Abstract
The neurohypophysial hormones oxytocin (OT) and vasopressin (VP) originate from hypothalamic neurosecretory cells in the paraventricular and supraoptic (SON) nuclei. The firing rate and pattern of action potentials arising from these neurones determine the timing and quantity of peripheral hormone release. We have used immunochemical identification of biocytin-filled SON neurones in hypothalamic slices in vitro to uncover differences between OT and VP neurones in membrane and synaptic properties, firing patterns, and plasticity during pregnancy and lactation. In this review we summarise some recent findings from this approach: 1) VP neuronal excitability is influenced by slow (sDAP) and fast (fDAP) depolarising afterpotentials that underlie phasic bursting activity. The fDAP may relate to a transient receptor potential (TRP) channel, type melastatin (TRPM4 and/or TRPM5), both of which are immunochemically localised more to VP neurones, and especially, to their dendrites. Both TRPM4 and TRPM5 mRNAs are found in the SON, but single cell RT-PCR suggestsTRPM4 might be the more prominent channel. Phasic bursting in VP neurones is little influenced by spontaneous synaptic activity in slices, being shaped largely by intrinsic currents. 2) The firing pattern of OT neurones ranges from irregular to continuous, with the coefficient of variation determined by randomly distributed, spontaneous GABAergic, inhibitory synaptic currents (sIPSCs). These sIPSCs are 4–5 fold more frequent in OT vs. VP neurones, and much more frequent than spontaneous excitatory synaptic currents. 3) Both cell types express Ca++-dependent afterhyperpolarisations (AHPs), including an apamin-sensitive, medium duration AHP and a slower, apamin-insensitive AHP (sAHP). In OT neurones, both AHPs are enhanced during pregnancy and lactation. During pregnancy, the plasticity of the sAHP is blocked by antagonism of central OT receptors. AHP enhancement is mimicked by exposing slices from Day 19 pregnant rats to OT and oestradiol, suggesting central OT and sex steroids program this plasticity during pregnancy by direct hypothalamic actions. In conclusion, the differences in VP and OT neuronal function are underlain by differences in both membrane and synaptic properties, and differentially modulated by reproductive state.
Keywords: afterhyperpolarisation, depolarising afterpotential, electrophysiology, pregnancy, lactation
Introduction
The magnocellular neurosecretory system is ideal for understanding the mechanisms and consequences of patterned activity in the brain as it relates to peptide release (1). The system is formed from vasopressin (VP) and (OT) cells within the supraoptic (SON), paraventricular (PVN) and accessory neurosecretory nuclei of the mammalian hypothalamus that send axons to the posterior pituitary gland to terminate at the neurohemal contact zone. There, VP and OT are released into the blood stream for effects at distant organs, such as the kidney, vascular smooth muscle, mammary gland and uterus. The quantity and timing of VP and OT release are locked to the pace and pattern of action potential activity initiated from the parent neurones in the SON and PVN (3). Action potentials invade the axon terminals to increase Ca++ influx and instigate classical stimulus-secretion coupling (3). With the electrical activation of VP and OT neurones, hormone release has essentially two phases. In the first, or facilitation phase, clusters of action potentials are highly efficient at releasing hormone due to enhanced Ca++ influx at the terminal. However, with continued activation and repetitive firing, a second phase of fatigue sets in, strongly diminishing the amount of hormone released per spike. Thus for both cell types, a phasic, bursting pattern of activity maximises hormone release, allowing short periods of robust firing and its attendant facilitation, interspersed with periods of relative quiescence. This pattern avoids release fatigue and allows recovery for the next bout of activity (4–7).
In vitro preparations are essential to study membrane and synaptic properties of VP and OT neurones, because intracellular recordings from these cells in vivo are notoriously brief due to vascular pulsation (8, 9). Thus, understanding the mechanisms underlying firing has benefited greatly from in vitro preparations developed in the last 20–30 years, such as the acute hypothalamic slice (10, 11), hypothalamic explant (12, 13), and cultured organotypic slices (14). In addition to recording stability, these preparations have an added advantage in that many aspects of the in vivo firing patterns of both cell types are spontaneously expressed. VP and OT neurones can also be studied in primary culture (15), or after an acute dissociation that allows the best voltage clamp conditions (16). As an added bonus, many of the axonal varicosities/endings of these neurones within the neural lobe are large enough (~10–15 μm) for different types of recordings. Patch clamp recordings from such swellings can be obtained after acute dissociation (17), or from slices of the neural lobe using visual guidance (18). Even sharp electrode intracellular recordings from neurohypophysial terminals and axons are possible in the neural stalk as well as neural lobe (19).
These seminal forays in vitro instigated a rich assortment of subsequent investigations, such that many of the electrical and synaptic properties of VP and OT neurones are now known. In this article we will review some recent contributions of this lab. As the title suggests, these studies have been aimed at the VP and OT neuronal firing patterns (performance), their underlying properties (focusing on spike after potentials), and for OT neurones the plasticity associated with reproductive state. Although this review will focus on studies largely from rat, in vitro studies of magnocellular neurosecretory neurones studies have been performed in guinea pigs (20–22), mice (23, 24), and cats (25).
Firing Patterns in vitro
VP Neurone Firing
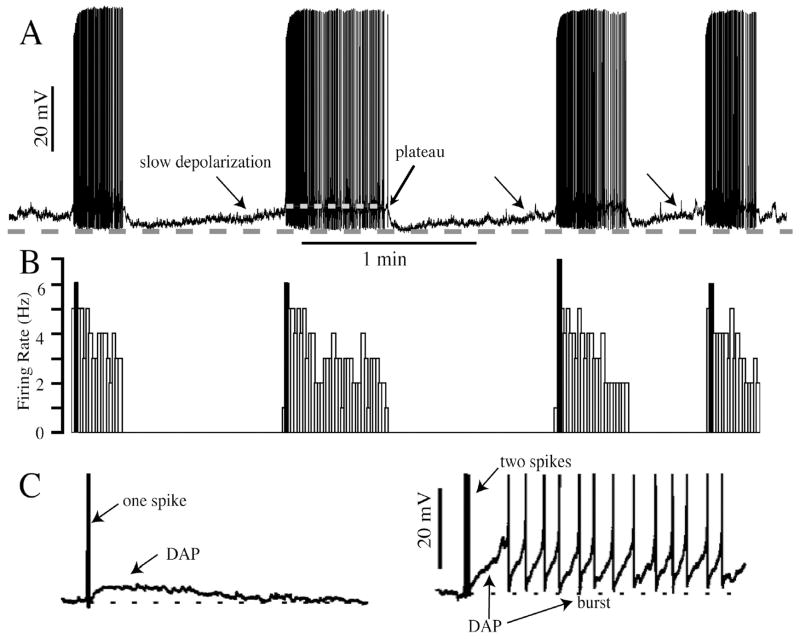
The firing patterns of antidromically-identified VP and OT in vivo have been studied intensely under a variety of conditions (2, 26, 27, 28, 29). OT neurones can be identified either by their association with milk ejection in lactating females, or in male or virgin female rats by their excitation with intravenously administered cholecystokinin (CCK) and a lack of response to acute hypertension (30, 31). In contrast, VP neurones are typically identified by a lack of association with milk ejection in lactating females, an abrupt inhibition to acute hypertension, and are either unresponsive or mildly inhibited by CCK (30, 32, 33). Both cell types are activated by hyperosmotic, hypovolemic and hypotensive challenges, albeit the form of the response differs. Thus in the face of VP-releasing stimuli such as dehydration (a hypovolemic and osmotic challenge), hyperosmolality, or hemorrhage (a hypovolemic and hypotensive challenge), VP neurones move from a slow, irregular pattern (< 1.5 Hz) with a Poisson spike interval distribution to a phasic bursting pattern of activity consisting of bursts and interburst intervals each on the order of 20–30 sec (Fig. 1). In general, the amount of VP is coded most particularly by changes in intraburst firing rate, but changes in burst length have also been noted. While a phasic pattern is most characteristic of activated VP neurones, a tonic pattern is not uncommon. This tonic activity may be transitional to, or from, the phasic bursting pattern (28, 34). However, even during tonic spiking, clustered spiking can occur (28), and this should facilitate hormone release (6).
Figure 1. Phasic bursting VP neurone and DAP.
