Abstract
Despite the central role of memory B cells (MBC) in protective immune responses, little is understood about how they are acquired in naïve individuals in response to antigen exposure, and how this process is influenced by concurrent activation of the innate immune system’s Toll-like receptors (TLR). In this longitudinal study of malaria-naïve individuals, we examined the MBC response to two candidate malaria vaccines administered with or without CpG, a TLR9 ligand. We show that the acquisition of MBC is a dynamic process in which the vaccine-specific MBC pool rapidly expands and then contracts, and that CpG enhances the kinetics, magnitude, and longevity of this response. We observed that the percentage of vaccine-specific MBC present at the time of re-immunization predicts vaccine-specific Ab levels 14 days later; and that at steady state, there is a positive correlation between vaccine-specific MBC and Ab levels. An examination of the total circulating MBC and plasma cell (PC) pools also suggests that MBC differentiate into PC through polyclonal activation, independent of antigen specificity. These results provide important insights into the human MBC response which can inform the development of vaccines against malaria and other pathogens that disrupt immunological memory.
Keywords: Human, B cells, Memory, Parasitic-Protozoan, Vaccination
INTRODUCTION
The adaptive immune system encodes the ability to “remember” an initial encounter with an antigen and to respond to that antigen upon re-exposure in a rapid and robust fashion for the life time of the individual. This phenomenon of immunological memory is a fundamental property of the adaptive immune system and is the basis for all vaccine development. For most vaccines, neutralizing Ab plays a critical role in protective immune responses (1), and thus the mechanisms that underlie the generation and maintenance of humoral memory are of considerable interest. Long-term humoral immunity is encoded in memory B cells (MBC) and long-lived plasma cells (LLPC) which are generated as a part of the primary immune response (2, 3). LLPC are terminally differentiated cells that reside in the bone marrow and are responsible for the long-term maintenance of serum Ab levels which play a key role in the initial control of pathogens and their toxins upon reinfection. MBC are capable of mounting an antigen-induced response by proliferating and differentiating into plasma cells (PC) resulting in rapid, high titer secondary Ab responses upon re-exposure to pathogens. Despite the central role of MBC in combating infections, our understanding of the cellular and molecular mechanisms that underlie the generation and maintenance of B cell memory is incomplete. Efforts to develop new vaccines would benefit from a more detailed knowledge of these processes, particularly vaccines against pathogens such as Plasmodium falciparum and HIV which appear to subvert immunological memory (4, 5).
A hallmark of immunological memory is its longevity. The long-lived nature of PC in humans has been inferred from the stability of serum Ab induced by vaccination or infection. Virus-specific Ab levels were shown to be maintained for longer than 60 years after smallpox vaccination (6–8). A recent longitudinal study provides evidence for the remarkable stability of Ab responses following infection with half-lives ranging from 50 years for varicella-zoster virus (VZV) to over 200 years for measles and mumps viruses (9). Recently developed quantitative assays have allowed analyses of the longevity of antigen-specific MBC in immunized individuals. Vaccinia-specific MBC have been detected for over 50 years after smallpox vaccination and represented approximately 0.1% of total circulating MBC (6). These MBC were apparently functional and produced a robust Ab response upon re-vaccination. MBC specific for a variety of other viral and non-replicating antigens including measles, mumps, rubella, Epstein-Barr Virus, VZV and tetanus and diphtheria toxins were also found to be remarkably stable in a recent cross-sectional analysis of adults (9). An inherent limitation to the analysis of MBC in humans has been the restricted access to tissues other than peripheral blood and the unknown relationship between the relative frequency of MBC in the peripheral blood and lymphoid tissue. However, a recent study of individuals decades after smallpox vaccination showed that although the majority of vaccinia-specific MBC were in the spleen, their frequency in the spleen reflected the frequency in peripheral blood (10).
Although the longevity of PC and MBC is a central feature of humoral memory, our understanding of the mechanisms that underlie the maintenance of these cell populations for the life time of an individual is only partial. A key question is the role of antigen exposure in maintaining immunological memory in humans. There are several historical examples of the maintenance of Ab-mediated immunity in the absence of antigen exposure including immunity to measles on the Faroe Islands, yellow fever in the U.S., and polio in remote Eskimo villages [reviewed in (2)]. The detection of antigen-specific MBC for over 50 years after smallpox vaccination also provides evidence for the persistence of MBC in the absence of antigen (6). If antigen-specific activation is not required to maintain MBC, the question is whether or not nonspecific polyclonal activation plays a role. One study shows that diphtheria vaccination does not increase the frequency of tetanus-specific MBC (11), suggesting that MBC do not increase in numbers in response to bystander polyclonal activation.
For PC two mechanisms have been proposed to account for their longevity—namely, that they are inherently long-lived, or alternatively that they are replenished periodically by MBC that differentiate into PC following stimulation with antigen or polyclonal activators, such as TLR ligands and bystander T cell help [(12), reviewed in (13)]. That MBC are responsible for replenishing PC is suggested by the correlation observed between tetanus- and measles-specific MBC and their respective Ab levels at steady state (12). This study also notes a small increase in PC to non-cognate antigens following tetanus toxoid vaccination, an observation the authors interpreted as polyclonal activation and differentiation of non-tetanus MBC. Correlations between MBC and Ab levels have also been observed in smallpox and anthrax immunized adults (6, 14). A recent study in infants shows an association between MBC and Ab levels 1 month after immunization with the serogroup C meningococcal conjugate vaccine, and also an association between the number of MBC prior to a booster vaccination at 5 months and the Ab levels at 1 year (15). However, correlations between Ab levels and MBC have not always been observed (10, 16, 17), suggesting that LLPC may be regulated independently of MBC. For example, a recent cross-sectional study showed that MBC and serum Ab levels correlated for some but not all antigens (9). Indeed, MBC in individuals with the highest rate of revaccination or latent infections showed the weakest correlation with Ab titers, an indication that LLPC maintenance is not dependent on MBC. Consistent with this is the observation that PC-sparing B cell depletion through Rituximab treatment impaired recall Ab responses to some antigens but did not result in significant decreases in serum Ab levels (18–21).
Although several studies have addressed the maintenance of MBC and LLPC, relatively little is known about how they are acquired in humans. Of particular interest in this process is the role of TLR9, a pattern recognition receptor that initiates innate immune responses. TLR9 detects microbial DNA with hypomethylated CpG motifs and in humans is preferentially expressed by plasmacytoid dendritic cells (PDC) and B cells [reviewed in (22)]. The net effect of TLR9 activation is the differentiation of Th1 cells and the induction of IgG isotype switching and Ab secretion. In a hepatitis B vaccine clinical trial the addition of CPG 7909, a B-class CpG oligodeoxynucleotide (ODN), accelerated the acquisition of specific Ab and increased peak Ab titers (23, 24). However, the impact of CpG on the MBC response to primary immunization has not been delineated. Here we describe the kinetics of antigen-specific MBC acquisition in naïve individuals in response to vaccination with malaria antigens and provide evidence that CPG 7909 enhances this process. This analysis was carried out in the context of two separate clinical trials of two candidate malaria subunit protein vaccines formulated on aluminum hydroxide gel (Alhydrogel), with and without CPG 7909, given to healthy malaria-naïve adults (25) (Martin et al., unpublished, www.clinicaltrials.gov #NCT00320658) . We observed that the acquisition of MBC is a highly dynamic process; within 7 days of the second and third vaccinations the vaccine-specific MBC pool first expanded to represent approximately 3–4% of all IgG+ MBC in the periphery and then contracted to less than 1% within 6 months of the first vaccination. CPG 7909 clearly enhanced the kinetics, magnitude and longevity of this response. We also describe an antigen-independent effect of vaccination on pre-existing MBC that suggests a role of the innate immune system in regulating the behavior of existing B cell memory.
