Abstract
The development of neural crest cells involves an epithelial-mesenchymal transition (EMT) associated with the restriction of cadherin 6B expression to the pre-migratory neural crest cells (PMNCCs), as well as a loss of N-cadherin expression. We find that cadherin 6B, which is highly expressed in PMNCCs, persists in early migrating neural crest cells and is required for their emigration from the neural tube. Cadherin 6B-expressing PMNCCs exhibit a general loss of epithelial junctional polarity and acquire motile properties before their delamination from the neuroepithelium. Cadherin 6B selectively induces the de-epithelialization of PMNCCs, which is mediated by stimulation of BMP signaling, whereas N-cadherin inhibits de-epithelialization and BMP signaling. As BMP signaling also induces cadherin 6B expression and represses N-cadherin, cadherin-regulated BMP signaling may create two opposing feedback loops. Thus, the overall EMT of neural crest cells occurs via two distinct steps: a cadherin 6B and BMP signaling-mediated de-epithelialization, and a subsequent delamination through the basement membrane.
Keywords: EMT, Cadherin 6B, N-cadherin, BMP, Neural Crest, Chicken
INTRODUCTION
The epithelial-mesenchymal transition (EMT) is an important morphogenetic process that underlies many key developmental events and mediates an important step in tumor invasion and metastasis. Cadherins are known to play crucial roles in the EMT. It is widely believed that a loss of E-cadherin expression is a key regulator of the EMT and a hallmark of the progression of tumor cells to an invasive state (Yang and Weinberg, 2008). According to this view, the loss of adhesion due to the lack of E-cadherin allows cells to escape from the epithelium and migrate away. Despite the strong appeal of this idea, however, the roles of cadherins in the EMT are not yet well understood.
The roles of cadherins in morphogenetic processes that entail enhanced cell motility are much more varied and complex, and can mediate dynamic behaviors of cells as well as stable interactions (Brieher and Gumbiner, 1994; Chihara et al., 2003; Geisbrecht and Montell, 2002; Matsunaga et al., 1988; Medioni et al., 2008; Pollack et al., 1998; Shimizu et al., 2005). Moreover, a loss of the expression of one cadherin during an EMT or similar morphogenetic process is often associated with the increased expression of a different cadherin. For example, many epithelial cells undergoing oncogenic transformation gain the expression of N-cadherin even as they lose E-cadherin (Li et al., 2001; Tomita et al., 2000; Tran et al., 1999; Wheelock et al., 2008). This type of cadherin switching has been thought to bring about changes in the strength of cell adhesion, but intrinsic differences in their adhesive activities are probably insufficient to account for changes occurring during the EMT. Importantly, cadherins are also known to mediate signaling events that can be important for the EMT and other diverse morphogenetic cell behaviors (Gumbiner, 2005; Wheelock and Johnson, 2003; Wheelock et al., 2008), including their modulation of growth factor receptor signaling (Qian et al., 2004; Rudini et al., 2008; Suyama et al., 2002).
We have chosen a classic example of an EMT in vivo, the formation of the neural crest from the neural tube epithelium (Hay, 2005; Yang and Weinberg, 2008), to investigate the mechanisms by which cadherins participate in the EMT. A well-studied hierarchy of transcription factors (including Sox9, Snail2/Slug, AP-2 and others) and signaling pathways (including BMP, Wnt, and others) specify the differentiation, location and timing of neural crest cell emigration from the dorsal region of the neural tube (Kalcheim and Burstyn-Cohen, 2005; Meulemans and Bronner-Fraser, 2004; Sauka-Spengler and Bronner-Fraser, 2008). The EMT is thought to be triggered by localized BMP signaling, which downregulates the expression of N-cadherin in the pre-migratory neural crest cells (PMNCCs). Although this loss of N-cadherin can potentially change adhesion properties, it has also been suggested to mediate signaling through cleavage and translocation of its cytoplasmic domain into the nucleus (Shoval et al., 2007). Concomitant with the loss of N-cadherin is the restriction of cadherin 6B expression to the PMNCCs (Nakagawa and Takeichi, 1998). Although cadherin 6 remains expressed in migrating neural crest of the mouse (Inoue et al., 1997), cadherin 6B is lost in migrating neural crest of the chick (Nakagawa and Takeichi, 1995). The loss of cadherin 6B expression has been reported to be associated with the EMT (Coles et al., 2007). However, because we observed continued cadherin 6B expression in early migrating neural crest just after cells delaminate form the neural tube (below), we decided to revisit the role of cadherin 6B in the EMT of the neural crest.
MATERIALS AND METHODS
Chicken embryo culture
Fertilized chicken (Gallus gallus) eggs were purchased form Charles River Laboratories (North Commons, CT) and incubated at 38°C in a humidified incubator (G.Q.F. Manufacturing, Savannah, GA, USA). Embryos were staged according to the number of somite pairs.
Tissue preparation, immunohistochemistry and β-galactosidase staining
Embryos were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 15 minutes and immersed in 30% sucrose solution at 4°C overnight. For cryosection, the embryos were embedded in OCT compound (Tissue-Tek) and sectioned at 10-16 μm. Immunohistochemistry was performed with the primary antibodies listed in Table 1. The following secondary antibodies (Molecular Probes) were used at a 1:1000 dilution: anti-mouse IgG-Alexa 488, anti-mouse IgG-Alexa 546, anti-rabbit IgG-Alexa 546, anti-rabbit IgG-Alexa 633, anti-rat IgG-Alexa 546, anti-mouse IgM-Alex-546 and anti-mouse IgM-Alex-633. When required, Phalloidin-Texas Red or Phalloidin-Alexa 633 (Molecular Probes) was used for actin staining and DRAQ5 (Biostatus) was used for nucleus staining. Whole-mount β-galactosidase staining was performed as described (Baumer et al., 2002) and embryos were processed using a general procedure for cryosection. Images were collected using a Nikon Eclipse TE2000 confocal microscope or using Openlab software and a Hammatsu digital camera on a Zeiss Axioplan 2.
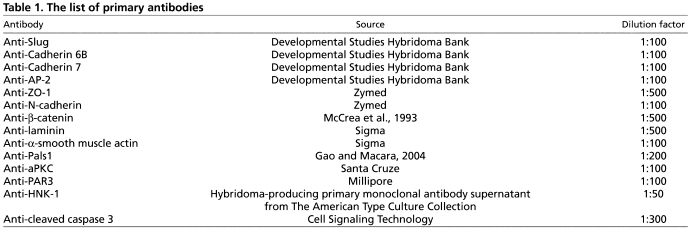
Table 1.