A. Voltage record of phasic bursting VP neurone recorded with the whole cell patch technique in the SON of a hypothalamic slice made from an adult female rat. Bursts are underlain by a plateau potential (white dashed line), and separated by silent periods consisting of a slow depolarisation. The grey dashed line indicates the peak of the post-burst hyperpolarisation. B. Ratemeter histogram of the spike activity shown in A. Note the highest firing rate occurs in the first 2 sec of the burst (blackened line). C. DAP activated by one spike (left) summates after two spikes, instigating a burst.
Remarkably, spontaneous phasic bursting was observed in early in vitro recordings from the PVN or SON, both in hypothalamic slices (11, 35, 36, 37) and in acutely prepared hypothalamic explants (12, 38), with features similar to those observed in vivo. In four such studies, phasic bursting parameters have been similarly evaluated, both in hypothalamic explants (12, 39) and slices (35, 39, 40). While there is a wide range in key bursting parameters (burst duration, intraburst firing rate, interburst interval duration) both within and across these studies, a similar variability is present within in vivo studies (28). As shown in Table 1, there is reasonable correspondence in the reported mean intraburst firing rates and interburst intervals across in vitro studies, with the greatest differences occurring in average burst length, and these values are not too different from some in vivo studies. Phasic bursting neurones are immunoreactive for VP or VP-associated neurophysin (37, 40, 41, 42, 43), but a small percentage of identified OT neurones can exhibit phasic bursting indistinguishable from VP neurones (41). This is also true of a few milk-ejection neurones in vivo (2, 44).
Table 1.
Properties of phasic bursting neurones in vivo and in vitro.
| Authors | Preparation, recording | Burst Length (s) | Intraburst Firing Rate (Hz) | Silence Interval (s) |
|---|---|---|---|---|
| Armstrong and Sladek, 1982 (12)† | acute explant, extracellular | 14.8 ± 0.8 | 7.9 ± 0.8 | 36.9 ± 2.3 |
| Mason, 1983 (35) | acute slice, intracellular | 16.7 ± 4.5 | 3.1 ± 0.3 | 28.2 ± 9.2 |
| Sabatier et al., 2004 (39)† | acute slice, extracellular | 41 ± 11 | 5.3 ± 0.7 | 35 ± 5 |
| Li et al., 2007 (40)†† | acute slice, whole cell | 39.9 ± 1.5 | 3.4 ± 0.1 | 31.4 ± 0.9 |
| Wakerley et al, 1978 (55)* | in vivo, extracellular | 20.4 | 6.3 | 17.4 |
| Sabatier et al., 2004 (39)††,** | in vivo, extracellular | 62 ± 6 | 7.1 ± 0.3 | 37 ± 4 |
| Nissen et al., 1993 (119)†,** | in vivo, extracellular | 44.8 ± 7.8 | 8.1 ± 0.5 | 32.4 ± 6.7 |
All values x̄ ± s.e.m.
Dorsal approach, lactating females with minimal (6 hrs) dehydration. Normalized data, standard errors unreported.
Transpharyngeal approach.
Transpharyngeal approach.
male rats.
virginfemale rats.
Historically, the similarities between in vivo and in vitro studies coupled with the reduced neuronal connectivity present in vitro argued that phasic bursting was primarily the result of intrinsic membrane properties of VP neurones. Further proof came from Hatton (45), who reported that phasic bursting activity in the PVN remained after blocking chemical synaptic transmission in slices, from Andrew (46), who found phasic activity could still be observed even with Ca++ spikes after blocking the fast sodium channels underlying action potentials, and from Oliet and Bourque (47), who reported phasic bursting in a small number of acutely isolated SON neurones.
Although the basic mechanisms required for phasic bursting are intrinsic, synaptic activity certainly modulates this behaviour. First, this pattern is expressed with low incidence in unstimulated animals (2), where synaptic activity appears necessary to instigate and maintain it. Blockade of excitatory synaptic input in vivo abolishes phasic bursting (48), and even renders SON neurones insensitive to acute osmotic stimulation (49), despite their intrinsic osmosensitivity. This is consistent with the necessity of intact afferent inputs from additional osmosensitive regions such as the organum vasculosum of the lamina terminalis for a full VP response in response to hyperosmotic challenge (50, 51). Furthermore, blockade of local GABAergic activity blocks the inhibition of VP neurones to acute hypertension, suggesting that the brainstem and forebrain pathways implicated in this response ultimately target perinuclear GABA neurones projecting to the SON (52).
Second, intraburst firing rate increases with the increased strength of a peripheral signal (34, 53, 54, 55), and synaptic activity is likely to participate in this coding. Stronger stimulation, whether it be direct depolarisation in vitro (41, 56) or acute osmotic stimulation (34) or haemorrhage (44) in vivo, can prolong burst duration as well, even induce continuous activity.
Third, bursting is very sensitive to strong, transient inputs. Briefly imposed trains of action potentials triggered either antidromically (57) or orthodromically (58) can quickly instigate individual bursts, and when given late in a burst, foreshorten them. Together, these data suggest that the intrinsic processes governing phasic activity are highly regulated by synaptic inputs. Since some key properties underlying phasic bursting are voltage-dependent (see below), excitatory inputs would be critical to elevating the membrane potential to burst threshold.
OT Neurone Firing
OT neurones may fire in a slow irregular pattern in vivo in a resting state, but are also characterised by a continuous firing pattern with a Gaussian distribution of spike times. This tonic discharge pattern largely characterises OT neuronal activity in many release states, save those of parturition and lactation (2). However, the irregular-continuous continuum also describes the background firing of OT neurones between the explosive milk ejection bursts during lactation. Both irregular and continuous firing patterns can be observed in identified OT neurones in vitro (40, 41, 59). The firing rate response of OT neurones to such graded stimuli as increasing osmolality is relatively linear (60). During lactation, the strength of the milk ejection burst can be modulated by the strength of suckling (i.e., number of pups) (61), which may be contribute to an increased local release of OT in the SON and PVN that is critical to OT bursting during lactation (62).
Milk-ejection type bursts are not observed spontaneously in acute slices. However, the alpha1 adrenergic receptor agonist phenylephrine can induce unsynchronised short bursts in OT neurones (63), as can OT (64). The relationship of these bursts to those observed during lactation is not clear. Not only is this bursting unsynchronised, it can be induced in male and virgin female rats where it has never been reported in vivo. And with phenylephrine at least, this activity appears to require an unphysiologically low amount of Ca++ in the extracellular fluid. In contrast to acute slices, in organotypic slices of hypothalamus from early postnatal animals, synchronised bursting of identified OT neurones has been observed, and is entirely dependent on periodic bursts of glutamatergic transmission (65). This bursting is also remarkably similar in periodicity (minutes) to that during lactation. There are nevertheless questions, such as why in vivo OT neurone bursting is only observed in female animals during lactation and parturition, and to a much smaller extent during late pregnancy? And since the pups are not sexed before culturing the hypothalamus, the in vitro behaviour is undoubtedly found in male brains as well. Regardless, these data strongly suggest that intrinsic hypothalamic circuits are critical to this behaviour. Perhaps the absence of activity from normally restraining afferent inputs in these explants is permissive for the expression of local circuits, especially when coupled with the increase in local synaptic formation that would be available without competition from the normal brainstem and forebrain afferents as the cultures develop.
Effect of Synaptic Inputs on Firing Patterns
For both cell types, the statistical distribution of spikes during activity is relevant to hormone release, as clustered spiking (i.e., groups of spikes with short interspike intervals, or ISIs), such as occurs at burst onset, facilitates hormone release (6, 28). In this regard there is a marked difference between in vivo and in vitro activity for VP neurones. In hypothalamic slices, ISIs within bursts largely fit a deterministic, regular process that suggests intraburst firing is controlled by an intrinsic mechanism, such as the depolarising after potential (DAP), with a degree of superimposed spike frequency adaptation (39, 40, 66). This is in contrast to bursts in vivo, where following a brief post-spike period of increased excitability spikes are irregularly, nearly randomly distributed (28, 39).