MATERIALS AND METHODS
Study population and vaccination procedure
The acquisition of MBC was evaluated in malaria-naïve adults enrolled in 2 separate phase 1 clinical trials of the blood stage malaria vaccine candidates, Apical Membrane Antigen 1-Combination 1 (AMA1-C1) and Merozoite Surface Protein 142-Combination 1 (MSP142-C1), both formulated on Alhydrogel and mixed with 564 µg of CPG 7909 (25) (Martin et al., unpublished, www.clinicaltrials.gov #NCT00320658). Both vaccines contained an equal mixture of antigen from 2 different clones of P. falciparum (FVO and 3D7) produced separately as recombinant proteins. Both trials were conducted under Investigational New Drug Applications reviewed by the US FDA, and both were reviewed and approved by the NIAID IRB and by the IRBs at their respective sites and funding agencies. Written informed consent was obtained from all participants. Samples from 40 individuals, 20 from each trial, were randomly selected for analysis, half of whom had been vaccinated with CPG 7909-containing vaccines. Individuals received intramuscular vaccinations on days 0, 28, and 56. For the AMA1-C1 trial, individuals received 80 µg of AMA1-C1 protein with the exception of 4 volunteers in the CPG 7909 group who received 20 µg. Since the dose of AMA1-C1 was not associated with a difference in the magnitude of the AMA1-C1-specific MBC response at any time point (p>0.100 for all time points) the high and low dose groups were analyzed as a single group. For MSP142-C1, all individuals received 80 µg of protein.
PBMC isolation, cryopreservation and recovery
Peripheral venous blood samples were drawn into heparanized tubes (BD). PBMC were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences) and frozen at ten million cells/ml in 90% heat-inactivated FBS (Gibco) and 10% DMSO (Sigma-Aldrich) using “Mr. Frosty” freezing containers (VWR) to control the rate of freezing to −80 °C for 24 h before transfer to liquid nitrogen. Liquid nitrogen storage freezers were connected to an automatic fill system and were outfitted with an external temperature alarm system. For each individual, frozen PBMC from all available time points were thawed and assayed simultaneously. PBMC were rapidly thawed in a 37°C water bath and then added to complete media [RPMI-1640 plus L-glutamine (Gibco) supplemented with 10% heat-inactivated FBS, penicillin (10,000 IU/ml) streptomycin (10,000µg/ml) (Gibco), and 2-ME (50µM) (Gibco)] warmed to 37°C. Cells were washed, resuspended in complete media, and counted using trypan blue (BioWhittaker) dye exclusion to detect viable cells.
Multiparameter flow cytometry and cell sorting
To verify that the ELISPOT assay specifically detects MBC, PBMC obtained from malaria-naïve healthy donors at the NIH blood bank were isolated from elutriated mononuclear cells by Ficoll-Hypaque density gradient centrifugation. Cells were washed in PBS and platelets were removed by low-speed centrifugation through FBS. Cells were stained with fluorescently-labeled Ab to CD19 (PE-Cy5.5, Caltag), CD27 (PE, Caltag), and CD38 (APC, BD) and sorted on a Becton-Dickinson FACSAria cell-sorting system (BD). B cell subsets were defined as naïve (CD19+ CD27−), memory (CD19+ CD27+CD38−) and plasma cells (CD19+ CD27+CD38+++). Sorted B cells were cultured in triplicate with or without non-B cells in complete media or stimulation media (see below) for 5 days, washed in complete media warmed to 37°C, and transferred directly onto 96 well filter-bottom ELISPOT plates (Millipore Multiscreen-HA) prepared for the MBC ELISPOT assay with IgG-specific capture Ab and antigens, as described below.
The kinetics of the PC response to vaccination was examined in a subset of individuals from the AMA1-C1/Alhydrogel study. The lymphocyte fraction of peripheral venous blood samples was separated by Ficoll-Hypaque density gradient centrifugation, and 1 × 106 fresh PBMC were placed in each well of a 96-well plate and stained at 4°C for 30 min with fluorescently-labeled Ab to CD19 (PE-Cy5.5, Invitrogen), CD3 (Alexa 405, Invitrogen), CD27 (PE, Invitrogen), CD38 (PE-Cy7, Invitrogen), and IgD (FITC, Invitrogen), and then washed with PBS. Stained cells were resuspended in PBS, fixed with 1% paraformaldehyde and analyzed on a Becton-Dickinson LSR-II (BD). Data was processed by Flow Jo software (TreeStar). To visualize the B cell response to vaccination in the peripheral blood, a broad lymphocyte gate was applied to the flow cytometry data, and then CD3 negative, CD19 positive events were selected and analyzed for expression of CD27 and CD38. Contour and density plots were used to set uniform regions around CD27+CD38+ and CD27+CD38+++ events to calculate percentages (among the CD3−CD19+ cells).
Memory B cell ELISPOT Assay
Antigen-specific MBC and total IgG+ MBC were quantified by an assay in which MBC were stimulated in vitro to differentiate into antibody secreting cells (ASC) (26). One million thawed PBMC were placed in each well of a 24-well plate (Corning) containing 1 ml of complete media alone or complete media plus a cocktail of polyclonal activators which included 2.5 µg/ml of CpG ODN-2006 (Operon), SAC at 1/10,000 dilution (Sigma-Aldrich), and PWM at 1/100,000 dilution (Sigma-Aldrich). Cells were kept at 37°C in a 5% CO2 incubator for 5 days, washed twice with complete media warmed to 37°C, counted, and distributed onto 96 well plates that had been prepared for the MBC ELISPOT assay, as described below.
For the AMA1-C1- and MSP142-C1-specific ELISPOT assays, filter-bottom 96-well plates (Millipore Multiscreen-HA) were coated with a 1:1 mixture of AMA1-C1-FVO (2.5 µg/ml) and AMA1-C1-3D7 (2.5 µg/ml) or a 1:1 mixture of MSP142-C1 -FVO (2.5 µg/ml) and MSP142-C1-3D7 (2.5 µg/ml) for a final concentration of 5 µg/ml in PBS (KD Medical). These recombinant proteins were clinical grade and lot matched to the vaccines administered to the study participants. For the detection of total IgG-secreting cells, wells were coated with polyvalent goat anti-human IgG (Caltag) at 10 µg/ml in PBS. As a control, wells were coated with the irrelevant antigen keyhole limpet hemocyanin (KLH; Pierce) at 2.5 µg/ml in PBS. Plates were incubated overnight at 4°C. They were then washed once with PBS-0.05% Tween-20 (Fisher), and 3 times with PBS. Plates were blocked with 1% BSA (Sigma-Aldrich) in RPMI-1640 for 2 h at 37°C. Stimulated PBMC resuspended in 200 µl of fresh complete media were distributed onto coated 96-well plates in triplicate as follows: AMA1-C1- and MSP142-C1-coated wells at 5 × 105 cells/well, and anti-human IgG-coated wells at 1 × 104 cells/well. Both were followed by serial 2-fold dilutions. As controls, stimulated PBMC resuspended in 200 µl of fresh complete media were distributed onto KLH-coated wells at 2 × 104 cells/well and unstimulated PBMC at 2 × 104 cells/well on anti-human IgG-coated wells. Plates were kept at 37°C in a 5% CO2 incubator for 5 h and then washed 4 times with PBS and 4 times with PBS-0.05% Tween-20. The detection Ab, goat anti-human IgG Fc-alkaline phosphatase (Jackson) diluted in PBS-0.05% Tween-20 with 1% FBS, was added to wells and incubated overnight at 4°C. Plates were washed 4 times with PBS-0.05% Tween-20, 3 times with PBS and 3 times with distilled water, before developing with the substrate BCIP/NBT (Calbiochem). Spots were counted with the ImmunoSpot Series 4 Analyzer (Cellular Technology Ltd). Laboratory investigators were blinded to the CPG 7909 status of study participants. Antigen-specific MBC data is expressed as follows:
ELISA
The ELISA protocol and Ab data from the AMA1-C1 trial were published previously (25) . ELISA data shown represent the mean ELISA units for AMA1-C1-FVO and AMA1-C1-3D7, and MSP142-C1-FVO and MSP142-C1 -3D7, respectively. It is noteworthy that there was a strong correlation between AMA1-C1-FVO and AMA1-C1-3D7 titers (25), as well as MSP142-C1-FVO and MSP142-C1-3D7 titers (Martin et al, unpublished, www.clinicaltrials.gov #NCT00320658).