The list of primary antibodies
Electroporation and DNA constructs
DNA constructs were diluted to 1-3 μg/μl in Tris EDTA buffer containing 0.05% Fast Green (Sigma) and injected into the lumen of the neural tube of 10-15 somite pair stage (ss) embryos at the level of the first somite pair. Five 50 msecond pulses were applied at 30 V by use of ECM 830 electroporator (BTX, San Diego, CA, USA). cDNAs encoding full-length chicken Slug, chicken N-cadherin and chicken cadherin 6B were provided by Angela Nieto (Instituto de Neurociencias), Masatoshi Takeichi (RIKEN) and Jeh-Ping Liu (University of Virginia), respectively. The coding regions were cloned into pCIG (a gift from Jing Yu, University of Virginia), which contains an IRES-GFP. pCAGGS-chick SOX9-IRES-nls-GFP (from Martin Cheung, University of Hong Kong), pCIG-BMP4 (from Jing Yu, University of Virginia), pCAGGS-Noggin-CD4-IRES-GFP (from Jing Yu, University of Virginia), pCAβ-chick Smad6-IRES-GFP (from Claudio Stern, University College London), pCAβ-Xenopus truncated BMPR-IRES-GFP (from Claudio Stern, University College London), pCAβ-IRES-mGFP (from Andrea Streit, King's College London), were used for each ectopic expression experiment. BRE-Hspa1a-LacZ (from Leif Oxburgh, Maine Medical Center Research Institute) was used as a BMP signaling reporter. For the GPI-cadherin 6B-expressing construct, a chimera cDNA containing the cadherin 6B extracellular domain (amino acid 1-615) and a glycosyl-phosphatidylinositol (GPI) anchor (amino acid 345-381) of human CD55 (a gift from Judith White, University of Virginia) was inserted into pCIG. The shRNA expression vector for chick cadherin 6B (pSilencer-2.0-U6-cadherin 6B) was made by subcloning annealed oligonucleotides for the target sequence into pSilencer-2.0-U6 (Ambion). For the shRNA of chick cadherin 6B, we chose a target sequence that is highly conserved with a target sequence of a shRNA used to knockdown canine cadherin 6 (Capaldo and Macara, 2007). The sequences of oligonucleotides for constructing the shRNA are shown in Table S1 in the supplementary material.
In ovo injection of type I collagen
The Type I collagen matrix solution (4.2 mg/ml) was a mixture (5:1:2:2) of type I collagen solution (8.4 mg/ml, BD Biosciences), reconstitution buffer (2.2 g NaHCO3 and 200 mM HEPES in 0.05 N NaOH), PBS (×5) and 5 mM CaCl2. The collagen matrix solution was injected into the neural tube of 11-14 ss embryos at the level of the first somite pair. If necessary, a DNA construct(s) was electroporated before collagen injection. Embryos were fixed with 4% paraformaldehyde after 7-9 hours. The axial level posterior to the ninth somite pair was examined to rule out any potential physical damage of collagen injection on cells of the neural tube.
RESULTS
Cadherin 6B is expressed in delaminating neural crest cells and is required for the overall EMT
We carefully examined the patterns of expression of N-cadherin and cadherin 6B in the trunk neural tube of chick embryos. The pre-migratory neural crest cells (PMNCCs) in the dorsal neural tube were identified by expression of the transcription factor Slug (Fig. 1A-F) (del Barrio and Nieto, 2002; LaBonne and Bronner-Fraser, 2000; Nieto et al., 1994). The level of N-cadherin protein is low in the dorsal region of the neural tube where the PMNCCs arise (Fig. 1G-L), with a reciprocally high expression of cadherin 6B in the same region (Fig. 1M-R and Fig. 2A), as previously described (Nakagawa and Takeichi, 1998). Strikingly, however, we observed that cadherin 6B continues to be expressed in early migrating neural crest cells that have already delaminated from the neural tube epithelium through the basement membrane, as delineated by laminin staining (Fig. 2A, parts b,b′). Therefore, an overall loss of cadherin-mediated adhesion may not be responsible for delamination from the epithelium.
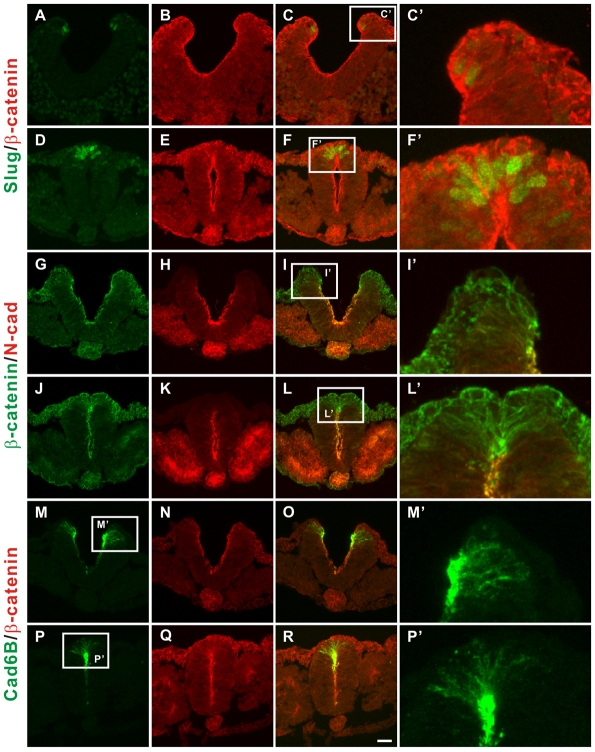
Fig. 1.
Nonpolarized distributions of cadherin 6B and β-catenin in pre-migratory neural crest cells. Immunohistochemistry was performed on cryosections of 10-13 ss embryos for pairs of proteins in each panel. Both the open neural plate at posterior levels (A-C,G-I,M-O) and the closed neural tube at more anterior levels (D-F,J-L,P-R) were examined. Immunohistochemistry for Slug (green)/β-catenin (red) (A-F), β-catenin (green)/N-cadherin (N-cad, red) (G-L), cadherin 6B (Cad6B, green)/β-catenin (red) (M-R) were performed. The white boxed regions in C,F,I,L,M,P are magnified in C',F',I',L',M'P', respectively. The most representative images are shown (at least three random sections per each embryo and at least seven embryos were analyzed). Scale bar: 25 μm.
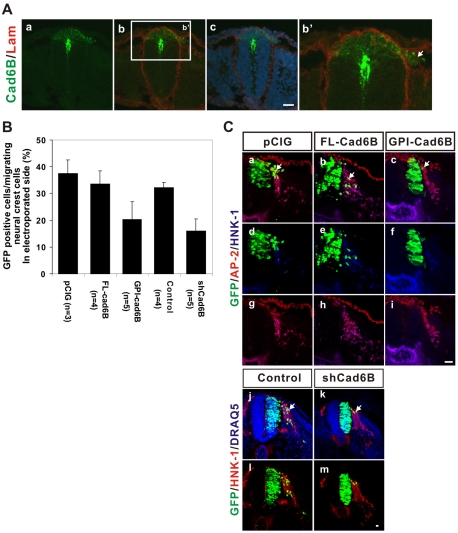
Fig. 2.
Expression of cadherin 6B in early migrating neural crest cells and its requirement for the overall EMT. (A) Early migrating neural crest cells express cadherin 6B. Immunohistochemistry for cadherin 6B (green) and laminin (Lam) to delineate the basement membrane (red) was performed on cryosections of 10-13 ss embryos. The white box region in b is magnified in b' and an arrow indicates cadherin 6B-positive migrating neural crest cells. Nuclei were stained blue by DRAQ5 (c). The most representative images are shown (at least three random sections per each embryo and at least eight embryos were analyzed). Scale bar: 25 μm. (B) Twelve to 15 somite pair stage embryos were electroporated with one of the following constructs, which contains an IRES-GFP to mark cells; a control vector (pCIG), a wild-type cadherin 6B (FL-Cad6B), dominant-negative cadherin 6B (GPI-Cad6B), pCIG + an shRNA expression vector to knockdown cadherin 6B (shCad6B), or pCIG + a control shRNA vector (control). The embryos were incubated for 20-24 hours. Immunohistochemistry for AP-2 and HNK-1 (colors indicated by color of labels) was used to identify differentiated migrating neural crest cells. In order to measure the efficiency of neural crest cell emigration of transfected cells, GFP-positive migrating cells were counted in a series of 10-23 serial sections exhibiting high electroporation efficiencies of the dorsal neural tube (the thickness of each serial section is 14-16 μm and the total thickness of a series is at least 210 μm). The percentage of GFP-positive cells in the total pool of migrating neural crest cells (AP-2 positive/HNK-1 positive cells or HNK-1 positive cells) is expressed as mean ± s.d. for each experimental treatment. (C) Representative immunostained sections for B are shown. Arrows indicate cells that are positive both for GFP and for markers of migrating neural crest cells. Scale bar: 25 μm.
