Abstract
Low levels of reactive oxygen species (ROS) production are necessary to optimize muscle force production in unfatigued muscle. In contrast, sustained high levels of ROS production have been linked to impaired muscle force production and contraction-induced skeletal muscle fatigue. Using genetically engineered mice, we tested the hypothesis that the independent transgenic overexpression of catalase (CAT), copper/zinc superoxide dismutase (CuZnSOD; SOD1) or manganese superoxide dismutase (MnSOD; SOD2) antioxidant enzymes would negatively affect force production in unfatigued diaphragm muscle but would delay the development of muscle fatigue and enhance force recovery after fatiguing contractions. Diaphragm muscle from wild-type littermates (WT) and from CAT, SOD1 and SOD2 overexpressing mice were subjected to an in vitro contractile protocol to investigate the force–frequency characteristics, the fatigue properties and the time course of recovery from fatigue. The CAT, SOD1 and SOD2 overexpressors produced less specific force (in N cm−2) at stimulation frequencies of 20–300 Hz and produced lower maximal tetanic force than WT littermates. The relative development of muscle fatigue and recovery from fatigue were not influenced by transgenic overexpression of any antioxidant enzyme. Morphologically, the mean cross-sectional area (in μm2) of diaphragm myofibres expressing myosin heavy chain type IIA was decreased in both CAT and SOD2 transgenic animals, and the percentage of non-contractile tissue increased in diaphragms from all transgenic mice. In conclusion, our results do not support the hypothesis that overexpression of independent antioxidant enzymes protects diaphragm muscle from contraction-induced fatigue or improves recovery from fatigue. Moreover, our data are consistent with the concept that a basal level of ROS is important to optimize muscle force production, since transgenic overexpression of major cellular antioxidants is associated with contractile dysfunction. Finally, the transgenic overexpression of independent endogenous antioxidants alters diaphragm skeletal muscle morphology, and these changes may also contribute to the diminished specific force production observed in these animals.
Cellular alterations resulting in the disruption of balance between reactive oxygen species (ROS) production and endogenous antioxidants result in redox disturbances (Reid, 2006) that have been linked to the pathogenesis of numerous clinical maladies as well as the progression of skeletal muscle wasting due to disuse or disease. The classical idea that ROS production is always detrimental to cells has recently been challenged, however. Numerous studies have begun to unravel the complex, multifunctional roles of varying levels of ROS in skeletal muscle. Indeed, it is now clear that: (1) ROS are necessary for optimal muscle contractile function (Reid, 2008); (2) low (i.e. below basal) or high (pathological) levels of ROS impede muscle force production (Reid, 2008); and (3) increased ROS production during contractions is critical to exercise-induced adaptations in skeletal muscle (Gomez-Cabrera et al. 2008). Taken together, these studies have led to the developing theory that ROS production in skeletal muscle conforms to the principle of hormesis. This theory predicts that low levels of contraction-induced ROS are important to skeletal muscle function, gene expression and adaptation to activity, while high levels can become toxic or exert catabolic influences on the myofibres.
Numerous pathways are capable of ROS production in skeletal muscle, and endogenous cellular antioxidants provide the first line of defense in reducing potentially deleterious levels of oxidants. Key endogenous antioxidant enzymes include superoxide dismutases (SOD) and catalase, and these enzymes work co-operatively to protect cells against ROS-mediated damage (Powers & Jackson, 2008). Copper/zinc superoxide dismutase (CuZnSOD; SOD1) is the most abundant of the SOD moieties andexertsthemajorityofits protectiveeffectsinthe cytosol and nucleus of eukaryotic cells (Powers & Jackson, 2008). Manganese superoxide dismutase (MnSOD; SOD2) is found primarily in the mitochondrial matrix and functions in the maintenance of redox balance during mitochondrial respiration (Powers & Jackson, 2008). The superoxide dismutases dismutate superoxide radicals to the less reactive hydrogen peroxide (H2O2) and molecular oxygen (O2). Catalase is a peroxisomal enzyme that catalyses two molecules of H2O2 to O2 and two molecules of H2O. The abundance of ROS during sustained or intense contractions may overwhelm the reducing capacity of the cellular defenses and pose serious negative effects on both skeletal muscle performance and the exacerbation of myofibre wasting during disease and disuse-related maladies (Powers et al. 2007). Therefore, a commonly tested therapeutic measure to counteract high levels of ROS in skeletal muscle involves increasing available antioxidants in an attempt to restore redox balance.
Recent research has focused on exploring antioxidant upregulation (via exogenous administration) in the restoration of homeostatic redox muscle balance during intense muscle contractions. In this regard, exogenous antioxidant supplementation attenuates ROS-mediated disuse atrophy and contractile dysfunction in skeletal muscle (Betters et al. 2004; McClung et al. 2007; Servais et al. 2007) as well as delaying the development of fatigue during intense contractions (Reid, 2008). Unfortunately, increasing the reducing capacity of the cell by exogenously administering antioxidants also inhibits baseline muscle force production and exercise-induced muscular adaptations (Coombes et al. 2001; Gomez-Cabrera et al. 2008). It is currently unknown, however, whether the genetic induction of a single endogenous antioxidant would alter skeletal muscle force production or delay the development of muscular fatigue in a manner similar to that observed with exogenous administration. We tested the hypothesis that, in unfatigued skeletal muscle, transgenic overexpression of catalase, CuZnSOD or MnSOD antioxidant enzymes would negatively affect diaphragm muscle force production. We also hypothesized that transgenic overexpression of endogenous antioxidant enzymes would delay the development of skeletal muscle fatigue and enhance force recovery following fatiguing contractions. Our findings reveal that transgenic overexpression of antioxidant enzymes impairs force production in unfatigued muscle. Surprisingly, however, transgenic overexpression of endogenous antioxidants does not alter the development of contraction-induced muscle fatigue nor does it speed recovery from fatigue.
