Abstract
Coordinated transfer of information between the brain hemispheres is essential for function and occurs via three axonal commissures in the telencephalon: the corpus callosum (CC), hippocampal commissure (HC) and anterior commissure (AC). Commissural malformations occur in over 50 human congenital syndromes causing mild to severe cognitive impairment. Disruption of multiple commissures in some syndromes suggests that common mechanisms may underpin their development. Diffusion tensor magnetic resonance imaging revealed that forebrain commissures crossed the midline in a highly specific manner within an oblique plane of tissue, referred to as the commissural plate. This specific anatomical positioning suggests that correct patterning of the commissural plate may influence forebrain commissure formation. No analysis of the molecular specification of the commissural plate has been performed in any species, therefore we utilized specific transcription factor markers to delineate the commissural plate, and identify its various sub-domains. We found that the mouse commissural plate consists of four domains, and tested the hypothesis that disruption of these domains might affect commissure formation. Disruption of the dorsal domains occurred in strains with commissural defects such as Emx2 and Nfia knockout mice but commissural plate patterning was normal in other acallosal strains such as Satb2. Finally, we demonstrate an essential role for the morphogen, Fgf8 in establishing the commissural plate at later developmental stages. The results demonstrate that correct patterning of the commissural plate is an important mechanism in forebrain commissure formation.
Keywords: Commissural plate, Nuclear factor I (Nfi), Empty spiracles homeobox (Emx), Sine oculis-related homeobox 3 homolog (Six3), Zinc finger protein of the cerebellum 2 (Zic2), Fibroblast growth factor 8 (Fgf8)
Introduction
Early anatomical studies described a unique anatomical region within the brains of many mammalian species, where all telencephalic commissures initially cross the interhemispheric midline; this region was termed the commissural plate (Rakic and Yakovlev, 1968 and references therein). Rakic and Yakovlev (1968) proposed that the human commissural plate could be anatomically divided into dorsal and ventral domains, respectively termed the massa commissuralis (MC) where the corpus callosum (CC) and hippocampal commissure (HC) cross, and the area septalis (or septal area, SA), where the anterior commissure (AC) crosses the midline. To date, there has been no anatomical characterization of the commissural plate in the mouse, nor any molecular characterization in any species.
During early embryogenesis, patterning centers of morphogens such as fibroblast growth factors (Fgf), sonic hedgehog, bone morphogenic proteins and the secreted Wnt proteins divide the brain into various sectors (Hebert, 2005; Takahashi and Liu, 2006), including the pallium and the subpallium (Rubenstein et al., 1998). One such forebrain patterning center is the commissural plate, which expresses Fgf8 (Shimamura and Rubenstein, 1997; Fukuchi-Shimogori and Grove, 2001; Shimogori et al., 2004; Storm et al., 2006) and patterns the cerebral cortex into functionally specific domains (O’Leary and Sahara, 2008). Although the commissural plate has been identified as an early patterning center, it is not clear whether this region corresponds to that described by Rakic and Yakovlev (1968) as the area where all forebrain commissures cross the midline. A more detailed analysis of this region throughout development is required to connect the two developmental functions of the commissural plate, namely patterning and commissure formation.
To examine the development of all midline commissures in the same brain in three dimensions we used diffusion tensor magnetic resonance imaging (DTMRI), which measures the direction and microstructural properties of white matter tracts. DTMRI was used to virtually section the embryonic brain at any angle, revealing that the three commissures formed initially within a single oblique coronal plane of tissue, which we could then section histologically at the same angle. This allowed us to observe this “plate” of tissue in a single section and to identify molecular expression domains that could delineate each region of the commissural plate. Transcription factors and anatomical correlates were used to define four domains of the mouse commissural plate. These included the nuclear factor I family (Nfia), empty spiracles homologs (Emx1), sine oculis-related homeobox 3 (Six3) and the zinc finger protein of the cerebellum 2 (Zic2) (Yoshida et al., 1997; Ogura et al., 2001; Shu et al., 2003; Steele-Perkins et al., 2005; Lavado et al., 2008). Patterning of these domains was disrupted in mice with commissural defects and deficient in NFIA and EMX2 proteins. However, patterning was not disrupted in the Satb2 knockout mice, which also displays commissural defects. Finally, we found that Fgf8 is required for patterning the dorsal domains of the commissural plate. Overall, this analysis establishes a framework for understanding the development of this important brain region and its role in commissure formation.
Materials and Methods
Animals
Wildtype C57Bl/6J, and litters of Emx2 (Pellegrini et al., 1996), Nfia knockout mice (Steele-Perkins et al., 2005) and Fgf8flox/flox (Meyers et al., 1998) crossed with Emx1Cre (Iwasato et al., 2004) animals, were bred on site at the University of Queensland under approval from the institutional Animal Ethics Committee. Mice knockout for the special AT-rich sequence binding protein 2 (Satb2; Britanova et al., 2006) were maintained at the Max-Planck-Institute for Experimental Medicine in accordance with German law and approved by the Bezirksregierung Braunschweig; the brains were shipped to the University of Queensland for experimental analysis. Targeted deletion of Fgf8 in the dorsal forebrain was performed by crossing the Fgf8flox/flox and Emx1Cre strains (both conditional alleles with wildtype activity) to generate Emx1-cre;Fgf8flox/flox mice. Mapping Emx1 Cre recombinase in the cortex was performed by crossing Emx1Cre mice with those expressing green fluorescent protein (GFP) following loxP recombination (B6;129-Gt(ROSA)26Sortm2Sho/J known here are ROSA26-eGFPflox (Mao et al., 2001)). Transgenes used to produce the genetically modified mice are detailed in Table 1. Timed-pregnant dams were identified by the presence of a vaginal plug, this being designated as embryonic day 0. Embryos were genotyped by PCR as previously described for the Nfia−/− (Steele-Perkins et al., 2005), Emx2−/− (Pellegrini et al., 1996), Fgf8flox/flox (Meyers et al., 1998), Emx1Cre (Iwasato et al., 2004), ROSA26-eGFPflox (according to The Jackson Laboratory genotyping protocol for this strain) and Satb2−− strains (Britanova et al., 2006). For embryo perfusion, pregnant dams at the appropriate gestational stages were first deeply anesthetized (6.5 mg/ml sodium pentobarbitone, i.p.) and embryos recovered by cesarian section. Embryonic day 14 (E14) heads were immersion fixed in 4% w/v paraformaldehyde (PFA; ProSciTech, Australia) in phosphate buffered solution (PBS; pH 7.4; Lonza, USA). Embryos from stages E15 to E17 were transcardially perfused using 0.9% saline solution (0.9% w/v NaCl in double-distilled H2O) for 2 min, followed by 4% PFA for 4 min. At least three animals were used per experiment.
Table 1.
Mouse strains used.
| Mouse strain | Transgene | Reference |
|---|---|---|
| Emx2 | 250 bp fragment deletion in the 5′ part of the homeobox with Neo insert | Pellegrini et al., 1996 |
| Nfia | 700 bp deletion including all but 9 bp of exon 2, with LacZ/Neo insert | Steele-Perkins et al., 2005 |
| Fgf8flox | loxP sites were inserted in the intron upstream of exon 2 and another in the 3′-UTR allowing excision of exons 2 and 3 | Meyers et al., 1998 |
| Emx1cre | A nuclear localization signal-Cre-poly (A) signal was inserted, in the sense orientation, immediately 5′ to the Emx1 translational initiation site (ATG) into a PAC-Emx1 clone | Iwasato et al., 2004 |
| Satb2 | Deletion of exon 2 and Cre insertion (null allele) | Britanova et al., 2006 |
| ROSA26-eGFPflox | a loxP-flanked STOP fragment lies between the eGFP gene and the Gt(ROSA)26Sor promoter | Mao et al., 2001 |
Diffusion tensor magnetic resonance imaging (DTMRI)
Imaging of E14 to E17 C57Bl/6 brains (in heads) was performed using an 11.7 Tesla magnetic resonance spectrometer (Biospin; Bruker, Billerica, MA) following incubation in 1 μM Magnevist (Berlex Imaging, USA). Diffusion-weighted (DW) images were acquired with a three-dimensional DW fast spin echo sequence (Mori and van Zijl, 1998) as previously described (Ren et al., 2007). Briefly, the imaging involved a repetition time of 700 ms, echo time of 27 ms, imaging resolution of 0.09 × 0.09 × 0.09 mm, signal averages of 4, and twin navigator echoes for eddy current correction. Two non-DW images and six DW images (b = 1200 s/mm2) were acquired. The total imaging time was approximately 15 h. The diffusion tensor was calculated using a log-linear fitting method, with three pairs of eigenvalues and eigenvectors calculated for each pixel. The eigenvector associated with the largest eigenvalue was referred to as the primary eigenvector. Files were reassembled using DTI-Studio (www.mristudio.org) and color maps were generated using fractional anisotropy and primary eigenvector analyzes. An angle of zero degrees for calculation of the oblique coronal plane was taken from the base of the brain along the ventral hypothalamus. Tractography was performed using DTI-Studio.
Antibodies
Antibodies, sources and their concentrations are shown in Table 2.
Table 2.