The persistence of cadherin 6B in early emigrating neural crest cells suggests that it might have a positive role in the overall EMT of neural crest cells. Therefore, we performed experiments to determine whether endogenous cadherin 6B is required for the production of migratory neural crest cells. Because of the mosaic nature of electroporation, especially in the dorsal region of the neural tube, we focused on the cell-autonomous effects of the expression of various constructs by scoring only GFP-positive cells. We counted GFP positive/AP-2 positive/HNK-1 positive cells or GFP positive/HNK-1 positive cells outside the neural tube as a measure of the production of migratory neural crest cells; an example of the assay is shown in Fig. 2C. Because the emigration of endogenous neural crest cells occurs periodically along the anterior-posterior axis (Loring and Erickson, 1987; Rickmann et al., 1985; Teillet et al., 1987), we carried out the analysis using serial sections exhibiting high electroporation efficiencies of the dorsal neural tube.
To inhibit endogenous cadherin 6B, we used both a dominant-negative cadherin 6B construct (GPI-cad6B) as well as shRNA-mediated knockdown. The dominant-negative cadherin 6B is a GPI-anchored form of the extracellular domain, which is highly selective for cadherin 6B relative to N-cadherin, because type II cadherins (cadherin 6B) do not interact with type I cadherins (N-cadherin) (Patel et al., 2006). Expression of GPI-cad6B significantly reduced the amount of the neural crest cell emigration compared with pCIG controls (Fig. 2B; 20.34±6.64%, n=5 embryos versus 37.48±5.12%, n=3 embryos, P<0.032 using Wilcoxon rank sun test). By contrast, the effect of ectopic expression of a wild-type cadherin 6B (FL-cad6B) was similar to control GFP expression (Fig. 2B; 33.60±4.83%, n=4 embryos versus 37.48±5.12%, n=3 embryos). We also inhibited cadherin 6B using shRNA-mediated knockdown. Cadherin 6B shRNA selectively decreases the level of cadherin 6B expression and does not induce apoptosis (see Fig. S1 in the supplementary material). Cadherin 6B knockdown decreased the amount of emigrating neural crest cells (Fig. 2B; 16.06±4.37%, n=5 embryos for shCad6B versus 32.27±1.87% for control shRNA, n=4 embryos) even more than did the GPI-cad6B (∼20%). Therefore, both the dominant-negative cadherin 6B and knockdown show that cadherin 6B contributes positively to the overall EMT of the trunk neural crest.
Pre-migratory neural crest cells lose epithelial cell polarity and gain motile properties prior to delamination
The subcellular distribution of cadherin 6B in the PMNCCs appeared to be relatively nonpolarized (Fig. 1, Fig. 2A). Therefore, we compared the distribution of cadherin 6B in the PMNCCs to the distribution of N-cadherin in the adjacent neural epithelial cells. We examined the more posterior region at the level of the last third somite pair of 10-13 ss embryos, before neural crest cells begin delamination. The distribution of N-cadherin is highly localized in the apical junctional region at the lumen of the neural tube where it colocalizes with β-catenin (Fig. 1G-L). By contrast, cadherin 6B appears all along the lateral cell membranes, as well as being concentrated in apical junctions at the lumen (Fig. 1M-R). The distribution of β-catenin in the cadherin 6B-expressing cells is similar (Fig. 1A-R). Slug positive, cadherin 6B-positive neural crest precursors are also present in the two lateral neural tips of the posterior open neural plate prior to neural tube fusion (Fig. 1A-C,M-O). Even at this early stage of neural crest specification, the distributions of cadherin 6B and β-catenin appear relatively nonpolarized (Fig. 1M,M′ for cadherin 6B and Fig. 1C,C′ for β-catenin). These observations suggest that PMNCCs in both the dorsal neural tips and the dorsal neural tube exhibit a partial loss of their polarized epithelial characteristics.
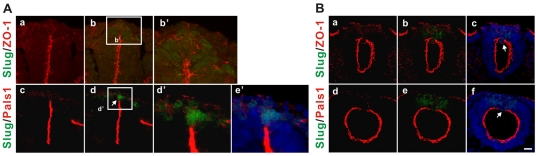
We also examined the subcellular distribution of the tight junction protein ZO-1 and the apical junctional Crumbs complex protein Pals1 (Iden and Collard, 2008; Shin et al., 2006; Suzuki and Ohno, 2006). ZO-1 and Pals1 are highly localized in apical cell junctions of most (Slug negative) neuroepithelial cells and clearly line the lumen of trunk neural tube (Fig. 3A; see also Fig. S4C in the supplementary material for tight junction associated proteins, Par3 and aPKC). By contrast, ZO-1 and Pals1 are also distributed all along the lateral cell membranes of Slug-expressing PMNCCs (Fig. 3A, parts b′,d′). The lack of polarized apical junctional localizations of ZO-1 and Pals1 are more apparent in cranial neural crest cells, in which the lumen is more open and expanded than in the trunk (Fig. 3B). ZO-1 and Pals1 staining disappear from the luminal surface of the most dorsal region of the cranial neural tube where the Slug-expressing cells reside, and ZO-1 and Pals1 are present in lateral cell membranes of these Slug-expressing neural crest cells (Fig. 3B). It is uncertain whether the disruption of polarized localization of tight junctional protein and apical junctional proteins was due to a redistribution concomitant with a loss of cell polarity, a downregulation of their levels of expression, or both. Therefore, the distributions of apical junctional proteins indicate that the PMNCCs are poorly polarized, even while they remain in the neural tube well before they delaminate.
Fig. 3.
Nonpolarized distributions of ZO-1 and Pals1 in pre-migratory neural crest cells. Immunohistochemistry was performed on cryosections of 10-13 ss embryos. (A) ZO-1 and Pals1 staining in trunk regions. The white boxed regions in b and d are magnified in b' and d', respectively. e' shows nuclei staining of d'. An arrow in d indicates the less polarized expression of Pals1. Slug (green)/ZO-1 (red) (a,b,b') and Slug (green)/Pals1 (red) (c,d,d'). (B) ZO-1 and Pals1 staining in cranial regions. Arrows indicate the absence of polarized junctional expression of ZO-1 (c) and Pals1 (f) in the most dorsal region in the neural tube. Slug (green)/ZO-1 (red) (a-c) and Slug (green)/Pals1 (red) (d-f). Nuclei were stained blue by DRAQ5 (e',c,f). The most representative images are shown (at least three random sections per embryo and at least four embryos were analyzed). Scale bar: 25 μm.