Methods
Animals
Details of the transgenic strains used have been described previously (Rader et al. 2006). Male wild-type (WT) and transgenic mice were obtained from the University of Texas Health Science Center at San Antonio. The experiment was performed on male mice (19 months old), with eight mice per group. Mice of C57BL/6 wild-type background served as control animals. The Tg(SOD1) and Tg(CAT) mice were generated on a C57BL/6 background using large fragments of human genomic DNA (>60 kb) containing the gene and the 5′- and 3′-flanking sequences (Chen et al. 2003, 2004). The Tg(SOD2) transgenic mice were generated on a C57BL/6 background using 13 kb of endogenous mouse DNA containing the gene and 2 kb of the flanking sequence (Raineri et al. 2001). The flanking regions of the transgenes contained the regulatory sequences required for a tissue-specific expression pattern not different from the endogenous genes. The transgenic mice were bred and raised in a barrier facility at the University of Texas Health ScienceCenteratSan Antonio.Themicewereshipped to the University of Florida, where they were housed in a barrier facility for 30 days prior to experimentation. All experiments were conducted in accordance with the policies contained in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and were approved by the University of Florida Animal Care and Use Committee.
Measurement of in vitro diaphragmatic contractile properties
Diaphragm muscle was selected for this study for the following reasons. First, the diaphragm is a mixed fibre muscle that contains all four major myosin heavy chain (MHC) isoforms found in mouse skeletal muscle. Second, the mouse diaphragm is a thin muscle with parallel fibres that makes it an ideal muscle (i.e. without diffusion limitations and complex architecture) for in vitro muscle function studies.
Contractile properties of the diaphragm were measured as previously described (Powers et al. 2002; Staib et al. 2002; Shanely et al. 2003) with the following modifications. Briefly, the animals were anaesthetized with an I.P. injection of sodium pentobarbitone (80 mg kg−1). After reaching a surgical plane of anaesthesia, mice were killed by exsanguination and the entire diaphragm was removed and placed in a dissecting chamber containing a Krebs–Henseleit solution (Sigma-Aldrich, St. Louis, MO, USA) equilibrated with a 95% O2–5% CO2 gas. A muscle strip, including the tendinous attachments at the central tendon and ribcage (length, 8 ± 1 mm), was dissected from the midcostal region. The strip was suspended vertically between two lightweight Plexiglass clamps, with one end connected to an isometric force transducer (model FT-03, Grass Instruments, Quincy, MA, USA) within a jacketed tissue bath. The force output was recorded via a computerized data-acquisition system (Super Scope II, GW Instruments Somerville, MA, USA). The tissue bath was filled with Krebs–Henseleit solution and 12 μM d-tubocurarine to produce complete neuromuscular blockade. The saline within the bath was aerated with gas (95% O2–5% CO2), pH was maintained at 7.4, and the osmolality of the bath was ~290 mosmol (kg H2O)−1. After a 15 min equilibration period (at 37°C), in vitro diaphragmatic contractile measurements were made. The muscle strip was stimulated along its entire length with platinum wire electrodes (modified S48 stimulator, Grass Instruments) by using supramaximal (~150%) stimulation voltage to determine the optimal contractile length (Lo). The value of Lo was determined by systematically adjusting the length of the muscle using a micrometer while evoking single twitches. Thereafter, all contractile properties were measured isometrically at Lo. To measure the force–frequency response, each strip was stimulated supramaximally with 120 V pulses at 10–300 Hz (total of ~9 contractions). The duration of each train was 500 ms to achieve a force plateau. Contractions were separated by a 3 min recovery period. For comparative purposes, diaphragmatic (bundles of fibres) force production was normalized as specific Po. Five minutes after completion of the last peak tetanic tension measurement, the diaphragm strip was subjected to a 30 min fatigue protocol. To evaluate fatigue resistance, the strip was stimulated submaximally at 2 s intervals (30 Hz; 250 ms train duration) for 30 min. Tolerance to fatigue (fatigue index) was assessed by the percentage of initial force generated by the strip compared with the tension developed at various time intervals during the protocol. Muscle fatigue was defined as the rate of decline in muscle force production. The ratio of the period of muscle contraction to rest (duty cycle) was 12.5%. Recovery from the fatigue protocol was determined by the measurement of seven maximal tetanic contractions at 1, 5, 10, 15, 20, 25 and 30 min postfatigue. These times were chosen because our preliminary experiments revealed that the healthy adult mouse diaphragm regains ~80% of initial force-generating capacity within 10 min following this type of fatigue protocol (data not shown). The total muscle cross-sectional area (CSA) at right angles to the long axis was calculated by the following algorithm (Segal et al. 1986):
where 1.056 is the density of muscle (in g cm−3). Fibre length was expressed in centimetres measured at Lo (Powers et al. 2002).