Primary antibodies used in immunohistochemistry analysis
| Antibody | Host, isotype | Source, Cat. No. | Antigen | Protocol, dilution |
|---|---|---|---|---|
| NFIA | Rb, IgG | Active Motif, 39329 | 478-492aa of human NFIA | IHC-P, 1:1000 |
| EMX1 | Rb, serum | Gift: | hEmx1 cDNA full length protein | IHC-P, 1:500; IHC- FrFl, 1:1000 |
| SIX3 | Gp, IgG | Rockland, 200-201-A26 | 270-289aa of mouse Six3 | IHC-P, 1:200 |
| ZIC2 | Rb, serum | Brown et al., 2003 | 2–109aa peptide from hZic2 cDNA | IHC-P, 1:1000 |
| GAP-43 | Ms, Clone 9-1E12 | Chemicon, MAB347 | purified rat brain GAP-43 | IHC-P, 1:100 |
| GFAP | Rb, IgG | DAKO, Z0334 | Bovine spinal cord isolate | IHC-FrFl, 1:30,000 |
| GFP | Rb, IgG | Invitrogen, A11122 | A. victoria isolate | IHC-FrFl, 1:1000 |
IHC-P, paraffin section immunohistochemistry; IHC-FrFl, vibratome-sectioned free-floating immunohistochemistry; Gp, guinea pig; Ms, mouse; Rb, rabbit.
The GAP-43 (Clone 9-1E12; Chemicon; MAB347) antibody specifically detects a single band in Western blots at ~45kDa on rat brain and nerve tissues (Schreyer and Skene, 1991) and on mouse brain extracts and mouse brain membrane fractions of growing neurons (manufacturer’s information).
The NFIA antibody was purchased from Active Motif (39329) and used at 1:1000. The specificity of anti-NFIA in the mouse has been previously confirmed by Western blot analysis with HA-tagged NFIA, peptide blocking experiments and using Nfia knockout mice (Plachez et al., 2008).
Anti-GFP is a rabbit polyclonal IgG antibody raised against GFP that was isolated directly from A. victoria and purified by ion-exchange (manufacturer’s information). Specificity of the antibody was confirmed in the present study by immunohistochemistry (1:1000) in a non-GFP-expressing mouse line (C57Bl/6; data not shown) and in a GFP-expressing mouse line (Emx1cre-ROSA26; see Results section).
Rabbit anti-glial fibrillary acidic protein (GFAP) IgG (Dako, USA; Z0334) was produced from purified bovine spinal cord isolate (manufacturer’s information). Specificity of this antibody in mouse brain has been confirmed by immunohistochemistry in GFAP knockout mice (Hanbury et al., 2003).
The rabbit anti-ZIC2 antibody was produced by Brown et al. (2003) using the protein produced from a cloned human ZIC2 fragment that corresponded to amino acids 2-109. The anti-serum was reactive by immunohistochemical and immunoblot methods only in CHO cells transfected with Zic2 at the expected 55kDa weight, but not Zic1 or Zic3 expression plasmids. As expected, immunoreactivity was localised to the nucleus in cultured CHO cells (Brown et al., 2003).
The rabbit anti-EMX1 antibody was produced from recombinant human EMX1 and characterized by Briata et al. (1996). In that study, the antiserum identified a single band corresponding to EMXl (31 kDa) in protein extracted from E13.5 mouse telencephalon, and a single ~31kDa band from Sf9 cells expressing EMX1, but not EMX2, in which no band was observed. During this characterization the preadsorption control produced no staining. A Western blot of total lysates of the telencephalon of an E13.5 and a new-born mouse reacted with the EMX2-adsorbed anti-EMXl antiserum revealing a single ~31kDa band.
A synthetic peptide corresponding to amino acids 270–289 of the mouse SIX3 protein (GenBank AAH94426) was used as the antigen to produce the protein A-purified polyclonal antibody in guinea pigs (Rockland Immunochemicals, USA; 200-201-A26). Titration experiments at concentrations between 1:100 and 1:1000 revealed optimal immunoreactivity at 1:200. Preadsorption control immunohistochemistry was performed using the synthetic peptide at 50× the antibody concentration (Supp. Fig. 1), and revealed selective immunoreactivity in the mouse E17 forebrain, corresponding to known Six3 expression (Oliver et al., 1995).
Goat, affinity-purified fluorescent secondary antibodies included anti-mouse Alexa Fluor 633 (1:100; A21126, Invitrogen, Australia), anti-rabbit Alexa Fluor 488 (1:200; A11034, Invitrogen, Australia) and anti-guinea pig Alexa Fluor 546 (1:200; A11074, Invitrogen, Australia). Additional chromogenic immunohistochemistry was performed for GAP43 (as above) with donkey anti-mouse biotin-SP-conjugated secondary antibody (715-065-150, Jackson ImmunoResearch Laboratories, USA), and for rabbit anti-GFP (A11122, Invitrogen, Australia) using goat anti-rabbit biotin-conjugated secondary antibody (BA-1000, Vector laboratories, USA).
Immunohistochemistry and image acquisition
Paraffin tissue infiltration and paraffin-embedded sectioning were utilized to obtain 5 μm sections. As DTMRI revealed the commissural plate to be an oblique structure, we developed a technique to obtain sections spanning the commissural plate at the oblique coronal orientation as well as in a standard sagittal plane. This novel sectioning angle was achieved by melting paraffin-embedded tissue blocks onto an angled wooden chuck to achieve an oblique complementary cutting angle. Sections were deparaffinized and rehydrated with a series of xylene and ethanol washes. Antigen retrieval was performed under pressurized heat in sodium citrate buffer (10mM C6H5Na3O7 . 2H2O, 0.05% v/v Tween 20 in MilliQ™ H2O, pH 6.0) using an antigen de-cloaking chamber (Biocare Medical, USA). Following antigen retrieval, all steps were performed in 0.0025% v/v Triton X-100 and PBS. Immunofluorescence analysis was performed following 10% normal goat serum blocking. Primary antibodies were incubated overnight at 4°C, followed by repeated washes and secondary antibody incubation for 2 h at room temperature. All sections were also stained for the nuclear marker DAPI (1:1000) (Invitrogen, Australia). Sections were coverslipped in PVA/DABCO mounting medium (Sigma-Aldrich, Australia). Immunofluorescence analysis was performed such that GAP43, SIX3 and ZIC2 were revealed on the same section (as described above), with EMX1 and NFIA (each with GAP43) immunohistochemistry performed on the preceding and following serial section, allowing overlay of images. Chromogenic immunohistochemistry was performed using avidin-biotin amplification (PK-6100, Vector laboratories, USA) and 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, Australia) visualization. Bright field imaging and fluorescence imaging were performed with a Zeiss upright Axio-Imager Z1 microscope fitted with AxioCam HRc and HRm cameras. Images were pseudocolored to permit overlay, cropped, sized and brightness-enhanced for presentation with Adode Photoshop.
In situ hybridization
For in situ hybridization, embryos were transcardially perfused with 4% PFA and vibratome sectioned at 45μm. Sections were then mounted onto Superfrost slides (Menzel-Glaser, Brunswick, Germany) and allowed to dry. Sections were fixed with 4% PFA, permeablised with Proteinase K (10μm/mL in PBS; Roche, Mannheim, Germany), re-fixed with 4% PFA, followed by acetylation in a solution of 1.33% triethanolamine, 0.064% hydrochloric acid, and 0.375% acetic anhydride. All hybridizations were performed at 68°C overnight. Sense and antisense riboprobes were generated for a 266bp sequence extending from 20bp 3′ of exon 2 to the stop codon in exon 3 (courtesy of Prof Gail Martin, UCSF, USA). Probes were digoxigenin-labeled (DIG RNA Labeling Mix; Roche, Mannheim, Germany), detected with anti-digoxigenin-AP at 1:4000 (Roche, Mannheim, Germany), and visualized with the color substrate BM Purple (Roche, Mannheim, Germany).
Measurements and statistical analysis
Oblique coronal angles were determined using exported DICOM sections from DTI-Studio and ImageJ software. Measurements of anatomical and molecular domains were taken from the AC (point zero) using ImageJ software. Graphs were prepared of the mean ± S.D. using GraphPad Prism v.4 (GraphPad Software, USA). Wildtype littermates served as controls for the knockout mice and all controls were pooled for graphical representation. All experimental procedures were performed on at least three individual mice of each genotype. Statistical differences between conditions were established using two-way ANOVA with Bonferroni’s post-hoc t-test whereby p < 0.05 (GraphPad Prism v.4).
Results
DTMRI reveals that all commissures cross the midline in a common oblique-coronal plane
Investigating the embryonic development of multiple axon tracts in the same brain has previously proven challenging. Live tract-tracing techniques, and even tract-tracing in fixed tissue using carbocyanine dyes, do not allow the unbiased labeling of all axons comprising a single tract, due to the nature of the dye or tracer placement (usually at a single injection site). Moreover, multiple injections of different tracts in a single brain are difficult to perform and to analyze in three-dimensions. We wanted to examine the development of the AC, HC and CC in the same brain to gain an understanding of how these tracts develop in relation to one another and to determine where they first cross the midline. To do this, we used DTMRI to generate a three-dimensional map of the fiber tracts and their orientation (Fig. 1). DTMRI, which can be used on fixed (or living) brains, analyzes the diffusion of water-molecules along ordered structures in the brain. The orientation of this diffusion can then be color-coded to provide a color-map in three dimensions of all the fiber tracts in the brain. Color orientation maps were generated from fractional anisotropy calculations and primary eigenvector maps using DTI-Studio. The advantage of this approach is that imaging data can be viewed in any plane of optical section, providing novel ways of looking at how multiple fiber tracts develop in relation to one another. As previously described, the AC was the first forebrain commissure to cross the midline and is visible by DTMRI at E14 (Fig. 1A). At E15, the HC was similarly identified both dorsal and rostral to the AC (Fig. 1B). At E15, both commissures crossed the midline at an oblique coronal angle of approximately −58° to the base of the brain. The AC and HC increased in size in the sagittal plane (Fig. 1C) at E16, but with brain growth they now crossed at an angle of approximately −65° to the ventral surface of the brain. Although CC axons are known to cross at this stage (Rash and Richards, 2001), they either could not be detected at the current imaging resolution or could not be distinguished from the HC axons by DTMRI until E17 (Fig. 1D). The CC then extended rostrally. At E17, the AC, HC and caudal extent of the CC formed an angle of −68° to the ventral surface of the brain, or 22° from the traditional coronal plane. Therefore, the oblique angle of the commissural plate became increasingly obtuse during embryonic development to reach −68° at E17, at which stage all three forebrain commissures had crossed the midline.