Given the substantially larger amount of synaptic activity in vivo compared with in vitro preparations (13), synaptic inputs would be the primary candidate for this difference in spike distribution. In slices, we found that random, spontaneous GABAergic synaptic activity (most of which derives from miniature synaptic potentials) dominates 4–5 fold over excitatory activity, and is sufficient to impart irregularity to the spike trains of both VP and OT neurones that are firing along the irregular/continuous continuum (40). Interestingly, for phasic bursting VP neurones the parameters of burst duration, silence duration, intraburst firing rate and intraburst ISI coefficient of variation were not modified when blocking fast inhibitory or excitatory transmission in slices (40). Sabatier et al. (39) suggested that the relative paucity of synaptic activity in vitro allows the DAP to provide a powerful, deterministic driving force for spike initiation. In contrast, in vivo, tonic synaptic events that produce membrane potential fluctuations coupled with an averaged increased membrane conductance (reducing membrane time constant) can impart stochastic (e.g., irregular firing) behaviour; this increased conductance further enhances spike timing in response to synaptic inputs (67, 68). As mentioned earlier, strong, synchronous volleys of synaptic activity that evoke increased spiking in vivo appear to reset phasic patterning depending upon their occurrence, early vs. late, into a burst (58). Such transient inputs actually may have a reduced impact on bursting in states with lower amounts of tonic, background synaptic activity, such as that found in vitro.
During lactation, the background firing behaviour of OT neurones between milk-ejection bursts is not static, with the pattern becoming more irregular just prior to a burst (69, 70). This increased irregularity is accompanied by increasing coordination among OT neurones, a phenomena predicted by the facilitating, depolarising role of locally released OT in these neurones (71). Given the sensitivity of OT neuronal firing in particular to distributed, GABAergic synaptic inputs (40), we suggest that this dynamic change in firing pattern prefacing burst onset may result from a change in the strength and/or distribution of GABAergic activity during the interburst interval. In this regard, OT’s ability to directly depolarise OT neurones (59, 64) and its recently described ability to enhance post-inhibitory rebound depolarisations following inhibitory postsynaptic potentials (IPSPs) (72, also see below) might suggest that OT modulation during the interburst interval is dynamic.
It’s possible that IPSPs may actually play a greater role in regulating OT than VP neuronal behaviour. Based solely on the amount of miniature synaptic activity in adult slices, OT neurones receive many more spontaneous inhibitory currents compared to VP neurones (40). It remains to be determined whether this physiological estimate is correlated with a difference in morphological, synaptic input.
Spike Afterpotentials Regulate Firing Pattern
VP Neurone Afterpotentials
As mentioned above, intrinsic membrane properties including DAPs are critical to generate the phasic bursting pattern in VP neurones, notwithstanding the role of synaptic modulation. DAPs are kinetically complex and consist of at least two potentials. The best studied is a slow (1–2 sec) afterpotential (sDAP) typically activated by spikes and is more prevalent in VP than in OT neurones (41). Spikes close in succession produce summating sDAPs and generate a plateau potential that drives burst duration. These two features were originally described by Andrew and Dudek (56, 73) and were later shown by Bourque (74) to result from a voltage- and Ca++-dependent, TTX-resistant current that appeared as an increased conductance near spike threshold. This was in contrast to an early current-clamp study finding in increased input resistance during plateau potential (46). A later study however found that sDAPs were associated with a decreased conductance and a reversal potential near the K+ equilibrium potential (75). While these disparate findings have not been reconciled, modelling studies from this lab indicate both scenarios are equally compatible with the generation of regenerative sDAPs, plateau potentials and phasic bursting (66, 76). However, the reaction of VP neurones to synaptic inputs could be markedly different if a plateau potential were generated by an increased vs. decreased tonic conductance, with one scenario shortening, and the other lengthening, the membrane time constant. One difficulty with conductance measurements, raised by Andrew (46), is the possibility that the measuring pulse could itself induce a conductance change, for example by briefly deactivating the salient current.
sDAPs are blocked by low mM amounts of external Cs+ (77, 79) and the non-specific cation channel blocker flufenamic acid (78). Histamine, via H1 histamine receptors, enhances the sDAP and (80) also promotes bursting (81). In contrast, kappa opiate receptor activation suppresses sDAPs (82) and chronic inactivation of these receptors abolishes bursting altogether (83). Complementarily, transient inhibition of kappa receptors prolongs bursts and can lead to continuous firing (82, 84), whilst simultaneously enhancing the sDAP and plateau potentials (85).
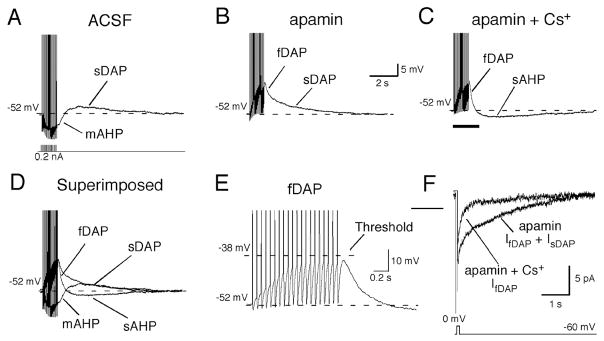
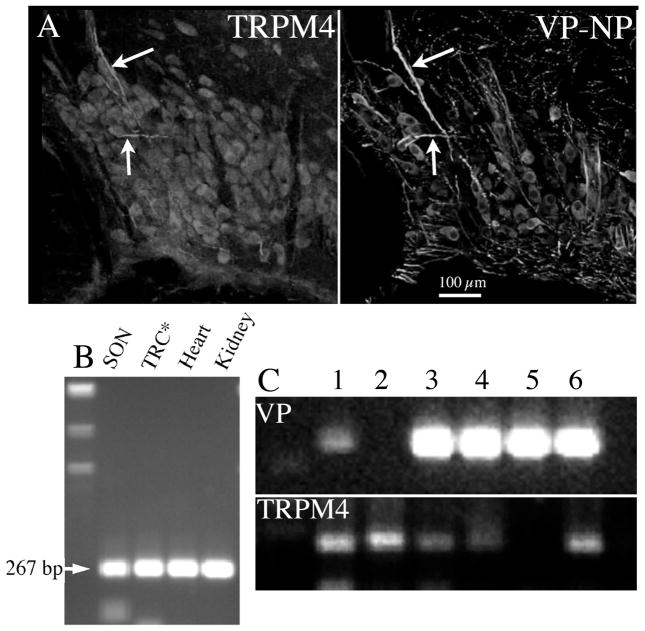
Recently we have determined that DAPs in VP neurones are complex, and comprised of at least two kinetically and pharmacologically distinguishable potentials (79) (Fig. 2). A faster DAP (fDAP) precedes the sDAP, with a tau of ~200 msec (79). The fDAP is also largely restricted to VP neurones, and further shares with the sDAP a marked Ca++- and activity-dependence. The fDAP is suppressed by flufenamic acid, which has also been reported for the sDAP (78). However unlike the sDAP, the fDAP is insensitive to extracellular Cs+. Although the nature of the current underlying the sDAP is still unresolved due to conflicting reports (74, 75), ion-substitution and voltage clamp experiments suggest that a mixed cation, outwardly rectifying current underlies the fDAP. Many of the IfDAP properties are reminiscent of transient receptor potential channels of the melastatin variety (TRPM4 or TRPM5) (86). In support of this possibility, we have found mRNA for both TRPM4 and TRPM5 in the SON, with TRPM4 mRNA in amounts sufficient to appear in single cell reverse transcriptase-polymerase chain reactions (Fig. 3). It’s not yet understood what determines how these two DAPs interact to control bursting. However, since it can be active at potentials negative to those activating the sDAP, the fDAP may bootstrap the membrane potential to the range where the slower sDAP would come into play. In addition, the faster time course of the fDAP more closely matches the time course of immediate postspike excitability in VP neurones (39), suggesting it might be critical to regenerative spiking during bursts. Unfortunately, at this time there are no pharmacological tools to further dissect the relative contributions of the fDAP and sDAP to VP neuronal excitability. A modelling approach similar to that used for understanding of how sDAPs underlie bursts (66, 76) could be insightful in this regard.
Figure 2. DAPs and AHPs in MNCs.
