Abstract
Objectives
Subclinical coronary atherosclerosis is increased in patients with rheumatoid arthritis (RA) and is associated with insulin resistance. Adipocytokines are associated with obesity, insulin resistance, inflammation and coronary heart disease in the general population. We examined the hypothesis that adipocytokines affect insulin resistance and coronary atherosclerosis among patients with RA.
Methods
Coronary calcium, insulin resistance (HOMA) and serum adipocytokine concentrations (leptin, adiponectin, resistin and visfatin) were measured in 169 patients with RA. The independent effect of each adipocytokine on HOMA and coronary artery calcification determined by electron beam CT was assessed adjusting for age, race, sex, BMI, traditional cardiovascular risk factors and inflammatory mediators. We also examined whether the effect of HOMA on coronary calcium is moderated by adipocytokines throughan interaction analysis.
Results
Leptin was associated with higher HOMA, even after adjusting for age, race, sex, BMI, traditional cardiovascular risk factors and inflammatory mediators (p<0.001), but visfatin (p=0.06), adiponectin (p=0.55) and resistin (p=0.98) were not. None of the adipocytokines were independently associated with coronary calcium (all p>0.05). Serum leptin concentrations interacted with HOMA (multivariate p interaction=0.02). Increasing leptin concentrations attenuated the increased risk of coronary calcification related to HOMA. The other adipocytokines and HOMA did not interact significantly (p>0.05).
Conclusion
Leptin is associated with insulin resistance in patients with RA but paradoxically attenuated the effects of insulin resistance on coronary calcification.
Keywords: Rheumatoid Arthritis, Adipocytokines, Atherosclerosis, Insulin Resistance, Leptin, Adiponectin, Resistin, Visfatin
Introduction
Patients with rheumatoid arthritis (RA) have an increased prevalence of premature atherosclerosis(1). We have reported that insulin resistance was increased in patients with RA and associated with accelerated coronary atherosclerosis(2) and that concentrations of adipocytokines, mediators common to inflammation, insulin resistance and atherosclerosis, were higher in patients with RA than in control subjects(3).
Adipocytokines are cytokines associated with adipose tissue; the most widely studied are leptin, adiponectin, resistin and visfatin. Leptin plays a key role, not only in the regulation of appetite and body weight, but also in the modulation of immune responses. Circulating leptin concentrations are increased in obesity, and are associated with inflammation, insulin resistance and subclinical coronary atherosclerosis(4,5). Leptin also interacts with insulin in several ways that may act to either facilitate or inhibit atherosclerosis(6,7). Resistin and visfatin are also associated with increased inflammation, insulin resistance and cardiovascular risk(4,8). In contrast, adiponectin is anti-inflammatory, and is inversely associated with obesity, insulin resistance and cardiovascular risk(4).
Although adipocytokines play a key role in the interface between obesity, inflammation, insulin resistance and atherosclerosis, little is known about their contribution in RA. In a previous study investigating the effects of adipocytokines on radiological joint damage we found that concentrations of leptin, adiponectin, resistin and visfatin were increased in patients with RA, independent of BMI(3). However, there is no information about the relationship between adipocytokine concentrations, insulin resistance, and coronary atherosclerosis in RA. This is important because we have shown that insulin resistance is increased in patients with RA and is associated with coronary atherosclerosis(2). Thus, we examined the hypothesis that serum adipocytokine concentrations are associated with insulin resistance and may modify its effects on the risk of coronary calcification in RA.
Materials and Methods
Patients
One hundred and sixty-nine patients with RA were recruited and details regarding recruitment and methodological procedures have been described previously(1–3). The study was approved by the Vanderbilt University Institutional Review Board and subjects gave written informed consent.
Clinical Measurements and Indices / Scores
Clinical information including BMI, history of hypertension, diabetes, smoking, laboratory data including insulin resistance (HOMA index), and Agatston coronary calcium scores were obtained as described in detail previously(1–3). C-reactive protein (CRP) concentrations were determined in the hospital clinical laboratory. Before 2003, the laboratory did not use a high-sensitivity CRP assay, and low concentrations were reported as <3 mg/l. In 40 patients with CRP concentrations <3 mg/l, concentrations were measured using Lincoplex® Multiplex Immunoassay (Millipore). Serum concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), leptin, resistin, and adiponectin were measured using Lincoplex® Multiplex Immunoassay (Millipore). Visfatin was measured by Visfatin C-terminal ELISA kit (Phoenix Pharmaceuticals).