Figure 1.
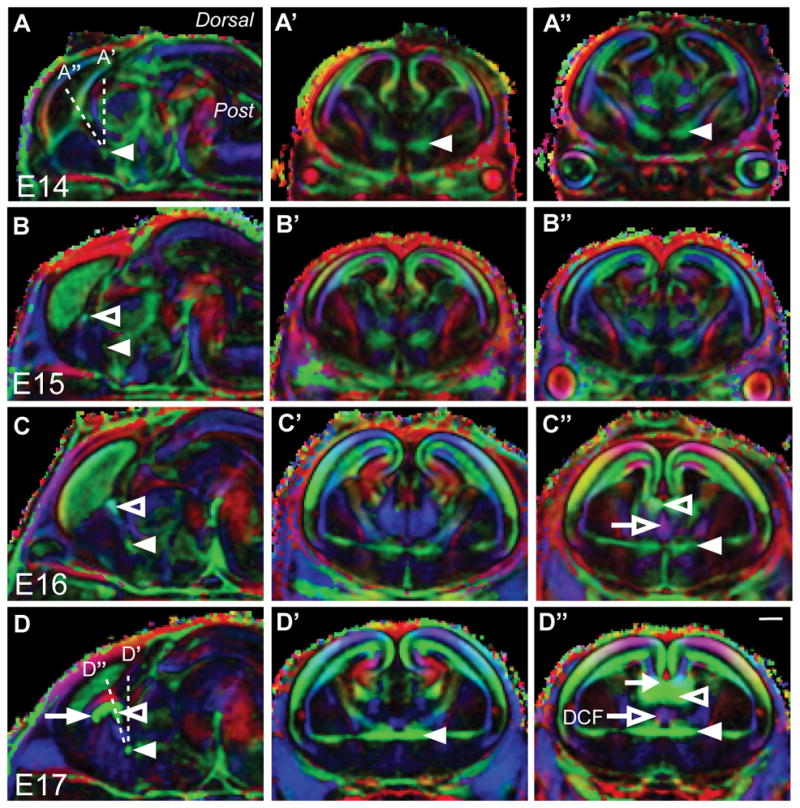
DTMRI analysis of the developing mouse commissural plate shows the midline crossing of the commissures occurs at an oblique coronal angle. A-D″, Color maps generated from fractional anisotropy values and primary eigenvectors. Diffusion directions: green, medial to lateral; blue, anterior to posterior; red, dorsal to ventral. The CC, HC and AC are indicated by the arrow, open arrowhead and closed arrowhead, respectively. In A, the difference in sectioning angle between the coronal plane (A′) and the oblique coronal plane (A″) can be appreciated by the dashed lines. Development of the commissures can be observed in the sagittal plane (A, B, C, D). The AC can be visualized by DTMRI proximal to the midline at E14 (A). B: By E15, the HC can also be visualized. At E16, the AC and HC have grown (C). D: By E17 all forebrain commissures have crossed the midline. The medial to lateral development of these commissures cannot be appreciated simultaneously in the traditional coronal plane alone (A′, B′, C′, D′). However, in an oblique coronal plane, determined from the sagittal images, all three commissures can be observed (A″, B″, C″, D″). For example, the CC (closed arrow) and HC (open arrowhead) are not present in the coronal plane at E17 (D′), but appear together in the oblique coronal plane (D″). In both C″ and D″ the descending columns of the fornix (DCF) can be seen in purple (open arrow), dorsal to the AC (closed arrowhead). Scale bar = 200 μm.
A comparison of traditional coronal images (Figs. 1A′-D′) and oblique coronal images (Figs. 1A″-D″) demonstrated that the commissures crossed within a single plane of tissue, not visible in traditional coronal sections. This single plane of tissue is the commissural plate.
Analysis of commissural tracts within the commissural plate
To further validate the identification of the commissural plate by DTMRI, we performed a histological analysis at E17, when each forebrain commissure could be identified as a substantial tract. A detailed anatomical analysis of all forebrain commissures in the oblique plane was also necessary in order to identify how different regions of the commissural plate related to the different commissural projections. Paraffin-embedded tissue sections at 5 μm were stained with anti-GAP43 to identify axon tracts (Shen et al., 2002). Sectioning the brain at an oblique coronal angle (68°) allowed the full dorsoventral axis of the commissural plate, including all forebrain commissures, to be viewed in a single section, which was not possible by sectioning in the traditional coronal plane.
The rostral commissural plate was dominated by the CC (Fig. 2C), which crosses the midline at the boundary between the cingulate cortex and septum. More caudally however, the CC, HC and AC crossed the midline at an oblique angle of 68° (Figs 2D, E). Moreover, two distinct components of the HC could be easily observed: the dorsal HC (DHC) and the ventral HC (VHC). Further ventrally, axons forming the dorsal columns of the fornix (DCF) were apparent (Fig. 2F′), dorsal to the AC.
Figure 2.
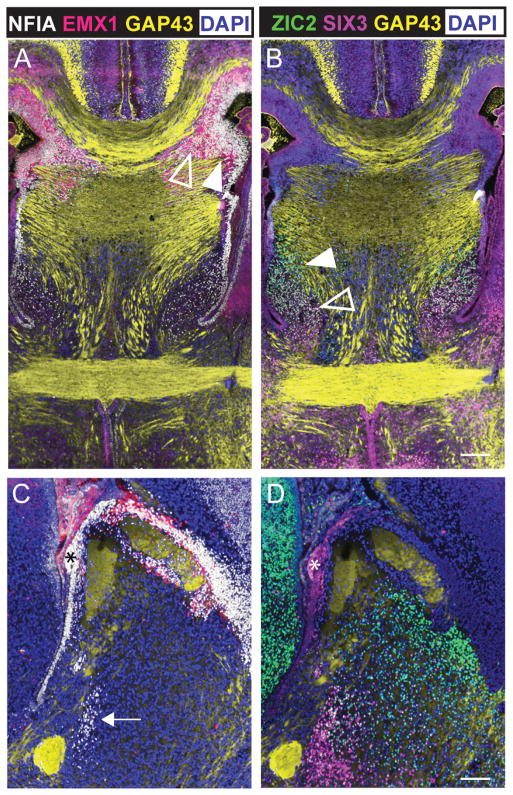
Four distinct fiber tracts emerge within the mouse commissural plate by E17. A: Representative photomicrograph of a mid-sagittal section of E17 C57Bl/6 brain stained with hematoxylin. B: A 2.5× enlargement of the commissural plate in A. Axon tracts of the corpus callosum (CC, green), dorsal hippocampal commissure (DHC, blue), ventral hippocampal commissure (VHC, red) and anterior commissure (AC, pale blue) are outlined. The putative commissural plate has been outlined in black (A). Dashed vertical lines indicate the oblique coronal angle used to obtain sections in C-E. C-E: Representative rostral to caudal sections of the commissural plate at E17, immunolabeled with anti-GAP43 (brown) for growing neuronal processes and counterstained with hematoxylin (blue). C′, D′, E ′: Enlargements of highlighted regions in C-E. C′: In a rostral plane of the commissural plate, the CC crosses the midline at the boundary between the cingulate cortex (CgCtx) and the septum. CC crossing at this boundary continues caudally. D′: In addition to the CC, ipsilateral descending columns of the fornix (arrows) and dorsal and ventral segments of the HC begin to emerge (arrowheads). E′: At the most caudal extent of the commissural plate all four forebrain commissures cross the midline within the same oblique plane. From dorsal to ventral these are the CC, DHC (arrowheads), VHC (arrowheads) and AC. Also seen in E′ is the caudal extent of the descending columns of the fornix (arrows) projecting ventrally between the VHC and the AC. Fibers of the AC are in direct contact with the ventricular zone of the third ventricle at the midline (open arrowhead). Scale bar in A = 1 mm, scale bars in C-E′ = 500 μm.
The commissural plate consists of multiple molecular domains
The anatomy of the commissural plate had previously been described in human fetal brain (Rakic and Yakovlev, 1968) as having dorsal and ventral domains, but nothing was known about the molecules expressed in these domains. To begin to address this, we first investigated the expression of genes at E17, in relation to where the different forebrain commissures formed. A dorsal MC domain and a ventral SA domain were tentatively designated in the mouse according to the anatomical model originally proposed for the human commissural plate (Rakic and Yakovlev, 1968). By screening known candidate genes we determined that the MC could be identified by the expression of the pallial markers NFIA (Plachez et al., 2008) and EMX1 (Boncinelli et al., 1993). The ventral SA could be identified by the subpallial markers SIX3 (Oliver et al., 1995) and ZIC2 (Brown et al., 2003). Importantly, these markers are expressed early during development and could provide molecular landmarks throughout the embryonic development of the commissural plate.
