Abstract
The cardioprotective effects of 17β-estradiol (E2) in women are hypothesized to be partially mediated by its metabolites, 2-hydroxyestradiol (2-HOE) and 2-methoxyestradiol (2-MeOH). Therefore, the purpose of our study was to determine the acute effects of E2, 2-HOE, and 2-MeOH to inhibit coronary arterial constriction. Right coronary arteries were dissected out of hearts obtained from breeding sows, cut into 4 mm rings, and suspended in organ baths. Incubation of the rings with E2, 2-HOE, and 2-MeOH (10 μmol/L) for 60 min attenuated a subsequent KCl-induced contraction by ~ 50%; the protein synthesis inhibitor, cycloheximide, and the estrogen receptor antagonists, ICI 182,780 and tamoxifen, did not effect the attenuation. Moreover, E2, 2-HOE, and 2-MeOH antagonized the contraction induced by the vasospasm agonist, endothelin-1 (0.1μmol/L) by ~ 36%; when the L-type Ca2+ channel blocker, nifedipine, was added at the conclusion of the experiment no additional contractile attenuation was present. Our results suggest that E2, 2-HOE, and 2-MeOH demonstrate a similar nongenomic inhibition of agonist-induced extracellular Ca2+-dependent contractions.
Keywords: 17β-estradiol, 2-hydroxyestradiol, 2-methoxyestradiol, endothelin-1, acetylcholine, artery, coronary, calcium
Introduction
Because the development of coronary heart disease dramatically increases after women reach menopause this suggests that 17β-estradiol (E2) protects women against the development of vascular disease (Orshal and Khalil, 2004). Rosano et al. (1997) even demonstrated that the short-term administration of E2 has an anti-ischemic effect in postmenopausal women with coronary artery disease. As suggested by Dubey et al. (2004), the cardiovascular benefits of E2 may also be mediated by its biologically active metabolites, 2-hydroxyestradiol (2-HOE) and 2-methoxyestradiol (2-MeOH). Thus far no studies have directly compared coronary artery reactivity between E2, 2-HOE, and 2-MeOH. Kitazawa et al. (1997) did measure the ability of E2, 2-HOE, and 2-MeOH to relax pre-constricted (using a high KCl solution) denuded rat femoral arteries and found the potency order to relax a KCl-induced contraction was E2 = 2-MeOH ≫ 2-HOE.
Our interest in the ability of the E2 metabolites to regulate coronary arterial diameter is based upon the findings that 2-MeOH does not demonstrate the mitogenic effects associated with E2 (Gui and Zheng, 2006; Nishigaki et al., 1995); therefore, our long-term hypothesis is that these metabolites may help maintain blood flow through the coronary vasculature without promoting abnormal cell growth and proliferation. E2 does appear to suppress the occurrence of coronary arterial spasms and the resultant reduction in the development of myocardial ischemia (Kawano and Ogawa, 2005). However, there are a lack of studies evaluating the ability of E2, 2-HOE, and 2-MeOH to inhibit a subsequent coronary artery contraction and/or spasm. Sudhir et al. (1997) initially found that the intracoronary infusion of E2 can reduce coronary vasospasms mediated by the atherosclerotic and vasoconstrictive peptide, endothelin-1 (ET-1). Moreover, Dubey et al. (2001) reported that 2-MeOH is able to inhibit the synthesis of ET-1. Based upon these reports by Sudhir et al. (1997) and Dubey et al. (2001) this study tested the hypothesis that a short-term exposure to E2, 2-HOE, and 2-MeOH is able to similarly attenuate coronary arterial vasoconstriction. Because the generation of coronary arterial tone is principally due to Ca2+ influx through voltage-gated Ca2+ channels (Quignard et al., 1997), we predict that our hypothesized contractile inhibition is due to blockage of extracellular Ca2+ influx.