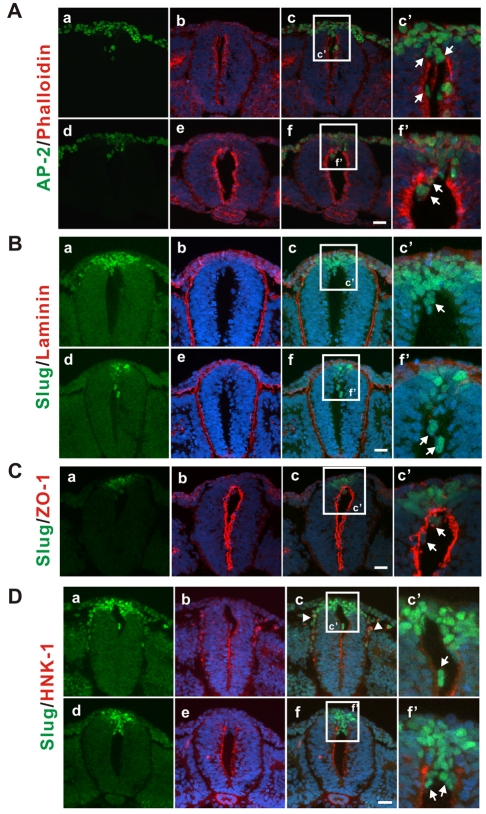
To better understand the state of the PMNCCs, we examined their motile properties, because enhanced motility is a typical phenotype of mesenchymal cells that have undergone an EMT. The neural tube is completely surrounded by a basement membrane, as illustrated by laminin staining (Fig. 2A, Fig. 4B), which could restrict the movement of the PMNCCs. Therefore, we filled the lumen of the neural tube with type I collagen gel by in ovo injection (see Fig. S3A in the supplementary material) to provide a substrate to support cell migration without the barrier of the basement membrane. We then examined the locations of PMNCCs after a period of incubation using Slug and AP-2 as markers for PMNCCs (Fig. 4). At the axial level of the embryo, where both migratory and pre-migratory neural crest cells exist, AP-2- (Fig. 4A, parts a,b,c,c′, arrows) or Slug- (Fig. 4B, parts a,b,c,c′, arrows) expressing cells were found in the lumen of the collagen filled neural tube, as well as outside of neural tube (due to normal migration). Moreover, at the axial level of the embryo with only PMNCCs, AP-2- (Fig. 4A, parts d,e,f,f′, arrows) or Slug- (Fig. 4B, parts d,e,f,f′, arrows) expressing cells were also found in the lumen. The migration of Slug-expressing cells into the lumen was accompanied by a loss of the polarized distribution of ZO-1 (Fig. 4C).
Fig. 4.
Ectopic collagen allows premature migration of the pre-migratory neural crest cells into the lumen of the neural tube. Type I collagen was injected into the lumen of the neural tube of 11-14 ss embryos and the embryos were incubated for 7-9 hours before fixation. (A) AP-2 (green)-positive cells enter the lumen of neural tube at the axial level where both migratory and pre-migratory neural crest cells normally exist (a-c) and at the axial level, which has only pre-migratory neural crest cells (the segmental plate, d-f). Arrows (c',f') indicate AP-2-positive cells in the lumen. Phalloidin stains actin (red). The most representative images are shown (at least three random sections per embryo and at least seven embryos were analyzed). (B) Slug (green)-expressing cells enter the lumen of neural tube at the axial levels where there are only pre-migratory neural crest cells (between the three last formed somite pairs, d-f) and at axial levels where both migratory and pre-migratory neural crest cells are found (a-c). Arrows (c',f') indicate Slug-positive cells in the lumen. Red staining is for laminin. The most representative images are shown (at least three random sections per embryo and at least 10 embryos were analyzed). (C) Slug (green)-positive cells residing in the lumen (arrows in c') have nonpolarized distribution of ZO-1 (red), which normally demarcates the apical-lumenal boundary. The most representative images are shown (at least three random sections per embryo and at least five embryos were analyzed). (D) Slug-positive cells inside of the lumen do not express HNK-1 (c',f', arrows). This is the case both at axial levels that do not have endogenous migratory neural crest (between the three last formed somite pairs, d,e,f,f') and at axial levels with endogenous migratory neural crest (a,b,c,c'). By contrast, the endogenous migratory neural crest cells express both Slug (green) and HNK-1 (red) (a-c; arrowheads). The most representative images are shown (at least three random sections per each embryo and at least eight embryos were analyzed). For all samples, blue is DRAQ5 nuclear staining and the white boxed regions in c and f are magnified in c' and f', respectively. Scale bars: 25 μm.
Migration of cells into the collagen filled lumen was selective to the PMNCCs and did not seem to occur for the polarized neuroepithelial cells from the lateral region of the neural tube, because they appeared to arise most from the dorsal region and all of the cells that move into the lumen were positive for Slug or AP-2. Moreover, type I collagen did not stimulate migration by inducing the differentiation of the PMNCCs into migratory neural crest cells, because the cells appearing in the lumen did not express either of two markers for differentiated migratory neural crest cells, HNK-1 (Fig. 4D) (Bronner-Fraser, 1986) or cadherin 7 (data not shown) (Bronner-Fraser, 1986; Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998). Even the early stage PMNCCs in the dorsal neural tips of the posterior open neural plate appeared to have motile properties. This was observed as a result of either in ovo injection of type I collagen, which filled the neural groove of the posterior open neural plate (see Fig. S3B,C in the supplementary material) or explant culture of whole embryo segments containing the unclosed neural tube in type I collagen gels (see Fig. S3D,E in the supplementary material). The acquisition of motile properties along with the loss of polarity, as well as expression of α-smooth muscle actin (α-SMA) in dorsal region of neural tube (see Fig. S2 in the supplementary material), support the conclusion that the PMNCCs undergo de-epithelialization in a distinct step prior to delamination for the overall EMT.
Cadherin 6B induces the de-epithelialization step of the EMT
The association of cadherin 6B expression with the nonpolarized and motile properties of the PMNCCs raised the issue of the role of this cadherin in de-epithelialization. We therefore examined the effect of ectopic cadherin 6B expression on the properties of neuroepithelial cells in the neural tube. Ectopic expression of wild-type cadherin 6B (FL-Cad6B) caused cells of the electroporated neural tube halves to round up and accumulate in the lumen of the neural tube (Fig. 5A, parts a,b). In addition, the polarized localization of the tight junction protein ZO-1, N-cadherin, p120 or β-catenin at the luminal surface was strongly disrupted in the region where cadherin 6B was expressed (Fig. 5A, parts c,d; see Fig. S4A in the supplementary material). This accumulation of cells in the lumen is probably not due to the active migration of cells [like the previously described migration of the endogenous PMNCCs into the collagen gel (Fig. 4)], but rather due to a gross disruption of epithelial integrity. De-epithelialization and accumulation of the cells was not caused by a dominant-negative cadherin 6B (GPI-Cad6B) (Fig. 5A, parts e-h). Importantly, overexpression of wild-type N-cadherin (N-cad) induced the accumulation of only a small number of cells in the lumen of the neural tube (Fig. 5A, parts i-l), similar to a previous report (Nakagawa and Takeichi, 1998), but the effect was slight and not comparable with the effect of cadherin 6B expression. Thus, the de-epithelialization induced by cadherin 6B is selective, consistent with the notion that its upregulation in the PMNCCs provides a cadherin type selective function on the PMNCCs.
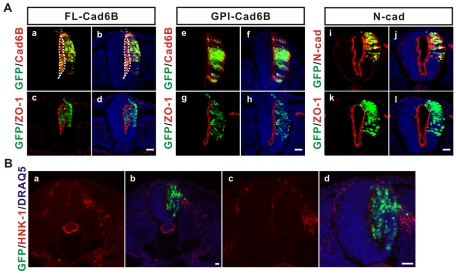
Fig. 5.