The pattern of NFIA immunoreactivity followed the ventricular zones of the dorsal and ventral reaches of the lateral ventricles, and was particularly intense ventral to the CC and dorsal to the DHC in the region of the glial wedge (Fig. 3A, B) (Shu et al., 2003). NFIA immunoreactivity was intercalated with the fibers of the DHC, but not in the VHC, and became more dispersed in the septum, ventral to the VHC, and with a few immunoreactive cells present ventral to the AC. In addition, a cluster of NFIA-immunoreactive cells was present dorsal and rostral to the AC as revealed in the sagittal plane (arrow in Fig. 3C), which corresponds to the ventral diagonal band of Broca. NFIA immunoreactivity also bordered the caudal commissural plate, with NFIA being expressed in the epithelium and ventricular zone of the rostral wall of the third ventricle (asterisk in Fig. 3C). EMX1 immunoreactivity was located dorsal and ventral to both the DHC and the CC similar to the pattern seen with NFIA, but did not extend ventrally past the VHC.
Figure 3.
Molecular delineation of the mouse commissural plate at E17 reveals four subdomains. Fluorescence immunohistochemistry of the dorsal telencephalic markers NFIA (closed arrowhead, A) and EMX1 (open arrowhead, A) at E17 in the oblique coronal plane and sagittal plane, where two (5μm think) adjacent sections have been overlaid (A, C). The ventral telencephalic markers SIX3 (open arrowhead) and ZIC2 (closed arrowhead) are expressed in the same sections (B, D). GAP43 labeling is shown in yellow to delineate the commissural axons. DAPI counterstain is shown in blue. While both NFIA and EMX1 encircle the rostral to caudal extent of the CC and surround the DHC, only NFIA expression spans the commissural plate ventrally to end rostral and dorsal of the AC (arrow). From the VHC towards the AC, the ventral markers ZIC2 and SIX3 are expressed in opposing gradients. ZIC2 is predominantly expressed around the fornix, while SIX3 is predominantly expressed around the AC. Laterally, ZIC2 is expressed within the septum, bounded by the lateral ventricles. However, SIX3 is expressed along the mediolateral extent of the AC. Immunoreactivity is also present in the choroid plexus and the epithelium of the rostral wall of the third ventricle (asterisks in C and D). These features are not considered part of the commissural plate. From these expression patterns dorsal domains (MC) and ventral domains (SA) are characterized as follows: MC or MC1, NFIA+ and EMX1+; MC2 NFIA+ and ZIC2+; SA1, NFIA+ and ZIC2+ and SIX3+; SA2, SIX3+. Scale bars: A-B = 200 μm, C-D = 150 μm.
Two ventral markers, ZIC2 AND SIX3 were preferentially expressed in the SA. ZIC2 was present around the lateral aspects of the VHC and dorsal to the AC whereas SIX3 expression was more ventral and surrounded the AC. Similar to NFIA, ZIC2 was expressed in the epithelium of the rostral wall of the third ventricle between the commissural plate and the diencephalon (Fig. 3D). This expression analysis demonstrated that EMX1 and NFIA were expressed in the MC, but NFIA was also expressed in the dorsal SA (designated SA1). ZIC2 expression overlapped with that of NFIA in the ventral MC (MC2), and overlapped with NFIA and SIX3 expression in the dorsal SA (SA1). Only SIX3 was expressed in the ventral SA (designated SA2).
In summary, based on the above data, the mouse commissural plate can be divided more usefully into four molecular domains, each associated with a specific commissural projection. From dorsal to ventral these are: MC1, associated with the CC labeled with NFIA and EMX1+; MC2, associated with the DHC and VHC labeled with NFIA+, EMX1+ and ZIC2+; SA1, associated with the fornix labeled with NFIA+, ZIC2+ and SIX3+; and SA2, associated with the AC labeled with SIX3+ (summarized in Fig. 10). Since NFIA and EMX1 overlapped in the MC, either could be used to label this region.
Figure 10.
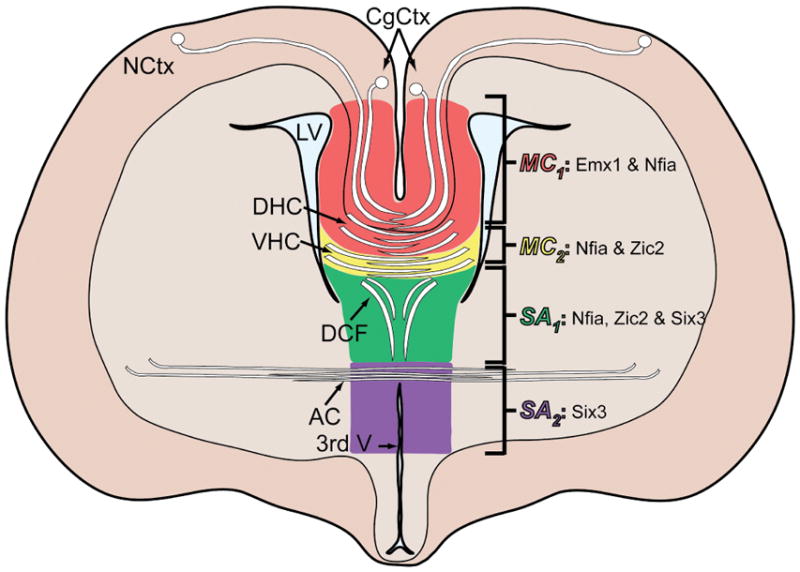
Schematic of the commissural plate at an oblique coronal angle showing the commissures and their associated molecular domains. Abbreviations: 3rd V, third ventricle; CgCtx, cingulate cortex; LV, lateral ventricle; NCtx, neocortex.
Molecular development of the commissural plate
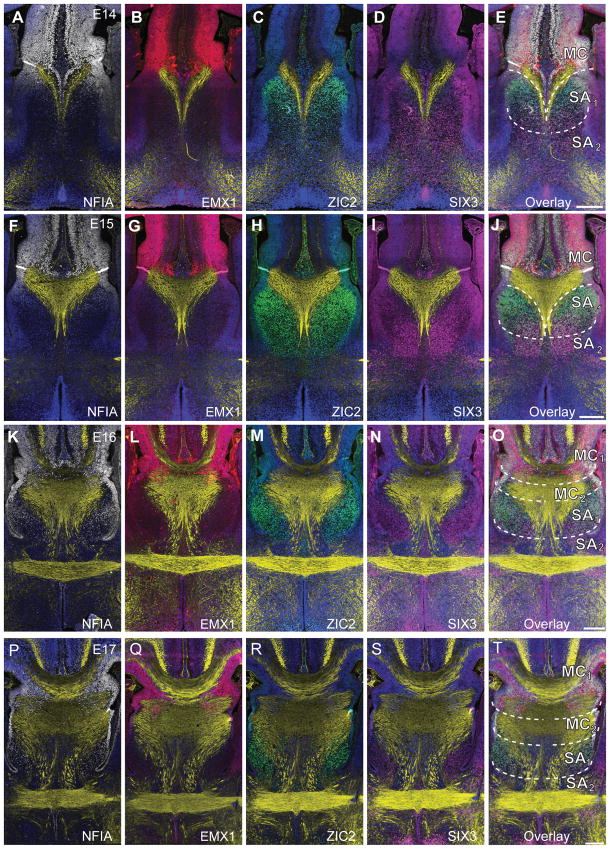
Having established the basic expression of genes in different domains within the commissural plate, we wanted to investigate earlier developmental stages to determine if these markers defined the different regions of the commissural plate before, and during, the time at which the first commissural axons cross the midline. This information would be useful in determining whether commissural plate formation could play an instructive role in commissure development and positioning. A series of oblique coronal sections of the dorsoventral length of the commissural plate was prepared from E14–E17 brains and fluorescence immunohistochemistry performed (Fig. 4).
Figure 4.
Molecular patterning and morphology of the developing mouse commissural plate demonstrates the emergence of the four subdomains. Fluorescence immunohistochemistry for the markers NFIA (white), EMX1 (red), SIX3 (magenta) and ZIC2 (green) throughout the developmental oblique coronal plane of the commissural plate in mouse embryos at E14 (A-E), E15 (F-J), E16 (K-O) and E17 (P-T). GAP43 staining is shown in yellow. DAPI counterstain is shown in blue. An overlay of this molecular patterning is shown with boundaries indicated by dashed lines, defining dorsal domains (MC) and ventral domains (SA), characterized as follows: MC or MC1, NFIA+ and EMX1+; MC2 NFIA+ and ZIC2+; SA1, NFIA+ and ZIC2+ and SIX3+; SA2, SIX3+. Scale bar = 200 μm.
At E14 (Fig. 4A–E), the dorsal-most fibers of the fornix converged at the midline within MC2 and passed ventrally through SA1. Therefore, at E14, the fornix delineates the boundary between the MC and the SA. However, ventrally, ZIC2- and SIX3-positive domains defined a molecular boundary in the SA, delineating SA1 and SA2 respectively, even at this early developmental stage. SIX3 was weakly expressed in the dorsal midline at this age (Fig. 4D).