Myofibre cross-sectional area
Sections from frozen diaphragm samples were cut at 8 μm using a cryotome (Shandon Inc., Pittsburgh, PA, USA). Sections for cross-sectional area analysis were then dried at room temperature for 30 min and incubated in a phosphate-buffered saline (PBS) solution containing 0.5% Triton X-100. Sections were then rinsed in PBS and simultaneously exposed to primary antibodies specific to dystrophin protein (rabbit host, no. RB-9024-R7, Lab Vision Corp., Fremont, CA, USA), myosin heavy chain type I [mouse host, immunoglobulin M (IgM) isotype, A4.840, Developmental Studies Hybridoma Bank, Iowa City, IA, USA; Jergovic et al. 2001] and MHC type IIA [mouse host, immunoglobulin G (IgG1) isotype, N2.261, Developmental Studies Hybridoma Bank] in a dark humid chamber at room temperature for 1 h. Sections were subsequently rinsed three times in PBS and exposed to Rhodamine Red anti-rabbit secondary antibody (R6394, Molecular Probes, Eugene, OR, USA), Alexa Fluor 350 goat anti-mouse IgM isotype specific secondary antibody (AB1552, Molecular Probes) and Alexa Fluor 488 goat anti-mouse IgG isotype specific secondary antibody (A11011, Molecular Probes) diluted in PBS containing 0.5% Pierce Super Blocker (57535, Pierce, Rockford, IL, USA) in a dark humid chamber at room temperature for 1 h. Sections were then washed in PBS and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). After mounting, slides were coverslipped and sealed for viewing via an inverted fluorescence microscope (Carl Zeiss Axiovert 200). Fibre typing using this method permits visualization of the myofibre membrane protein dystrophin using the Rhodamine filter (red), type I myosin using the 4′,6-diamidino-2-phenylindole (DAPI) filter set (blue), type IIA myosin using the fluorescein isothiocyanate filter (green) and type IIB/IIX myofibres (non-stained/black). Images were obtained at ×10 magnification, and approximately 250 myofibres were analysed for myofibre cross-sectional area (in μm2) using Scion Image software (Scion Technologies, Frederick, MD, USA) by a blinded investigator. The decision to analyse ~250 fibres per muscle was based on the finding that increasing fibre sample size above 250 does not alter the standard deviation around the mean.
Assessment of non-contractile tissue in diaphragm muscle
Sections from frozen diaphragm samples were cut at 8 μm using a cryotome (Shandon Inc.). Haematoxylin and Eosin staining was performed on sections taken from diaphragm in all animals. Six digital images at ×40 magnification were analysed by overlaying an 18 × 14 grid on each image. Each dot was counted by a blinded investigator as part of the non-contractile tissue if it was not on a muscle fibre as previously described (McClung et al. 2005). Dots at least 75% outside myofibres were included in our analysis, whereas dots that were not clearly distinguishable were omitted from the count. Results are presented as both the percentage non-contractile tissue (%NCT) and the inverse, percentage contractile tissue (%CT). Specific force of costal diaphragm strips is also presented as corrected for the %CT in an attempt to determine the potential contribution of non-contractile tissue changes to force production (Criswell et al. 1997).
Total glutathione (GSH) and protein carbonyls
Total glutathione and protein carbonyls were assayed as indicators of oxidative stress in the diaphragm. Total glutathione was measured using a commercially available spectrophotometric kit (Cayman Chemical, Ann Arbor, MI, USA). Briefly, 40–50 mg costal diaphragm was analysed for GSH according to the manufacturer's recommendations. Sample protein content was assayed using the Bradford method (Sigma, St Louis, MO, USA). Protein carbonyls were measured in 40–50 mg total costal diaphragm muscle using a commercially available enzyme-lined immunosorbent assay (ELISA; Biocell PC Test, Northwest Life Science Specialties, LLC, Vancouver, WA, USA) according to the manufacturer's instructions.
Western blotting
Crude diaphragm muscle protein extracts were isolated and assayed using the Bradford method (Sigma). Proteins (60 μg) were then separated by polyacrylamide gel electrophoresis via 4–15% gradient and transferred to nitrocellulose membranes (100 V for 3 h at 4°C). The membrane was then stained with Ponceau S. Images of each Ponceau S stained membrane were analysed using computerized image analysis (Scion Image, Scion Technologies) to verify equal loading and transfer between lanes (data not shown). Membranes were then washed and blocked in PBS–Tween buffer containing 5% skim milk and 0.05% Tween for 2 h and subsequently incubated with antibodies against manganese superoxide dismutase (MnSOD; SOD-111; Stressgen; Victoria, BC, Canada), copper/zinc superoxide dismutase (CuZnSOD; SOD-101; Stressgen) and catalase (Ab16731; Abcam, Cambridge, MA, USA). Primary antibodies were diluted 1:500 in blocking buffer and applied to the membranes with gentle rocking overnight at 4°C. Membranes were then incubated with horseradish peroxidase–antibody conjugate (1:2000) directed against the primary antibody for 2 h. Membranes were then treated with chemiluminescent reagents (luminol and enhancer; ECL Plus, Amersham Biosciences, Piscataway, NJ, USA) and exposed to light-sensitive film. Film images were captured and subsequently analysed using computerized image analysis (Scion Image, Scion Technologies).
