Abstract
The ‘executive’ regions of the prefrontal cortex (PFC) such as the dorsolateral PFC (dlPFC) and its rodent equivalent medial PFC (mPFC) are thought to respond in concert with the ‘limbic’ regions of the PFC such as the orbitofrontal (OFC) cortex to orchestrate behavior that is consistent with context and expected outcome. Both groups of regions have been implicated in behavioral abnormalities associated with addiction and psychiatric disorders, in particular, schizophrenia and mood disorders. Theories about the pathophysiology of these disorders, however, incorporate abnormalities in discrete PFC regions independently of each other or assume they are one and the same and, thus, bunch them under umbrella of ‘PFC dysfunction.’ Emerging data from animal studies suggest that mPFC and OFC neurons display opposing patterns of plasticity during associative learning and in response to repeated exposure to psychostimulants. These data corroborate clinical studies reporting different patterns of activation in OFC versus dlPFC in individuals with schizophrenia or addictive disorders. These suggest that concomitant but divergent engagement of discrete PFC regions is critical for learning stimulus-outcome associations, and the execution of goal-directed behavior that is based on these associations. An atypical interplay between these regions may lead to abnormally high or low salience assigned to stimuli, resulting in symptoms that are fundamental to many psychiatric and addictive disorders, including attentional deficits, improper affective response to stimuli, and inflexible or impulsive behavior.
Keywords: schizophrenia, substance abuse, working memory, orbitofrontal cortex, electrophysiology, learning
INTRODUCTION
Historically, the operations of the cerebral cortex have been attributed to two distinct patterns of neuronal processing. One is to divide cortex into ‘elementary units’ such as cortical columns (Pandya and Kuypers, 1969; Jones and Powell, 1970; Nauta, 1971). This view originated from early discoveries of discrete language centers in the brain and has expanded to include current ‘phrenology’-like assignment of specific cognitive processes to exact cortical locations. Behavioral correlations of discrete cortical lesions in human and animal studies, as well as neurophysiological findings of distinct integrative representations in different subregions, have supported this modular view of cognitive processing (Mishkin and Pribram, 1955; Goldman and Rosvold, 1970; Stuss and Benson, 1984; Passingham et al, 2000). The other is the connectionist view that emphasizes parallel distributed neural networks in mediation of complex cognitive processes (Edelman and Mouncastle, 1978; Goldman-Rakic, 1988; Damasio, 1989; Mesulam, 1990; Fuster, 2003). This view is supported by substantial morphological evidence (eg, Selemon and Goldman-Rakic, 1988) as well as electrophysiological recordings in behaving animals and functional-imaging studies in humans (Fuster and Alexander, 1971; Batuev et al, 1985; Goldman-Rakic, 1988; Treue and Maunsell, 1996; Dolan et al, 1997; Friston, 2002; Gruber et al, 2007). Contemporary approaches to cortical function consider both types of processing relevant and generally assume that modular processing is a manifestation of parallel computational networks. These networks may be maintained by separate sets of distributed structures and are responsible for different forms of associative processing (Fodor and Pylyshyn, 1988; Kimberg and Farah, 1993; Mesulam, 1998).
Based on this literature, we assume that the proper functioning of the cerebral cortex depends on the successful dynamic interactions between distributed cortical networks and advance the hypothesis that disorders that involve abnormal cognitive–affective interface result from disturbed interactions between these parallel networks. This view predicts concomitant disruptions in several cortical networks in disease states and, therefore, differs from most existing perspectives about the role of cerebral cortex in disease, which focus on a single ‘cortical organ’ as being dysfunctional in specific diseases; for example, orbitofrontal dysfunction in addiction, dorsolateral prefrontal cortex (dlPFC) dysfunction in schizophrenia. Fundamental to our hypothesis is that parallel cortical networks are critical for learning of proper stimulus-outcome associations and that disturbance in this form of plasticity leads to aberrant processing of these associations and disruption in execution of goal-directed behavior. The focus of the reviewed literature that supports this hypothesis will be on the ‘limbic’ orbitofrontal cortex (OFC) and the ‘executive’ dlPFC and medial prefrontal cortex (mPFC). We briefly review the evidence to suggest that although these prefrontal cortex (PFC) subregions subserve different functions, they are both vulnerable structures in major psychiatric disorders, in particular schizophrenia, and in addiction. We will then examine the electrophysiological evidence in laboratory animals that suggest that these subregions of the PFC display parallel but distinct patterns of plasticity during associative learning. We will conclude with a review of the data on the parallel but dissimilar patterns of plasticity in these cortical regions in an animal model of psychosis and addiction. Together, these new findings will support the idea that the interplay between these cortical structures may provide a substrate for maladaptive processes that contribute to major psychiatric disorders, addiction, and the prevalent comorbidity among these disorders.
FUNCTIONS OF THE PFC: PLASTICITY VERSUS FLEXIBILITY
The principal function of the cerebral cortex in general and the PFC in particular is thought to be the temporal organization of behavior (eg, Damasio, 1979; Fuster, 2001). Specifically, frontal cortical regions are thought to (1) detect situations that demand mediation; (2) direct selective attention to stimuli relevant to this situation; (3) suppress distractions caused by irrelevant stimuli; (4) bring on-line relevant past memories; (5) plan a behavioral sequence based on these memories and the present relevant stimuli; and (6) encode the preparatory set that leads to motor execution of the appropriate behavior. Thus, functions associated with the PFC involve working in the ‘present’ to coordinate behavior based on cross-temporal contingencies. Accordingly, most of the research focused on understanding PFC function has involved assessing the response of neurons during dynamic events, such as attention or working memory (eg, Fuster and Alexander, 1971; Wilkins et al, 1987; Funahashi et al, 1993).
The idea that plasticity, or experience-induced lasting changes in synaptic strength or structural features, is a component of PFC function has historically received little attention because a lasting effect is somewhat inconsistent with the dynamic and flexible function of the PFC. Experience-induced synaptic and structural plasticity have generally been localized to the hippocampus and basal ganglia regions, such as the striatum. Nevertheless, studies performed in primate somato-sensory and auditory cortex have long shown plasticity or ‘reorganization’ in cortical columns in response to obliteration of sensory input to these regions (Harrington and Merzenich, 1970). Somatosensory cortical systems also show experience-induced plasticity by increasing neuronal representation to a sensory event after the animals learn to associate that event with a reinforced outcome (Blake et al, 2005). More recent electrophysiological studies in primates and rodents have shown that this form of plasticity generalizes to OFC as well as medial and lateral PFC by demonstrating that neurons in these regions encode novel visuo-motor, audio-motor, and olfacto-motor associations (Rolls et al, 1996; Asaad et al, 1998; Passingham et al, 2000; Schoenbaum et al, 2003; Boettiger and D’Esposito, 2005). Collectively, these findings indicate that neurons in different PFC subregions are plastic and play a role in associative learning. Thus, the PFC—in addition to its classic executive functions that include assignment of attentional reserves, shifting between competing behavioral sets, conflict monitoring, and sensorimotor integration—also partakes in the acquisition of simple associations. This dual function suggests that the same PFC neuronal networks that represent the stimulus-outcome associations may subserve the executive functions such as attentional tuning and decision making that are related to these associations. This is significant in the context of major psychiatric disorders and addiction, which involve cognitive deficits as well as behavioral anomalies indicative of disrupted stimulus (or action)-outcome associations, in that a faulty learning of associations by PFC networks may be fundamental to aberrant encoding of cognitive processes by these same networks. As would be expected, however, the ‘normal’ pattern of PFC participation in associative learning is not simple and, as described in the following section, appears to involve shifting dynamics between at least two PFC regions, underscoring the interactive role of multiple PFC regions in the pathophysiology of above-mentioned disorders.