At E15, a small number of callosal pioneering axons projected from the cingulate cortex towards the midline within the dorsal MC1 (Fig. 4F–J). The HC also crossed the midline within the ventral MC2 domain. Ventrally, ZIC2 and SIX3 expression continued to define the SA1 and SA2 regions, respectively, although the border between these regions was not sharp. Furthermore, the AC crossed the midline within the SIX3-positive domain such that a region of SIX3 expression bordered this commissure later in development (Fig. 4K–T).
Anatomically, the dorsal and ventral components of the HC can be distinguished at E16 and this became important for delineating the domains of the MC. The DHC and VHC crossed the midline within MC2, whereas the CC crossed within the MC1. We next addressed whether the delineation of these domains conferred any functional relevance to the formation of the forebrain commissural projections.
Patterning of sub-domains within the commissural plate affects commissure formation
To test the hypothesis that correct commissural plate development may be a requirement for forebrain commissural formation, we analyzed a number of mouse mutants (Nfia−/−, Emx2−/−, Satb2−/− and Emx1-cre;Fgf8flox/flox mice) in which forebrain commissure formation is known to be disrupted, and analyzed the development of the commissural plate in these animals using the markers we had defined above. Nfia and Emx2 knockout mice were chosen because these mice have previously been shown to exhibit defects in midline development (Pelligrini et al., 1996; Shu et al., 2003; Piper et al., 2009), specifically in the size of the cingulate cortex, whose most ventral region comprises part of MC1. Fgf8 conditional knockout mice were investigated because Fgf8 is known to be expressed in the commissural plate, prior to commissure formation, when this region serves as the rostral patterning center (Tole et al., 2006; Cholfin and Rubenstein, 2008). If differences in commissural plate patterning were observed in these mutants we could not be sure whether the commissural plate was disrupted as a consequence of defects in commissure formation. Therefore, as a comparison, we also examined patterning of the commissural plate in a mouse model that displays commissural defects, but contains a mutation for a gene which is not expressed in the septum (the likely main adult derivative of the commissural plate), and is not know to have midline defects. As Satb2 is expressed within callosal axons and determines their choice of trajectory during development in a cell-autonomous manner (Alcamo et al., 2008; Britanova et al., 2008), in this way analysis of Satb2 knockout mice served as a comparison for commissural patterning as a result of commissural defects.
To examine commissural plate development, we labeled brains from each mutant strain with the key commissural plate markers EMX1, NFIA, ZIC2 and SIX3. This also allowed us to measure the changes in commissural plate domains in Nfia knockout mice and to measure the size of the expression domains in each mutant (as demonstrated in Supp. Fig. 2). Measurements were made using the sagittal plane because, in the absence of commissural projections, it was difficult to identify the exact oblique-coronal plane of tissue for comparison in the absence of the axonal tracts.
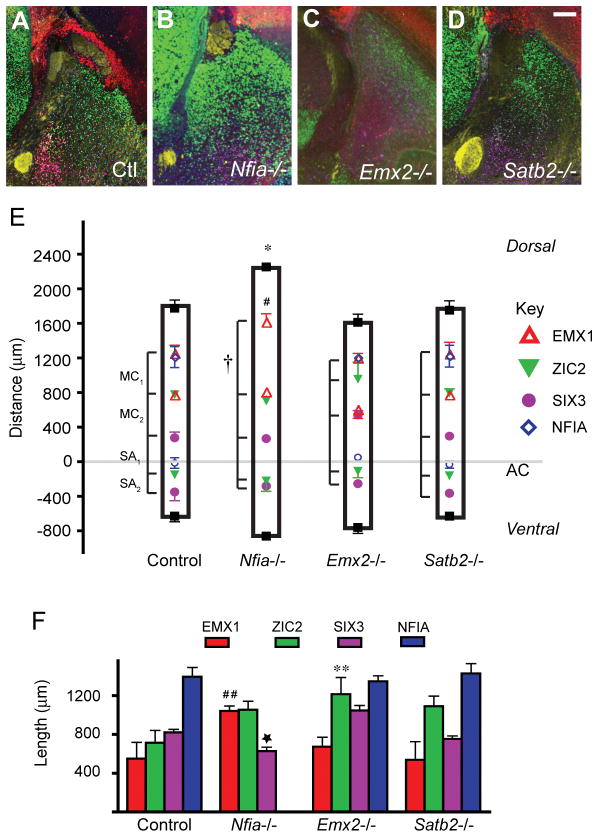
Nfia is important for the formation of glial populations at the midline (Shu et al., 2003), and the subsequent development of the CC and HC. Measurements of the commissural plate domains indicated a significant expansion of the MC in Nfia knockout mice (Fig. 5F). An increase of approximately 475 μm was observed in the total distance from the AC to the dorsal surface of the brain (p < 0.001). Although a reduction in the size of the SA2 domain was also observed, this was not significantly different from that of wildtype littermates. Emx2 is a transcription factor required for correct arealization of the cortex (Yoshida et al., 1997; Bishop et al., 2000; Mallamaci et al., 2000) and the formation of commissural projections, as well as the development of the cingulate cortex (Pellegrini et al., 1996; Yoshida et al., 1997). Emx2−/− mice displayed a reduction in the size of the MC but a significant expansion in SA1 compared with control animals (p < 0.05). In Emx2−/− mice, the AC is disrupted to varying extents, but exists, thus allowing measurement of the commissural plate domains using the AC as a reference point. As illustrated in Fig. 5C, the AC of Emx2−/− mice was thin and defasciculated. These results demonstrate that either an expansion or a reduction in the size of the MC is correlated with defects in CC and HC formation, and that an expansion of the SA2 domain may affect AC development.
Figure 5.
Commissural plate domains are disrupted in Emx2−/ − and Nfia−/−, but not Satb2−/ −mice. Fluorescence immunohistochemistry in the sagittal plane for GAP43 (yellow), EMX1 (red), ZIC2 (green) and SIX3 (magenta) reveals the anatomical and molecular features of the commissural plate in wildtype (A), Nfia−/ − (B), Emx2−/− (C), and Satb2−/ − mice (D). NFIA immunoreactivity is not shown here because measurements were taken from additional sequential, but not adjacent, sagittal sections that did not allow overlay. Loss of the CC is seen in Nfia−/− mice (B), where DHC and VHC dysgenesis is also apparent. Agenesis of the CC and HC is apparent in Emx2−/ − (C) and Satb2−/ − mice (D). The AC is enlarged in Satb2−/ − mice (D). E: Quantification of the commissural plate molecular boundaries as dorsoventral distances from the center of the AC. The MC, SA1 and SA2 domains are indicated to the left of the bars. Key to symbols: squares, dorsal and ventral surfaces of the brain; open upward triangles, EMX1; closed downward triangles, ZIC2; closed circles, SIX3. Wildtype littermates served as controls for the mutant mice. * p < 0.05 compared to the dorsal height of control. # p < 0.05 compared to the dorsal extent of EMX1 expression of control. † p < 0.05 compared to MC of control. F: Molecular domain lengths for each of the mouse strains. Domain length calculations were based on linear distance along the oblique angle of the commissural plate, as demonstrated in Supp. Fig. 2. ## p < 0.05 compared to EMX1 of control. ** p < 0.05 compared to ZIC2 of control. Star = p < 0.05 compared to SIX3 of control. Scale bar = 150 μm.
Thus far, our results had correlated disruptions in commissural projections with disruptions in commissural plate domains. However, it is possible that defects in the size of commissural plate domains occur as a consequence of the lack of formation of commissural projections. One argument against this is that we based our analyses on differences in protein expression domains and not differences in axonal projections. However, to address this issue further we analyzed the size of the commissural plate domains in Satb2 knockout mice, which display commissural defects through the cell-autonomous regulation of cortical lamination and axonal targeting, rather than the regulation of the development of the midline.
SATB2 is a chromatin remodeling protein that regulates commissural neuron identity in the neocortex (Britanova et al., 2008; Gyorgy et al., 2008). As Satb2−/− mice have agenesis of the CC, a larger AC and a thinner cortex, we reasoned that there may be a change in the size of the MC. Unlike Nfia and Emx2, the absence of Satb2 had no effect on the sizes of the expression domains of EMX1/NFIA or SIX3 (Fig. 5D), despite the mutants having no CC and a larger AC. There was a slight dorsal expansion of the expression domain of ZIC2 due to the absence of the CC. To further investigate midline development in the Satb2−/− mice we stained oblique coronal sections with GFAP to label midline glial populations. Three such populations were present at the midline, the glial wedge, the indusium griseum glia (IGG) and the midline zipper glia (MZG) (Fig. 6A). The only difference from control brains was that the IGG and MZG had not been split by the formation of the CC (Fig. 6B), thus Satb2−/− mice display normal development of the commissural plate and the midline glial structures associated with it, despite their midline commissural defects. This phenotype demonstrates that disruptions in commissural projections do not affect the gross size of the commissural plate domains per se and instead supports the hypothesis that the correct patterning of the commissural plate is a mechanism regulating forebrain commissure formation.
Figure 6.