Statistical analysis
Biochemical and morphological data were analysed using ANOVA. Where significant differences existed, a Bonferroni post hoc analysis was used. Repeated-measures ANOVAs were implemented to compare force–frequency responses, fatigue, and force recovery indexes. Significance was established apriori at P < 0.05.
Results
Verification of transgenic overexpression
Transgenic overexpression of catalase (34.3 ± 1.69 g), CuZnSOD (32.6 ± 0.81 g) or MnSOD (31.3 ± 0.91 g) did not alter experimental animal body weights from WT (31.6 ± 0.85 g). The mRNA abundances and activities of each of the antioxidant enzymes with transgenic overexpression have been previously described in detail (Raineri et al. 2001; Chen et al. 2003; Rader et al. 2006). Western blotting was performed to verify the overexpression of major antioxidant enzyme proteins in the diaphragm of Tg(CAT), Tg(SOD1) and Tg(SOD2) mice. Diaphragm catalase protein abundance (optical density; OD) was significantly higher (+3933%) in the catalase overexpressing animals compared+with WT (WT, 12793 ± 111 OD; CAT, 515957 ± 1613 OD). Importantly, catalase overexpression did± not alter diaphragm CuZnSOD or MnSOD protein abundances. Diaphragm CuZnSOD protein abundance increased 280% from WT values (3828 ± 966 OD) with transgenic overexpression (14533 ± 536 OD). Overexpression of CuZnSOD did not alter diaphragm catalase or MnSOD protein abundances. Finally, diaphragm MnSOD protein abundance increased by 169% from WT values (51370 ± 659 OD) with transgenic overexpression (138265 ± 1569 OD), and MnSOD overexpression did not alter diaphragm catalase or CuZnSOD protein abundances.
Contractile properties
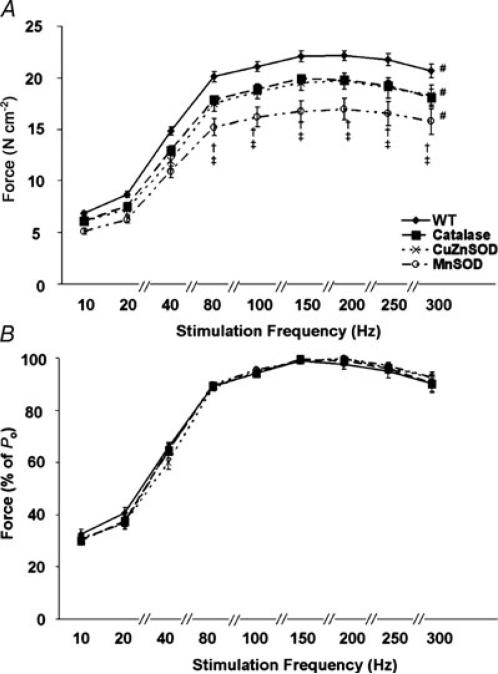
Compared with WT, the force–frequency curve of the diaphragms from catalase, CuZnSOD and MnSOD overexpressors was shifted downwards and to the right (Fig. 1A). This indicates a reduction in force generation at stimulation frequencies tested between 20 and 300 Hz. Compared with the catalase and CuZnSOD overexpressing animals, diaphragms from MnSOD overexpressors demonstrated the greatest downwards and right shift of the force–frequency curve at stimulation frequencies between 80 and 300 Hz. Note, however, that the group differences in diaphragm force production were eliminated when force was normalized as a percentage of Po (Fig. 1B). This finding indicates that the right shift in the force–frequency curve observed in Fig. 1A was due to reduced muscle force production and not to a change in the muscle force–stimulation frequency relationship.
Figure 1. Force–frequency curves of in vitro diaphragm strips from WT, catalase, CuZnSOD and MnSOD overexpressing mice.
A, force–frequency characteristics expressed as a function of specific force production (in N cm−2). B, force–frequency characteristics expressed as a percentage of maximal specific tension (% of Po). Values expressed are means ± s.e.m. # Significantly different (P < 0.05) reductions in force generation at stimulation frequencies tested between 20 and 300 Hz compared with WT. † Significantly different (P < 0.05) from catalase. ‡ Significantly different (P < 0.05) from CuZnSOD.