DIVERGENT ROLES OF OFC AND mPFC IN ENCODING ASSOCIATIVE LEARNING
Although an integrative approach to PFC’s executive functions has been argued convincingly by Fuster and others (Shallice, 1982; Baddeley, 1996; Fuster, 2001; Miller and Cohen, 2001), the general tendency in the biological psychiatry and addiction fields has been to assign specific functions to discrete PFC regions, in particular dlPFC and OFC. This functional differentiation approach is supported by notable differences in the pattern of anatomical connections to these regions. For example, the primate dlPFC and its rat equivalent of mPFC receive extensive innervations from the mediodorsal nucleus of thalamus and send prominent projections to dorsal striatum, nucleus accumbens, and ventral tegmental area (VTA) (Groenewegen and Uylings, 2000; Ongur and Price, 2000; Brown and Bowman, 2002). This pattern of connections is consistent with a key role at the top of the executive hierarchy and as the ultimate regulator of goal-directed behavior (Fuster, 2001). Accordingly, these regions are critical for key executive functions such as set-shifting (Ragozzino et al, 1998; Brown and Bowman, 2002; Stefani et al, 2003) and inhibitory control over behavior (Watanabe, 1986). In addition, dlPFC and mPFC have been strongly implicated in the processing of working memory primarily because lesions of dlPFC in primates and mPFC in rats impair working memory function (Mishkin and Pribram, 1955; Sakurai and Sugimoto, 1985). Electrophysiological studies in both species as well as imaging studies in humans also show neuronal activation in these regions during the delay period of working memory tasks (Fuster and Alexander, 1971; Funahashi et al, 1989; Jung et al, 1998). Another dorsal PFC region, the anterior cingulate, receives similar reciprocal projections as the dlPFC and mPFC but has been primarily implicated in attentional processes and in conflict monitoring (Dalley et al, 2004).
The OFC receives prominent inputs from sensory associative cortices, particularly olfactory, gustatory, and visual areas, as well as from hypothalamus and amygdala, positioning it at a suitable level to integrate potentially salient information about environmental contingencies (Rolls, 1996; Groenewegen and Uylings, 2000; Ongur and Price, 2000). OFC has reciprocal connections with primate dlPFC (rat mPFC) and may relay the integrated and ‘value-tagged’ summary of sensory inputs to these regions of PFC (Rolls, 1998). Lesion and electrophysiological studies in experimental animals indicate that OFC is involved in assessment of the rewarding value of salient stimuli and in prediction of such events (Rolls et al, 1996; Jentsch et al, 2002; Schoenbaum et al, 2003). OFC is also critical for reversal learning because subjects and animals with OFC lesions have profound difficulty during reversal of stimuli–response contingencies (Rolls, 1998). In addition, OFC (and ventral PFC) exercise inhibitory control over impulsive and socially inappropriate behaviors (Anderson et al, 1999; Iversen and Mishkin, 1970).
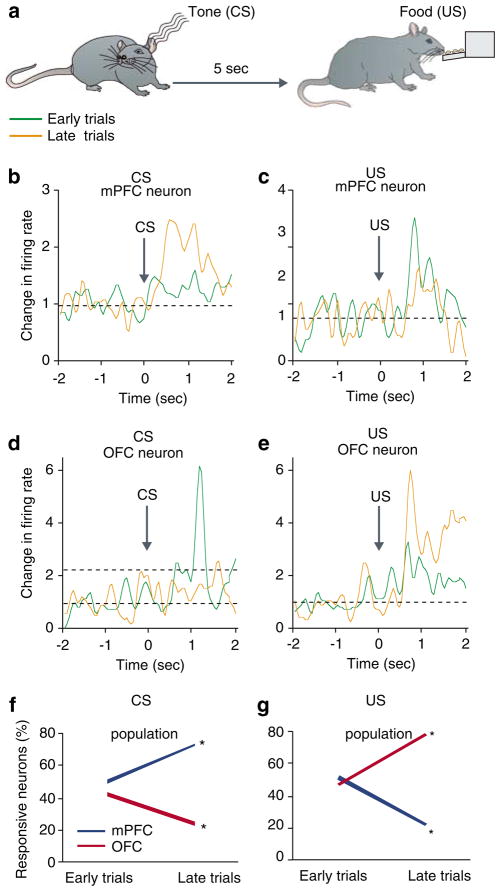
The anatomical and functional distinctions between OFC vs dorsal regions of the PFC have led to a prevailing view that these subregions subserve different functions and organize behavior through their separate projections to the basal ganglia in particular dorsal and ventral striatum (Robbins and Everitt, 2002; Haber et al, 2006). A complementary view is that different subregions participate in parallel monitoring of similar events and that the dynamic interaction of these parallel networks is critical for proper organization of behavior. Evidence for this level of processing is beginning to emerge. Human imaging studies have shown dissociative pattern of activation in dlPFC and OFC during working memory (Perlstein et al, 2002). In animal studies, simultaneous recordings from dorsomedial PFC and motor cortex neurons show functional coupling during performance of a working memory task (Narayanan and Laubach, 2006). Recent studies from our lab suggest that although multiple cortical regions demonstrate plasticity during associative learning, the pattern of plasticity is region and modality specific (Homayoun and Moghaddam, 2005). We recorded simultaneously from mPFC and OFC neurons of rats (Figure 1) during a form of classical conditioning where a conditioned stimulus (CS, tone) was followed by an unconditioned stimulus (US, food) (Figure 2). This experimental design allowed us to compare the dynamics of neuronal activity in the two PFC subregions beginning from the very first encounters with CS and US. As the training session began, neurons in both regions exhibited an active representation of various task components, including the tone and reward consumption (depicted as ‘early trials’ in Figure 2). Toward the end of the conditioning session, both regions modified their representations of these salient events. The pattern of this modification was different in each region. In the mPFC, representation of rewarding events gradually decreased, whereas representation of the task rule (tone) increased. In the OFC, the representation of reward was enhanced and that of task rule contingency was reduced (Figure 2). Changes in representation included the number of neurons that were recruited to represent each event as well as the strength by which individual neurons encoded for that event. Thus, at early stages of associative learning, both regions appear to actively and concomitantly engage in learning of simple stimulus-outcome associations in an opposing manner.