Statistical Analysis
Descriptive statistics were calculated as median with the interquartile range (median [IQR]).Wilcoxon rank-sum tests were used to compare continuous variables.
The independent association between each adipocytokine and insulin resistance was assessed using a multiple linear regression model with HOMA as the outcome variable and the concentration of each adipocytokine as the predictor. We examined the additional contribution of adipocytokines beyond that provided by clinical and demographic variables including BMI, traditional cardiovascular risk factors, and inflammation. Those models adjusted for: 1) Base model: age, race, sex and BMI; 2) the base model + traditional cardiovascular risk factors (hypertension, diabetes, serum HDL and LDL cholesterol concentrations and current smoking); 3) the base model + major inflammatory mediators associated with cardiovascular risk (serum TNF-α, IL-6 and CRP concentrations); and 4) full model: the base model + cardiovascular risk factors + inflammatory mediators.
The independent association between each adipocytokine and coronary calcium was assessed using a proportional odds logistic regression model using coronary calcium as the outcome variable and the adipocytokine concentration as the predictor. Statistical adjustment was performed similarly using the 4models described previously. To prevent possible overfitting in the full model, principal component analysis was used to include the 3 markers of inflammation (serum TNF-α, IL-6 and CRP concentrations) as one variable that was used along with age, sex, race, BMI and cardiovascular risk factors.
Interaction analyses were performed between each adipocytokine and insulin resistance on coronary calcification by including a cross-product term in a proportional odds logistic regression model (each adipocytokine concentration x HOMA). Full model adjustments were similar to the proportional odds model described above. Sensitivity analyses restricted to RA patients without diabetes yielded similar results.
Concentrations of each adipocytokine, TNF-α, IL-6, CRP and HOMA were log-transformed to normalize the skewed distribution. The effect size of each adipocytokine on HOMA was expressed as a multiple linear regression coefficient per IQR adipocytokine difference with 95% confidence intervals (CI). The effect size of each adipocytokine on coronary calcium was expressed as odds ratios (OR) per IQR adipocytokine difference with 95% CI. Statistical significance was determined using a two-sided 5% significance level (p value <0.05). Statistical analysis was done with R 2.9.1 (http://www.r-project.org).
Results
Demographic characteristics and adipocytokine concentrations
Descriptive information including demographics, clinical data, traditional cardiovascular risk factors, and concentrations of adipocytokines and inflammatory mediators in this cohort of patients with RA have been reported previously(3) and are shown in Table 1. Serum leptin concentrations were higher in females than males (30.9 [17.3–44.9] ng/ml vs. 9.4 [4.5–18.2] ng/ml, p<0.001) but adiponectin concentrations did not differ significantly among women (25.0[15.1–39.4] ug/ml) and men (20.5[13.8–35.7] ug/ml)(p=0.35). Visfatin and resistin concentrations did not differ significantly among men and women.
Table 1.
Descriptive Data of Patients with Rheumatoid Arthritis*
| Factor | RA (N=169**) |
|---|---|
| Sex (Males%) | 31.1% |
| Age (Years) | 54[45–63] |
| Race (Caucasian%) | 88.1% |
| Hypertension (%) | 53.3% |
| Diabetes (%) | 11.2% |
| Smokers (%) | 24.3% |
| Systolic blood pressure (mmHg) | 133.5[117.5–145.5] |
| Diastolic blood pressure (mmHg) | 75.0[67.5–82.0] |
| HDL cholesterol (mg/dl) (n=168) | 43.0[37.0–54.0] |
| LDL cholesterol (mg/dl) (n=168) | 112.5[88.8–135.2] |
| Glucose (mg/dl) | 87.0[83.0–94.0] |
| Height (m) | 1.68[1.61–1.75] |
| Weight (kg) | 78.9[69.6–91.2] |
| BMI (kg/m2) | 28.3[24.0–33.23] |
| HOMA (n=164) | 2.4 [1.2–4.5] |
| Coronary Calcium (Agatston Score) (n=164) | 1.8 [0.0–150.3] |
| Leptin (ng/ml) (n=164) | 23.0 [9.5–38.9] |
| Resistin (ng/ml) (n=166) | 7.6 [5.6–11.4] |
| Adiponectin (ug/ml) (n=166) | 22.8 [14.8–38.7] |
| Visfatin (ng/ml) (n=166) | 6.0 [4.4–8.4] |
Some data (demographics and adipocytokine concentrations) have been presented previously(3). Data are described as either percentages or median[IQR].