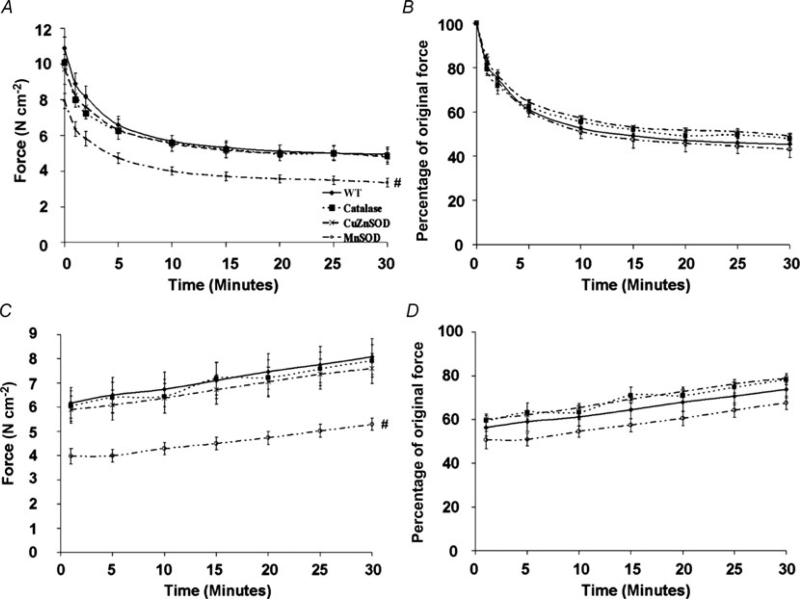
The fatigue protocol, in absolute terms (N cm−2; Fig. 2A), produced curves of similar shape for all groups although diaphragms from the MnSOD overexpressors generated significantly less force compared with WT and with catalase and CuZnSOD overexpressor groups. In relative terms (percentage of original force; Fig. 2B), no differences were seen in the curve shape or the percentage of force produced between diaphragms from animals in different experimental groups. The recovery protocol, in absolute terms (N cm−2; Fig.2C), produced curves of similar shape for all groups, although diaphragms from the MnSOD overexpressors generated a significantly lower amount of force compared with WT and with catalase and CuZnSOD overexpressor groups where indicated. In relative terms (percentage of original force; Fig. 2D), there were no differences in the curve shapes between diaphragms from animals in different experimental groups, but diaphragms from MnSOD overexpressors produced a smaller percentage of original force than catalase or CuZnSOD overexpressors where indicated. Finally, maximal tetanic force production was reduced from WT values by 8, 11 and 22% in diaphragms from catalase, CuZnSOD and MnSOD transgenic overexpressors, respectively.
Figure 2. Comparison of fatigue development and recovery of in vitro diaphragm strips from WT mice and from catalase, CuZnSOD and MnSOD overexpressing mice.
A, absolute fatigue development. B, relative fatigue development. C, absolute force recovery from fatigue. D, relative force recovery from fatigue. Values expressed are means ± s.e.m. # Significantly different (P < 0.05) from all at all time points.
Oxidative stress
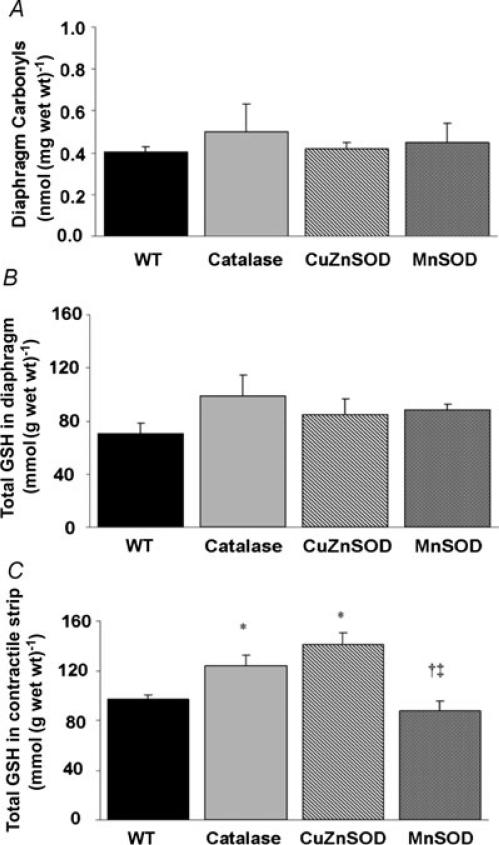
Basal levels of biomarkers of oxidative damage as well as the development of contraction-induced diaphragmatic oxidative injury were determined by measuring protein carbonyl formation and total GSH. Compared with WT, diaphragmatic protein carbonyl levels (in nmol (mg tissue wet weight)−1) were not altered by transgenic overexpression of catalase, CuZnSOD or MnSOD (Fig. 3A) at baseline. In addition, compared with WT, total GSH concentrations (in mmol (g tissue wet weight)−1) in the diaphragm were not altered by transgenic overexpression of catalase, CuZnSOD or MnSOD (Fig. 3B) at baseline. Following the contractile protocol, total GSH increased by 27 and 45% in the diaphragms of catalase and CuZnSOD overexpressors, respectively (Fig. 3C).
Figure 3. Effects of transgenic overexpression of catalase, CuZnSOD and MnSOD on diaphragmatic markers of oxidant stress.
A, protein carbonyl formation. B, total diaphragm glutathione (in mmol (g wet weight)−1). C, total glutathione (in mmol (g wet weight)−1) in diaphragm strips subjected to fatigue–recovery contraction protocol. Values are means + s.e.m/ * Significantly different (P < 0.05) from WT. † Significantly different from catalase. ‡ Significantly different from CuZnSOD.
Fibre type distribution
The distribution of myofibres expressing type I, type IIA and type IIB/X MHC was determined in the diaphragm of all experimental groups (Table 1). The percentage of diaphragm myofibres expressing type I MHC was not altered from WT mice with transgenic overexpression of catalase, CuZnSOD or MnSOD. The percentage of diaphragm myofibres expressing type IIA MHC increased by 19 and 12% from WT values with transgenic overexpression of catalase and CuZnSOD, respectively. The percentage of diaphragm myofibres expressing type IIB/X MHC decreased 16% from WT values with transgenic overexpression of catalase.
Table 1.