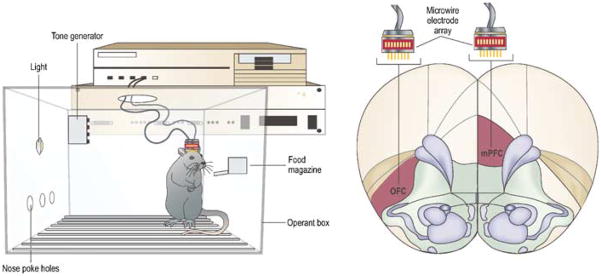
Figure 1.
Microwire arrays for electrophysiological recordings can be implanted in several cortical regions. Using this method, unit activity and field potentials that are time locked to specific behavioral events can be measured simultaneously in multiple regions, while rats are performing tasks in operant boxes.
Figure 2.
PFC subregions display differential patterns of plasticity during a classical conditioning paradigm. (a) Rats were trained inside an operant chamber to associate a tone (CS) with food reward (US) delivered 5 s later. Each rat received 18 presentation of CS with an inter-CS interval of 120 s. (b–e) Single neurons recorded from the mPFC or OFC exhibited opposite plastic responses to task relevant events of CS and US. Examples compare the phasic peri-event time histograms (window=±2 s around event, bin=50 ms) of individual neurons during early and late eight trials within the same learning session. Note that the mPFC neuron in (b) amplified its representation of CS, whereas the other mPFC neuron in (d) loses its initial response to US. In contrast, the OFC neuron in (c) diminishes its representation of CS, whereas the OFC neuron in (e) amplifies its response to UCS. Firing rates are normalized to pre-tone baseline period. (f–g) Plasticity in the population response of the two regions assessed by the proportion of neurons with a phasic response. The mPFC and OFC display opposite trajectories; the mPFC enhanced its representation of CS and OFC that of US. *p<0.05 compared to early trials (based on 136 mPFC and 94 OFC neurons).
These findings suggest that the nature of the interaction of mPFC and OFC networks during learning is not ‘in parallel’ in the classical sense, meaning that different networks process the same events similarly but in varying intensities, or ‘in series’, meaning that response in one network follows the response in another. Although both regions behaved similarly during the early stages of learning, the pattern of plasticity in each region was divergent and modality specific, suggesting that different modulatory mechanisms mediate the learning of associations in each region. Taken together, these findings suggest that opposing pattern of activation in OFC and mPFC may play a critical role in learning and other cognitive processes. This dynamic relationship may be a functional substrate at the circuitry level for convergence of various parameters that regulate normal and pathophysiological neuroplasticity processes at the molecular and cellular level.
MULTIPLE CORTICAL REGIONS ARE IMPLICATED IN PSYCHIATRIC DISORDERS AND ADDICTION
In recent years, functional imaging studies have implicated similar regions of the PFC, including dlPFC and OFC, in the pathophysiology of addiction and many psychiatric disorders, including schizophrenia, mood disorders, posttraumatic stress disorder (PTSD), attention-deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and autistic spectrum disorders (eg, Volkow and Fowler, 2000; Shin et al, 2005; Friedlander and Desrocher, 2006; Seidman et al, 2006; Silk et al, 2006; Waltz and Gold, 2007). At a first glance, the wide range of the symptoms associated with these disorders questions a shared pathophysiology. On the other hand, the high incidence of comorbidity among these disorders, and the notion that at a fundamental level, most of their symptoms involve faulty processing of stimulus (or action)–outcome associations and the planning of behavior that is guided by these associations, supports the notion that there are shared cortical pathophysiological processes across these illnesses. Based on our hypothesis, a critical component of this pathology is the disrupted dynamics between OFC and mPFC (or dlPFC) during associative learning.
As reviewed in articles in this issue of Neuropsychopharmacology Reviews, a shared characteristic of addictive and most psychiatric disorders is that they involve neuroplastic processes, either in terms of a developmental time course as in case of schizophrenia, ADHD, and autism, or in terms of adaptations to environmental factors, as in case of the mood disorders, PTSD, and addiction. The PFC is strongly implicated in these processes because it is a site where the majority of postmortem and functional abnormalities are described. In most cases, however, the hypothesized role of PFC in mediating the faulty neuroplasticity has focused on a single subregion (eg, Lewis et al, 2005) and very few studies have systematically compared one cortical region with another. Below, we will briefly review the limited evidence that these abnormalities occur in multiple cortical sites underscoring the idea that the pathophysiology in these disorders involves disrupted dynamics among several PFC subregions as opposed to localized anomalies within a single region.
Schizophrenia
Schizophrenia is considered a neurodevelopmental disorder where interaction between susceptibility genes and environmental factors leads to symptom onset during early adulthood or late adolescence (Harrison, 1997). The ontogenic trajectory of PFC subregions, including late maturation relative to other brain structures and a delayed pruning of dendritic branches of pyramidal cells in this region, fits the time course of disease development in schizophrenia (Weinberger, 1987). A similar pattern and time course of development is assumed to occur in all PFC subregions (Rakic, 2002); therefore, the developmental abnormalities associated with schizophrenia would be expected to affect all of these structures. Functional imaging studies performed in individuals with schizophrenia, however, have focused primarily on the dlPFC region of the frontal lobe, because this region of the primate is critical for sustaining constructs such as working memory and behavioral flexibility, that are the hallmark of the cognitive deficits associated with schizophrenia (Goldman-Rakic, 1994). Earlier functional studies indicated a state of dlPFC hypofunction (Weinberger et al, 1986), whereas later studies indicated both excessive hyperactivation and failure to adequately activate PFC (Manoach et al, 2000; Ramsey et al, 2002; Callicott et al, 2003) during performance of PFC-dependent tasks. These functional imaging studies have prompted the postmortem research to focus primarily on disease-specific pathologies in the dlPFC of individuals with schizophrenia (see article by Lewis in this volume). Postmortem studies, in turn, have revealed subtle changes in cellular architecture within dlPFC that are now the basis of leading theories related to pathophysiology of schizophrenia. For example, reduction in the markers of GABA synthesis (GAD67) and reuptake (GAT1) in a subset of inhibitory interneurons, parvalbumin-immunoreactive cells have been reported in dlPFC (Akbarian et al, 1995; Volk et al, 2000). This deficiency in GABAergic transmission is associated with a compensatory upregulation of GABAA receptors at axon initial segments of pyramidal neurons (Volk et al, 2002). Other groups have found changes in markers of glutamatergic transmission in dlPFC, including increased expression of NMDA receptor NR1 subunits (Grimwood et al, 1999; Dracheva et al, 2001) and increased level of the endogenous glutamate receptor ligand NAAG (N-acetylaspartyl glutamate) (Tsai et al, 1995). Altogether, these deficits may constitute the basis for morphometric alterations reported in schizophrenia including decreased cortical thickness and gyrification (Kulynych et al, 1997; Vogeley et al, 2000; Kuperberg et al, 2003) and reduced gray matter volume (Zipursky et al, 1998).
