arterioscler Thromb Vasc Biol. 2005;25:1641–1646. doi: 10.1161/01.ATV.0000171983.95612.90. [DOI] [PubMed] [Google Scholar]
- 18.Tennent GA, Hutchinson WL, Kahan MC, Hirschfield GM, Gallimore JR, Lewin J, Sabin CA, Dhillon AP, Pepys MB. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis. 2008;196:248–255. doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Torzewski M, Reifenberg K, Cheng F, Wiese E, Kupper I, Crain J, Lackner KJ, Bhakdi S. No effect of C-reactive protein on early atherosclerosis in LDLR−/− / human C-reactive protein transgenic mice. Thromb Haemost. 2008;99:196–201. doi: 10.1160/TH07-10-0595. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs A, Tornvall P, Nilsson R, Tegner J, Hamsten A, Bjorkegren J. Human C-reactive protein slows atherosclerosis development in a mouse model with human-like hypercholesterolemia. Proc Natl Acad Sci U S A. 2007;104:13768–13773. doi: 10.1073/pnas.0706027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepys MB, Baltz M, Gomer K, Davies AJ, Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278:259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther. 2003;99:261–282. doi: 10.1016/s0163-7258(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 23.Kushner I, Feldmann G. Control of the acute phase response. Demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J Exp Med. 1978;148:466–477. doi: 10.1084/jem.148.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson HC, Mc CM. The occurrence in the rabbit of an acute phase protein analogous to human C reactive protein. J Exp Med. 1951;93:25–36. doi: 10.1084/jem.93.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A. 1994;91:8724–8728. doi: 10.1073/pnas.91.18.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, Gaszner M, Felsenfeld G. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc Natl Acad Sci U S A. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 2001;276:40071–40079. doi: 10.1074/jbc.M105456200. [DOI] [PubMed] [Google Scholar]
- 28.Chiesa G, Hobbs HH, Koschinsky ML, Lawn RM, Maika SD, Hammer RE. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a) J Biol Chem. 1992;267:24369–24374. [PubMed] [Google Scholar]
- 29.Fan J, Ji ZS, Huang Y, de Silva H, Sanan D, Mahley RW, Innerarity TL, Taylor JM. Increased expression of apolipoprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J Clin Invest. 1998;101:2151–2164. doi: 10.1172/JCI1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 31.Hirata M, Watanabe T. Regression of atherosclerosis in normotensive and hypertensive rabbits. A quantitative analysis of cholesterol-induced aortic and coronary lesions with an image-processing system. Acta Pathol Jpn. 1988;38:559–575. [PubMed] [Google Scholar]
- 32.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 33.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. Jama. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H, Koike T, Ichikawa T, Hatakeyama K, Shiomi M, Zhang B, Kitajima S, Morimoto M, Watanabe T, Asada Y, Chen YE, Fan J. C-reactive protein in atherosclerotic lesions: its origin and pathophysiological significance. Am J Pathol. 2005;167:1139–1148. doi: 10.1016/S0002-9440(10)61202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 37.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, Fay WP, Simon DI, Edelman ER. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–515. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 39.Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbits) Arterioscler Thromb Vasc Biol. 2003;23:1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.