Glial populations form normally in Satb2 knockout mice. Glial populations within the commissural plate at E17 include the indusium griseum glia (IGG) at the dorsal limit of the MC1, the glial wedge (GW) at the ventral limit of the MC1, and the midline zipper glia (MZG) also at the boundary of the MC1 and MC2. A small population of glia surrounds the AC at the boundary of the SA1 and SA2. These populations can be seen in the wildtype CoP in A. In Satb2−/− mice all glial populations are present and appear normal (B). The indusium griseum glia and midline zipper glia are usually split by the formation of the corpus callosum but this does not occur due to the absence of the corpus callosum in Satb2−/− mice. Scale bar = 400 μm.
Fgf8 is required for formation of the commissural plate
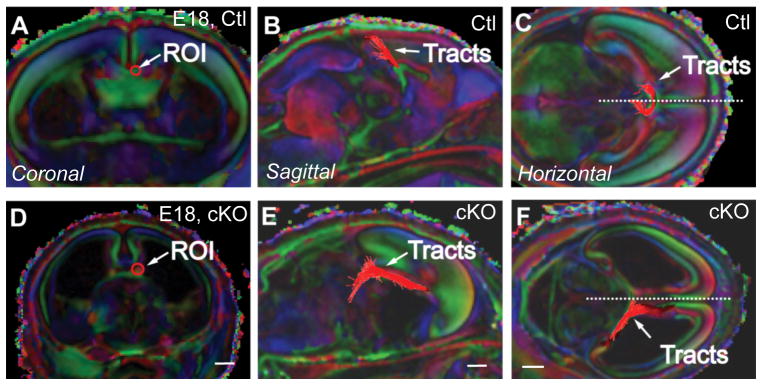
Fgf8 is expressed in the rostral patterning center during early embryogenesis (Maruoka et al., 1998), and later at the midline within the presumptive commissural plate (Crossley and Martin, 1995; Yaylaoglu et al., 2005). In situ hybridization for Fgf8 demonstrated that Fgf8 mRNA expression was confined to the border between the broader MC and SA domains in the MC2 and SA1 domains of the commissural plate (Fig. 7). Since knockout of Fgf8 results in early embryonic lethality and gross telencephalic dysmorphology, conditional knockout lines have been generated to study middle to late embryonic development (Meyers et al., 1998). Similarly, conditional knockout lines for Fgf receptor 1 (Fgfr1) used to study brain development have identified that this receptor participates in midline and commissural defects later in development (Smith et al., 2006; Tole et al 2006). Here, we used a Cre/loxP recombinase system to remove Fgf8 (Meyers et al., 1998) in the CoP following activation of the Emx1 promoter (Iwasato et al., 2004) (Fig. 7). This conditional knockout activity is expected to occur from E10.5 when both Fgf8 and Emx1 are expressed in the most rostral aspect of the forebrain (see Iwasato et al., 2004 and Kawauchi et al., 2005). Successful targetting of the Cre/loxP system is demonstrated in Fig. 7 at E14. In Emx1-cre;Fgf8flox/flox mutants, Fgf8 is absent from the commissural plate (Fig. 7F), compared to controls (Fig. 7D). Emx1-cre;Fgf8flox/flox embryos developed throughout gestation but did not survive postnatally. At E14, mutants exhibited hypoplasia of the olfactory bulbs and loss of the septum (Fig. 8), as previously described for Foxg1-cre;Fgfr1 mutants (Tole et al., 2006). At E18, communication between the lateral ventricles was evident in the Emx1-cre;Fgf8flox/flox mutant due to the lack of septum development (Fig. 8H). Furthermore, as described for Fgfr1 mutants, Emx1-cre;Fgf8flox/flox mutants displayed agenesis of the CC and HC (Fig. 8J–L). Dysgenesis of the AC was apparent in some embryos, although AC midline crossing remained (Fig. 8L). In addition to the structural MRI images, hematoxylin staining demonstrated a loss of almost the entire commissural plate, with the exception of the caudal MC1 domain (Fig. 8J-J′). In this region, some tissue remained and cortical axons approached, but did not cross the midline (Fig. 8L-L′). The hippocampus was slightly smaller than control (data not shown). Only a few fimbria glia were present in Emx1-cre;Fgf8flox/flox mutants (Fig. 8K-K′). Axonal tractography of the corpus callosum in Emx1-cre;Fgf8flox/flox mutants demonstrated that callosal axons grew in a rostro-caudal trajectory, rather than a medio-lateral trajectory (Fig. 9). Although NFIA, SIX3, EMX1 and ZIC2 expression remained where tissue was present (Fig. 8M–P), the dramatic phenotype meant that no measurements of the medial commissural plate subdomains were possible, hence demonstrating an absolute requirement for FGF8 in formation of the commissural plate.
Figure 7.
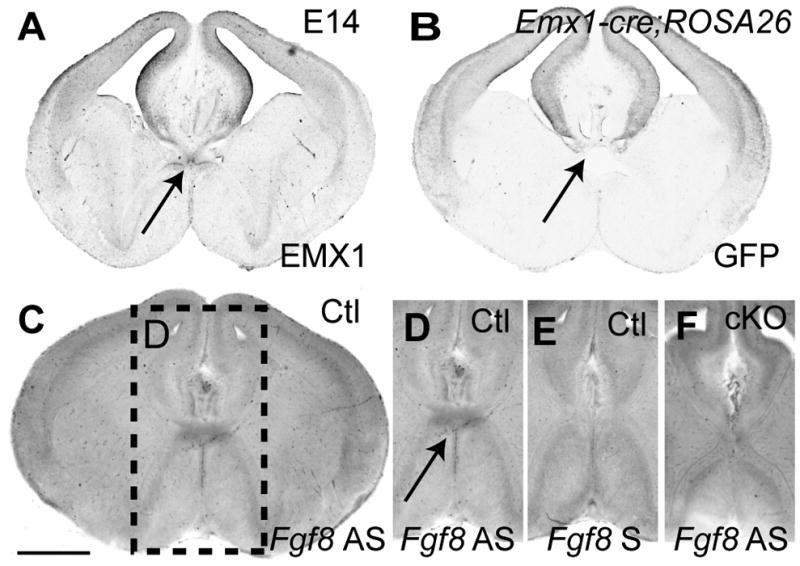
Absence of Fgf8 at the commissural plate in the Emx1-cre;Fgf8flox/flox mice. A: Immunohistochemistry shows EMX1 expression in the pallium and medial telencephalon extending ventrally to the lamina terminalis. B: Emx1-cre and ROSA26flox/flox mice were crossed to obtain a green fluorescent protein (GFP) profile of the extent of Emx1-cre recombinase activity. Immunohistochemistry for GFP at E14 reveals recombinase activity in the pallium. In particular, recombinase activity occurs around the dorsal part of the lamina terminalis (arrow). C and D: In situ hybridization in Emx1+/+;Fgf8flox/flox (Ctl) mice demonstrates that Fgf8 is expressed in the commissural plate at E14 at the border of the MC and SA domains. By contrast, sense in situ hybridization in control mice (E) and antisense in situ hybridization in Emx1-cre;Fgf8flox/flox (cKO) mice (F) reveal no Fgf8 expression. Due to the restricted overlay of expression domains, the telencephalon develops without major deficit until E14. Scale bar = 400 μm.
Figure 8.
Agenesis of the commissural plate in Fgf8 hypomorphs. Fractional anisotropy images of the Emx1-cre;Fgf8flox/flox (cKO) heads and littermate controls (Ctl) at E14 (A-D) and E18 (E-I) where the intensity of white features indicates tissue and white matter tracts, while black indicates fluid spaces such as ventricles. At E14, cKO mutants show absence of the commissural plate (arrowhead in D), failure of midline fusion (arrow in B) and hypoplasia of the olfactory bulbs (arrows in C and D). At E18, further dysmorphologies are seen, including agenesis of the corpus callosum and hippocampal commissure (arrowhead, I), displacement of the corticoseptal boundary in the anterior telencephalon (compare hatched lines in E and F) and communication of the lateral ventricles can be seen (asterisk, H). H and I correspond to hatched lines in F. J-K: Hematoxylin stain shows the architecture of the brain at the coronal plane approximate to I. Hypoplasia of the neocortex can be seen in J, and hypoplasia of the commissural plate is evident J′. K-K′: All but few glia are eliminated following conditional knockout of Fgf8 as shown by immunohistochemistry for GFAP at the coronal plane approximate to I. A few fimbrial glia are indicated by the arrow in K′. L-L′: While axons of the fimbria stained with anti-GAP43 and the AC are present in cKO mice, midline crossing only occurs for the AC. M-P: Expression of the commissural plate markers are disrupted, but not eliminated by conditional knockout of Fgf8, as shown by immunohistochemistry for commissural plate markers EMX1, NFIA, ZIC2 and SIX3 in the mid-sagittal plane of E17 cKO mice. Scale bars: A-D = 600 μm; E-I = 700 μm; J, L, N = 1 mm; K, M, O = 500 μm; P-S = 300 μm.
Figure 9.
DTMRI tractography in E18 brains of Emx1-cre;Fgf8flox/flox mice (cKO). From a region of interest (ROI) in the corpus callosum (A-F), tractography in mutants shows profound misprojections that fail to cross the midline (hatched line) compared to wildtype littermates (Ctl). Probst bundles are apparent from an ROI in the corpus callosum (E and F). Tractography was performed with DTI-Studio (tracking thresholds: angle <70 degrees, FA>0.3). Scale bar A, D = 0.7 mm; B, E = 0.8 mm; C, F = 1 mm.