Because of feasibility issues with postmortem studies such as tissue availability and the time consuming nature of morphometric and other anatomical methods, regional selectivity of the changes reported in dlPFC is not well characterized. Functional imaging studies, on the other hand, have reported a dissociation in the aberrant pattern of activity in schizophrenia across subregions of PFC. Several recent studies have now found overactivation of OFC and nearby ventral PFC regions in schizophrenia that correlate with behavioral impairment (Sevy et al, 2007; Waltz and Gold, 2007; however see, Wilder et al, 1998). An event-related fMRI study by Gur and co-workers demonstrated simultaneous hypoactivation of dlPFC and hyperactivation of OFC and other termoral-limbic structures during a word encoding and retrieval task (Ragland et al, 2004). Another notable recent study compared the pattern of activity in dorsal and ventral PFC in normal controls and subjects with schizophrenia during N-back test of working memory (Tan et al, 2006). Although control subjects responded to incremental working memory load with disproportionately greater dorsal, but not ventral, PFC activation and greater functional connectivity between dorsal PFC and parietal cortex, the schizophrenia patients displayed load-dependent overactivation in ventral PFC and enhanced functional connectivity between ventral PFC and parietal cortex. This finding is consistent with several other recent studies that indicate opposite patterns of activity in subregions of PFC during performance of cognitively engaging tasks (Bertolino et al, 1998; Callicott et al, 2003; Tan et al, 2005). The overactivation of OFC or other ventral PFC regions, is often interpreted as a compensatory reaction aimed to engage extra neuronal networks to supplement higher executive functions (Callicott et al, 2003; Tan et al, 2005). An alternative interpretation is that simultaneous disturbances in multiple cortical networks underlie the symptoms.
Addiction
Addiction is often viewed as a disorder of neuroplasticity that is caused by excessive drug use (Wolf, 2002; Nestler, this volume). Accordingly, most animal models of addiction involve repeated exposure to drugs of abuse in various experimental settings. A frequently reported effect of repeated exposure to many drugs of abuse in rodents is the progressive enhancement of their motor effects. This phenomenon of locomotor sensitization has been advanced as a model for plastic processes responsible for development of addiction (Robinson and Berridge, 1993; Wolf, 1999). Extensive studies at the molecular and cellular levels have identified a host of mechanisms that mediate the adaptive response of brain to different classes of drugs of abuse, including psychostimulants, opioids and nicotine (Nestler, 2001; Wolf, 2002). Much of the work delineating the cellular and molecular basis of psychostimulant-, opioid-, and nicotine-induced sensitization has focused on the so-called ‘reward’ circuitry of brain. This is mostly due to the critical role of the neurotransmitter dopamine both in the processing of reward-related information (Wise and Rompre, 1989) and in the mediation of locomotor responses of these drugs (Robinson and Berridge, 1993; Pierce et al, 1997). These studies have shown that mesoaccumbens dopaminergic projections from VTA to nucleus accumbens is necessary for development and expression of drug-induced locomotor sensitization and have identified a host of drug-related midbrain and striatal plasticity processes that can subserve the progression towards addiction (Robbins and Everitt, 2002; Bonci et al, 2003; Kalivas et al, 2005). Other models of addiction in rodents involving anhedonia and relapse models using self-administration procedures (Koob and Le Moal, 2001; Robbins and Everitt, 2002) have also identified similar critical subcortical structures for the maintenance of the modeled behavior.
Research focused on individuals with substance abuse disorder, however, has revealed that the pathophysiology of addiction is not limited to subcortical processes, and that cortical structures, particularly PFC subregions, may play a key part in addiction and relapse. Functional imaging studies show altered patterns of PFC activity in psychostimulant addicts during baseline conditions and after challenge by either drug or drug-related cues (Volkow et al, 1992; Grant et al, 1996; London et al, 2000; Bolla et al, 2003; Ersche et al, 2005). Notably, many of these studies demonstrate a non-uniform pattern of abnormal activity, with foci of hyper- and hypo-activity in various subregions of PFC (Childress et al, 1999; Kilts et al, 2001; Goldstein and Volkow, 2002; Arana et al, 2003; Bolla et al, 2003). For example, OFC shows strong abnormal activity in many studies with substance abusers. During baseline and when challenged with drug-related cues, this region shows over-activation, as compared to normal controls, in a manner that correlates with relevant behavioral measures such as craving for drug, propensity to disinhibited behavior or risky decision-making (Volkow et al, 2005). On the other hand, Volkow and colleagues have reported a different pattern of change in the metabolic activity of dorsal and mPFC in psychostimulant abusers (Goldstein et al, 2007a, b).
Mood Disorders
Several studies have reported disruptions in multiple cortical regions in mood disorders including a reduction in mPFC and OFC gray matter (Drevets et al, 1998; Kumar et al, 1998; Sax et al, 1999; Strakowski et al, 1999). There have also been reports of layer-specific changes in the density and size of PFC neurons in mood disorders (Rajkowska et al, 1999). Functional-imaging studies have led to characterization of parallel neural circuitry involving ventral and dorsal PFC, as well as amygdala, insula, anterior cingulate, and hippocampus, that regulate emotional behavior and are dysregulated in mood disorders (Phillips, 2003; Carlson and Meyer, 2006). Although, similar to other fields, few studies have examined simultaneous changes in different cortical regions, studies of individual regions have reported disrupted activation of OFC and dlPFC (Matsuo et al, 2007). A recent study by Phillips and co-workers showed that during performance of a cognitive task, there is dissociation between activation of OFC and dlPFC in bipolar patients (Kronhaus et al, 2006). These limited studies suggest that similar to schizophrenia and addictive disorders, the pathophysiology of PFC in mood disorders may involve abnormal dynamic interactions of ventral and dorsal regions of the PFC.
NEUROPLASTICITY OF PFC CIRCUITRY IN AN ANIMAL MODEL
The work reviewed so far suggests that interplay between OFC and the mPFC (or dlPFC) may be critical to normal associative learning and that disruption of this interaction may be a component of the pathophysiology associated with addictive and psychiatric disorders. To investigate how the diverging patterns of responsiveness between these two cortical regions may be altered in pathological states, we recently recorded the impact of repeated amphetamine exposure on the firing rate and pattern of neurons in mPFC and OFC of behaving rats (Homayoun and Moghaddam, 2006). Repeated amphetamine is a pharmacological model for both schizophrenia and addiction. The model has face validity for schizophrenia because individuals exposed to repeated amphetamine and related stimulants exhibit schizophrenia-like symptoms, such as psychosis and cognitive deficits (Snyder, 1973; Castner and Goldman-Rakic, 1999). The model also has predictive validity in that the clinical psychotic symptoms as well as behavioral activation of laboratory animals by amphetamine are ameliorated by antipsychotic drugs (Geyer and Moghaddam, 2002). As reviewed above, the locomotor sensitization effects of repeated amphetamine and the corresponding molecular and cellular changes have also been presented, as a plausible model for the plasticity associated with development of addiction (Robinson and Berridge, 1993; Wolf, 2002). The brain regions studied in the context of this plasticity have primarily involved subcortical regions, in particular dopamine-containing cell body and terminal regions, such as VTA and ventral striatum (eg, Kalivas et al, 1993; Bonci et al, 2003).