Data Analysis
The correlation between different continuous measures was determined by using the Spearman correlation coefficient. To account for the correlation among multiple measurements for the same subject, the generalized estimating equations (GEE) method (27) was employed to study the association between continuous outcomes and covariates of interest. The GEE method yields valid inferential results even when the correlation structure of the repeated measurements is not correctly specified. GEE models with the study day as a categorical covariate were used to compare repeated measurements with the baseline measurement within the same group. Comparisons between the two groups were conducted by using GEE models with the study day indicators and the CPG 7909 group indicator, as well as their interaction terms as covariates. An “exchangeable” correlation structure was used as the working assumption for all GEE analyses. All P values were two-sided, and P values of less than 0.05 were considered to be statistically significant. Data analyses were performed with STATA, version 10.0 (StataCorp LP) and GraphPad Prism version 5.01 for Windows (GraphPad Software).
RESULTS
Verification of the specificity of the MBC ELISPOT assay
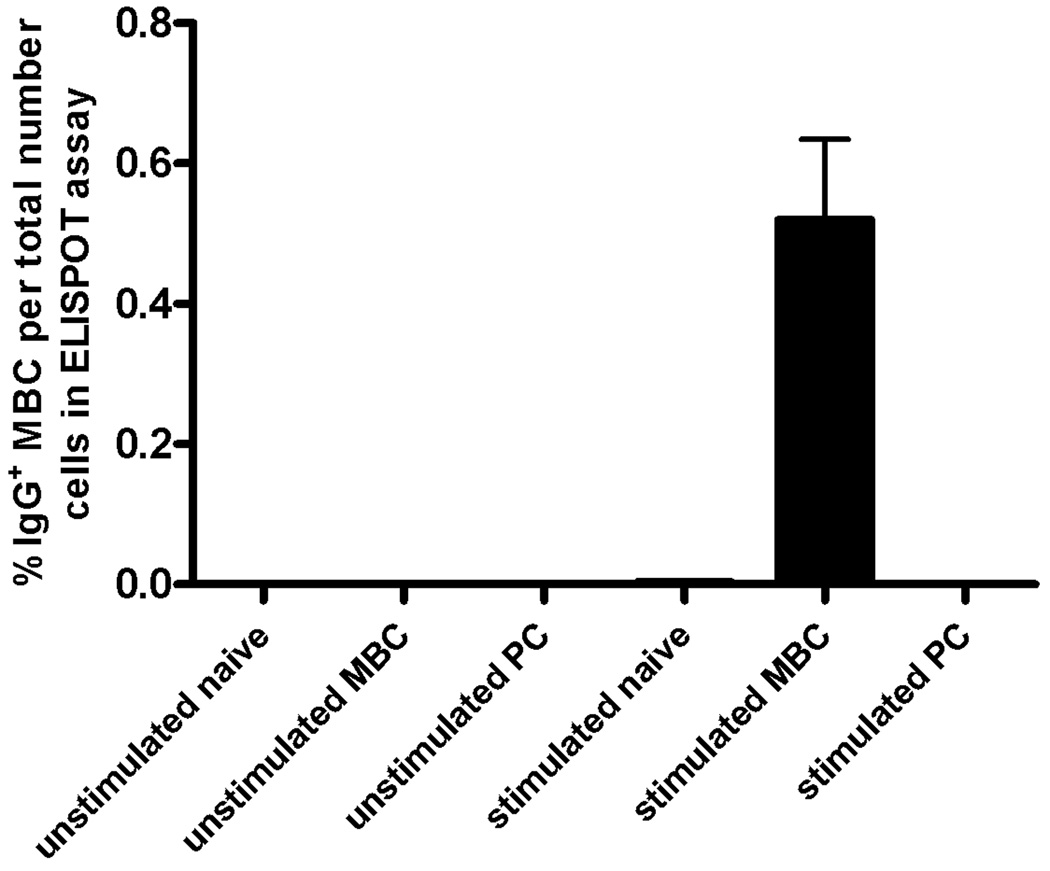
MBC were quantified in peripheral blood samples by the method of Crotty et al.(28), which is based on the differentiation of MBC (CD19+CD27+) but not naïve B cells (CD19+CD27−) into ASC in response to polyclonal activators. To verify that this assay specifically detects MBC, the lymphocyte fraction from elutriated PBMC obtained from healthy volunteers was separated by fluorescence-activated cell sorting into the following subpopulations: CD19− non B cells, CD19+CD27− naïve B cells, CD19+CD27+ MBC, and CD27+CD38+++ PC. Each subpopulation was stimulated with polyclonal activators for 5 days and assayed for IgG-secreting cells, which were only detected in cultures of CD19+CD27+ MBC (Figure 1).
Figure 1. Only MBC differentiate into ASC in response to polyclonal activators in vitro.
The lymphocyte fractions from elutriated PBMC obtained from healthy volunteers were subjected to fluorescence activated cell sorting based on the expression of CD19, CD27 and CD38 into naïve B cells, memory B cells (MBC), and plasma cells (PC). Subpopulations were cultured for 5 days in the presence of the polyclonal activators PWM, SAC and CpG (stimulated) or in their absence (unstimulated).The total number of IgG+ ASC was quantified in an ELISPOT assay using human IgG-specific Ab to capture secreted IgG. Shown are the mean numbers of IgG+ ASC expressed as a percent of the number of cells used in the ELISPOT assay after 5 days in culture. Error bars indicate SEM (n=3 independent experiments).
The acquisition of MBC in naïve individuals
To determine the kinetics, magnitude and longevity of the MBC response to primary immunization, as well as the impact of TLR9 activation on this process, we examined the acquisition of P. falciparum-specific MBC in malaria-naïve individuals enrolled in two Phase 1 clinical trials of two malaria subunit protein vaccine candidates, AMA1-C1 and MSP142-C1. Both AMA1-C1 and MSP142-C1 were formulated on Alhydrogel with and without CPG 7909. Samples from 40 individuals, 20 from each trial, were randomly selected for analysis, half of whom had been vaccinated with CPG 7909-containing vaccines. Individuals were vaccinated on days 0, 28 and 56, and peripheral blood samples were collected at the times shown in Table 1. For both trials the mean viability of PBMC after thawing was similar in the CPG and non-CPG groups, 92.3% and 95.9%, respectively (p=0.165).
Table 1.
Sample size and mean vaccine-specific MBC percentage by vaccine type, CpG group, and study day.