Afterpotentials in a VP neurone were generated by a train of action potentials evoked by intracellular current injections (20, 5 ms depolarising pulses, 100–250 pA, 20Hz). A. In artificial cerebrospinal fluid, the train was followed by the mAHP, which was subsequently followed by the sDAP. B. Bath application of apamin (100 nM) completely blocked the mAHP and unmasked the fDAP, which was followed by the sDAP. C. Additional application of Cs+ (5 mM) blocked the sDAP and revealed the sAHP. D. Superimposed traces of A–C illustrate the temporally overlapping, multiple afterpotentials. E: Expanded portion of the trace in C (indicated by underline) revealed that the onset of the fDAP occurred after the 1st action potential and its amplitude increased with each subsequent action potential until a plateau was reached after 12 spikes. F. Inward tail currents underlying the DAPs (IDAPs) were generated by 50 ms steps to 0 mV from a holding potential of −60 mV. Tail currents with similar time courses as the fDAP and sDAP were obtained, and the application of 5 mM Cs+ blocked only IsDAP. With permission from Teruyama and Armstrong (80).
Figure 3. Localization of TRM4 type channels in the SON.
A. Left panel: immunochemical localisation of TRPM4 in a 40 μm coronal section of SON of adult male rat. Most neurones are lightly stained, as are thick processes characteristic of dendrites (arrows). Labelling from polyclonal antibody (1:7500) raised in rabbit against TRPM4 (Abcam, Cambridge, UK), and followed by goat-anti-rabbit antibody conjugated to Alexa 568 (Invitrogen, Carlsbad, CA). Right panel: immunochemical localization of VP-associated neurophysin (VP-NP) in the same tissue section shown in left panel. VP-NP was localised using a monoclonal antibody (1:500) relatively specific for VP-NP (PS41, provided by H. Gainer, NIH), followed by goat-anti-mouse antibody conjugated to Alexa 488 (Invitrogen). Images from 2 μm optical sections taken on a confocal microscope (BioRad 1024, Hercules, CA). Note the double labelling of the dendrites (arrows) and many neurones. B. mRNA coding for TRPM4 is expressed in the SON. Total RNA was extracted from SON tissue punched out from brain slices, and reverse transcribed. Amplified products of the expected size were obtained for TRPM4 (267 bp). Rat kidney, heart, and tongue epithelial tissues (TRC) known to express TRPM4 served as positive controls. C. Individual SON neurones were collected from dissociated SON tissue. Following total RNA extraction and reverse-transcription from these single cells, PCRs for VP and TRPM4 were performed to verify the collected cells are MNCs. The cDNA from each neurone was amplified by PCR for TRPM4 and TRPM5. Amplified products of expected size for TRPM4 were obtained from 5 out of 7 cDNA libraries derived from those single MNCs. Of these 5, 4 neurones also contained VP mRNA.
DAPs temporally overlap and engage in mutual masking with intrinsic spike hyperpolarising potentials (AHPs) to shape bursting. VP neurones possess at least three AHPs, two of which clearly contribute to burst duration and intraburst frequency. The medium duration AHP (mAHP) (100–200 ms) is Ca++-dependent and mediated by small conductance K+ channels of the SK3 variety. These channels are sensitive to the bee venom apamin, which blocks the mAHP (25, 41, 43, 80, 87, 88). The mAHP underlies spike frequency adaptation in both VP and OT neurones (41, 89). Paradoxically, the mAHP appears not to mediate burst termination in VP neurones, but in fact allows burst duration to reach 20–30 sec by gating firing. Kirkpatrick and Bourque (89) found that while apamin blocked spike frequency adaption at burst onset as predicted, it also produced pronounced increases in intraburst firing rates and much shorter bursts! This result coincides with data showing that burst length is finely regulated in an activity-dependent manner by the autoinhibitory factor dynorphin, which is released somatodendritically during bursts to act on kappa opiate receptors on VP neurones, suppressing the sDAP and thereby terminating bursts (84). Since blockade of the mAHP also unmasks DAP onset (41, 87), the initiation of bursting depends critically on the interplay of these two opposing potentials.
A much slower (2–4 sec), Ca++-dependent AHP (sAHP) also regulates SON firing and gains importance with higher firing rates (88, 90, 91). Although clearly mediated by a K+ current, the channel underlying the sAHP has not been identified. Inhibition of the sAHP with muscarine elevates the plateau potential and increases firing rate, but in contrast to blockade of the mAHP, muscarine prolongs bursts (90). As the influence of the sDAP wanes through a burst (and eventually collapses) from its activity-dependent inhibition by dynorphin (66, 84, 85), the sAHP would become paramount and assist in the precipitous hyperpolarisation observed at burst termination. Thus while both the mAHP and sAHP suppress steady state firing during bursts (89, 90), the mAHP appears to play no significant role in burst termination. However, it should be noted that the basal mean intraburst firing rate observed by Kirkpatrick and Bourque (89) of ~11 Hz was almost twice that observed in the study of Ghamari-Langroudi and Bourque (90), and the elevated firing rates associated with AHP inhibition were proportionately greater (to ~20 Hz after mAHP blockade, to ~11 Hz after sAHP blockade). It’s possible that the greater level of activity reported by Kirkpatrick and Bourque (89) allowed burst foreshortening due to a combination of strong sAHP activation coupled with activity-dependent sDAP inhibition. In contrast, the lower intraburst activity reported by Ghamari-Langroudi and Bourque (90) may have been insufficient to strongly invoke activity-dependent inhibition of the sDAP. Regardless of the precise interpretation, the presence of multiple Ca++-dependent AHPs and DAPs with contrasting kinetics and pharmacological sensitivity suggests that bursting could be finely controlled by afferent inputs selectively targeting different afterpotentials. At burst onset, DAPs must overcome AHPs, and the membrane potential must be depolarised enough for DAPs to summate. The sensitivity of DAPs to Ca++ may assist in this tuning; sDAPs can be observed following single spikes (56, 73, 76), whereas the mAHP, and especially the sAHP, need trains of spikes for full activation. Thus at depolarised membrane potentials, VP neurones are poised to burst. The act of bursting itself, both through activation of the sAHP and by deactivating the sDAP through autoinhibition, then generates the onset of the interburst interval. This interval is needed for recovery of full VP releasing capacity (4–6), as the membrane potential slowly depolarises to once again allow full expression of a DAP-instigated burst. While the mechanism of the slow depolarisation is unknown, it probably does not relate to the dynorphin/kappa receptor activity that controls burst termination (84).
OT Neurone Afterpotentials
Although we have reported DAPs in a small number of OT neurones (41, 79, 92, 93), their role in OT firing patterns is unclear. While most putative OT neurones fail to show the phasic bursting pattern of VP neurones, a few do, both in vivo and in vitro (26). The DAPs (fDAP and sDAP) found in a few OT neurones could underlie this activity. Interestingly, there is an increased incidence of DAPs in OT neurones during pregnancy and lactation (93) which undoubtedly involves the sDAP, but may involve the fDAP too. Although most OT neurones that do express DAPs do not exhibit the 20–30 sec bursts of VP neurones, some do. And DAPs in OT neurones can lead to a brief (a few seconds) period of postspike excitability (41). Thus although superficially similar, the DAPs observed in OT neurones may not result from the same mechanisms as those in VP neurones, and/or additional OT-specific properties may prevent sDAP summation and plateau potential formation.
OT neurones express other currents that may assist bursting during lactation. One such current is a sustained outward rectifier (SOR) that is strongly activated at membrane potentials near spike threshold and is deactivated by even small (5–15 mV) hyperpolarisations (94, 96). Following its deactivation, OT neurones show a rebound depolarisation that can generate brief spike trains. While these trains are not long enough to mimic milk ejection bursts, they nevertheless uncover a mechanism for excitability via inhibitory, GABAergic transmission. This is of interest because during lactation, GABA promotes the onset of a burst even while inhibiting background discharge (96). The SOR is a K+ current that puts OT neurones in a high conductance state. Much like M-current behaviour, at the offset of a small hyperpolarisation that deactivates the SOR the membrane potential again depolarises, and a rebound depolarization (RD) develops with the time necessary to reactivate the SOR. In addition, the RD is partially Ca++ dependent, suggesting its amplitude may be further shaped by the activation of Ca++ currents. Recently, OT has been shown to promote low threshold Ca++ currents activated at the offset of GABA-mediated inhibitory synaptic potentials in organotypic explants exhibiting milk-ejection type bursts (72). We speculate this excitability is also strongly influenced by the SOR-RD sequence (84, 96) and may contribute to the ability of randomly occurring IPSPs to impart irregularity to OT (and VP) neuronal behaviour when firing along the continuous–irregular continuum (40). Finally, the high conductance state and faster membrane time constant imparted by the SOR also may have consequences for the irregular-continuous firing continuum in OT neurones and its interaction with synaptic inputs, much as a continuous bombardment of synaptic potentials, as suggested above (39, 67).