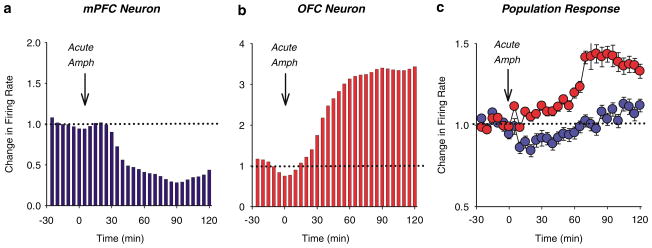
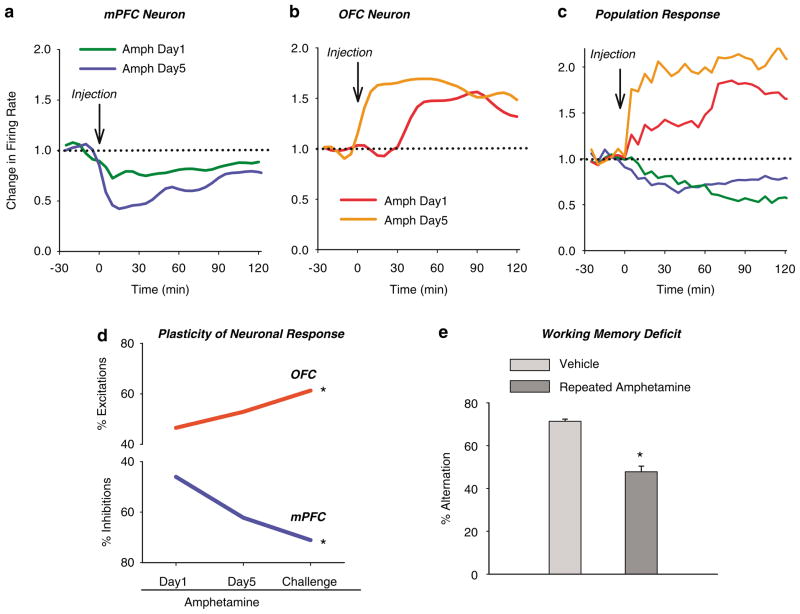
As clinical imaging studies have provided increasing evidence for abnormal pattern of PFC activation in individuals addicted to psychostimulants (Volkow et al, 1992; Grant et al, 1996; London et al, 2000; Bolla et al, 2003; Ersche et al, 2005), rodent studies have followed suit and have reported psychostimulant-induced sustained changes in the pattern of dendritic morphology, membrane excitability, and gene expression (Lu et al, 1997; Sorg et al, 1997; Crombag et al, 2004; Robinson and Kolb, 2004; Dong et al, 2005). In response to the first exposure to amphetamine, we have observed a profound effect on spontaneous activity of neurons in both the mPFC and OFC (Homayoun and Moghaddam, 2006). To our surprise, the direction of these phasic responses was opposite: the mPFC neurons were primarily inhibited, whereas the OFC neurons were primarily excited by amphetamine (Figure 3). Animals then received repeated daily injections of amphetamine for five days and then weekly challenge injections up to nearly a month later. Neurons in both cortical regions displayed remarkable plasticity to amphetamine in that the initial inhibitory mPFC response and OFC excitatory response was gradually amplified. The amplification was sustained nearly a month later, meaning that both cortical subregions developed ‘sensitization’ in their neuronal response to amphetamine (Figure 4). This injection regimen and daily recordings were repeated in animals that were performing an operant instrumental task. Once more, we observed a divergent pattern of firing activity changes in mPFC and OFC that became progressively exaggerated with repeated treatment, suggesting that the disruption of cortical activity by amphetamine is not a confound of different behavioral state. In the same group of rats, we observed an impairment of working memory, as assessed by spontaneous alternation task, 1 month after the repeated amphetamine treatment, supporting the notion that this mechanism disrupts the behaviors that are supported by the PFC. These findings are consistent with recent animal and clinical studies showing drug-induced persistent impairment of PFC-related functions (Grant et al, 2000; Bolla et al, 2003; Hester and Garavan, 2004; Kubler et al, 2005; Castner et al, 2005; Ersche et al, 2005; Schoenbaum et al, 2006).
Figure 3.
Acute amphetamine exerts differential effects on mPFC and OFC ensemble unit activity. (a–b) Representative firing rate histograms of individual neurons responding to acute systemic amphetamine (2 mg/kg). Arrow indicates the time of injection and dashed line signifies change from pre-injection baseline. Acute amphetamine inhibited the mPFC neuron (a) but excited the OFC neuron (b). (c) Population firing rate response (mean±SEM) of all neurons in each region to acute amphetamine. Acute amphetamine caused an excitatory response in OFC and an inhibitory response to mPFC (adapted from Homayoun and Moghaddam, 2006).
Figure 4.
Differential plasticity of mPFC and OFC in response to repeated amphetamine. Animals underwent daily amphetamine (2 mg/kg) injections for 5 days followed by a same dose challenge 10 days later. (a–b) Plasticity of individual neuronal responses to repeated amphetamine. Response of the same individual neurons during first day of amphetamine injection has been compared to day 5. Note the potentiation of amphetamine-induced inhibition in the mPFC neuron (a) and excitation in OFC neuron (b). (c) Repeated amphetamine exposure leads to amplification of the predominant population response in each region; amphetamine challenge causes a greater mPFC inhibition and OFC excitation than the first day dose. Each line depicts the average normalized firing rate of all neurons with the predominant response type in each region. (d) Differential plasticity of mPFC and OFC neurons in response to repeated amphetamine. The percentage of neurons with a significant response to amphetamine increased from day 1 to day 5 and challenge in both regions. However, the direction of responses differed between the two regions with predominant excitations in OFC and inhibitions in the mPFC. *p<0.05 compared to day 1 (adapted from Homayoun and Moghaddam, 2006). (d) Repeated exposure to amphetamine impairs PFC-dependent working memory. One month after the end of the repeated amphetamine treatment schedule, rats used for recording studies were tested in drug-free condition using spontaneous alternation task. Repeated exposure to amphetamine impaired working memory, assessed by % alternation in the maze, as compared to repeated or acute vehicle treatment. Total arm entries did not change (data not shown) indicating that the effect was not related to changes in the general motor activity (*p<0.05 compared to control).