| AMA1-C1A | MSP142-C1B | |||||||
|---|---|---|---|---|---|---|---|---|
| Study day | −CPG 7909 | +CPG 7909 | −CPG 7909 | +CPG 7909 | ||||
| n | % MBCC | n | % MBC | n | % MBC | n | % MBC | |
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | |||||
| 0 | 10 | 0.03 | 10 | 0.02 | 11 | 0.01 | 9 | 0.01 |
| (−0.16, 0.22) | (−0.77, 0.80) | (−0.05, 0.07) | (−0.38, 0.39) | |||||
| 3D | 10 | 0.02 | 8 | 0.04 | ||||
| (−0.21, 0.26) | (−0.74, 0.82) | |||||||
| 7 | 10 | 0.01 | 9 | 0.05 | 11 | 0.01 | 9 | 0.004 |
| (−0.20, 0.21) | (−0.73, 0.83) | (−0.05, 0.07) | (−0.38, 0.39) | |||||
| 28 | 10 | 0.02 | 9 | 0.09 | 10 | 0.04 | 9 | 0.07 |
| (−0.19, 0.23) | (−0.76, 0.94) | (−0.02, 0.10) | (−0.32, 0.45) | |||||
| 31 | 8 | 0.03 | 9 | 0.54 | 11 | 0.04 | 9 | 0.06 |
| (−0.18, 0.24) | (−0.27, 1.36) | (−0.01, 0.10) | (−0.32, 0.44) | |||||
| 35D | 10 | 0.09 | 8 | 2.94* | ||||
| (−0.14, 0.32) | (2.12, 3.75) | |||||||
| 56 | 10 | 0.13 | 8 | 1.79* | 10 | 0.10* | 9 | 0.97* |
| (−0.09, 0.35) | (0.97, 2.60) | (0.04, 0.15) | (0.59, 1.35) | |||||
| 59 | 8 | 0.11 | 9 | 1.58* | 10 | 0.08* | 8 | 1.20* |
| (−0.11, 0.33) | (0.72, 2.43) | (0.02, 0.14) | (0.80, 1.60) | |||||
| 63D | 7 | 0.26 | 8 | 3.45* | ||||
| (0.04, 0.48) | (2.55, 4.35) | |||||||
| 84D | 8 | 0.75* | 7 | 1.52* | ||||
| (0.51, 0.98) | (0.67, 2.37) | |||||||
| 140 | 10 | 0.59* | 9 | 1.44* | 11 | 0.13* | 9 | 0.83* |
| (0.37, 0.81) | (0.62, 2.26) | (0.08, 0.19) | (0.45, 1.21) | |||||
| 236D | 7 | 0.15 | 4 | 1.41* | ||||
| (−0.21, 0.50) | (0.45, 2.37) | |||||||
Missing data due to technical error (4.6%), individual lost to follow-up (5.8%), or unavailability of sample (7.5%).
Missing data due to unavailability of sample (2.9%).
Percent vaccine-specific MBC and 95% CIs are obtained by fitting GEE models described in the statistical method section.
Samples collected for AMA1-C1 trial only.
P value <0.05 vs. study day
In the AMA1-C1 trial, prior to vaccination (baseline) the mean percentage of IgG+ MBC that were AMA1-C1-specific was not significantly different from the irrelevant control antigen KLH (0.02% AMA1-C1-specific MBC vs. 0.07% KLH-specific MBC; p=0.44). The lower limit of detection of the ELISPOT assay in this study is the lowest percentage of antigen-specific spots that can be distinguished from the average percentage of spots on wells coated with KLH. As per Crotty et al. (28), by using best estimates of the relevant parameters (1.25×106 PBMC per ml of blood; B cells are 10% of PBMC; MBC are 30% of B cells; IgG+ MBC are 50% of MBC) 0.07% translates into a lower limit of detection in this study of approximately 13 antigen-specific IgG+ MBC per ml of blood.
Compared to baseline, individuals receiving AMA1-C1/Alhydrogel without CPG 7909 did not have a statistically significant increase in AMA1-C1-specific MBC until 28 days after the third vaccination, at which point 0.75% of all IgG+ MBC were AMA1-C1-specific (p<0.001 vs. baseline; Figure 2, top panel). AMA1-C1-specific MBC remained above baseline at 0.59% 84 days after the third vaccination (p<0.001 vs. baseline), and then decreased to baseline levels approximately 100 days later (p=0.57 vs. baseline). At all other time points, the percentage of AMA1-C1-specific MBC did not differ significantly from baseline levels. Thus, vaccination with AMA1-C1/Alhydrogel without CPG 7909 induced AMA1-C1-specific MBC only after repeated immunization and these MBC did not persist at detectable levels 6 months after the last vaccination.
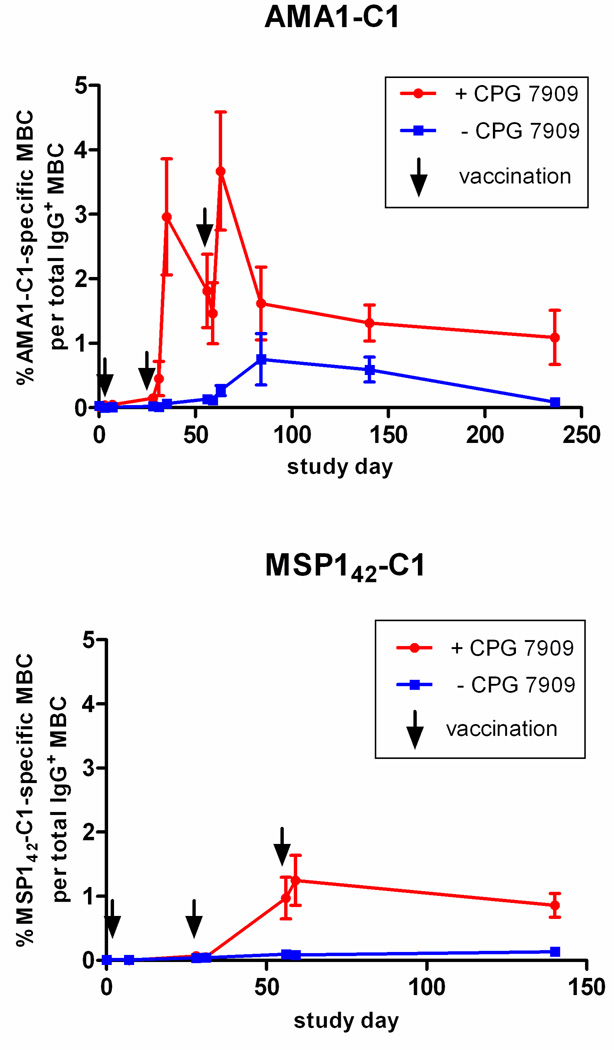
Figure 2. In malaria-naïve individuals, CPG 7909 enhances the kinetics, magnitude, and longevity of the AMA1-C1- and MSP142-C1-specific MBC response to vaccination.
(Top) Individuals vaccinated with AMA1-C1/Alhydrogel with CPG 7909 (red circles; n=10), or without CPG 7909 (blue squares; n=10). (Bottom) Individuals vaccinated with MSP142-C1/Alhydrogel with CPG 7909 (red circles; n=9) or without CPG 7909 (blue squares; n=11). Data are reported as mean percentage ± SEM. The sample size at each time point is given in Table 1.
In contrast, a dramatic increase in AMA1-C1-specific MBC was observed in individuals vaccinated with AMA1-C1/Alhydrogel plus CPG 7909. In this group AMA1-C1-specific MBC appeared in the peripheral circulation 3 days after the second vaccination and peaked 4 days later, at 2.94% of all IgG+ MBC, and then decreased to 1.79% 21 days later on the day of the third vaccination (Figure 2, top panel). Seven days after the third vaccination the percentage peaked again at 3.45% and then contracted to 1.41% by the end of the study (236 days after the first vaccination), a rate of decline of approximately 0.4% per month. AMA1-C1-specific MBC were significantly increased over baseline at all time points beginning 1 week after the second vaccination through the end of the study period (p<0.010 vs. baseline). Compared to individuals who received AMA1-C1/Alhydrogel without CPG 7909, the mean percentage of AMA1-C1-specific MBC was higher at all time points after vaccination, reaching statistical significance on days 35, 56, 59 and 63 (all p<0.010) and marginal statistical significance on days 140 and 236 (p=0.092 and p=0.061, respectively). Thus, the inclusion of CPG 7909 enhanced the kinetics, magnitude, and longevity of the AMA1-C1-specific MBC response.