Diaphragm percentage fibre types
| Genotype | Type I MHC (%) | Type IIA MHC (%) | Type IIB/X MHC (%) |
|---|---|---|---|
| WT | 11.9 ± 0.9 | 42.3 ± 1.6 | 45.6 ± 1.1 |
| Tg(CAT) | 11.6 ± 1.0 | 50.2 ± 3.0* | 38.2 ± 2.7* |
| Tg(SOD1) | 10.0 ± 0.8 | 47.4 ± 12* | 42.6 ± 1.3 |
| Tg(SOD2) | 12.3 ± 0.3‡ | 42.2 ± 1.0†‡ | 45.5 ± 1.1† |
Percentages of type I, type IIA and type IIB/X myosin heavy chain-expressing myofibres in diaphragm from control animals (WT) and from mice overexpressing catalase (Tg(CAT)), copper/zinc superoxide dismutase (Tg(SOD1)) and manganese superoxide dismutase (Tg(SOD2)). Values are means ± s.e.m.
Significantly different (P < 0.05) from WT.
Significantly different (P < 0.05) from Tg(CAT).
Significantly different (P < 0.05) from Tg(SOD1).
Diaphragm morphology
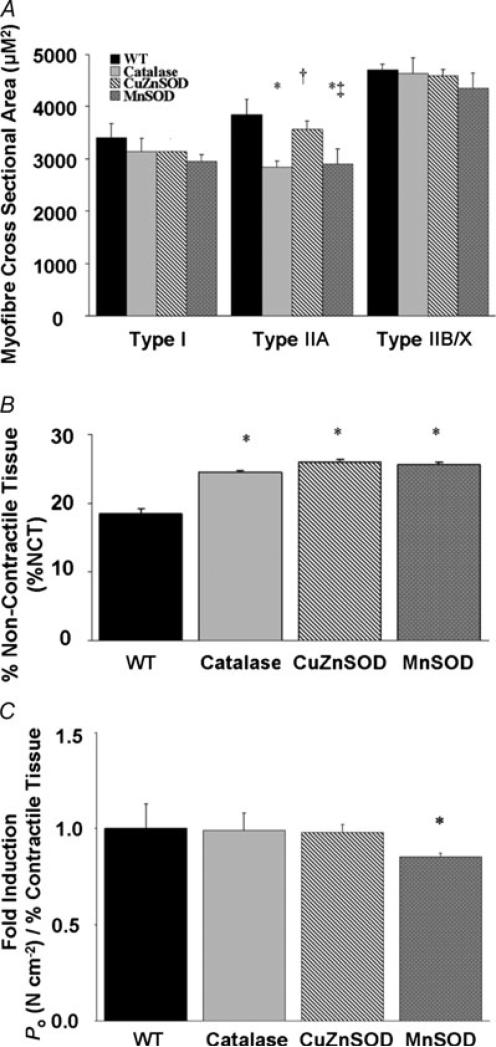
To determine whether transgenic overexpression of individual antioxidant enzymes alters muscle fibre size, we measured the myofibre cross-sectional area (in μm2) of fibres in cross-sections of diaphragm muscle from each experimental group (Fig. 4A). Transgenic overexpression of catalase, CuZnSOD or MnSOD did not influence the CSA of type I myofibres in the diaphragm. In contrast, compared with WT, type IIA myofibre CSA decreased by 26 and 25% with transgenic overexpression of catalase and MnSOD, respectively. Transgenic overexpression of catalase, CuZnSOD or MnSOD did not alter the CSA of type IIB/X myofibres in the diaphragm. Finally, the percentage of non-contractile tissue was also analysed in the diaphragms from animals of all experimental groups (Fig. 4B). Compared with WT, the percentage of non-contractile tissue in the diaphragm increased by 33% with catalase overexpression, by 41% with CuZnSOD overexpression and by 40% with MnSOD overexpression.
Figure 4. Diaphragm morphology.
A, mean + s.e.m. cross-sectional area (in μm2) in diaphragm skeletal muscle myofibres expressing myosin heavy chain (MHC) types I, IIA and IIB/X. * Significantly different (P < 0.05) from all other treatment groups within MHC type. † Significantly different from catalase. ‡ Significantly different from CuZnSOD. B, percentage of diaphragm non-contractile tissue. * Significantly different (P < 0.05) from WT. C, maximal tetanic Po (in N cm−2) produced by transgenic diaphragm strips corrected for the percentage of diaphragm contractile tissue (%CT). Values are presented as (N cm−2)/%CT and are expressed as multiples of the WT values (fold induction). * Significantly different (P < 0.05) from all other treatment groups.
Figure 4C compares the maximal tetanic Po produced by transgenic diaphragm strips normalized to the amount of contractile material in the muscle; hence, this assessment eliminates the influence of diaphragm non-contractile tissue expansion on the calculation of maximal tetanic Po. The catalase and CuZnSOD transgenic overexpression-induced maximal tetanic Po deficits were significantly attenuated by correcting for the relative percentage of contractile tissue. This correction did not completely attenuate the MnSOD transgenic overexpression-induced deficit in maximal tetanic Po. The mechanism(s) for these differential findings remain unclear.
Discussion
Overview of principal findings
Several new and important findings emerged from these experiments. First, transgenic overexpression of the individual antioxidant enzymes catalase, CuZnSOD or MnSOD is associated with diminished skeletal muscle specific force production, resulting in a right and downwards shift in the force–frequency curve. Second, transgenic overexpression of catalase, CuZnSOD or MnSOD produces important morphological alterations to the diaphragm, including an increase in noncontractile material and alterations in the myosin heavy chain phenotype. Furthermore, the individual transgenic overexpression of catalase, CuZnSOD or MnSOD is insufficient to delay muscular fatigue during prolonged submaximal contractions. Finally, our data reveal that transgenic overexpression of key antioxidant enzymes does not enhance recovery of the diaphragm following a fatiguing contraction protocol. A brief discussion of these key results follows.