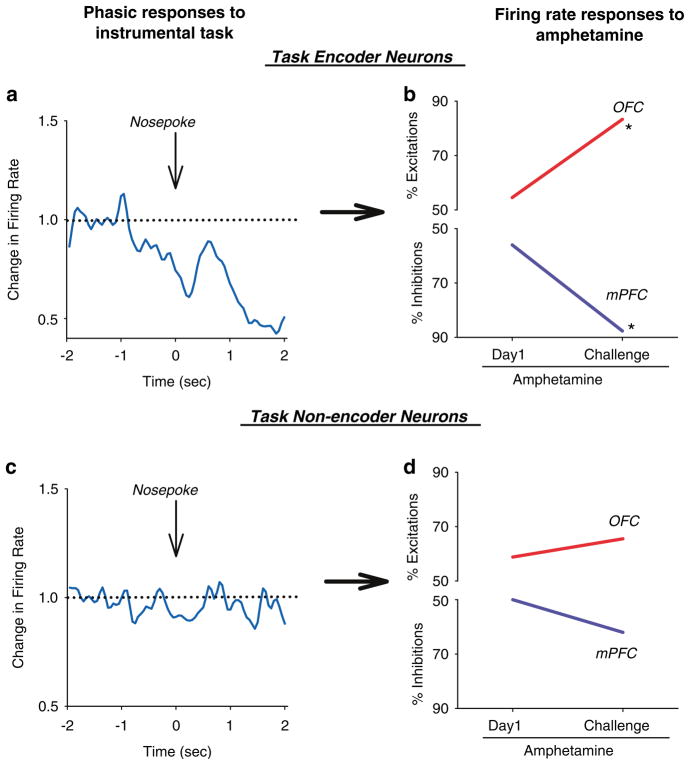
Other studies also support the idea that psychostimulants produce a pattern of divergent plasticity in PFC networks. Morphological studies have shown an opposite effect of amphetamine self-administration on dendritic spines (Crombag et al, 2004), with spine density enhancing in mPFC and decreasing in OFC, likely a compensation for the altered activity patterns in these regions. Robbins and co-workers (Winstanley et al, 2006) have described a double dissociation effect of amphetamine on regulation of impulsive behavior by mPFC and OFC such that amphetamine increases the premature responses in the mPFC-dependent 5-choice serial reaction task (Muir et al, 1996; Harrison et al, 1997) but decreases the impulsive choice in the OFC-dependent delayed discounting paradigm (Richards et al, 1999). In our studies, we also assessed the effect of repeated amphetamine on OFC and mPFC neuronal activity, while animals were engaged in a behavioral task. We observed that the effect of amphetamine was more prominent on the neuronal ensembles that actively participated in representing task events as compared to the non-encoder ensembles (Figure 5). Thus, mechanisms that are responsible for the different pattern of plasticity in OFC and mPFC neurons may preferentially disrupt those sets of cortical ensembles that are engaged in encoding goal-directed behavior.
Figure 5.
Amphetamine plasticity is selective for functionally engaged neurons. Rats received repeated amphetamine treatment (2 mg/kg daily for 5 days followed by same dose challenge 10 days later) during performance of an instrumental responding task in which nosepoke into a lit hole led to a food pellet delivery in a food magazine. Each session consisted of 10 min of baseline task performance and 40 min of post-amphetamine performance. (a–b) Based on their pre-injection activity, neurons were divided into task encoders, that is, those with a significant phasic response to one of the task relevant events (cue, nosepoke, consumption), or task non-encoders. Examples of individual neurons from each group are displayed on the left panels ((a) a task encoder mPFC neuron, (b) a task non-encoder OFC neuron). (c–d) Proportion of amphetamine-responsive neurons in each subgroup. Amphetamine-induced plasticity selectively targeted the task encoder neurons, increasing the proportion of inhibitory responses in the mPFC and excitatory responses in OFC. In contrast, the task non-encoder neurons did not display plasticity to repeated amphetamine.
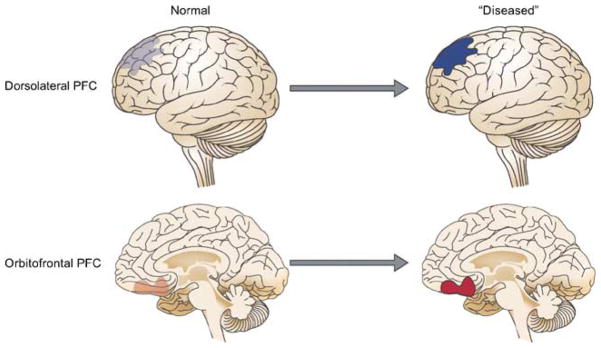
The divergent pattern of plasticity in response to amphetamine in OFC and mPFC may model the heterogeneity in metabolic activity of PFC in imaging studies of drug abusers (Volkow and Fowler, 2000; Volkow et al, 2004) and patients with schizophrenia (Ragland et al, 2004). It is noteworthy that the opposing pattern of plasticity in OFC and mPFC neurons after repeated amphetamine mimicked some aspects of the plasticity displayed by these regions during classical conditioning (Figure 1), suggesting that the abnormal plasticity in a disease state taps into existing mechanisms that support normal learning but produces an exaggerated pattern of OFC-dlPFC divergent activation (Figure 6). In the context of addiction, the finding of a lasting deactivation of mPFC during amphetamine challenge fits with the notion that psychostimulants can hamper the mPFC (or dlPFC) function, leading to behavioral disinhibition. This would impair proper decision-making in favor of habitual impulses and previously conditioned responses (Robbins, 1996; Jentsch and Taylor, 1999; Bechara et al, 2001). On the other hand, the enhanced and immediate response of the majority of OFC neurons to amphetamine challenge may be a representation of the higher salience assigned to the previously experienced drug. Together, deficits in the executive control of behavior (Everitt and Robbins, 2005), combined with a progressive enhancement of the motivational value of the drug, can increase the propensity for relapse when the individual is challenged by drug or drug-associated cues. In the context of schizophrenia, abnormal interaction of OFC and dlPFC in addition to causing cognitive doficits may lead to improper encoding of stimulus-outcome contingencies period. This will result in excessive emphasis being placed on irrelevant stimuli leading to delusions and thought disorders, whereas insufficient emphasis is placed on relevant stimuli causing flattened affect and other so-called negative symptoms.
Figure 6.
The dlPFC and the OFC display opposing pattern of activation during normal learning and cognition (see text). In disorders such as addiction or schizophrenia, this normal pattern of opposed activation may be exaggerated.