OT neurones also have both mAHPs and sAHPs that regulate firing rate, and while similar in pharmacology and time course to those expressed in VP neurones (41, 93, 94), the currents underlying each are smaller in OT vs. VP neurones in virgin female rats. This may be simply related to the small density of high voltage Ca++ currents expressed by OT neurones (91). Whether or not the mAHP and sAHP simply gate firing rate, or whether they play a more complex role in pattern generation as suggested for each in VP bursting activity, is also unknown. But as will be discussed below, there is a marked enhancement of AHPs specifically in OT neurones in response to pregnancy and lactation.
Plasticity in OT Neurones During Pregnancy and Lactation
Plasticity in AHPs
There is a long and intriguing history of reversible morphological and physiological plasticity associated with OT neurones during the reproductive cycle. Landmark quantitative studies of SON ultrastructure revealed that by late pregnancy and extending into lactation, withdrawal of glial processes from between neurones and dendrites, cellular hypertrophy, increased close soma-somatic and dendro-dendritic appositions, and a marked increase in synaptic numbers, were all observed specifically on OT neurones, presumably in preparation for enhanced OT release needed during nursing (97, 98). These changes may well be triggered by the shifting plasma levels of the sex steroid hormones, oestradiol (E2) and progesterone during these periods, which in turn target CNS neurones, though perhaps not OT neurones directly. For example, both E2and progesterone rise during pregnancy, with progesterone falling dramatically two days prior to parturition. Mimicking this pattern in ovariectomised female rats induces maternal behaviour (99), and a similar regimen of progesterone followed by E2 induces the morphological plasticity in OT neurones with one caveat: there needs to be high central levels of OT (100). Although extracellular levels of OT in the SON or PVN do not appear to rise during pregnancy (101), mRNA for OT (102) and OT receptor binding in magnocellular nuclei (103) do increase during this period, and the steroid model of sustained E2 and progesterone followed by progesterone withdrawal mimics the increase in OT mRNA (104). Following on these findings, a recent in vitro model suggests that slices from (Day 19) pregnant rats (i.e., just prior to normal plasticity before the fall of P) treated with OT and/or OT + E2 express aspects of the morphological plasticity (increased soma-somatic apposition, increased synaptic activity) associated with parturition and lactation (105, 106). These results suggest that the act of making slices and washing may remove progesterone for a time sufficient to allow OT and E2 to exert direct hypothalamic effects. However, these studies reported a hyperosmotic media was required for full expression, suggesting that further excitation of OT neurones is needed to synergise with OT and E2.
We have investigated identified OT neurones during pregnancy and lactation with the goal of determining whether their electrical or synaptic properties underlie the dramatic changes in firing pattern associated with lactation (79, 91, 92, 107, 108). A consistently observed change is in the size of mAHP and sAHPs, the amplitude of which is larger in late pregnancy and in lactation. These changes are not due to an increased Ca++ current density, but could be related either to AHP channel density, to Ca++ handling, or in the case of SK channels, to some modification of the SK-calmodulin sensor’s interaction with Ca++ (91). Regardless, it appears OT neurones utilise enhanced braking mechanisms during periods of enhanced excitability, when highly sculpted, short synchronous bursts release a bolus of OT required for maximal mammary contraction and milk ejection.
Although previous in vivo data support the hypothesis that centrally applied OT can induce neuronal plasticity (98, 100), whether central OT receptor activity is required for this plasticity in vivo is unknown. Toward this end, we treated pregnant female rats with intracerebroventicular application of a specific OT antagonist or vehicle for 7–9 days during the last week of pregnancy before making hypothalamic slices at the end of pregnancy (days 19–22) (108). Administration of the OT antagonist completely blocked the normal plasticity of AHPs observed in pregnant rats, without effecting VP neurones. A similar treatment was found to disrupt the timing of OT release to suckling and pup weight gain (109). Whether or not these abnormalities are the result of the AHP suppression is of course undetermined, but together the studies suggest that central OT activity during pregnancy is an essential component in programming OT neurones for milk-ejection related release.
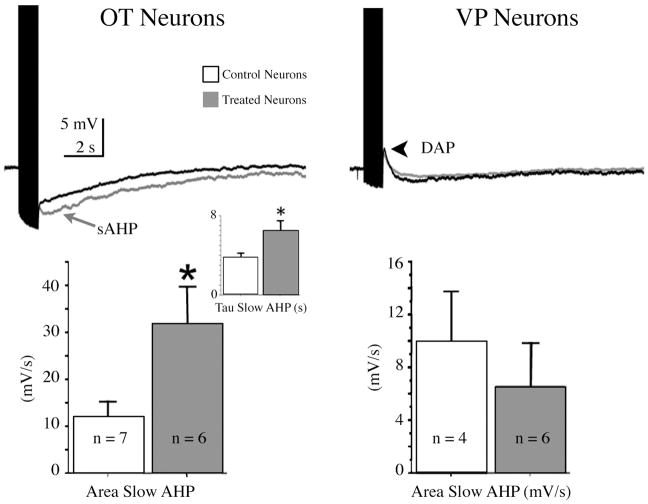
In recent preliminary experiments, we used the in vitro model of Langle et al (105) and Theodosis et al. (106) to determine whether or not OT and/or E2 work directly on hypothalamus to enhance AHPs during pregnancy. As shown in Fig. 4, OT neurones in coronal slices taken from rats at day 19 of pregnancy and treated with a combination of OT (500 nM) and E2 (500 pM) for 2 hrs showed strong, tonic enhancement of the sAHP, whereas VP neurones were unaffected. Many experiments are needed to determine the specific roles of OT and E2, their receptor specificity, and whether these results are limited to pregnant rats. And perhaps most importantly, how, and where, do OT and/or E2 receptor transduction lead to long lasting changes? For example, recent studies show that OT released centrally during pregnancy and lactation may exert effects on long-term potentiation and memory via the activation of a mitogen-activated protein kinase cascade and phosphorylation of cAMP-responsive element binding protein (110). Thus in addition to its well known function in contracting the myoepithelium in the mammary gland, and uterine smooth muscle, OT may centrally exert growth factor type effects with long term consequences for cellular and synaptic plasticity.
Figure 4. OT and E2 enhance AHPs in OT neurones from pregnant rats.
Slices from Day 19 pregnant rats were exposed either to a vehicle control or a combination of E2 (500 pM) and OT (10 nM) for 2 hrs. After washing, whole cell recordings were made in the SON and tested for AHPs, then later identified as VP or OT types with immunocytochemistry using standard procedures (e.g., Teruyama and Armstrong, 2007). Left top panel: The AHPs following a 1 sec train of spikes at 20 Hz in treated (grey) and control (black) slices. Traces represent averages of 7 control and 6 treated OT neurones. Treated neurones had a much larger sAHP. Left bottom panel: Histogram showing the difference in the means (± s.e.m.) of treated and untreated OT neurones of sAHP area. Inset: the increase in the sAHP was also accompanied by a longer decay constant for the sAHP. Right top panel: AHPs in VP neurones were not affected. Note the presence of DAPs. Right bottom panel: Histogram showing the difference in the means (± s.e.m.) of treated and untreated VP neurones of sAHP area.
In addition, dendritically released OT could promote bursting and spike clustering during lactation, via its ability to release Ca++ from intracellular stores (64, 111). This priming effect may further underlie OT’s ability to facilitate its own release and contribute to the depolarisation critical to milk-ejection bursts (70, 71). Ca++ itself may act as a second messenger and/or transcription factor for modulation and long term signaling, and my thus contribute to OT neuronal plasticity as well as a real time adjustment in firing pattern.