CPG 7909 had a similar impact on the acquisition of MBC in response to vaccination with MSP142-C1/Alhydrogel (Figure 2, bottom panel). Compared to the AMA-C1 trial, fewer PBMC samples were collected in the MSP142-C1 trial, namely, on the day of each vaccination (days 0, 28 and 56), 7 days after the first vaccination, 3 days after the second and third vaccination, and on day 140 (Table 1). Prior to vaccination (baseline) the mean percentage of IgG+ MBC that were MSP142-C1-specific was not significantly different from KLH (0.01% MSP142-C1-specific MBC vs. 0.07% KLH-specific MBC; p=0.89). Vaccination with MSP142-C1/Alhydrogel without CPG 7909 did not generate statistically significant levels of MSP142-C1-specific MBC until 28 days after the second vaccination, reaching 0.10% of all IgG+ MBC (p=0.005 vs. baseline). The percentage of MSP142-C1-specifc MBC remained greater than baseline at 3 and 84 days after the third vaccination (day 3 after third vaccination, 0.08% p=0.022 vs. baseline; day 84 after third vaccination, 0.13%, p<0.001 vs. baseline). Vaccination with MSP142-C1/Alhydrogel with CPG 7909, by contrast, generated a mean percentage of MSP142-C1-specific MBC of 0.97% on the day of the third vaccination (p<0.001 vs. baseline) and remained at 1.20% and 0.83% at 3 and 84 days after the third vaccination (p<0.001 and p=0.001, respectively, vs. baseline). Because samples were not collected 7 days after the second and third vaccination in the MSP142-C1 trial, we do not know whether the MBC percentage reached higher peak levels, as observed in the AMA1-C1 trial. Thus, despite the difference in the ability of AMA1-C1 and MSP142-C1 to generate vaccine-specific MBC in the absence of CPG 7909, the CPG 7909-containing vaccines resulted in similar levels of vaccine-specific MBC at the end of each study, approximately 1% of total IgG+ MBC, compared with approximately 0.1% for the non-CPG 7909-containing vaccines.
The acquisition of MBC mirrors and predicts Ab responses
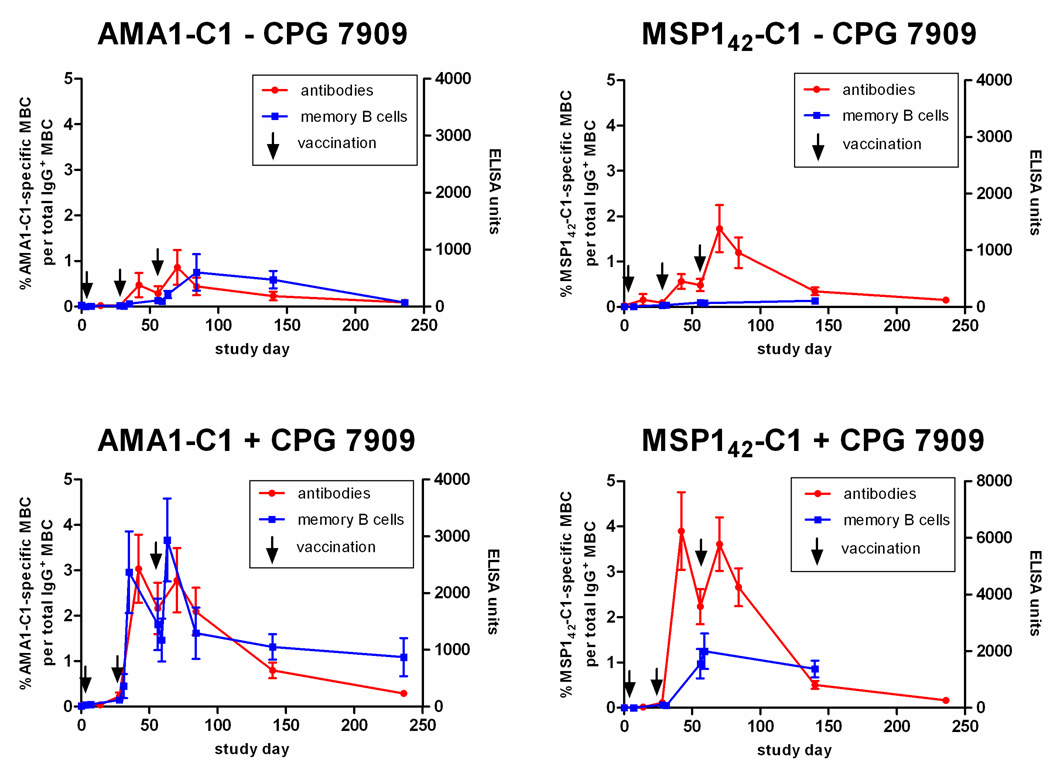
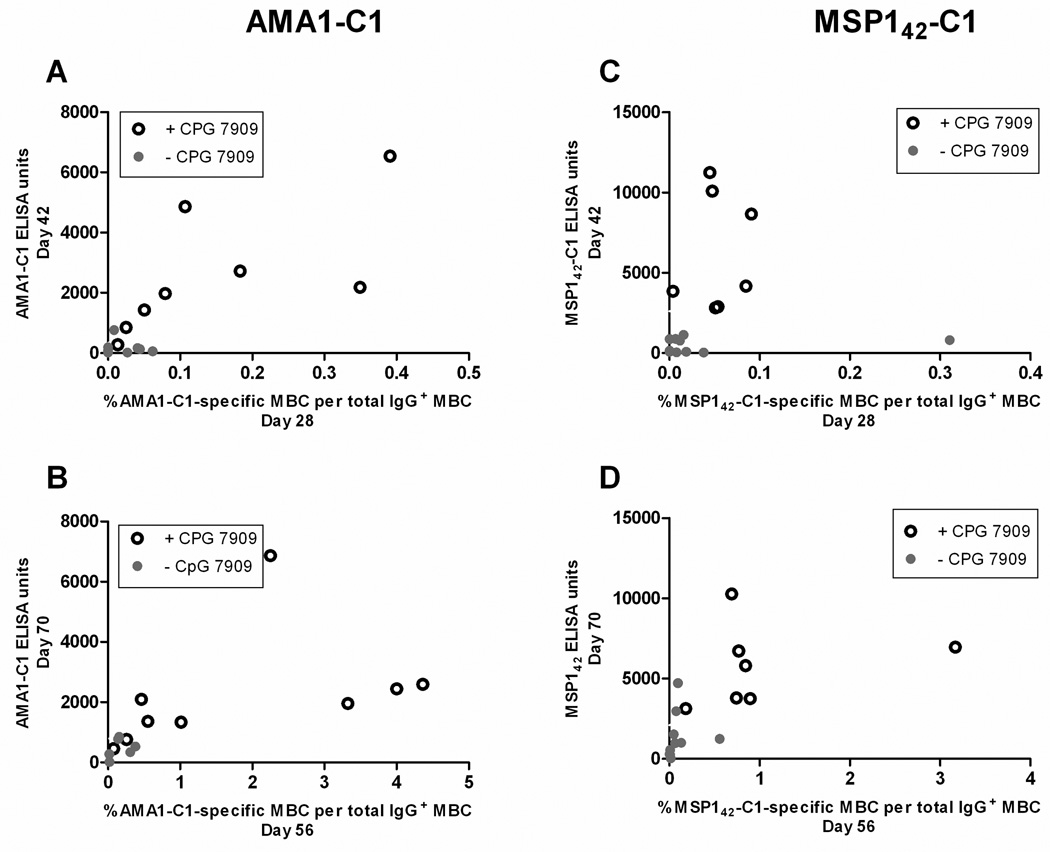
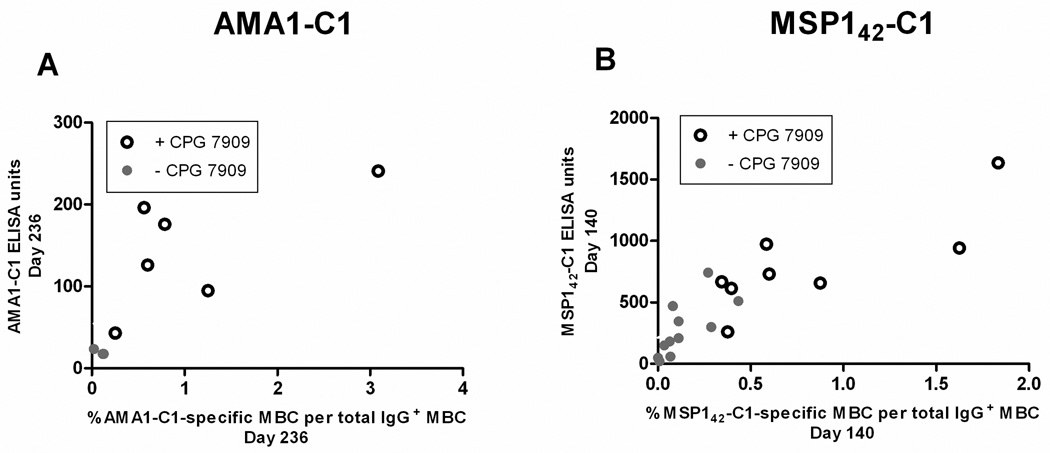
In humans the role MBC play in maintaining Ab titers and LLPC remains unclear. The longitudinal design of this study allowed an examination of the relationship between MBC and Ab titers in a manner not possible in cross-sectional analyses. In general we observed that vaccination with either AMA1-C1 or MSP142-C1 on Alhydrogel generated Ab levels that correlated with the vaccine-specific MBC response, and for both vaccines the inclusion of CPG 7909 induced higher levels of Ab and MBC (Figure 3). We also observed that the percentage of AMA1-C1-specific MBC on the day of the second and third vaccination (days 28 and 56) was highly correlated with the levels of AMA1-C1 antibodies 14 days later (days 42 and 70) (Figure 4A, second vaccination, r=0.70, p=0.003; Figure 4B, third vaccination, r=0.87, p=<0.001). The majority of the Ab response likely represents the differentiation of MBC into short-lived PC given the rapid decline in titers that followed. In the MSP142-C1 trial we observed a similar relationship between MBC at the time of re-vaccination and antibody titers 14 days later in the MSP142-C1 trial (Figure 4C, second vaccination r=0.47, p=0.057; Figure 4D, third vaccination r=0.83, p<0.001). To determine the relationship between antigen-specific MBC and antibody titers at steady state (approximately 3 and 6 months after the last MSP142-C1 and AMA1-C1 vaccination, respectively), the last time point with corresponding ELISPOT and ELISA data was analyzed in cross-section. We observed a positive correlation between antigen-specific MBC and Ab titers in both trials (Figure 5A, AMA1-C1, r=0.80, p=0.003; Figure 5B, MSP142-C1, r=0.86, p<0.001). Since LLPC are the likely source of antibody titers at this later time point, the correlation between MBC and antibody titers suggests that the maintenance of LLPC may be linked to MBC.