FUTURE DIRECTIONS AND CLINICAL IMPLICATIONS
There is a growing recognition that circuit-level mechanisms are the proximal cause of disease-related symptomatology. Although the emphasis of this line of reasoning has been on cortical–subcortical interactions (Fujii and Graybiel, 2005; Pasupathy and Miller, 2005), the evidence reviewed above argues for the importance of intra-PFC circuit-level reorganization during learning and as a substrate for pathological vulnerability related to psychiatric disorders. The functional attributes of the PFC as a whole depends on active interactions of multiple parallel distributed networks where modular properties of specific PFC subregions and the nature of neuromodulatory afferents may determine the specific function served (Fuster, 2001). Abnormal plasticity in any of the PFC subregions may disrupt the dynamic interplay between these structures, leading to cognitive deficits, exaggerated or understated response to stimuli and an overall improper planning of goal-directed behaviors.
The relevance of these mechanisms to addiction and psychiatric disorders can be tested by future research focused on concomitant measures of different PFC sub-region activation in various contexts. In addition to functional imaging studies, the interactions between these regions can be evaluated by studying large-scale mechanisms that maintain the ability of various cortical regions to dynamically interact with each other, as well as with subcortical structures. These include measuring oscillatory coherence and frequency-band phase locking among large numbers of neurons on a dynamic and task-relevant basis (Varela et al, 2001; Grunze et al, 1996). These methods provide excellent translational tools because of their relevance to human EEG recordings and recent reports of cortical oscillatory disruptions in individuals with schizophrenia (Spencer et al, 2004; Cho et al, 2006; Heinks-Maldonado et al, 2007). So far, these methods have been successfully applied to investigate the circuitry-level mechanisms that are important for PFC’s interactions with other regions. For example, Hyman et al (2005) revealed network-level mechanisms that allow dynamic coordination between the mPFC and hippocampus during performance of two distinct tasks. They found that a subset of mPFC neurons dynamically entrained their firing to hippocampal theta rhythm based on the ongoing behavior. The same group of neurons prominently encoded for the task-relevant events by modulating their firing, suggesting that hippocampal oscillations selectively coordinate the mPFC circuits that are engaged in task performance. It is important to determine whether similar mechanisms support the concomitant dynamics of interacting PFC subregions.
The idea that disrupted dynamics of dlPFC–OFC interaction contributes to some brain disorders also provides insights about the therapeutic efficacy of existing drugs and design of novel approaches. Pharmacological manipulation of neuromodulatory systems, such as the monoaminergic systems, may work by fine tuning interacting PFC networks and optimizing their function. For example, we recently compared the effects of typical antipsychotic drug haloperidol with its superior atypical counterpart clozapine on the firing activity of mPFC pyramidal neurons in vivo (Homayoun and Moghaddam, 2007). Clozapine produced a state-dependent ‘fine-tuning’ of mPFC neuronal activity in which less active ensembles were boosted while more active ensembles were inhibited. This effect was not produced by haloperidol, suggesting that such a fine-tuning of PFC networks may contribute to higher clinical efficacy of clozapine.
A non-pharmacological strategy to modify maladaptive network-level dynamics may rely on utilization of deep brain stimulation, repetitive transcranial magnetic stimulation and vagus nerve stimulation, which can potentially reorganize disrupted network interactions. Of note, some of these modalities, as well as their predecessors such as electric shock therapy, have proved effective against refractory cases of mood disorder and schizophrenia (Mayberg et al, 2005; Fitzgerald et al, 2006; O’reardon et al, 2007). Future research should focus on how dynamics of PFC circuitry, in particular OFC interactions with mPFC and dlPFC, are affected by these interventions and identify the parameters that can optimize their effectiveness.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that this work was funded by the National Institute of Mental Health, Tourette Syndrome Association, and Pittsburgh Life Sciences Greenhouse. Authors do not have personal financial holdings and have not received financial compensations from individual or corporate entities over that past 3 years for research or professional service that could be perceived as constituting a potential conflict of interest.
References
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr, et al. GABAa receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cerebral Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Arana F, Parkinson J, Hinton E, Holland A, Owen A, Roberts A. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. This is one of the first studies demonstrating that prefrontal cortex neuron demonstrate plasticity during associative learning. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuev AS, Shaefer VI, Orlov AA. Comparative characteristics of unit activity in the prefrontal and parietal areas during delayed performance in monkeys. Behav Brain Res. 1985;16:57–70. doi: 10.1016/0166-4328(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Elman I, Mattay VS, Tedeschi G, Frank JA, et al. Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1998;43:641–648. doi: 10.1016/s0006-3223(97)00555-6. [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol. 2005;94:2239–2250. doi: 10.1152/jn.00172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, D’Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, London E, Kiehl K, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Bernardi G, Grillner P, Mercuri N. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci. 2003;24:172–177. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Brown V, Bowman E. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Meyer SE. Phenomenology and diagnosis of bipolar disorder in children, adolescents, and adults: complexities and developmental issues. Dev Psychopathol. 2006;18:939–969. doi: 10.1017/S0954579406060470. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–751. doi: 10.1016/j.biopsych.2004.12.019. This study demonstrated that the amphetamine sensitization model may have face validity for cognitive deficits and prefrontal cortex functional abnormalities associated with schizophrenia. [DOI] [PubMed] [Google Scholar]
- Childress A, Mozley P, McElgin W, Fitzgerald J, Reivich M, O’Brien C. Limbic activation during cue-induced cocaine craving. Am J Psychiat. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Gorny G, Li Y, Kolb B, Robinson T. Opposite effects of amphetamine self-administration experience on dendriticspines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2004;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Dalley J, Cardinal R, Robbins T. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The frontal lobes. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. Oxford University Press; New York: 1979. pp. 360–411. [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neur Comp. 1989;1:123–132. [Google Scholar]
- Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RS, et al. How the brain learns to see objects and faces in an impoverished context. Nature. 1997;389:596–599. doi: 10.1038/39309. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif F, Tsui J, Ju W, Cooper D, Hu X, et al. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortexpyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Marras S, Elhakem S, Kramer F, Davis K, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontalcortex of elderly patients with schizophrenia. Am J Psychiat. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Mouncastle VB. The Minful Brain. Plenum Press; New York: 1978. [Google Scholar]
- Ersche K, Fletcher P, Lewis S, Clark L, Stocks-Gee G, London M, et al. Abnormal frontal activations related to decision making in current and formeramphetamine- and opiate-dependent individuals. Psychopharmacol. 2005 doi: 10.1007/s00213-005-2205-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits tocompulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Benitez J, de Castella AR, Brown TL, Daskalakis ZJ, Kulkarni J. Naturalistic study of the use of transcranial magnetic stimulation in the treatment of depressive relapse. Aust N Z J Psychiatry. 2006;40:764–768. doi: 10.1080/j.1440-1614.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- Fodor JA, Pylyshyn ZW. Connectionism and cognitive architecture: a critical analysis. Cognition. 1988;28:3–71. doi: 10.1016/0010-0277(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clin Psychol Rev. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Fujii N, Graybiel AM. Time-varying covariance of neural activities recorded in striatum and frontal cortex as monkeys perform sequential-saccade tasks. Proc Natl Acady of Sciences of USA. 2005;102:9032–9037. doi: 10.1073/pnas.0503541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce C, Goldman-Rakic P. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;80:3392–3397. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce C, Goldman-Rakic P. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic ‘scotomas’. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J, Alexander G. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. A seminal review on the temporal organization of behavior by prefrontal cortex sub-regions. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and the Mind: Unifying Cognition. Oxford University Press; New York: 2003. [Google Scholar]