For technical or logistic reasons, adiponectin, visfatin, resistin (n=3 each), Agatston score (n=5), HOMA and leptin (n=5 each), and HDL and LDL (n=1 each) values were not available in all subjects.
We have reported previously in this cohort(3) that age correlated positively with adiponectin (Spearman rho=0.26, p<0.01) but not with the other adipocytokines. BMI correlated positively with leptin (rho=0.58, p<0.01) and negatively with adiponectin (rho=-0.39, p<0.01), but did not correlate significantly with resistin (rho=0.01, p>0.05) and visfatin (rho=0.02, p>0.05). IL-6 and CRP correlated significantly with leptin, and TNF-α, IL-6 and CRP correlated with visfatin(all p values < 0.05)(3).
Relationship between adipocytokines and HOMA
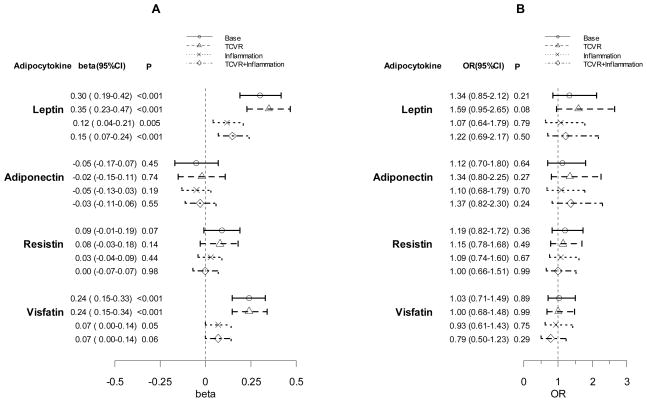
The independent association between each adipocytokine and HOMA is shown in Figure 1 (Panel A). Leptin was significantly associated with HOMA after adjustment for age, race, sex and BMI (base model), and after additional adjustment for traditional cardiovascular risk factors, inflammatory mediators, or both (beta coefficient 0.15 (95%CI 0.07–0.24), p<0.001). In a sensitivity analysis excluding patients with diabetes, the positive association of leptin with HOMA remained significant in the full model (p<0.001). Adiponectin and resistin were not associated with HOMA (Figure 1A). Visfatin was associated with HOMA when adjusted for age, race, sex and BMI, and also after additional adjustment for traditional cardiovascular risk factors (beta coefficient 0.24 (95%CI 0.15–0.34), p<0.001), but the association was attenuated when additionally adjusted for inflammation (p=0.06).
Figure 1.
Independent Association between Adipocytokines and Insulin Resistance (HOMA) (Panel A) and Coronary Calcium (Panel B)
TCVR: Traditional Cardiovascular Risk Factors. See methods for adjustment method details. A: Beta (95%CI) refers to the multiple linear regression coefficients for the effect of log-adipocytokine per IQR difference in increment with 95% CI on log-HOMA.B: Results are ORs for coronary calcium score per IQR log-adipocytokine increment with 95% CI estimated by a proportional odds logistic regression model.