Figure 3. The AMA1-C1- and MSP142-C1-specific Ab response mirrors the corresponding MBC response and is enhanced by CPG 7909.
The Ab levels (red circles) determined by ELISA are given for individuals vaccinated with AMA1-C1/Alhydrogel (left panels) or MSP142-C1/Alhydrogel (right panels) without CPG 7909 (top) or with CPG 7909 (bottom). Ab levels for each vaccine are the average of the 3D7 and FVO responses. The corresponding percentages of antigen-specific MBC are given for comparison (blue squares). Data are reported as mean percentage (MBC) or mean ELISA units (Ab) ± SEM. The sample size at each time point is given in Table 1.
Figure 4. The level of antigen-specific MBC at the time of booster vaccination predicts the Ab response 14 days later.
For AMA1-C1 the percentage of antigen-specific MBC at the time of the second and third vaccinations predicted the levels of AMA1-C1 Ab 14 days later (A, second vaccination, r=0.70, p=0.003; B, third vaccination, r=0.87, p=<0.001). A similar relationship was observed for the MSP142-C1 vaccine (C, second vaccination, r=0.47, p=0.057; D, third vaccination, r=0.83, p<0.001). At these time points corresponding ELISPOT and ELISA data were available for 15 individuals in the AMA1-C1 trial and 17 individuals in the MSP142-C1 trial. Ab levels for each vaccine are the average of the 3D7 and FVO responses.
Figure 5. At steady state, levels of antigen-specific MBC and Ab are highly correlated.
To determine the correlation between antigen-specific MBC and Ab titers closer to steady state, the last time point with corresponding ELISPOT and ELISA data (AMA1-C1 trial day 236, MSP142-C1 trial day 140) was analyzed in cross-section (A and B). A positive correlation between antigen-specific MBC and Ab titers was observed in both trials at steady state (AMA1-C1 r=0.80, p=0.014; MSP142-C1 r=0.86, p<0.001). Corresponding ELISPOT and ELISA data were available for 9 individuals in the AMA1-C1 trial and 19 individuals in the MSP142-C1 trial at this time point. Ab levels for each vaccine are the average of the 3D7 and FVO responses.
Vaccination influences MBC and PC independently of antigen specificity
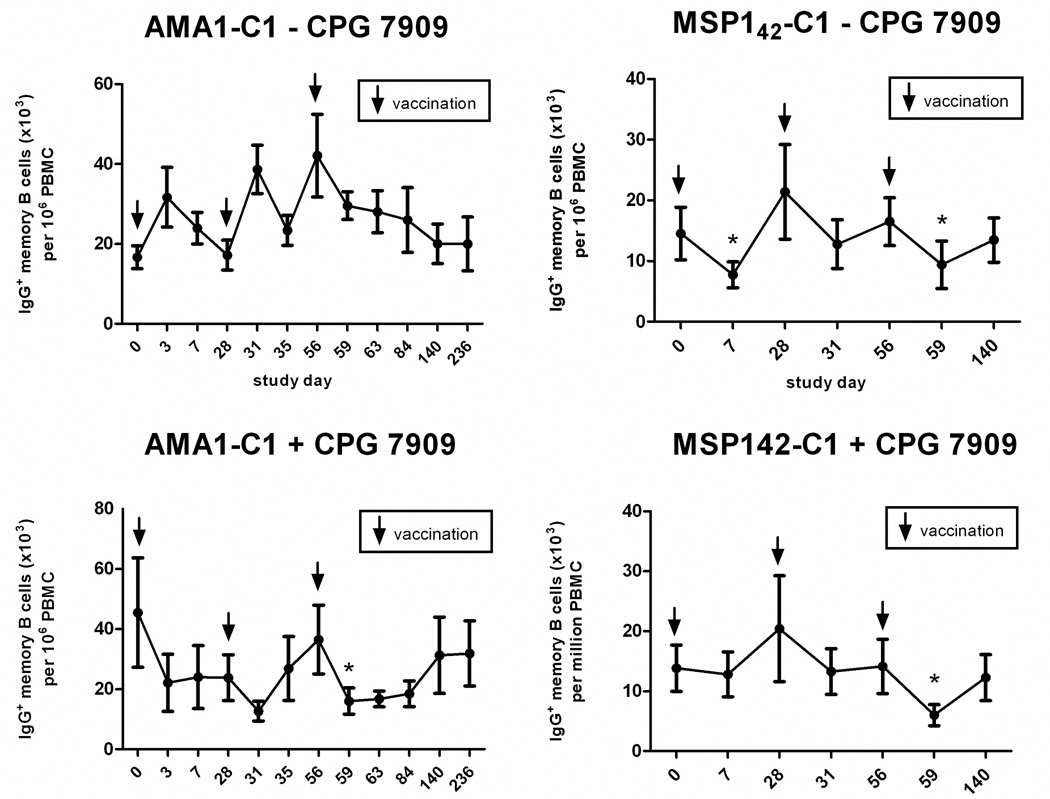
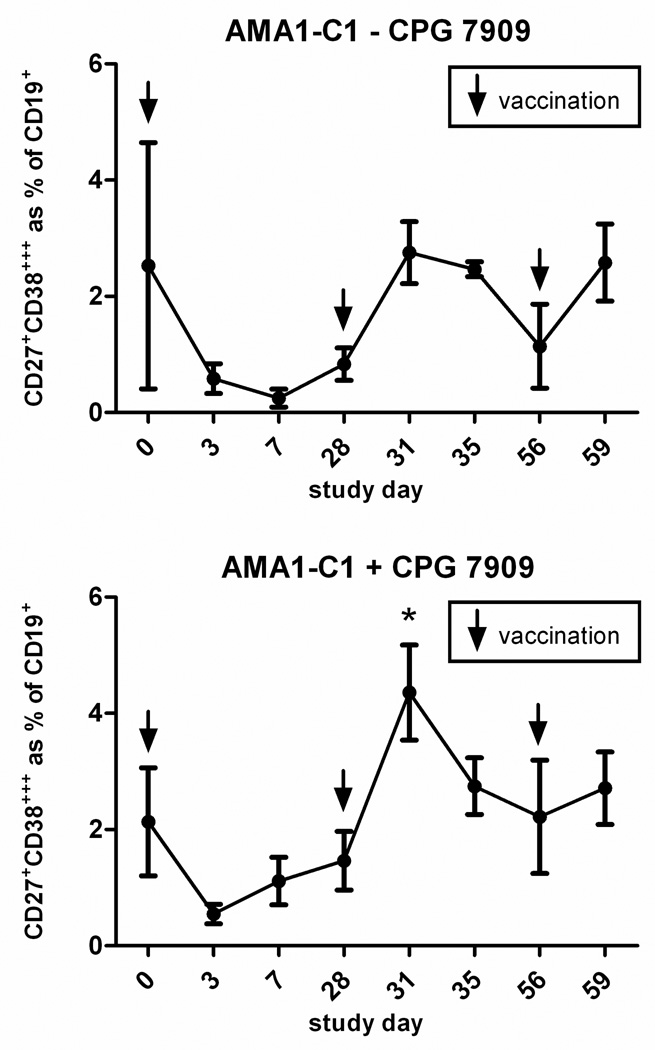
An examination of total IgG+ MBC circulating in the periphery showed that vaccination affected this population independently of antigen (Figure 6). Vaccination with AMA1-C1/Alhydrogel plus CPG 7909 was associated with a decrease in the frequency of IgG+ MBC 3 days after each vaccination, followed by a gradual return to baseline (Figure 6, lower left), although the decrease was only statistically significant after the third vaccination (p=0.012) At the same time points, the frequency of IgG+ MBC in those vaccinated with AMA1-C1/Alhydrogel without CPG 7909 did not show a consistent response (Figure 6, upper left). Vaccination with MSP142-C1 with or without CPG 7909, was associated with a decrease in the frequency of IgG+ MBC 7 days after the first vaccination, and 3 days after the second and third vaccinations, followed by a return to baseline (Figure 6. upper and lower right). The decrease was statistically significant after the third vaccination in the MSP142-C1 with CPG 7909 group (p=0.018), and after the first and third vaccinations in the MSP142-C1 without CPG 7909 group (p=0.021 and p=0.037, respectively). Thus, MBC appear to transiently leave the circulation after vaccination. To determine if there was a concomitant increase in total PC numbers indicating a polyclonal activation of MBC, fresh PBMC from a subset of individuals in the AMA1-CI study were analyzed by flow cytometry (fresh PBMC from the MSP142-C1 trial were not available). Irrespective of the CPG 7909 status, 3 days after the second and third vaccination with AMA1-C1/Alhydrogel, there was an increase in CD27+CD38+++ PC as a percentage of total CD3−CD19+ B cells (Figure 7). The increase was statistically significant after the second vaccination in the CPG 7909 group (p<0.01). Thus it appears that vaccination with adjuvant-containing vaccines has the potential to influence MBC through polyclonal activation, independently of antigen, and that this activation may drive the differentiation of MBC into PC (3).
Figure 6. Vaccination appears to have an antigen-independent effect on the total IgG+ MBC pool.
Shown is the total IgG+ MBC response expressed as the mean frequency for individuals immunized with AMA1-C1/Alhydrogel (left) or MSP142-C1/Alhydrogel (right) without CPG 7909 (top) or with CPG 7909 (bottom). Days on which there was a statistically significant (p<0.05) decrease in the total IgG+ MBC frequency, as compared to the total IgG+ MBC frequency on the day of the preceding vaccination, are indicated with an asterisk (*). For AMA1-C1 there were 10 individuals in both the non-CPG 7909 and CPG 7909 groups. For MSP142-C1 there were 11 individuals in the non-CPG 7909 group and 9 individuals in the CPG 7909 group. Note: x axis intervals are not proportional to the time intervals indicated.