- Geyer M, Moghaddam B. Animal models relevant to schizophrenia disorder. In: Davis KL, Charney C, Coyle JT, Nemeroff C, editors. Psychopharmacology: the Fifth Generation of Progress. Lippincott Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- Goldman P, Rosvold H. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Exp Neurol. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. A classic review on the pioneering work that provided the anatomical basis for regulation of cognition by parallel distributed cortical networks. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007b;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. NeuroImage. 2007a;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London E. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grant S, London E, Newlin D, Villemagne V, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. This seminal study was one of the first reports of on cognitive deficits and abnormal cortical circuits in addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood S, Slater P, Deakin JF, Hutson PH. NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neurorep. 1999;10:461–465. doi: 10.1097/00001756-199902250-00004. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Gruber O, Muller T, Falkai P. Dynamic interactions between neural systems underlying different components of verbal working memory. J Neural Transm. 2007;114:1047–1050. doi: 10.1007/s00702-007-0634-7. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Haber S, Kim K, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primate that interface with associative cortical connections, providing a substrate for incentive -based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T, Merzenich MM. Neural coding in the sense of touch: human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innvervating the hairy skin of monkeys. Exp Brain Res. 1970;10:251–264. doi: 10.1007/BF00235049. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. Schizophrenia: a disorder of neurodevelopment? Curr Opin Neurobiol. 1997;7:285–289. doi: 10.1016/s0959-4388(97)80018-9. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Society for Neuroscience Abstract. Washington, DC: 2005. Cortical dynamics of learning: how do prefrontal and orbitofrontal cortical ensembles differentially encode acquisition of Pavlovian and instrumental learning? #997.15. [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. This recent study describes long-lasting and opposing pattern of plasticity in two cortical regions in amphetamine sensitized rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Fine-tuning of awake prefrontal cortex neurons by clozapine: comparison with haloperidol and N-desmethylclozapine. Biol Psychiatry. 2007;61:679–687. doi: 10.1016/j.biopsych.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Jentsch J, Taylor J. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, II, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Jung M, Qin Y, McNaughton B, Barnes C. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cerebral Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Sorg B, Hooks M. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- Kalivas P, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kilts C, Schweitzer J, Quinn C, Gross R, Faber T, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: the role of working memory in complex, organized behavior. J Exp Psychol Gen. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kulynych J, Luevano L, Jones D, Weinberger D. Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry. 1997;41:995–999. doi: 10.1016/S0006-3223(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci USA. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G, Broome M, McGuire P, David A, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- London E, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lu W, Chen H, Xue C, Wolf M. Repeated amphetamine administration alters the expression of mRNA for AMPAreceptor subunits in rat nucleus accumbens and prefrontal cortex. Synapse. 1997;26:269–280. doi: 10.1002/(SICI)1098-2396(199707)26:3<269::AID-SYN8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Manoach D, Gollub R, Benson E, Searl M, Goff D, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Pribram K. Analysis of the effects of frontal lesions in monkey. I. Variations on delayed alternation. J Comp Physiol Psychol. 1955;48:492–495. doi: 10.1037/h0040318. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta W. The problem of the frontal lobe: a reinterpretation. J Psychiatry Res. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- O’reardon J, Solvason H, Janicak P, Sampson S, Isenberg K, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.01.018. (in press) [DOI] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HG. Cortico–cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Passingham R, Toni I, Rushworth M. Specialization within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Exp Brain Res. 2000;133:103–113. doi: 10.1007/s002210000405. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller E. Different time course of learning-related acitvity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proc Natl Acad Sci USA. 2002;99:1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Impaired DRL 30 performance during amphetamine withdrawal. Behav Brain Res. 2003;143:101–108. doi: 10.1016/s0166-4328(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Phillips ML. Understanding the neurobiology of emotion perception: implications for psychiatry. Br J Psychiatry. 2003;182:190–192. doi: 10.1192/bjp.182.3.190. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacol. 1997;133:188–195. doi: 10.1007/s002130050390. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M, Adams S, Kesner R. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent preforntal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolving concepts of cortical radial and areal specification. Prog Brain Res. 2002;136:265–280. doi: 10.1016/s0079-6123(02)36023-0. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Koning HA, Welles P, Cahn W, van der Linden JA, Kahn RS. Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain. 2002;125:1793–1807. doi: 10.1093/brain/awf188. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology. 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Robbins T. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Robbins T, Everitt B. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Robinson T, Berridge K. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson T, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–520. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E, Critchley H, Mason R, Wakeman E. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The Prefrontal Cortex. Oxford University Press; New York: 1998. pp. 67–86. [Google Scholar]
- Sakurai Y, Sugimoto S. Effects of lesions of prefrontal cortex and dorsomedial thalamus on delayed go/no-go alternation in rats. Behav Brain Res. 1985;17:213–219. doi: 10.1016/0166-4328(85)90045-2. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, Del Bello MP, Keck PE, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92:74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Transact R Soc Lond. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, et al. Visuospatial processing and the function of prefrontal–parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. 2006;163:1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- Snyder S. Amphetamine psychosis: a ‘model’ schizophrenia mediated by catecholamines. Am J Psychiat. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Sorg B, Davidson D, Kalivas P, Prasad B. Repeated daily cocaine alters subsequent cocaine-induced increase of extra-cellular dopamine in the medial prefrontal cortex. J Pharm Exp Thera. 1997;281:54–61. [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer T, Bogerts B, et al. Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiat. 2000;157:34–39. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cerebral Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Volkow N, Hitzemann R, Wang G, Fowler J, Wolf A, Dewey S, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Prefrontal unit activity during delayed conditional Go/No-Go discrimination in the monkey. II. Relation to Go and No-Go responses. Brain Res. 1986;382:15–27. doi: 10.1016/0006-8993(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Weinberger D, Berman K, Zec R. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow (rCBF) evidence. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr Res. 1998;30:169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cerebral Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wolf M. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. NMDA receptors and behavioural sensitization: beyond dizocilpine. Trends Pharmacol Sci. 1999;20:188–189. doi: 10.1016/s0165-6147(99)01356-5. [DOI] [PubMed] [Google Scholar]
- Zipursky R, Lambe E, Kapur S, Mikulis D. Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry. 1998;55:540–546. doi: 10.1001/archpsyc.55.6.540. [DOI] [PubMed] [Google Scholar]