Synaptic Plasticity
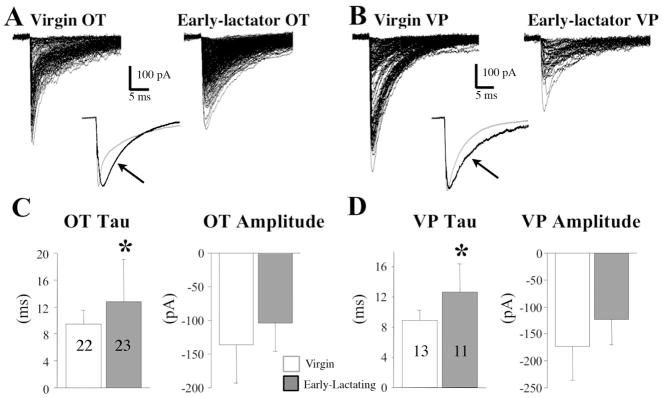
The degree to which changes in physiological synaptic function correlate with the aforementioned morphological synaptic plasticity is under debate. Brussaard et al. (112, 113) originally reported an increase in miniature IPSCs during pregnancy and early lactation, as well as a prolonged IPSC decay and a loss of neurosteroid sensitivity that appeared mediated by changes in GABAA-receptor alpha subunit ratios. More recent studies instead suggest that differences in GABAA-receptor alpha subunit clustering may mediate slow vs. fast decaying IPSCs (114). While these changes were specific to putative OT neurones, in our own preliminary studies (115) we found that both OT and VP neurones exhibited prolonged IPSCs during early lactation (Fig. 5). The precise impact of inhibitory synaptic plasticity on OT neuronal firing pattern during pregnancy and lactation is at this time undetermined. It also remains possible that increases in axo-somatic synaptic numbers during lactation are in part compensatory for cellular hypertrophy, rather than reflecting a novel functional importance.
Figure 5. The decay of IPSCs was slower during early lactation in OT and VP neurones.
A, superimposed traces of monoquantal, mIPSCs from OT neurones in slices taken from a virgin (left) and early-lactating rat (right). Inset: In the inset, traces from each set are averaged, and the averaged traces scaled to the peak and superimposed. Note the longer decay of mIPSCs in the OT neurone from the lactating rat (black trace, arrow). B, superimposed traces of monoquantal, mIPSCs from VP neurones in slices taken from a virgin (left) and early-lactating rat (right). Inset: In the inset, traces from each set are averaged, and the averaged traces scale to the peak and superimposed. Note the longer decay of mIPSCs in the VP neurone from the lactating rat (black trace, arrow). C, Left Panel: the decay tau of mIPSCs in OT neurones is significantly slower in early-lactating (Days 2–6) compared to virgin rats. Numbers represent the number of neurones for which averaged mIPSCs were taken (*p = 0.0045). Right Panel: mIPSC amplitudes in OT neurones were not significantly different in virgin compared to lactating rats. D, Left Panel: the decay tau of mIPSCs is significantly slower in VP neurones in early-lactating (Days 2–6) compared to virgin rats. Numbers represent the number of neurones for which averaged mIPSCs were taken (*p = 0.0041). Right panel: mIPSC amplitudes in VP neurones were not significantly different in virgin compared to lactating rats. Modified with permission from Li (115).
An increase in mEPSC frequency during mid-lactation, as well as a change in paired-pulse ratio of evoked IPSCs suggesting an increased probability of glutamate release in identified OT neurones, was first reported by Stern et al. (107). A later study found exactly the opposite result for probability, namely, a decreased probability of glutamate release in unidentified SON neurones during lactation (116). In this latter study, the decreased release probability appeared mediated by increased presynaptic inhibition via the activation of metabotropic glutamate receptors (mGluRs). The authors speculated that the withdrawal of astrocyte processes during lactation promoted diffusion of glutamate by effectively moving glutamate transporter activity away from synapses, where it would restrict glutamate diffusion and rapidly remove it from the juxtasynaptic, extracellular space. This was supported by the lack of effect of glutamate transporter inhibition during lactation vs. its effectiveness in reducing release probability in virgin rats. At this juncture, there is no resolution to the contrasting results of Stern et al. (107) and Oliet et al. (116) on the changes in paired-pulse ratio estimating release probability. Later studies by Oliet and others indicated that glial withdrawal did not alter ambient presynaptic inhibition of GABAergic synapses mediated by mGluRs (117), but during enhanced glutamate release, heterosynaptic inhibition was observed, again promoted by the enhanced diffusion afforded by glial withdrawal (118). Astrocytes are also a source for d-serine, which is a co-agonist at the NMDA receptor in the SON at the glycine site, and which regulates the relative degree of activity dependent potentiation vs. depression during repetitive afferent activation of glutamate synapses (119). Interestingly, the SON of lactating rats only shows potentiation to high frequency activity, when d-serine would be reduced near synapses due to glial withdrawal (119).
Concluding Remarks
The implementation of antidromic identification of magnocellular neurons in vivo allowed the first characterization of OT and VP firing patterns during evoked hormone release almost 40 years ago. Uncovering the precise membrane and synaptic properties of these two neuron types would then await intracellular filling, immunochemical identification, and voltage clamp analyses. These studies confirmed the asynchronous phasic bursting pattern of VP neurons, and this pattern’s resilience in vitro allowed the identification of some properties, such as the DAP and plateau potential, as essential to the underlying pattern. However DAPs are kinetically and mechanistically complex, and their underlying currents are still not fully understood. Ongoing studies aimed at uncovering the molecular basis of these potentials should provide important tools for future dissection of their precise roles. In addition, while the phasic bursting pattern in vitro is macroscopically similar to that observed in vivo, serial analysis of spike trains within bursts in vivo suggests that synaptic activity imparts a stochastic spike distribution not present in vitro-this shift toward more irregular intraburst firing may be critical in producing maximal VP release.
While the behaviour of OT neurons in response to some stimuli, such as osmotic challenge, is represented in the irregular-continuous firing continuum observed in hypothalamic slices, the importance of most underlying currents to OT firing is still unknown. Shifts toward spike time irregularity are influenced by spontaneous GABAergic synaptic potentials, and these potentials are much more frequent in OT vs. VP neurons. Since irregular firing presages milk-ejection bursts, future studies need to address the importance of altered GABAergic activity in precipitating bursts, and especially, how this activity interacts with intrinsic membrane properties. While asynchronous, milk-ejection type bursts have been observed after pharmacological manipulation in hypothalamic slices, synchronized bursts like those observed in lactating rats have been recorded, to date, only in organotypic slices. Together these data suggest the mechanisms for the burst per se may be intrinsic, but that synchronization is controlled by synaptic input. OT neurons exhibit considerable plasticity during pregnancy and lactation, not only in synaptic properties, but also in the up regulation of Ca++-dependent AHPs that gate intraburst spike frequency and sculpt burst duration. An in vitro model for this regulation suggests that central OT, in combination with E2, may be sufficient to instigate changes in both synaptic and intrinsic membrane properties during late pregnancy.
Acknowledgments
Supported by NIH grants NS23941 (WEA) and HL093728 (RT).