Relationship between adipocytokines and coronary calcium
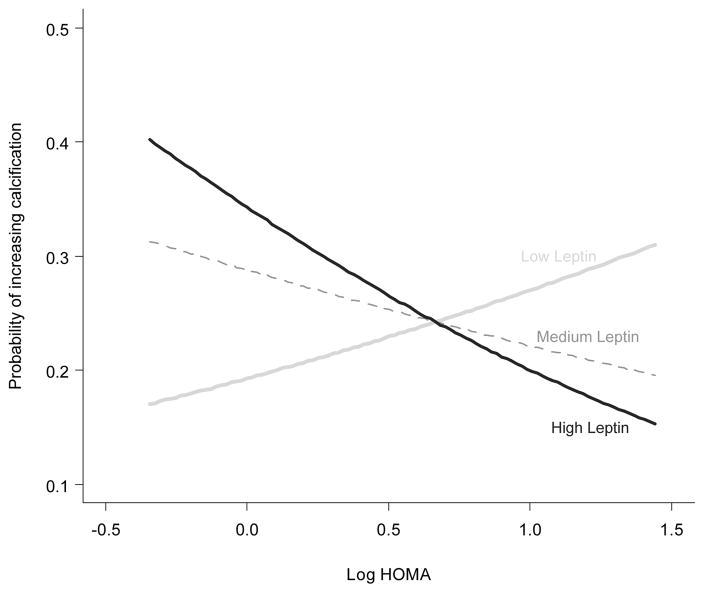
The independent associations between each adipocytokine and coronary calcium are shown in Figure 1 (Panel B). None of the adipocytokines was significantly independently associated with coronary calcium in any of the models examined. However, when each adipocytokine was tested for interaction with HOMA on the outcome of coronary artery calcium, leptin interacted significantly with HOMA after adjusting for age, race, sex and BMI (interaction p=0.01). As serum leptin concentrations increased, the effect of HOMA on increasing risk of coronary calcification was progressively attenuated (Figure 2). The leptin x HOMA interaction remained significant in the full model that includes base model covariates, cardiovascular risk factors and inflammatory mediators (interaction p=0.02). In a sensitivity analysis excluding patients with diabetes, the leptin x HOMA interaction on the outcome of coronary calcification remained significant in the full model (p=0.004). The interaction of adiponectin (interaction p=0.44), resistin (interaction p=0.62), and visfatin (interaction p=0.12) with HOMA on coronary calcification did not reach statistical significance in either the base model or full model.
Figure 2.
Interaction Plot Shows that High Leptin Concentrations Attenuate the Effect of Insulin Resistance on Severity of Coronary Calcification
Interaction p=0.02. Each curve for probability of severity of calcification by log (HOMA) is estimated using the median of each tertile of log(leptin). The interaction was adjusted for age, sex, race, BMI, inflammatory mediators, and cardiovascular risk factors using a proportional odds logistic regression model.
Discussion
The major findings of this study were that leptin was independently and positively associated with insulin resistance in patients with RA, and higher concentrations of leptin were associated with paradoxical attenuation of the pro-atherosclerotic effects of insulin resistance on coronary calcification. Visfatin was positively associated with insulin resistance in patients with RA; this association was attenuated when statistical adjustment for inflammation was performed.
Leptin, the oldest recognized adipocytokine, is best known for its association with obesity. The ob/ob leptin-deficient mouse is markedly obese; however, paradoxically most obese humans have increased concentrations of leptin, likely reflecting a state of leptin resistance(5). Leptin is also associated with inflammation in both the general population and in patients with RA(3,4) and, as we found, is associated with increased insulin resistance(4,6). Interestingly, in RA the association between leptin and insulin resistance was present after adjustment for many factors including BMI, inflammation and cardiovascular risk factors, suggesting an independent association between leptin and insulin resistance in RA.
Insulin and glucose stimulate leptin secretion, and leptin acts to decrease insulin secretion and improve insulin sensitivity(9). Thus, in patients with familial leptin deficiency or lipoatrophic diabetes, exogenous leptin administration improves insulin resistance and diabetes(9). The high concentrations of leptin associated with insulin resistance in obesity may reflect a state of leptin resistance since insulin sensitivity is not reversed by exogenous leptin administration(9). Such leptin resistance may be particularly likely to occur under conditions of increased inflammation because CRP can inhibit the binding of leptin to its receptor(10). Thus, the effects of leptin on insulin sensitivity are complex and, in the setting of RA, poorly defined.