Discussion
The current investigation characterized the mouse commissural plate as an oblique plane of tissue, within which all telencephalic commissures cross the midline into the contralateral hemisphere during embryonic development. This morphology is generally consistent with earlier histological studies of commissural plate anatomy in other mammals, including humans (Mihalkovics, 1877; Zuckerkandl, 1901; Hochstetter, 1929; Abbie, 1940; Rakic and Yakovlev, 1968). A significant advance in the analysis here is that we have used specific gene expression domains, as well as the dorso-ventral position of the three forebrain commissural projections, to identify that, at least in mouse, there are four, rather than two, distinct domains within the commissural plate. These expression domains are not abrupt, but rather, form gradients whereby either alone or in combination with another gene they become predominant occupiers of the CoP along its dorso-ventral aspect. In addition, anatomical correlations can be used to delineate different areas of the commissural plate. We have described how the MC1 is associated with the CC and DHC, the MC2 is associated with the VHC, the SA1 is associated with the fornix and the SA2 is associated with the AC. Although the molecular specification of the human commissural plate has not been investigated, species differences in the regions of the commissural plate may arise because of the notable presence of both a dorsal and ventral HC in mice, whereas in humans, the VHC is reduced to such an extent that its existence is questionable (Gloor et al., 1993). One probable reason for this species difference is that, in mouse, we have shown that the VHC lies in the MC2, a region comprising the ZIC2-immunopositive septum, and that relatively, the septum is much larger in the mouse than the human septum pellucidum. Therefore, the MC2 may be unique to the mouse brain commissural plate. In future, our analysis in mouse could be used to determine the molecular domains of the human commissural plate.
Nevertheless, the results demonstrate that there are four distinct domains in the mouse based on the protein expression of one, two, or three of the markers used (NFIA, EMX1, ZIC2 and SIX3) (Fig. 10). Our results therefore provide a method for understanding and analyzing the development of this region of the brain and how these domains regulate the crossing and dorso-ventral positioning of different forebrain commissural projections.
Having established that distinct molecular domains exist within the commissural plate, we investigated whether these genes regulate the size of the different commissural plate domains by examining commissural plate development in mouse mutants of four different genes, Nfia, Emx2, Fgf8 and Satb2. Nfia and Emx2 knockout mice were predicted to display defects in the MC based on previous work indicating an expansion or loss of the cingulate cortex respectively (Pellegrini et al., 1996; Shu et al., 2003; Piper et al., 2009), part of which makes up the MC1 domain. This was indeed the case, although in the case of Emx2, despite a tendency towards a smaller MC, our measurements did not reach statistical significance. However, we did find a statistically significant expansion of the SA domain labeled by ZIC2. Given that Emx2 is not expressed in the subpallium, this result indicates that Emx2 might normally restrict the size of the SA through as yet unidentified mechanisms. In contrast, NFIA deficient mice displayed an overall expansion in the size of the dorso-ventral midline. The Nfia mutation caused an expansion of the MC domain without causing an increase in the SA domains, indicating that Nfia may normally regulate the size of the MC through proliferation and/or differentiation.
To examine whether these differences in commissural plate domains were meaningful in terms of providing a mechanistic requirement for commissure formation, we utilized the Satb2 knockout (Britanova et al., 2006), where commissural projections are disrupted without the gene being expressed within the septum (Alcamo et al., 2008; Britanova et al., 2008). Disruption of callosal neuron identity by knockdown of Satb2 has been reported to affect neocortical thickness and to cause severe CC dysgenesis and misrouting of cortical axons towards the AC and corticospinal tract (Alcamo et al., 2008; Britanova et al., 2008). In the present study, Satb2−/− mice demonstrated normal commissural plate development demonstrating that commissural axon crossing of the midline does not influence commissural plate domain size per se. Thus, altered commissural plate patterning in other mutants such as Emx2−/− and Nfia−/− may provide an underlying cause for their commissural defects.
The mRNA of the morphogen FGF8 has been described as being notably expressed in the early rostral patterning center (Borello et al., 2008; Cholfin and Rubenstein, 2008) with ectopic expression of Fgf8 being reported to affect the expression of the midline patterning genes Zic2 and Lhx2 (Hayhurst et al., 2008; Okada et al., 2008), of which Lhx2 is postulated to coordinate forebrain development together with Six3 (Ando et al., 2005). Moreover, misexpression of both Zic2 and Six3 in humans is associated with holoprosencephaly (HPE) (Brown et al., 1998; Wallis et al., 1999; Brown et al., 2001; Rosenfeld et al., 2010). In addition to these candidates, a comparative genomic hybridization of individuals with HPE recently identified a deletion in FGF8 (Rosenfeld et al., 2010). However, except for a few expression studies (Yaylaoglu et al., 2005; Smith et al., 2006), the role of Fgf8 in commissural plate development has not been investigated. Here, we found that Fgf8 mRNA expression is found predominantly at the border of the MC and SA domains of the commissural plate, and consequently we found that loss of Fgf8 in this region particularly affects the MC2 and SA1 regions, resulting in loss of the septum and failure of CC and HC crossing. This tissue loss is consistent with reports that Fgf8 is required to maintain cell survival (Storm et al., 2006). In the absence of this tissue, re-specification of the tissue did not occur as the conditional Fgf8 knockouts retained the relative dorso-ventral expression of the four commissural plate patterning genes. The AC did form in these mutants, supporting the hypothesis that the SA2 is a separate domain of the commissural plate, which is not dominantly regulated by dorsal FGF8 expression within the Emx1-expressing telencephalon. Despite roles for Fgf8 regulation of Zic2 (Hayhurst et al. 2008; Okada et al. 2008), knockout of Fgf8 under these conditions did not eliminate ZIC2 and SIX3 expression from neighboring tissue. Part of the reason may be that Zic2 and Six3 expression arises earlier than that of Emx1 (Oliver et al., 1995; Elms et al., 2004) and hence these genes may be expressed before Cre-loxP recombination of the Fgf8 transcript. Alternatively, in the absence of Fgf8, another unknown genetic regulation pathway acts to maintain cell survival and expression of ZIC2 and SIX3. Finally, the SIX3 and ZIC2 expression domains lie outside the Fgf8-maintained tissue that became ablated in the current study. These results demonstrate an absolute requirement for FGF8 in the development of the central (MC2 and SA1) regions of the commissural plate.
An interesting question is how commissural plate development relates to the earlier events of midline formation and holoprosencephaly. From the present and previous studies ZIC2, SIX3, FGF8 and FGFR1/R3 contribute to the generation of midline tissue followed by its subsequent differentiation into the four domains of the commissural plate (including the septum; Tole et al., 2006). Loss of function mutations in ZIC2, SIX3, and FGF8 result in varying forms of HPE in mice and humans (Brown et al., 1998; Meyers et al., 1998; Wallis et al. 1999; Brown et al., 2001; Warr et al., 2008; Rosenfeld et al., 2010). Recent studies have elegantly elucidated regulatory mechanisms involving Zic, Six3 and Shh, and how these might be involved in HPE (Geng et al., 2008; Jeong et al., 2008; Warr et al., 2008; Marchal et al., 2009; Sanek et al., 2009). Mutations in Zic2 are thought to cause HPE during formation of the prechordal plate, prior to Fgf8 or Shh expression (Warr et al., 2008). Six3 mutations have been shown to decrease Shh expression (Geng et al., 2008; Jeong et al., 2008). While decreasing telencephalic Shh, Zic2, or both, diminishes Fgf8 expression in the rostral patterning centre, which precedes commissural plate formation (Warr et al., 2008). There is also evidence to suggest that Fgf8 expression is independent of Shh (Gutin et al., 2006). Given this data and the data presented here it is likely that these genes play roles in the patterning and formation of midline tissue in early stages of development and then also regulate the further differentiation of these regions into the commissural plate, allowing the formation of different commissural projections to occur in precise dorsovental regions of the midline. In addition, further cellular events such as midline fusion must occur in this region to allow crossing of the forebrain commissures. At present nothing is known about the molecules controlling midline fusion but it is possible that similar genes might be involved given that our Emx1-cre;Fgf8flox/flox mutants display some defects in midline fusion, but whether this is directly regulated by FGF8 is yet to be determined.
The mechanisms regulating commissure formation in the brain are complex and involve a large number of genes (reviewed in Paul et al., 2007). Such important developmental events are hypothesized to include fusion of the telencephalic midline, as well as later developmental events such axon guidance mechanisms (reviewed in Lindwall et al., 2007). Here, we demonstrate that patterning of the midline and specificity of the four domains of the commissural plate provide another mechanism by which forebrain commissural axon projections are regulated.
Supplementary Material
Acknowledgments
Funding Acknowledgements
RXM is a C.J. Martin Fellow and LJR is a Senior Research Fellow of the NHMRC, Australia. This work was funded by an NHMRC grant to LJR (456027), NIH grants to SM (AG 20012, EB 003543, ES 01266). VT was supported by DFG-Research Center for Molecular Physiology of the Brain and by DFG grant TA 303/4-1.
Other acknowledgements
The authors would like to thank Erica Little, John Baisden, Jane Ellis, Michael Piper, Charlotta Lindwall, Guy Barry, Sharon Mason, Manuela Schwark and Janette Thurley for technical advice and assistance. Thank you to Peter Gruss (Max Planck Society, Munich, Germany) for providing the Emx2 mutant line, Gail Martin (UCSF, USA) and Mike Lewandoski (Frederick Cancer Research and Development Center, MD, USA) for providing the FGF8flox/flox line, Shigeyoshi Itohara (Riken Brain Science Institute, Japan) for providing the Emx1Cre line, and Giorgio Corte (Advanced Biotechnology Center, Genova, Italy) for the anti-EMX1 antibody.