Figure 7. Vaccination has an antigen-independent effect on the total PC pool.
Shown is the CD27+CD38+++ PC response expressed as the mean percentage of CD3− CD19+ B cells for individuals immunized with AMA1-C1/Alhydrogel without CPG 7909 (top) or with CPG 7909 (bottom). Days on which there was a statistically significant (p<0.05) increase in the percentage of PC, as compared to the PC percentage on the day of the preceding vaccination, are indicated with an asterisk (*).There were 3 individuals in the non-CPG 7909 group and 8 individuals in the CPG 7909 group available for analysis. Due to the small sample size in the non-CPG 7909 group, a statistical test was not done. Note: x axis intervals are not proportional to the time intervals indicated.
DISCUSSION
B cell memory plays a central role in conferring protective immunity to many infectious diseases for which there are effective vaccines, and yet little is known about the generation of B cell memory in humans. In this longitudinal study we examined the effect of vaccination on MBC generation in naïve individuals and determined the impact of TLR9 activation on this process in vivo. The results presented here offer new insights into the kinetics of this process and provide evidence that the innate immune receptor TLR9 plays a significant role not only in the generation of MBC in naïve individuals but also in controlling the behavior of existing MBC. For the two protein subunit malaria vaccine candidates, AMA1-C1 and MSP142-C1, the inclusion of CPG 7909 had a dramatic effect, resulting in a more rapid acquisition of vaccine-specific MBC, in greater numbers, that persisted for longer.
The longitudinal design of this study permitted a detailed characterization of the kinetics of MBC generation and maintenance in response to primary and secondary vaccination. The capacity for a detailed characterization was most apparent in the analysis of the AMA1-C1 vaccine trial in which PBMC samples were collected at several time points after each vaccination. We observed that AMA1-C1-specific MBC peaked in the peripheral circulation 7 days after the second and third vaccinations, representing approximately 3–4% of the total IgG+ MBC pool. Although it has been reported for diphtheria vaccination that the magnitude of the peak MBC response decreased with each booster immunization (11), we did not observe a significant difference between peaks in this study [day 35 CPG 7909 group, 2.94% (95% CI, 2.12–3.75) vs. day 63 CPG 7909 group, 3.45% (95% CI, 2.44–4.35); p=0.328]. The differences between the studies may be due to the length of time between vaccination or the efficacy of the vaccines themselves. It is of interest that the second AMA1-C1 vaccination generated AMA1-C1-specific MBC at levels comparable to those observed after influenza (29, 30) and smallpox (31) booster vaccination. Irrespective of CPG 7909 status, the rate of decline of AMA1-C1-specific MBC was approximately 0.4% per month. If this rate held steady, within 2 years the level of AMA1-C1-specific MBC would approach pre-immune levels in the CPG 7909 group. However, we do not know whether, or at what level, the antigen-specific MBC pool reaches equilibrium. In a cross-sectional study 18 months after smallpox vaccination, antigen-specific MBC, as a percentage of the total IgG+ MBC, decreased to 0.1% from a peak of 1% 14 days after vaccination (31). Similarly, in individuals receiving influenza booster vaccinations, influenza-specific MBC increased from low levels before vaccination to 8.2% of IgG+ MBC 14 days after vaccination, and then declined rapidly to <1% 80 days post-vaccination (29). Based on these observations, AMA1-C1-specific MBC would be expected to reach equilibrium at ~0.3% within a year after the final vaccination.
Although TLR9 expression is known to be low in naïve B cells and constitutively high in MBC (32), the impact of this differential expression on the in vivo responsiveness to CpG in humans at the cellular level is not known. As measured by the MBC response, we observed no effect of CPG 7909 on primary immunization with AMA1-C1 or MSP142-C1, suggesting that CPG 7909 had little effect on naïve B cells directly, or indirectly through TLR9-expressing PDC. However, once generated by primary immunization, TLR9-expressing antigen-specific MBC responded dramatically to secondary immunization in the presence of CPG 7909. Although the relative impact of TLR9 activation in PDC versus MBC on the secondary response in vivo is not known, it is clear from the results of our in vitro experiments (Figure 1) that purified MBC differentiate into ASC upon TLR9 activation, as has been shown by others (28).