Cadherin 6B expression selectively induces de-epithelialization. (A) Twelve to 15 somite pair stage embryos were electroporated with the indicated cadherin constructs containing an IRES-GFP (green) to mark the transfected cells, and embryos were incubated for 20-24 hours prior to cryosectioning. Sections were also immunostained for either cadherin 6B (Cad6B, red), N-cadherin (N-cad, red) or ZO-1 (red), as indicated, and nuclei were stained blue by DRAQ5 (b,d,f,h,j,l). Wild-type cadherin 6B (FL-Cad6B) induced the accumulation of cells in the lumen of the neural tube (a,b, white broken line delineates the luminal surface) and the disruption of the polarized distribution of ZO-1 at the luminal surface (c,d) (11/11 embryos). Dominant-negative cadherin 6B (GPI-Cad6B, e-h, 7/7 embryos) and wild-type N-cadherin (N-cad, i-l, 7/7 embryos) had no effect on de-epithelialization or the polarized distribution of ZO-1. Scale bars: 25 μm. (B) De-epithelialized cells resulting from ectopic expression of cadherin 6B did not express HNK-1, a marker for differentiated migratory neural crest. Ten to 13 somite pair stage chick embryos were electroporated with pCIG-FL-cad6B containing an IRES-GFP (GFP, green) and incubated for 24 hours. The nuclei were stained blue by DRAQ5 (b,d); c and d are magnified images of a and b, respectively. The most representative images are shown (at least three random sections per each embryo and at least 10 embryos were analyzed). Scale bar: 25 μm.
We did not observe any stimulation of neural crest cell delamination through the basement membrane to the outside of the neural tube by ectopic cadherin 6B expression (see also Fig. 2B,C and below). This is clearly different from the striking ectopic delamination induced by co-expression of two transcription factors important for neural crest formation, Sox9 and Slug (see Fig. S4B in the supplementary material), as shown previously (Cheung et al., 2005). Nor was the de-epithelialized phenotype induced by cadherin 6B accompanied by expression of HNK-1 (Fig. 5B) or cadherin 7 (data not shown), two marker proteins of differentiated migratory neural crest cells. Sox9 expression alone caused a similar de-epithelialized phenotype (see Fig. S4B,C in the supplementary material); however, unlike cadherin 6B, Sox9 is known to induce expression of markers of neural crest cell differentiation, such as HNK-1 (Cheung and Briscoe, 2003). Therefore, de-epithelialization induced by cadherin 6B was not due to differentiation of PMNCCs into migratory neural crest cells. Thus, cadherin 6B expression selectively induces de-epithelialization independently of delamination or neural crest cell differentiation, suggesting that it contributes specifically to the first step of the overall EMT.
Cadherin 6B mediates de-epithelialization through stimulation of BMP signaling
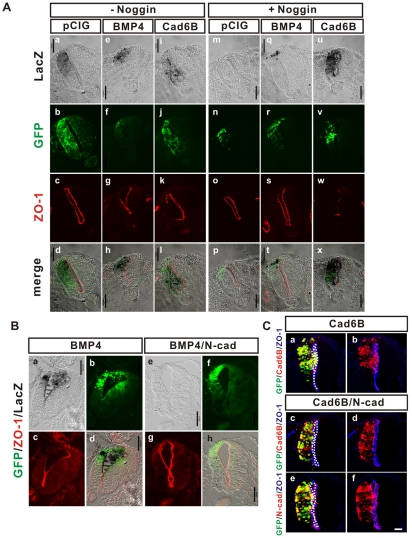
BMP4 positively regulates the EMT of avian neural crest cells (Burstyn-Cohen et al., 2004; Sela-Donenfeld and Kalcheim, 1999; Shoval et al., 2007) and induces the expression of cadherin 6B (see Fig. S5C in the supplementary material) (Liu and Jessell, 1998; Sela-Donenfeld and Kalcheim, 1999). Similar to cadherin 6B, BMP4 did not stimulate ectopic delamination but it induced the de-epithelialization of neuroepithelial cells, which was demonstrated by a disruption of the polarized distribution of ZO-1 and an accumulation of cells inside the lumen (Fig. 6A, parts e-h; see Fig. S5A, parts a-d in the supplementary material). We therefore determined whether ectopic cadherin 6B expression affects BMP signaling. We determined the pattern of BMP signaling with a reporter assay using β-galactosidase expression after electroporation of a BMP responsive element (BRE)-lacZ reporter, which has a concatamerized Smad1/5/8-binding site (Blank et al., 2008). Endogenous BMP signaling in the dorsal neural tube was shown by β-galactosidase (lacZ) staining after electroporation of BRE-lacZ reporter and pCIG (Fig. 6A, part a). This endogenous activity was blocked by co-expression of Noggin (Fig. 6A, part m), a protein that antagonizes BMP signaling by binding BMP and inhibiting its interaction with the BMP receptor (Zimmerman et al., 1996). Ectopic expression of BMP4 caused strong expression of β-galactosidase, which was detected in the de-epithelialized cells (Fig. 6A, parts e-h). Noggin co-expression partially inhibited BMP signaling (Fig. 6A, part q) and the de-epithelialized phenotype (Fig. 6A, parts r-t) induced by ectopic BMP4. Importantly, BMP signaling was also strongly activated by ectopic cadherin 6B expression (Fig. 6A, part i), and the de-epithelialized cells caused by cadherin 6B exhibited active BMP signaling, accompanying disruption of the polarized distribution of ZO-1 and accumulation of GFP-positive cells in the lumen (Fig. 6A, parts i-l). The effect of ectopic cadherin 6B expression was particularly striking in the presence of Noggin (Fig. 6A, parts u-x) compared with the expression of Noggin without cadherin 6B (Fig. 6A, parts m-p). Thus, cadherin 6B might activate BMP signaling in the absence of ligand, but detailed quantitative studies will be needed in order to determine whether activation is ligand independent. Importantly, stimulation of BMP signaling is specific to cadherin 6B because N-cadherin does not cause such stimulation; in fact N-cadherin inhibits both BMP signaling and de-epithelialization induced by either cadherin 6B or BMP4 (Fig. 6B,C). These results also suggest that intracellular BMP signaling may mediate de-epithelialization phenotype induced by cadherin 6B.
Fig. 6.
Cadherin 6B induces BMP signaling, while N-cadherin inhibits BMP signaling. Constructs were electroporated in 13-15 ss (A) or 10-13 ss (B,C) embryos. The effect of ectopic expression was analyzed at 18-24 hours post-electroporation. BRE-lacZ was transfected to serve as a β-galactosidase reporter for BMP signaling activity (A,B) and transfected cells are marked by the expression of GFP (green) from the IRES in the vectors. (A) Cadherin 6B stimulates BMP signaling. Ectopic cadherin 6B (i-l, 7/7 embryos) or BMP4 (e-h, 4/4 embryos) induces de-epithelialization and higher BMP signaling than endogenous BMP signaling (a, 5/5 embryos). Noggin expression inhibited endogenous BMP signaling (m, 4/4 embryos) and partially blocks stimulation of BMP signaling and de-epithelialization by ectopic BMP4 (q-t, 3/3 embryos). By contrast, Noggin does not inhibit stimulation of BMP signaling (u) or de-epithelialization (v-x) (4/4 embryos) induced by cadherin 6B. Scale bar: 100 μm. (B) Overexpression of N-cadherin inhibits BMP signaling and de-epithelialization due to ectopic BMP signaling. BMP4 induced expression of the BRE-lacZ reporter (e versus a), disruption of apical junctions (g versus c), and accumulation of cells in the lumen (f versus b) are all inhibited by ectopic N-cadherin (4/4 embryos). Scale bars: 100 μm. (C) De-epithelialization induced by ectopic cadherin 6B is blocked by overexpression of N-cadherin. Broken white line delineates the apical junctions revealed by ZO-1 staining (a,c,e). With overexpression of N-cadherin, ectopic cadherin 6B-expressing cells do not accumulate in the lumen (c,d, 3/3 embryos) and apical junctions are not disrupted (e,f, 3/3 embryos). Scale bar: 25 μm.