References
- Abbie AA. The origin of the corpus callosum and the fate of the structures related to it. J Comp Neurol. 1940;70:9–40. [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Ando H, Kobayashi M, Tsubokawa T, Uyemura K, Furuta T, Okamoto H. Lhx2 mediates the activity of Six3 in zebrafish forebrain growth. Dev Biol. 2005;287:456–468. doi: 10.1016/j.ydbio.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Barry G, Piper M, Lindwall C, Moldrich R, Mason S, Little E, Sarkar A, Tole S, Gronostajski RM, Richards LJ. Specific glial populations regulate hippocampal morphogenesis. J Neurosci. 2008;28:12328–12340. doi: 10.1523/JNEUROSCI.4000-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Boncinelli E, Gulisano M, Broccoli V. Emx and Otx homeobox genes in the developing mouse brain. J Neurobiol. 1993;24:1356–1366. doi: 10.1002/neu.480241008. [DOI] [PubMed] [Google Scholar]
- Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev. 1996;57:169–180. doi: 10.1016/0925-4773(96)00544-8. [DOI] [PubMed] [Google Scholar]
- Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, Tarabykin V. Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. Am J Hum Genet. 2006;79:668–678. doi: 10.1086/508214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnar Z, Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Brown LY, Kottmann AH, Brown S. Immunolocalization of Zic2 expression in the developing mouse forebrain. Gene Expr Patterns. 2003;3:361–367. doi: 10.1016/s1567-133x(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Brown LY, Odent S, David V, Blayau M, Dubourg C, Apacik C, Delgado MA, Hall BD, Reynolds JF, Sommer A, Wieczorek D, Brown SA, Muenke M. Holoprosencephaly due to mutations in ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Hum Mol Genet. 2001;10:791–796. doi: 10.1093/hmg/10.8.791. [DOI] [PubMed] [Google Scholar]
- Brown SA, Warburton D, Brown LY, Yu CY, Roeder ER, Stengel-Rutkowski S, Hennekam RC, Muenke M. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat Genet. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. doi: 10.1016/s0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol. 2008;509:144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Elms P, Scurry A, Davies J, Willoughby C, Hacker T, Bogani D, Arkell R. Overlapping and distinct expression domains of Zic2 and Zic3 during mouse gastrulation. Gene Expr Patterns. 2004;4:505–511. doi: 10.1016/j.modgep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Geng X, Speirs C, Lagutin O, Inbal A, Liu W, Solnica-Krezel L, Jeong Y, Epstein DJ, Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–47. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116:1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hébert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133(15):2937–46. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Gyorgy AB, Szemes M, de Juan Romero C, Tarabykin V, Agoston DV. SATB2 interacts with chromatin-remodeling molecules in differentiating cortical neurons. Eur J Neurosci. 2008;27:865–873. doi: 10.1111/j.1460-9568.2008.06061.x. [DOI] [PubMed] [Google Scholar]
- Hanbury R, Ling ZD, Wuu J, Kordower JH. GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J Comp Neurol. 2003;461(3):307–316. doi: 10.1002/cne.10667. [DOI] [PubMed] [Google Scholar]
- Hayhurst M, Gore BB, Tessier-Lavigne M, McConnell SK. Ongoing sonic hedgehog signaling is required for dorsal midline formation in the developing forebrain. Dev Neurobiol. 2008;68:83–100. doi: 10.1002/dneu.20576. [DOI] [PubMed] [Google Scholar]
- Hebert JM. Unraveling the molecular pathways that regulate early telencephalon development. Curr Top Dev Biol. 2005;69:17–37. doi: 10.1016/S0070-2153(05)69002-3. [DOI] [PubMed] [Google Scholar]
- Hines RJ, Paul LK, Brown WS. Spatial attention in agenesis of the corpus callosum: shifting attention between visual fields. Neuropsychologia. 2002;40:1804–1814. doi: 10.1016/s0028-3932(02)00032-5. [DOI] [PubMed] [Google Scholar]
- His W. Die Entwicklung des menschlichen Rautenhirns vom Ende des ersten bis zum Beginn des dritten Monats: I. Verlangertes Mark. Abhandlungen der Mathematisch-Physischen Klasse der Koniglich-Sachsischen Gesellschaft der Wissenschaften XV 1889 [Google Scholar]
- Hochstetter F. Beitrage zur Entwicklungsgeschichte des menschlichen Gehirns. Vienna: Deuticke; 1929. II Teil 3. Lieferung. Die Entwicklung des Mittel- und Rautenhirns; p. 170. [Google Scholar]
- Iwasato T, Nomura R, Ando R, Ikeda T, Tanaka M, Itohara S. Dorsal telencephalon-specific expression of Cre recombinase in PAC transgenic mice. Genesis. 2004;38:130–138. doi: 10.1002/gene.20009. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, El-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, Geng X, Oliver G, Epstein DJ. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet. 2008;40:1348–53. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Muzio L, Chan CH, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nat Neurosci. 2000;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Marchal L, Luxardi G, Thomé V, Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci U S A. 2009;106:17437–17442. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Mihalkovics VV. Entwicklungsgeschichte des Gehirns nach Untersuchungen an höheren Wirbelthieren und dem Menschen. Leipzig: Engelmann; 1877. [Google Scholar]
- Mori S, van Zijl PC. A motion correction scheme by twin-echo navigation for diffusion-weighted magnetic resonance imaging with multiple RF echo acquisition. Magn Reson Med. 1998;40:511–516. doi: 10.1002/mrm.1910400403. [DOI] [PubMed] [Google Scholar]
- Nielsen T, Montplaisir J, Lassonde M. Sleep architecture in agenesis of the corpus callosum: laboratory assessment of four cases. J Sleep Res. 1992;1:197–200. doi: 10.1111/j.1365-2869.1992.tb00038.x. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ogura H, Aruga J, Mikoshiba K. Behavioral abnormalities of Zic1 and Zic2 mutant mice: implications as models for human neurological disorders. Behav Genet. 2001;31:317–324. doi: 10.1023/a:1012235510600. [DOI] [PubMed] [Google Scholar]
- Okada T, Okumura Y, Motoyama J, Ogawa M. FGF8 signaling patterns the telencephalic midline by regulating putative key factors of midline development. Dev Biol. 2008;320:92–101. doi: 10.1016/j.ydbio.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Paul LK, Van Lancker-Sidtis D, Schieffer B, Dietrich R, Brown WS. Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain Lang. 2003;85:313–324. doi: 10.1016/s0093-934x(03)00062-2. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, Gu C, Richards LJ. Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb Cortex. 2009;19(Suppl 1):i11–21. doi: 10.1093/cercor/bhp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachez C, Lindwall C, Sunn N, Piper M, Moldrich RX, Campbell CE, Osinski JM, Gronostajski RM, Richards LJ. Nuclear factor I gene expression in the developing forebrain. J Comp Neurol. 2008;508:385–401. doi: 10.1002/cne.21645. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Ren T, Zhang J, Plachez C, Mori S, Richards LJ. Diffusion tensor magnetic resonance imaging and tract-tracing analysis of Probst bundle structure in Netrin1- and DCC-deficient mice. J Neurosci. 2007;27:10345–10349. doi: 10.1523/JNEUROSCI.2787-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LJ, Plachez C, Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin Genet. 2004;66:276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Ballif BC, Martin DM, Aylsworth AS, Bejjani BA, Torchia BS, Shaffer LG. Clinical characterization of individuals with deletions of genes in holoprosencephaly pathways by aCGH refines the phenotypic spectrum of HPE. Hum Genet. 2010 doi: 10.1007/s00439-009-0778-7. Published online 12 January, 2010. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Sanek NA, Taylor AA, Nyholm MK, Grinblat Y. Zebrafish zic2a patterns the forebrain through modulation of Hedgehog-activated gene expression. Development. 2009;136:3791–800. doi: 10.1242/dev.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci. 1991;11:3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003;23:203–212. doi: 10.1523/JNEUROSCI.23-01-00203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci. 2006;9:787–797. doi: 10.1038/nn1705. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol. 2005;25:685–698. doi: 10.1128/MCB.25.2.685-698.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Liu FC. Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors. Birth Defects Res C Embryo Today. 2006;78:256–266. doi: 10.1002/bdrc.20077. [DOI] [PubMed] [Google Scholar]
- Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet. 1999;22(2):196–8. doi: 10.1038/9718. [DOI] [PubMed] [Google Scholar]
- Warr N, Powles-Glover N, Chappell A, Robson J, Norris D, Arkell RM. Zic2-associated holoprosencephaly is caused by a transient defect in the organizer region during gastrulation. Hum Mol Genet. 2008;17(19):2986–96. doi: 10.1093/hmg/ddn197. [DOI] [PubMed] [Google Scholar]
- Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234:371–386. doi: 10.1002/dvdy.20441. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Suda Y, Matsuo I, Miyamoto N, Takeda N, Kuratani S, Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E. Zur Entwickelung des Balkens und des Gewolbes. Sitz-Ber dk Akad d Wissensch Math-Naturwissensch. 1901;110:233–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.