Overexpression of antioxidant enzymes depresses diaphragm specific force production
Our results support the hypothesis that transgenic overexpression of catalase, CuZnSOD or MnSOD antioxidant enzymes has a negative impact on diaphragmatic muscle specific force production at all stimulation frequencies. This hypothesis was formulated based on the knowledge that ROS have an important influence on force production in unfatigued skeletal muscle. Indeed, the low (i.e. optimal) levels of ROS normally present in skeletal muscle are a requirement for optimal force production (Reid, 2006, 2008). For example, depletion of ROS via antioxidants in unfatigued skeletal muscle results in a depression of muscle force production at all submaximal stimulation frequencies. In contrast, a modest increase in ROS in skeletal muscle fibres promotes an increase in force production (Reid et al. 1993). The positive impact of ROS on muscle force production is reversed at higher ROS concentrations as force production decreases in both a time- and dose-dependent manner (Reid, 2001). Based upon these findings, Reid et al. (1993) developed a theoretical model to describe the relationship between muscle redox balance and force production. This model forecasts that an optimal cellular redox state exists whereby conditions are ideal for muscle force production (Reid et al. 1993; Reid, 2001). It follows that a deviation from the optimal redox balance leads to a loss of force production. Therefore, in the present experiments, it appears that the individual overexpression of catalase, CuZnSOD or MnSOD in the diaphragm resulted in diminished ROS availability in the diaphragm and therefore contributed to the observed impairment in diaphragmatic specific force production. Moreover, since the MnSOD transgenic animals showed the largest force deficit, this finding suggests a potential role for mitochondrial ROS in the regulation of skeletal muscle force production. This is a testable hypothesis and is worthy of further investigation.
It is also feasible that myosin phenotype differences in diaphragm muscle between the WT animals and the transgenic (i.e. antioxidant overexpression) animals could contribute to the group differences in diaphragmatic specific force production. In this regard, our study revealed that the ubiquitous overexpression of antioxidant enzymes resulted in changes in diaphragmatic myosin heavy chain phenotype in two of the transgenic groups. Specifically, catalase and CuZnSOD overexpression resulted in an increase in myosin heavy chain IIA protein abundance in the diaphragm. In theory, shifts from faster IIB/X myosin heavy chain isoforms to the slower, more oxidative IIA isoform in the diaphragm of these animals could contribute to the observed reductions in diaphragm force production at maximal stimulation frequencies. Nonetheless, MnSOD overexpression did not alter the MHC phenotype of the diaphragm. Moreover, compared with WT animals, diaphragms from MnSOD overexpressing animals exhibited the largest reduction in maximal tetanic force production and the largest downwards and right shift of the force–frequency curve. This observation suggests that differences in myosin heavy chain phenotype between the experimental groups are not a major contributary factor to the disparity in muscle specific force production between groups.
Another potential explanation for the finding that overexpression of antioxidant enzymes results in a decrement in diaphragmatic specific force production is that marked morphological differences existed in diaphragm muscle structure between the experimental groups. Indeed, compared with WT animals, diaphragm muscle from the three groups of transgenic animals contained significantly higher levels of non-contractile tissue. Therefore, it is feasible that this increase in noncontractile tissue in skeletal muscle was a causal factor to the observed reduction in diaphragm specific force production. Normalizing force production of a muscle to contractile protein is effective in determining the force generated per relative proportion of contractile protein. Our data suggest that the expansion of the noncontractile tissue (reduction in contractile tissue proportion) accounts for the majority of the force deficits demonstrated in the catalase and CuZnSOD transgenic lines. In contrast, the normalization of muscle force production to the relative proportion of contractile protein does not eliminate the force deficits in the MnSOD transgenic animals. This finding suggests that the expanded non-contractile tissue is only a contributary factor to reduced force production in these animals (Fig. 4C).
In regard to non-contractile tissue in skeletal muscle, the development of fibrotic material in skeletal muscles is a common response to both ageing (Corsetti et al. 2008) and some pathologies (Zhu et al. 2007). Oxidative stress plays a key role in the regulation of fibroblast collagen synthesis and the activity of matrix metalloproteinase enzymes responsible for collagen breakdown/reorganization (Siwik et al. 2001). Therefore, the lifelong overexpression of these antioxidant enzymes in fibroblast cells could result in alterations in the normal collagen milieu of the diaphragm extracellular matrix, resulting in an increase in noncontractile material and potentially contributing to the reduction in specific force production.