Therefore, we examined whether de-epithelialization induced by cadherin 6B is dependent on BMP signaling. Smad6, an inhibitory Smad that binds the phosphorylated Smad1/5/8 to compete with Smad4 (Hata et al., 1998; Imamura et al., 1997; Linker and Stern, 2004), and dominant-negative form of the BMP receptor (DN-BMPR) (Linker and Stern, 2004; Suzuki et al., 1994) were used to inhibit intracellular BMP signaling steps cell autonomously. Co-expression of Smad6 or DN-BMPR by plasmid electroporation inhibited BMP signaling stimulated by ectopic BMP4 (see Fig. S5A, parts a,e,i in the supplementary material) as well as the de-epithelialized phenotype induced by ectopic BMP4 (see Fig. S5A, parts b-d,f-h,j-l in the supplementary material). The activation of BMP signaling induced by ectopic cadherin 6B (see Fig. S5A, part m in the supplementary material) was also inhibited by Smad6 (see Fig. S5A, part u in the supplementary material) and by DN-BMPR (see Fig. S5A, part q in the supplementary material). This was in contrast to the lack of inhibition by Noggin. Importantly, Smad6 and DN-BMPR inhibited the de-epithelialization induced by cadherin 6B (Fig. 7A). Therefore, it is very likely that cadherin 6B mediates de-epithelialization through stimulation of BMP signaling at the level of the BMP receptor.
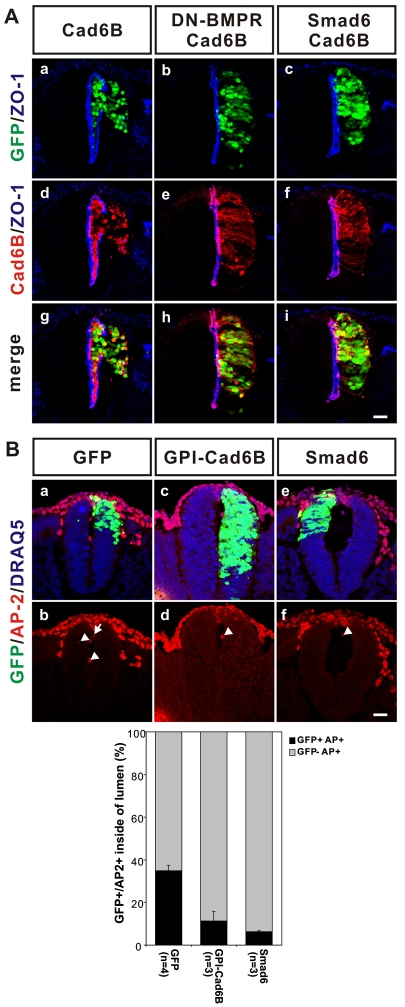
Fig. 7.
BMP signaling is required for cadherin 6B induced de-epithelialization. (A) BMP signaling is required for de-epithelialization induced by ectopic cadherin 6B. Thirteen to fifteen somite pair stage embryos were electroporated with the cadherin 6B construct containing an IRES-GFP (Cad6B) in all samples. In some samples, either a dominant-negative form of BMP receptor (DN-BMPR, b,e,h) or the inhibitory Smad, Smad6 (c,f,i), was co-expressed with cadherin 6B. Embryos were incubated for 22-24 hours. Ectopic expression of cadherin 6B (immunostained in red) induces de-epithelialization, as demonstrated by the disruption of the polarized distribution of ZO-1 (blue) (a,d,g). DN-BMPR (b,e,h, 5/6 embryos) and Smad6 (c,f,i, 4/5 embryos) inhibit de-epithelialization induced by cadherin 6B. Scale bar: 25 μm. (B) Premature migration of PMNCCs into collagen gel is dependent on cadherin 6B and BMP signaling. Embryos were electroporated with control vector pCIG-GFP (GFP), GPI-cadherin 6B-GFP (GPI-cad6B) or smad6-mGFP/pCIG-GFP (Smad6) at 11-14 ss. At 4-5 hours post-electroporation, type I collagen was injected into the neural tube lumen of embryos and embryos were incubated for another 7-8 hours. GFP (green) marks transfected cells. Graph below shows the percentage of GFP positive/AP-2-positive cells (transfected) out of the total number of AP-2 positive cells inside the lumen was determined for each experimental group. Only sections with high electroporation efficiency in dorsal cells were used. Representative images are shown (a-f). An arrow (b) indicates GFP-positive (green)/AP-2 positive (red) cells inside of the lumen and arrowheads (b,d,f) show GFP negative/AP-2-positive cells. Scale bar: 25 μm.
We also examined whether cadherin 6B/BMP signaling contributes to the de-epithelialized phenotype of the endogenous PMNCCs. Inhibition of BMP signaling by Smad6 expression increased the polarized localization of ZO-1 in the dorsal neural tube (see Fig. S6 in the supplementary material). Smad6 electroporated sides exhibited a decrease in the unpolarized ZO-1 in 35% of sections, but in only 11% of sections of the control mGFP electroporated group. We also examined whether cadherin 6B or BMP signaling contributes to the migratory potential of PMNCCs (Fig. 7B). Ectopic expression of either GPI-cad6B or Smad6 inhibited the migration of the PMNCCs into the collagen-filled lumen of the neural tube. The percentage of GFP-positive and AP-2-positive cells migrating into the lumen decreases in GPI-Cad6B or Smad6 electroporated embryos (11±4.6% or 6±0.5%, respectively, compared with 35% for control GFP electroporation). Therefore, it is very likely that cadherin 6B mediates the de-epithelialization (the migratory potential and lack of polarity) in the endogenous PMNCCs, at least in part, through stimulation of BMP signaling.
DISCUSSION
We have examined the formation of the neural crest as a significant in vivo model of a developmental EMT. A loss of N-cadherin expression in the dorsal region of the neural tube has been found to play a role in neural crest formation (Shoval et al., 2007), but at the same time high cadherin 6B expression becomes restricted to the PMNCCs. In chick embryos, cadherin 6B expression is also eventually lost in migratory neural crest cells (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998), and this has lead to findings suggesting that it has an inhibitory role in the EMT and must be lost for the EMT to proceed (Coles et al., 2007), similar to the proposed functions of E-cadherin in other EMTs and tumor suppression (Yang and Weinberg, 2008). However, we observed that cadherin 6B is not only expressed throughout the life of the PMNCCs, but continues for a period in early migrating neural crest just after they have undergone an EMT and emigrated from the neural tube. Similarly, cadherin 6 expression persists in early migrating neural crest of the mouse (Inoue et al., 1997). Indeed, we discovered that inhibiting the function or the expression of cadherin 6B interfered with the emigration of neural crest cells from the neural tube.