References
- 1.Armstrong WE. The neurophysiology of neurosecretory cells. J Physiol (Lond) 2007;585(Pt 3):645–47. doi: 10.1113/jphysiol.2007.145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 3.Douglas W, Poisner A. Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from the neurohypophysis. J Physiol (Lond) 1964;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicknell R. Downstream consequences of bursting activity in oxytocin neurones. In: Leng G, editor. Pulsatility in Neuroendocrine Systems. Boca Raton: CRC Press; 1988. pp. 62–74. [Google Scholar]
- 5.Bicknell REJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release from the rat neurohypophysis. Neuroendocrinology. 1981;33:295–99. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- 6.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol (Lond) 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutton A, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol (Lond) 1979;290:443–50. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Res. 1991;540(1–2):349–52. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- 9.Dyball REJ, Tasker J-G, Wuarin J-P, Dudek FE. In vivo intracellular of neurons in the supraoptic nucleus of the rat hypothalamus. J Neuroendocr. 1991;3(4):383–86. doi: 10.1111/j.1365-2826.1991.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 10.Dudek F, Hatton GI, Macvicar BA. Intracellular recordings from the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1980;301:101–14. doi: 10.1113/jphysiol.1980.sp013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatton GI, Armstrong WE, Gregory WA. Spontaneous and osmotically stimulated activity in slices of rat hypothalamus. Brain Res Bull. 1978;3:497–508. doi: 10.1016/0361-9230(78)90079-5. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong WE, Sladek CR. Spontaneous ‘phasic firing’ in supraoptic neurons recorded from hypothalamo-neurohypophysial explants in vitro. Neuroendocrinology. 1982;34:405–19. doi: 10.1159/000123336. [DOI] [PubMed] [Google Scholar]
- 13.Bourque CW, Renaud LP. A perfused in vitro preparation of hypothalamus for electrophysiological studies on neurosecretory neurons. J Neurosci Meth. 1983;7:203–14. doi: 10.1016/0165-0270(83)90002-x. [DOI] [PubMed] [Google Scholar]
- 14.Jourdain P, Poulain DA, Theodosis DT, Israel JM. Electrical properties of oxytocin neurons in organotypic cultures from postnatal rat hypothalamus. J Neurophysiol. 1996;76(4):2772–85. doi: 10.1152/jn.1996.76.4.2772. [DOI] [PubMed] [Google Scholar]
- 15.Legendre P, Cooke IM, Vincent J-D. Regenerative response of long duration recorded intracellularly from dispersed cell cultures of fetal mouse hypothalamus. J Neurophysiol. 1982;48:1121–41. doi: 10.1152/jn.1982.48.5.1121. [DOI] [PubMed] [Google Scholar]
- 16.Mason WT, Cobbett P, Inenaga K, Legendre P. Ionic currents in cultured supraoptic neurons: actions of peptides and transmitters. Brain Res Bull. 1988;20(6):757–64. doi: 10.1016/0361-9230(88)90088-3. [DOI] [PubMed] [Google Scholar]
- 17.Lemos JR, Nordmann JJ, Cooke IM, Stuenkel EL. Single channels and ionic currents in peptidergic nerve terminals. Nature. 1986;319(6052):410–12. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;88(2):380–84. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourque CW. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol (Lond) 1990;421:247–62. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe H, Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol (Lond) 1982;327:157–71. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson KR, Ronnekliev OK, Kelly MJ. Inward rectification (Ih) in immunocytochemically- identified vasopressin and oxytocin neurons of the guinea-pig supraoptic nucleus. J Neuroendocrinol. 1990;2(3):261–65. doi: 10.1111/j.1365-2826.1990.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 22.Hlubek MD, Cobbett P. Outward potassium currents of supraoptic magnocellular neurosecretory cells isolated from the adult guinea-pig. J Physiol (Lond) 1997;502(Pt 1):61–74. doi: 10.1111/j.1469-7793.1997.061bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inenaga K, Honda E, Hirakawa T, Nakamura S, Yamashita H. Glutamatergic synaptic inputs to mouse supraoptic neurons in calcium- free medium in vitro. J Neuroendocrinol. 1998;10(1):1–7. doi: 10.1046/j.1365-2826.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- 24.Stachniak TJ, Bourque CW. Visually guided whole cell patch clamp of mouse supraoptic nucleus neurons in cultured and acute conditions. Am J Physiol Regul Integr Comp Physiol. 2006;291(1):R68–76. doi: 10.1152/ajpregu.00830.2005. [DOI] [PubMed] [Google Scholar]
- 25.Fagan M, Andrew RD. Intracellular study of calcium-related events in cat magnocellular neuroendocrine cells. J Physiol (Lond) 1991;434:337–49. doi: 10.1113/jphysiol.1991.sp018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 27.Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16(9):727–39. doi: 10.1111/j.1365-2826.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 28.Poulain DA, Brown D, Wakerley JB. Statistical analysis of patterns of electrical activity in vasopressin and oxytocin-secreting neurones. In: Leng G, editor. Pulsatility in Neuroendocrine Systems. Boca Raton: CRC Press; 1988. pp. 120–54. [Google Scholar]
- 29.Richard P, Moos F, Dayanithi G, Gouzenes L, Sabatier N. Rhythmic activities of hypothalamic magnocellular neurons: autocontrol mechanisms. Biol Cell. 1997;89(9):555–60. [PubMed] [Google Scholar]
- 30.Leng G, Way S, Dyball RE. Identification of oxytoxin cells in the rat supraoptic nucleus by their response to cholecystokinin injection. Neurosci Lett. 1991;122(2):159–62. doi: 10.1016/0304-3940(91)90847-m. [DOI] [PubMed] [Google Scholar]
- 31.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253(4 Pt 2):R661–65. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- 32.Day TA, Ferguson AV, Renaud LP. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol (Lond) 1984;355:237–49. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris MC. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocr. 1979;82:115–25. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- 34.Brimble MJ, Dyball REJ. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977;271:253–71. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason WT. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B. 1983;217:141–61. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- 36.Pittman QJ, Hatton JD, Bloom FE. Spontaneous activity in perfused hypothalamic slices: dependence on calcium content of perfusate. Exp Brain Res. 1981;42:49–52. doi: 10.1007/BF00235728. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita H, Inenaga K, Kawata M, Sano Y. Phasically firing neurons in the supraoptic nucleus of the rat hypothalamus: immunocytochemical and electrophysiological studies. Neurosci Lett. 1983;37(1):87–92. doi: 10.1016/0304-3940(83)90509-8. [DOI] [PubMed] [Google Scholar]
- 38.Bourque CW, Renaud LP. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. J Physiol (Lond) 1984;349:631–42. doi: 10.1113/jphysiol.1984.sp015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558(Pt 1):161–80. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol (Lond) 2007;581(Pt 1):221–40. doi: 10.1113/jphysiol.2006.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol (Lond) 1994;475(1):115–28. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobbett P, Smithson KG, Hatton GI. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986;362:7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- 43.Erickson KR, Ronnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology. 1993;57(5):789–800. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- 44.Poulain D, Wakerley JB, Dyball REJ. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B. 1977;196:367–84. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- 45.Hatton GI. Phasic bursting activity of rat paraventricular neurones in the absence of synaptic transmission. J Physiol (Lond) 1982;327:273–84. doi: 10.1113/jphysiol.1982.sp014231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrew RD. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. J Physiol (Lond) 1987;384:451–65. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliet SH, Bourque CW. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol (Lond) 1992;455(Pt 1):291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol (Lond) 1995;484(Pt 2):415–24. doi: 10.1113/jphysiol.1995.sp020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown CH, Bull PM, Bourque CW. Phasic bursts in rat magnocellular neurosecretory cells are not intrinsically regenerative in vivo. Eur J Neurosci. 2004;19(11):2977–83. doi: 10.1111/j.0953-816X.2004.03408.x. [DOI] [PubMed] [Google Scholar]
- 50.Sladek CD, Johnson AK. Effect of anteroventral third ventricle lesions on vasopressin release by organ-cultured hypothalamo-neurohypophyseal explants. Neuroendocrinology. 1983;37(1):78–84. doi: 10.1159/000123519. [DOI] [PubMed] [Google Scholar]
- 51.Leng G, Blackburn RE, Dyball REJ, Russell JA. Role of anterior peri-third ventricular structures in the regulation of supraoptic neuronal activity and neurohypophysial hormone secretion in the rat. J Neuroendocrinol. 1989;1:35–46. doi: 10.1111/j.1365-2826.1989.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 52.Jhamandas JH, Renaud LP. A ©-aminobutyric-acid-mediated baroreceptor input to supraoptic vasopressin neurones in the rat. J Physiol (Lond) 1986;381:595–606. doi: 10.1113/jphysiol.1986.sp016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dreifuss J-J, Harris MC, Tribollet E. Excitation of phasically firing hypothalamic supraoptic neurones by carotid occlusion in rats. J Physiol (Lond) 1976;257:337–54. doi: 10.1113/jphysiol.1976.sp011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. J Physiol (Lond) 2009;587(Pt 23):5679–89. doi: 10.1113/jphysiol.2009.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakerley JB, Poulain DA, Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978;148:425–40. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- 56.Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984;51(3):552–66. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- 57.Dreifuss J-J, Tribollet E, Baertschi AJ, Lincoln DW. Mammalian endocrine neurones: control of phasic activity by antidromic action potentials. Neurosci Lett. 1976;3:281–86. doi: 10.1016/0304-3940(76)90055-0. [DOI] [PubMed] [Google Scholar]