Overexpression of a single antioxidant enzyme does not protect against contraction-induced muscle fatigue
Our hypothesis that overexpression of catalase, MnSOD or CuZnSOD would protect against muscle fatigue during prolonged and fatiguing contractions was based upon evidence indicating that ROS contribute to muscular fatigue and evidence that muscle fatigue can be delayed with pharmacological antioxidants (Reid, 2008). Similarly, recent work by Bruton and colleagues demonstrated that overexpression of MnSOD in mouse muscle improved fatigue resistance in single muscle fibres during repeated contractions (Bruton et al. 2008). Nonetheless, our findings do not indicate that the overexpression of a single antioxidant enzyme protects against contractile-induced fatigue. There are at least two potential explanations for our results. First, it is feasible that oxidative damage to skeletal muscle did not play a major role in the development of muscle fatigue in our experimental model. In this regard, our results do not provide definitive evidence regarding whether ROS production played a significant role in muscle fatigue during our fatigue protocol. For example, although no differences existed in total glutathione levels between control diaphragm muscle and diaphragm muscle strips exposed to our contractile protocol, this finding alone is not sufficient to rule out a potential role of ROS in the development of muscle fatigue in our experiments. Similar conclusions have been reached by others (Ferreira et al. 2009).
A second explanation for our findings could be that the overexpression of a single antioxidant enzyme in skeletal muscle is not be capable of providing protection against contraction-induced oxidative damage to muscle fibres. It is clear that cellular antioxidant defenses are not composed of a single antioxidant but consist of a co-operative network of multiple enzymatic and nonenzymatic antioxidants that are compartmentalized to provide optimal protection for cellular proteins, lipids and DNA against ROS-mediated oxidation (Powers & Jackson, 2008). Therefore, although increased expression of CuZnSOD or MnSOD would increase the ability of the cell to remove superoxide anions, this adaptation alone would not increase the cellular capacity to remove the oxidant byproduct of superoxide dismutation (i.e. hydrogen peroxide). Indeed, our results indicate that diaphragm levels of both catalase and total glutathione did not increase in diaphragm muscle of the MnSOD or CuZnSOD overexpressing animals. Similarly, the catalase overexpressing animals did not possess elevated levels of total glutathione, CuZnSOD or MnSOD. Hence, we conclude that the transgenic overexpression of a single antioxidant enzyme is not sufficient to provide protection against contraction-induced muscle fatigue.
Acknowledgements
This work was supported by the National Institutes of Health (RO1 HL072789 to S.K.P.).
References
- Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liang H, Van Remmen H, Vijg J, Richardson A. Catalase transgenic mice: characterization and sensitivity to oxidative stress. Arch Biochem Biophys. 2004;422:197–210. doi: 10.1016/j.abb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Chen X, Mele J, Giese H, Van Remmen H, Dolle ME, Steinhelper M, Richardson A, Vijg J. A strategy for the ubiquitous overexpression of human catalase and CuZn superoxide dismutase genes in transgenic mice. Mech Ageing Dev. 2003;124:219–227. doi: 10.1016/s0047-6374(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Coombes JS, Powers SK, Rowell B, Hamilton KL, Dodd SL, Shanely RA, Sen CK, Packer L. Effects of vitamin E and α-lipoic acid on skeletal muscle contractile properties. J Appl Physiol. 2001;90:1424–1430. doi: 10.1152/jappl.2001.90.4.1424. [DOI] [PubMed] [Google Scholar]
- Corsetti G, Pasini E, D'Antona G, Nisoli E, Flati V, Assanelli D, Dioguardi FS, Bianchi R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol. 2008;101:26E–34E. doi: 10.1016/j.amjcard.2008.02.078. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Powers SK, Herb RA, Dodd SL. Mechanism of specific force deficit in the senescent rat diaphragm. Respir Physiol. 1997;107:149–155. doi: 10.1016/s0034-5687(96)02509-1. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, Gilliam LA, Reid MB. l-2-Oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. J Appl Physiol. 2009;107:211–216. doi: 10.1152/japplphysiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve. 2001;24:1202–1212. doi: 10.1002/mus.1133. [DOI] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Mehl KA, Thompson RW, Lowe LL, Carson JA. Nandrolone decanoate modulates cell cycle regulation in functionally overloaded rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1543–R1552. doi: 10.1152/ajpregu.00285.2004. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- Rader EP, Song W, Van Remmen H, Richardson A, Faulkner JA. Raising the antioxidant levels within mouse muscle fibres does not affect contraction-induced injury. Exp Physiol. 2006;91:781–789. doi: 10.1113/expphysiol.2005.033043. [DOI] [PubMed] [Google Scholar]
- Raineri I, Carlson EJ, Gacayan R, Carra S, Oberley TD, Huang TT, Epstein CJ. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med. 2001;31:1018–1030. doi: 10.1016/s0891-5849(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Reid MB. Of balance and unbalance. J Appl Physiol. 2006;101:1011–1012. doi: 10.1152/japplphysiol.00539.2006. [DOI] [PubMed] [Google Scholar]
- Reid MB. Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Segal SS, White TP, Faulkner JA. Architecture, composition, and contractile properties of rat soleus muscle grafts. Am J Physiol. 1986;250:C474–479. doi: 10.1152/ajpcell.1986.250.3.C474. [DOI] [PubMed] [Google Scholar]
- Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42:627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanely RA, Coombes JS, Zergeroglu AM, Webb AI, Powers SK. Short-duration mechanical ventilation enhances diaphragmatic fatigue resistance but impairs force production. Chest. 2003;123:195–201. doi: 10.1378/chest.123.1.195. [DOI] [PubMed] [Google Scholar]
- Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Staib JL, Swoap SJ, Powers SK. Diaphragm contractile dysfunction in MyoD gene-inactivated mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R583–R590. doi: 10.1152/ajpregu.00080.2002. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J. Relationships between transforming growth factor-β1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]