Our findings of a positive role for cadherin 6B in the EMT of the neural crest appears contradictory to a previous report suggesting that cadherin 6B expression inhibits neural crest cell delamination from the neural tube (Coles et al., 2007). The basis for the discrepancy is not entirely clear; however, the previous study used electroporation of morpholinos (MOs) to knockdown cadherin 6B expression, while we used shRNA-mediated knockdown, which worked much better for us than morpholinos (K.-S.P. and B.M.G., unpublished), as well as a dominant-negative cadherin 6B construct to inhibit its function. In addition, we focused on trunk neural crest while they focused on cranial crest. Nonetheless, it is important to note that in neither study was an effect of cadherin 6B overexpression on neural crest cell delamination observed, an effect that would have been expected if loss of cadherin 6B expression was required for the EMT (the previous study reported an effect on only subsequent neural crest cell migration). Moreover, the normal expression pattern of cadherin 6B is more consistent with a positive role than an inhibitory role in the EMT; in contrast to N-cadherin it is expressed in the PMNCCs/dorsal neural tube continually throughout the period of the EMT and we find that its expression persists in neural crest cells that have just delaminated from the neural tube. Indeed this observation is what encouraged us to re-examine the role of cadherin 6B in the EMT. A previous study suggested that the Slug protein, which is also expressed in the PMNCCs, can repress cadherin 6B expression (Taneyhill et al., 2007). If such repression occurs in vivo during neural crest development, our findings suggest that it functions at a later stage after the EMT and neural crest emigration form the neural tube.
Although we found that cadherin 6B is required for the overall EMT, it is not sufficient to induce the emigration of neural crest cells from the neural tube, unlike the effects of SOX9+Slug expression. Rather, our findings suggest that cadherin 6B facilitates the EMT by promoting one step in the overall EMT, a loss of epithelial characteristics. Ectopic expression of cadherin 6B in the lateral regions of the neural tube caused the cells to lose junctional polarity, round up and accumulate inside the lumen of the neural tube. Thus, the differential expression of cadherins in the PMNCCs may alter the properties of the neural tube cells to make them more prone to be able to undergo an EMT.
The properties of the cadherin 6B-expressing PMNCCs in the dorsal region of the neural tube suggest that they have undergone de-epithelialization endogenously prior to their delamination from the tissue. The distribution of the cadherin 6B-catenin complex in the PMNCCs is much less localized in the polarized apical junctions at the lumenal surface than the distribution of the N-cadherin-catenin complex in the lateral and ventral regions of the neural tube. This is further illustrated by the nonpolarized distribution of the tight and apical junctional markers ZO-1 and Pals1, and of other proteins in these cells. Moreover, the PMNCCs appear to have motile properties, even though the presence of a basement membrane around the neural tube epithelium normally prevents them from moving. The acquisition of these nonpolarized and motile properties occurs well before the time that these cells would normally delaminate and emigrate from the neural tube, as it occurs in regions of the trunk neural tube well before the appearance of migratory neural crest cells. In fact, the nonpolarized and motile properties of the PMNCCs begins even earlier, before the fusion and closure of the neural tube, as they are observed in the Slug and cadherin 6B-expressing dorsal tips of the neural folds. Thus, we propose that the overall EMT of the neural crest consists of at least two distinct steps: de-epithelialization involving the loss of junctional polarity and the acquisition of motile properties; and the delamination of the cells through the basement membrane accompanying their directional migration away from the neural tube. A recent report focusing on the EMT of the primitive streak also proposed that the EMT proceeds in two distinct steps, although they proposed that the breakdown of the basement membrane occurs before the loss of epithelial junctions (Nakaya et al., 2008).
The mechanism underlying the loss of polarity caused by cadherin 6B may be due at least in part to stimulation of BMP signaling. VE-cadherin similarly has been shown to stimulate TGFβ signaling (Rudini et al., 2008). Ectopic expression of BMP4 caused de-epithelialization similar to cadherin 6B, consistent with the idea that BMP signaling could mediate its effects. Ectopic expression of cadherin 6B strongly induced BMP signaling in the neuroepithelial cells, as measured by a specific reporter assay. Most importantly, inhibition of either the BMP receptor or downstream events in the BMP signaling pathway blocked the de-epithelialization caused by cadherin 6B. BMP4 can stimulate signaling independently of cadherin 6B, as DN-cadherin 6B is unable to block de-epithelialization induced by ectopic BMP4 expression, even though it blocks de-epithelialization induced by cadherin 6B (see Fig. S7 in the supplementary material). Therefore, although we cannot rule out a role for the adhesive function of cadherin 6B, a major mechanism of specific de-epithelialization caused by cadherin 6B appears to be through stimulation of the BMP signaling pathway at or above the level of BMP receptor.
The de-epithelialization by cadherin 6B is cadherin type specific, because it was not elicited by overexpression of the cadherin normally expressed in these cells, N-cadherin, nor did N-cadherin expression stimulate BMP signaling. It is possible that de-epithelialization induced by cadherin 6B is caused by the loss of N-cadherin expression in the dorsal neural tube, as BMP signaling is known to inhibit N-cadherin expression (Shoval et al., 2007). Although we find that overexpression of N-cadherin inhibits de-epithelialization induced by cadherin 6B, we observed that it also inhibits BMP signaling. Because we also find that BMP signaling is involved in loss of junctional polarity of the PMNCCs, it is difficult to know whether N-cadherin affects epithelial polarity as an effector downstream of BMP signaling or upstream of BMP signaling. Indeed, cadherin 6B and N-cadherin seem to be involved in two reciprocal feedback loops regarding BMP signaling, with cadherin 6B-expressing cells associated with high BMP signaling and N-cadherin-expressing cells associated with low BMP signaling (Fig. 8). As differential cadherin expression mediates the sorting out of cell types, this may lead to formation of a high BMP signal domain in the dorsal neural tube. Overall, these results suggest that the differential expression of cadherins in the dorsal neural tube is in some way responsible for the de-epithelialized properties of PMNCCs.
Fig. 8.
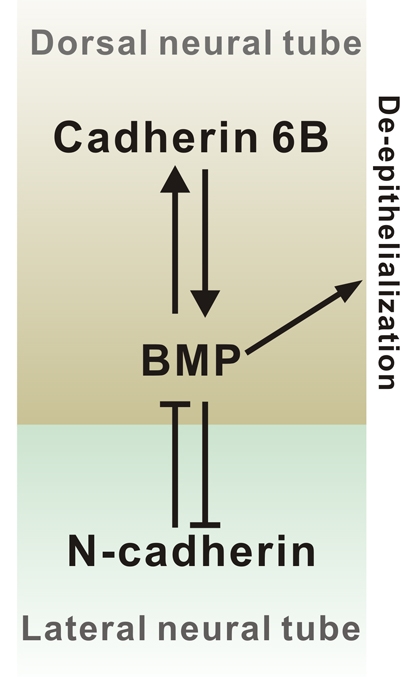
A model illustrating roles of cadherin 6B and N-cadherin in the regulation of BMP signaling. Cadherin 6B induces active BMP signaling and BMP signaling also induces the expression of cadherin 6B. This may create a positive-feedback loop to intensify BMP signaling locally. A reciprocal inhibitory loop may also exist between N-cadherin and BMP signaling, as BMP is known to inhibit N-cadherin expression in the dorsal neural tube (Shoval et al., 2007) and N-cadherin inhibits BMP signaling. As differential cadherin expression mediates the sorting out of cell types, this may lead to formation of a high BMP signal domain in the dorsal neural tube.