The mechanisms underlying the apparent expansion and contraction of circulating antigen-specific MBC still need to be elucidated. The contraction phase may represent migration of MBC to lymphoid tissue where newly generated MBC compete for limited homeostatic niches in the MBC compartment. Alternatively, in a manner analogous to T cell antigen-driven expansion and contraction, contraction may represent an activation-induced cell death phenomenon (33) . What remains unknown is which factors control the magnitude of the peak response and the subsequent steady state level.
The results presented here also address the controversy surrounding the relationship between MBC, LLPC, and serum Ab levels. In general, we observed a positive correlation between the magnitude of the vaccine-specific MBC response and Ab titers. We also observed that the percentage of vaccine-specific MBC present at the time of the second and third vaccinations predicted Ab titers 2 weeks later. The majority of this Ab was likely produced by short-lived PC given the rapid decline in titers that followed. Similar results were recently reported for infants immunized with the serogroup C meningococcal conjugate vaccine in which the frequency of specific MBC at the time of boosting correlated with post-vaccination titers (15). However, the Ab titers we observed closer to steady state (approximately 3 and 6 months after the last MSP142-C1 and AMA1-C1 vaccination, respectively) were likely produced by LLPC, and thus the correlation between MBC and Ab titers at steady state suggests that the maintenance of LLPC may be linked to MBC. The cellular and molecular nature of this relationship remains poorly understood.
In addition to the antigen-specific induction of MBC, we observed an approximately two fold antigen-independent decrease in the frequencies of total IgG+ MBC in circulation 3 days after the majority of vaccinations. This drop may reflect the migration of MBC into lymphoid tissues, apoptosis of MBC, differentiation of MBC into ASC, or a combination thereof. The concurrent increase in CD27+CD38+++ PC we observed 3 days after the second and third vaccinations in the AMA1-C1/Alhydrogel study suggests that the decline in MBC is due in part to their differentiation into PC. In a separate phase I study of AMA1-C1/Alhydrogel without CPG 7909, we observed a similar increase in PC 3 days after vaccination (unpublished). That polyclonal activation can drive the differentiation of MBC into PC is supported by other studies that have examined the antigen-independent effects of vaccination on PC. Bernasconi et al. observed an increase in ASC directed against Toxoplasma gondii and measles 6 days after vaccination with tetanus toxoid (12). These authors attributed this to the polyclonal activation and differentiation of all MBC into PC. Odendahl et al. also observed an increase in circulating ASC of unknown specificity 6 days after vaccination with tetanus toxoid, but interpreted the ASC to be LLPC displaced from the bone marrow by newly generated tetanus-specific PC that better competed for bone marrow PC niches (34). However, a recent study showed that up to one third of circulating ASC appearing after influenza vaccination were not vaccine-specific, and had recently divided, ruling out the possibility that these were LLPC displaced from the bone marrow (10). Collectively, these findings are consistent with a model in which the decrease in total IgG+ MBC (which we observed after vaccination) is due in part to their polyclonal activation and differentiation into PC (3).
An important question raised by these observations is the nature of the polyclonal activation of MBC in vivo. For the AMA1-C1 vaccine, CPG 7909 was required to induce a consistent decrease in total IgG+ MBC, presumably as a result of direct and/or indirect TLR9 signaling in MBC or PDC, respectively. However, for the MSP142-C1 vaccine, a decrease in total IgG+ MBC was observed in the absence of CPG 7909, presumably due in large part to the alum adjuvant. Recently, alum has been shown to activate an intracellular innate immune response through the Nalp3 inflammasome and to direct antibody responses by mechanisms that, although incompletely understood, likely involve activation of Th2 cells (35). Why AMA1-C1/Alhydrogel without CPG 7909 was not associated with a decrease in total IgG+ MBC is unclear, but may reflect a complex interplay between antigen-specific and polyclonal responses. Future studies might explore how activation of TLRs or inflammasomes influences MBC behavior.
The results of this study also provide an important baseline for further investigation of the B cell response to malaria vaccine candidates and natural infection with P. falciparum, the most lethal of human malaria species. Results of sero-epidemiological studies in malaria endemic areas indicate that humoral immunity to malaria in response to P. falciparum infection is slow to develop, incomplete, and short lived, all of which suggests that P. falciparum may have evolved mechanisms to subvert the generation and/or maintenance of immunological memory (4). It will therefore be of interest to compare the B cell response at the cellular level to candidate malaria vaccines in those with no exposure to P. falciparum, as in this study, to those living in a malaria endemic area. To that end we are performing similar analyses in individuals enrolled in Phase 1 clinical trials of candidate malaria vaccines in Mali. We are also conducting longitudinal studies in Mali (36) to understand the mechanisms that underlie protective immunity to malaria and how P. falciparum may modulate this response. An improved understanding of the B-cell response to malaria vaccine candidates and natural P. falciparum infection can help guide the development of vaccines that provide sustained protection against this important pathogen.
ACKNOWLEDGMENTS
We sincerely thank the volunteers that participated in this study. We also thank the trial site staff, and Jane Baer for her assistance in processing blood samples.
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; by contract N01-AI-25460 from the National Institutes of Health (to D.J.T and J.J.T.), and by UL1 RR024160 from the National Center for Research Resources (to URMC).
Nonstandard abbreviations used
- AMA1-C1
Apical Membrane Antigen 1-Combination 1
- ASC
antibody secreting cells
- GEE
generalized estimating equations
- KLH
keyhole limpet hemocyanin
- LLPC
long-lived plasma cells
- MBC
memory B cells
- MSP142-C1
Merozoite Surface Protein 142-Combination 1
- PC
plasma cells
- PDC
plasmacytoid dendritic cells
- PWM
pokeweed mitogen
- SAC
Protein A from Staphylococcus aureus Cowan
- VZV
varicella-zoster virus
REFERENCES
- 1.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Crotty S, Ahmed R. Immunological memory in humans. Semin. Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Understanding and making use of human memory B cells. Immunol. Rev. 2006;211:303–309. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 5.Cagigi A, Nilsson A, De Milito A, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 7.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 8.el-Ad B, Roth Y, Winder A, Tochner Z, Lublin-Tennenbaum T, Katz E, Schwartz T. The persistence of neutralizing antibodies after revaccination against smallpox. J. Infect. Dis. 1990;161:446–448. doi: 10.1093/infdis/161.3.446. [DOI] [PubMed] [Google Scholar]
- 9.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 10.Mamani-Matsuda M, Cosma A, Weller S, Faili A, Staib C, Garcon L, Hermine O, Beyne-Rauzy O, Fieschi C, Pers JO, Arakelyan N, Varet B, Sauvanet A, Berger A, Paye F, Andrieu JM, Michel M, Godeau B, Buffet P, Reynaud CA, Weill JC. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;111:4653–4659. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 11.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2002;20:498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 13.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–2213. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, Steward-Clark E, Stamey KL, Schmidt DS, Stinson KW, Freeman AE, Elie CM, Martin SK, Greene C, Aubert RD, Glidewell J, Perkins BA, Ahmed R, Stephens DS. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–1236. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, Borkowski A, Ceddia F, Borrow R, Siegrist CA, Pollard AJ. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J. Immunol. 2008;180:2165–2173. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 16.Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29:1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20:498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 18.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257–2259. [PubMed] [Google Scholar]
- 19.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 20.Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J. Clin. Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 21.Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 23.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–2467. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 24.Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine. 2006;24:20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- 25.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, Moretz S, Zhou H, Long CA, Miller LH, Treanor J. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS ONE. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 32.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 33.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2008 doi: 10.1007/s12026-008-8027-z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 35.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. Sickle Cell Trait is Associated with a Delayed Onset of Malaria: Implications for Time-to-Event Analysis in Clinical Studies of Malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]