- 58.Sabatier N, Leng G. Bistability with hysteresis in the activity of vasopressin cells. J Neuroendocrinol. 2007;19(2):95–101. doi: 10.1111/j.1365-2826.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita H, Okuya S, Inenaga K, Kasai M, Uesugi S, Kannan H, Kaneko T. Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro. Brain Res. 1987;416:364–68. doi: 10.1016/0006-8993(87)90920-6. [DOI] [PubMed] [Google Scholar]
- 60.Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21(17):6967–77. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lincoln DW, Wakerley JB. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975;250:443–61. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57(4):1027–38. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- 63.Wang YF, Hatton GI. Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. J Neurophysiol. 2004;91(5):2312–21. doi: 10.1152/jn.00697.2003. [DOI] [PubMed] [Google Scholar]
- 64.Wang YF, Ponzio TA, Hatton GI. Autofeedback effects of progressively rising oxytocin concentrations on supraoptic oxytocin neuronal activity in slices from lactating rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1191–98. doi: 10.1152/ajpregu.00725.2005. [DOI] [PubMed] [Google Scholar]
- 65.Israel JM, Le Masson G, Theodosis DT, Poulain DA. Glutamatergic input governs periodicity and synchronization of bursting activity in oxytocin neurons in hypothalamic organotypic cultures. Eur J Neurosci. 2003;17(12):2619–29. doi: 10.1046/j.1460-9568.2003.02705.x. [DOI] [PubMed] [Google Scholar]
- 66.Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci. 2004;24(20):4818–31. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4(9):739–51. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- 68.Zsiros V, Hestrin S. Background synaptic conductance and precision of EPSP-spike coupling at pyramidal cells. J Neurophysiol. 2005;93(6):3248–56. doi: 10.1152/jn.01027.2004. [DOI] [PubMed] [Google Scholar]
- 69.Brown D, Fontanaud P, Moos FC. The variability of basal action potential firing is positively correlated with bursting in hypothalamic oxytocin neurones. J Neuroendocrinol. 2000;12(6):506–20. doi: 10.1046/j.1365-2826.2000.00478.x. [DOI] [PubMed] [Google Scholar]
- 70.Moos F, Fontanaud P, Mekaouche M, Brown D. Oxytocin neurones are recruited into coordinated fluctuations of firing before bursting in the rat. Neuroscience. 2004;125(2):391–410. doi: 10.1016/j.neuroscience.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4(7):e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28(2):385–94. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221(4615):1050–52. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- 74.Bourque C. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986;70:204–09. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- 75.Li Z, Hatton GI. Reduced outward K+ conductances generate depolarizing after-potentials in rat supraoptic nucleus neurones. J Physiol (Lond) 1997;505(Pt 1):95–106. doi: 10.1111/j.1469-7793.1997.095bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roper P, Callaway J, Shevchenko T, Teruyama R, Armstrong W. AHP’s, HAP’s and DAP’s: how potassium currents regulate the excitability of rat supraoptic neurones. J Comput Neurosci. 2003;15 (3):367–89. doi: 10.1023/a:1027424128972. [DOI] [PubMed] [Google Scholar]
- 77.Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol (Lond) 1998;510(Pt 1):165–75. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol (Lond) 2002;545(Pt 2):537–42. doi: 10.1113/jphysiol.2002.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol. 2007;98(5):2612–21. doi: 10.1152/jn.00599.2007. [DOI] [PubMed] [Google Scholar]
- 80.Smith BN, Armstrong WE. Histamine enhances the depolarizing afterpotential of immunohistochemically identified vasopressin neurons in the rat supraoptic nucleus via H1-receptor activation. Neuroscience. 1993;53(3):855–64. doi: 10.1016/0306-4522(93)90630-x. [DOI] [PubMed] [Google Scholar]
- 81.Armstrong WE, Sladek CD. Evidence for excitatory actions of histamine on supraoptic neurons in vitro: mediation by an H1-type receptor. Neuroscience. 1985:16307–22. doi: 10.1016/0306-4522(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 82.Brown CH, Ghamari-Langroudi M, Leng G, Bourque CW. Kappa-opioid receptor activation inhibits post-spike depolarizing after- potentials in rat supraoptic nucleus neurones in vitro. J Neuroendocrinol. 1999;11(11):825–28. doi: 10.1046/j.1365-2826.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- 83.Brown CH, Ludwig M, Leng G. kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18(22):9480–88. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol (Lond) 2004;557(Pt 3):949–60. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95(5):3235–44. doi: 10.1152/jn.00062.2006. [DOI] [PubMed] [Google Scholar]
- 86.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280(8):6423–33. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 87.Bourque C, Brown D. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987:82185–90. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- 88.Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol (Lond) 1998;513(Pt 2):493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol (Lond) 1996;494(2):389–98. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24(35):7718–26. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol (Lond) 2005;566(Pt 2):505–18. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16(16):4861–71. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teruyama R, Armstrong WE. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J Neuroendocrinol. 2002;14(12):933–44. doi: 10.1046/j.1365-2826.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- 94.Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol (Lond) 1995;488(Pt 3):701–8. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. J Physiol (Lond) 1997;500(Pt 2):497–508. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moos FC. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol (Lond) 1995;488(Pt 1):103–14. doi: 10.1113/jphysiol.1995.sp020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004;25(3):403–11. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiological reviews. 2008;88(3):983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 99.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114(1):930–40. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 100.Montagnese C, Poulain DA, Theodosis DT. Influence of ovarian steroids on the ultrastructural plasticity of the adult supraoptic nucleus induced by central administration of oxytocin. J Neuroendocrinology. 1990;22:25–31. doi: 10.1111/j.1365-2826.1990.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 101.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 102.Horwitz MJ, Bloch KD, Kim NB, Amico JA. Expression of the endothelin-1 and oxytocin genes in the hypothalamus of the pregnant rat. Brain Research. 1994;64:859–64. doi: 10.1016/0006-8993(94)91905-4. [DOI] [PubMed] [Google Scholar]
- 103.Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(1):R53–58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- 104.Crowley RS, Insel TR, O’Keefe JA, Kim NB, Amico JA. Increased accumulation of oxytocin messenger ribonucleic acid in the hypothalamus of the female rat: induction by long term estradiol and progesterone administration and subsequent progesterone withdrawal. Endocrinilogy. 1995;136:224–31. doi: 10.1210/endo.136.1.7828535. [DOI] [PubMed] [Google Scholar]
- 105.Langle SL, Poulain DA, Theodosis DT. Induction of rapid, activity-dependent neuronal-glial remodelling in the adult rat hypothalamus in vitro. Eur J Neurosci. 2003;18(1):206–14. doi: 10.1046/j.1460-9568.2003.02741.x. [DOI] [PubMed] [Google Scholar]
- 106.Theodosis DT, Koksma JJ, Trailin A, Langle SL, Piet R, Lodder JC, Timmerman J, Mansvelder H, Poulain DA, Oliet SH, Brussaard AB. Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol Cell Neurosci. 2006;31(4):785–94. doi: 10.1016/j.mcn.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 107.Stern JE, Hestrin S, Armstrong WE. Enhanced neurotransmitter release at glutamatergic synapses on oxytocin neurones during lactation in the rat. J Physiol (Lond) 2000;526(Pt 1):109–14. doi: 10.1111/j.1469-7793.2000.t01-1-00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teruyama R, Lipschitz DL, Wang L, Ramoz GR, Crowley WR, Bealer SL, Armstrong WE. Central blockade of oxytocin receptors during mid-late gestation reduces amplitude of slow afterhyperpolarization in supraoptic oxytocin neurons. Am J Physiol Endocrinol Metab. 2008;295(5):E1167–71. doi: 10.1152/ajpendo.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lipschitz DL, Crowley WR, Bealer SL. Central blockade of oxytocin receptors during late gestation disrupts systemic release of oxytocin during suckling in rats. J Neuroendocrinol. 2003;15 (8):743–48. doi: 10.1046/j.1365-2826.2003.01052.x. [DOI] [PubMed] [Google Scholar]
- 110.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6(4):384–90. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 111.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418(6893):85–9. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 112.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19(5):1103–14. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 113.Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516(Pt 2):513–24. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koksma JJ, Fritschy JM, Mack V, Van Kesteren RE, Brussaard AB. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol Cell Neurosci. 2005;28(1):128–40. doi: 10.1016/j.mcn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Li C. The effects of GABAergic blockade on the firing patterns of supraoptic neurons [dissertation] Memphis (TN): University of Tennesse; 2006. p. 116. [Google Scholar]
- 116.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292(5518):923–26. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 117.Piet R, Bonhomme R, Theodosis DT, Poulain DA, Oliet SH. Modulation of GABAergic transmission by endogenous glutamate in the rat supraoptic nucleus. Eur J Neurosci. 2003;17(9):1777–85. doi: 10.1046/j.1460-9568.2003.02611.x. [DOI] [PubMed] [Google Scholar]
- 118.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101(7):2151–55. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–84. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]