Supplementary Material
Acknowledgments
We thank Angela Nieto, Masatoshi Takeichi, Jeh-Ping Liu, Jing Yu, Martin Cheung, Claudio Stern, Judith White, Leif Oxburgh and Andrea Streit for constructs. We thank Ian Macara for an antibody for Pals1. We also thank Douglas W. DeSimone, Xiaowei Lu and Jing Yu for critical reading of the manuscript and the members of the Gumbiner laboratory for helpful discussions and suggestions. This work was supported by NIH Grant R37GM37432 to Barry Gumbiner. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.050096/-/DC1
References
- Baumer N., Marquardt T., Stoykova A., Ashery-Padan R., Chowdhury K., Gruss P. (2002). Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129, 4535-4545 [DOI] [PubMed] [Google Scholar]
- Blank U., Seto M. L., Adams D. C., Wojchowski D. M., Karolak M. J., Oxburgh L. (2008). An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev. Biol. 8, 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher W. M., Gumbiner B. M. (1994). Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J. Cell Biol. 126, 519-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. (1986). Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev. Biol. 115, 44-55 [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Stanleigh J., Sela-Donenfeld D., Kalcheim C. (2004). Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 131, 5327-5339 [DOI] [PubMed] [Google Scholar]
- Capaldo C. T., Macara I. G. (2007). Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M., Briscoe J. (2003). Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681-5693 [DOI] [PubMed] [Google Scholar]
- Cheung M., Chaboissier M. C., Mynett A., Hirst E., Schedl A., Briscoe J. (2005). The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179-192 [DOI] [PubMed] [Google Scholar]
- Chihara T., Kato K., Taniguchi M., Ng J., Hayashi S. (2003). Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 130, 1419-1428 [DOI] [PubMed] [Google Scholar]
- Coles E. G., Taneyhill L. A., Bronner-Fraser M. (2007). A critical role for Cadherin6B in regulating avian neural crest emigration. Dev. Biol. 312, 533-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Barrio M. G., Nieto M. A. (2002). Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129, 1583-1593 [DOI] [PubMed] [Google Scholar]
- Gao L., Macara I. G. (2004). Isoforms of the polarity protein par6 have distinct functions. J. Biol. Chem. 279, 41557-41562 [DOI] [PubMed] [Google Scholar]
- Geisbrecht E. R., Montell D. J. (2002). Myosin VI is required for E-cadherin-mediated border cell migration. Nat. Cell. Biol. 4, 616-620 [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622-634 [DOI] [PubMed] [Google Scholar]
- Hata A., Lagna G., Massague J., Hemmati-Brivanlou A. (1998). Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 12, 186-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay E. D. (2005). The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 233, 706-120 [DOI] [PubMed] [Google Scholar]
- Iden S., Collard J. G. (2008). Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 9, 846-859 [DOI] [PubMed] [Google Scholar]
- Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M., Miyazono K. (1997). Smad6 inhibits signalling by the TGF-beta superfamily. Nature 389, 622-626 [DOI] [PubMed] [Google Scholar]
- Inoue T., Chisaka O., Matsunami H., Takeichi M. (1997). Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev. Biol. 183, 183-194 [DOI] [PubMed] [Google Scholar]
- Kalcheim C., Burstyn-Cohen T. (2005). Early stages of neural crest ontogeny: formation and regulation of cell delamination. Int. J. Dev. Biol. 49, 105-116 [DOI] [PubMed] [Google Scholar]
- LaBonne C., Bronner-Fraser M. (2000). Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 221, 195-205 [DOI] [PubMed] [Google Scholar]
- Li G., Satyamoorthy K., Herlyn M. (2001). N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 61, 3819-3825 [PubMed] [Google Scholar]
- Linker C., Stern C. D. (2004). Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 131, 5671-5681 [DOI] [PubMed] [Google Scholar]
- Liu J. P., Jessell T. M. (1998). A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125, 5055-5067 [DOI] [PubMed] [Google Scholar]
- Loring J. F., Erickson C. A. (1987). Neural crest cell migratory pathways in the trunk of the chick embryo. Dev. Biol. 121, 220-236 [DOI] [PubMed] [Google Scholar]
- Matsunaga M., Hatta K., Nagafuchi A., Takeichi M. (1988). Guidance of optic nerve fibres by N-cadherin adhesion molecules. Nature 334, 62-64 [DOI] [PubMed] [Google Scholar]
- McCrea P. D., Brieher W. M., Gumbiner B. M. (1993). Induction of a secondary body axis in Xenopus by antibodies to beta-catenin. J. Cell Biol. 123, 477-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C., Astier M., Zmojdzian M., Jagla K., Semeriva M. (2008). Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J. Cell Biol. 182, 249-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D., Bronner-Fraser M. (2004). Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291-299 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Takeichi M. (1995). Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121, 1321-1332 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Takeichi M. (1998). Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125, 2963-2971 [DOI] [PubMed] [Google Scholar]
- Nakaya Y., Sukowati E. W., Wu Y., Sheng G. (2008). RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10, 765-775 [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835-839 [DOI] [PubMed] [Google Scholar]
- Patel S. D., Ciatto C., Chen C. P., Bahna F., Rajebhosale M., Arkus N., Schieren I., Jessell T. M., Honig B., Price S. R., et al. (2006). Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124, 1255-1268 [DOI] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. (1998). Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 204, 64-79 [DOI] [PubMed] [Google Scholar]
- Qian X., Karpova T., Sheppard A. M., McNally J., Lowy D. R. (2004). E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 23, 1739-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickmann M., Fawcett J. W., Keynes R. J. (1985). The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J. Embryol. Exp. Morphol. 90, 437-455 [PubMed] [Google Scholar]
- Rudini N., Felici A., Giampietro C., Lampugnani M., Corada M., Swirsding K., Garre M., Liebner S., Letarte M., ten Dijke P., et al. (2008). VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 27, 993-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557-568 [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D., Kalcheim C. (1999). Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development 126, 4749-4762 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Yabe T., Muraoka O., Yonemura S., Aramaki S., Hatta K., Bae Y. K., Nojima H., Hibi M. (2005). E-cadherin is required for gastrulation cell movements in zebrafish. Mech. Dev. 122, 747-763 [DOI] [PubMed] [Google Scholar]
- Shin K., Fogg V. C., Margolis B. (2006). Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207-235 [DOI] [PubMed] [Google Scholar]
- Shoval I., Ludwig A., Kalcheim C. (2007). Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 134, 491-501 [DOI] [PubMed] [Google Scholar]
- Suyama K., Shapiro I., Guttman M., Hazan R. B. (2002). A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2, 301-314 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. (2006). The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979-987 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. (1994). A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc. Natl. Acad. Sci. USA 91, 10255-10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill L. A., Coles E. G., Bronner-Fraser M. (2007). Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134, 1481-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillet M. A., Kalcheim C., Le Douarin N. M. (1987). Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Dev. Biol. 120, 329-347 [DOI] [PubMed] [Google Scholar]
- Tomita K., van Bokhoven A., van Leenders G. J., Ruijter E. T., Jansen C. F., Bussemakers M. J., Schalken J. A. (2000). Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650-3654 [PubMed] [Google Scholar]
- Tran N. L., Nagle R. B., Cress A. E., Heimark R. L. (1999). N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am. J. Pathol. 155, 787-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Johnson K. R. (2003). Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19, 207-235 [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Shintani Y., Maeda M., Fukumoto Y., Johnson K. R. (2008). Cadherin switching. J. Cell Sci. 121, 727-735 [DOI] [PubMed] [Google Scholar]
- Yang J., Weinberg R. A. (2008). Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818-829 [DOI] [PubMed] [Google Scholar]
- Zimmerman L. B., De Jesus-Escobar J. M., Harland R. M. (1996). The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599-606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.