In addition to its effects on insulin sensitivity, leptin appears to promote atherosclerosis in some situations(5), and protect against it in others(11). The protective mechanisms involved are not well understood but may involve nitric oxide (NO)(6,7). We found that in patients with RA leptin concentrations were not associated with coronary calcification. However, higher leptin concentrations were associated with insulin resistance and attenuation of the effect of insulin resistance on coronary calcium. A possible interpretation is that the overall effects of leptin on atherosclerosis may be mediated through interactions with other risk factors for atherosclerosis rather than acting independently in RA. Alternatively, high leptin concentrations could reflect a feedback mechanism to improve insulin resistance and also ameliorate its effects on atherosclerosis in RA.
In the general population obesity is usually associated with higher concentrations of leptin and increased insulin resistance, coronary calcification and cardiovascular risk(5,12). However, in large-scale studies of obese patients with RA, all-cause(13) and cardiovascular mortality(14) were paradoxically decreased. Thus, our findings that higher concentrations of leptin appeared to ameliorate the effect of increasing insulin resistance on coronary calcium in patients with RA are concordant with those findings. RA may represent a disease that can provide insight into the biology of obesity and cardiovascular risk. Indeed, obesity may have beneficial effects in RA in terms of not only cardiovascular mortality but also bone damage, an effect that we have shown is mediated in part by leptin and BMI(3).
Adiponectin has anti-inflammatory and anti-atherogenic effects and is generally inversely correlated with obesity, inflammation(4) and leptin concentrations. However, in patients with RA, a population in whom leptin concentrations are increased, concentrations of adiponectin were paradoxically increased compared to control subjects(3). This raised the possibility that adiponectin, through its anti-atherogenic effects, could modify the risk of atherosclerosis in RA. However, we found that in patients with RA adiponectin was not associated with either coronary calcium or HOMA.
Resistin, a pro-inflammatory adipocytokine, has been associated with atherosclerosis(4). We found no association between resistin and HOMA or coronary calcium in patients with RA. It is possible that in patients with RA the effects of resistin on coronary calcium could have been obscured by pro-atherogenic effects of other inflammatory mediators that are increased in RA and associated with coronary calcification(15).
Visfatin, considered to be pro-inflammatory and associated with increased cardiovascular risk(8), was associated with HOMA but not with coronary calcification in our study. The association between visfatin and HOMA was attenuated markedly after adjustment for inflammatory markers, suggesting that the apparent effects of visfatin on insulin resistance in RA are mediated by inflammation.
The strengths of our study were that it used a well-validated outcome marker of coronary risk, coronary calcium, studied all four major adipocytokines as predictors, and adjusted for inflammatory mediators as well as traditional cardiovascular risk factors. The study also had some limitations. It was cross-sectional in design, thus a cause-effect relationship cannot be inferred; this will require additional studies. Furthermore, coronary calcification represents a late phase of atherosclerosis and we cannot exclude the possibility that adipocytokines may be a predictor of active, inflamed, developing or vulnerable plaques rather than atherosclerosis itself.
In conclusion, leptin is associated with increased insulin resistance and attenuation of the association between insulin resistance and coronary calcification in patients with RA.
Acknowledgments
Acknowledgements: None.
Sources of Funding: Supported by NIH grants HL65082, HL67964, GM07569, UL1 RR024975 from NCRR/NIH,P60 AR056116and the Dan May Chair in Medicine.
Footnotes
Disclosures: None of the authors has a conflict of interest related to this work.
References
- 1.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58(7):2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1906–14. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 5.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117(25):3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vecchione C, Aretini A, Maffei A, Marino G, Selvetella G, Poulet R, et al. Cooperation between insulin and leptin in the modulation of vascular tone. Hypertension. 2003;42(2):166–70. doi: 10.1161/01.HYP.0000082806.73530.68. [DOI] [PubMed] [Google Scholar]
- 8.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83(4):804–16. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz BO, Haznedaroglu IC. Rethinking leptin and insulin action: therapeutic opportunities for diabetes. Int J Biochem Cell Biol. 2006;38(5–6):820–30. doi: 10.1016/j.biocel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12(4):425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 11.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26(10):2883–9. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 13.Escalante A, Haas RW, del RI. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 14.Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50(11):3450–7. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- 15.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–5. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]