Materials and Methods
Isometric Tension Measurements
Pig hearts from female Yorkshire pigs 3 to 4 years of age (“retired” breeding sows) were freshly obtained from a local packing plant, placed on ice, and brought back to the laboratory. The right coronary artery was immediately dissected from each heart in a cold, modified low Ca2+ Kreb’s solution containing (in mmol/L) 138 NaCl, 5 KCl, 0.1 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and a pH of 7.4.
Coronary arteries were sectioned into 4 mm rings (endothelium intact) and suspended in 25 mL organ baths containing an oxygenated (95% O2: 5% CO2) modified Kreb’s solution (PSS; composition in mmol/L: 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose) having a pH of 7.4 at 37oC. The inner diameter of these arterial rings were ~ 250 μm, and the optimal length for each ring was determined as previously described (Hill et al., 2000). Endothelial integrity was confirmed by relaxation to bradykinin (0.1μmol/L). For each experiment we evaluated the effects of 17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), 2-methoxyestradiol (2-MeOH), and the EtOH solvent on different ring segments of the same artery. A total of 27 pigs were used to complete this study.
To determine the effective short-term incubation time to cross-compare E2, 2-HOE, and 2-MeOH we determined the relaxation kinetics for each of these estradiols. The amount of time (in minutes) to achieve a 25%, 50%, 75%, and 100% relaxation of a pre-constricted artery (using a 60 mmol/L KCl solution) is shown in Table 1. Based upon these data all incubation times were 60 minutes. This kinetic analysis is similar to the E2 kinetics reported by Kitazawa et al. (1997) using the rat portal vein. They found that E2 (5 μmol/L) induced a decline in a KCl constricted vein by ~80% in 60 minutes, and that a 60 minute pre-incubation before the administration of a high KCl solution reduced the KCl-induced contraction by a similar magnitude (~80% of control).
Table 1.
Percent relaxation kinetics (in minutes) for 30 μmol/L 17β-estradiol, 2-hydroxyestradiol, and 2-methoxyestradiol.
| Treatment | n | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 17β-Estradiol | 7 | 12.86 ± 1.20 | 24.60±1.37 | 38.59±1.50 | 56.27±1.32 |
| 2-Hydroxyestradiol | 7 | 12.66±1.75 | 24.82±1.65 | 38.44±1.46 | 53.99±1.16 |
| 2-Methoxyestradiol | 7 | 10.49±1.65 | 23.63±1.17 | 37.57±1.02 | 53.59±1.14 |
To evaluate the relaxation ability of E2, 2-HOE, and 2-MeOH, rings were initially pre-constricted with a depolarizing 60 mmol/L KCl solution (60K; composition in mmol/L: 83 NaCl, 60 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose; pH of 7.4) and a concentration-response relationship generated to each respective estradiol (0.3 μmol/L to 100 μmol/L) and its ethanol (EtOH) vehicle. Each estradiol concentration was allowed to equilibrate with the rings for 60 minutes before adding the next sequential concentration.
The ability of E2, 2-HOE, and 2-MeOH to attenuate vascular tone development was evaluated against known vasoconstrictive agonists, a depolarizing 60K solution, endothelin-1 (ET-1), and acetylcholine (ACH). The 60K-induced constriction was evaluated before and after incubation with E2, 2-HOE, and 2-MeOH (10 μmol/L). This was done in the presence and absence of the protein synthesis inhibitor, cycloheximide (10 μmol/L). Moreover, the reversibility of any estradiol-induced attenuation of the 60K contraction was further evaluated with a subsequent washout period (30 minutes) before exposing the rings to 60K for a third time.
To determine the vascular reactivity to ET-1 and ACH in the presence of the estradiols, a repeatable contractile response to 60K was initially obtained before the 60 minute incubation period with the estradiols or EtOH. Incubation with the estradiols or EtOH was done in the presence (PSS, 2 mmol/L Ca2+) and absence (0 mmol/L Ca2+) of extracellular Ca2+. The 0 mmol/L Ca2+ solution had the following composition (in mmol/L: 138 NaCl, 5 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 10 glucose) having a pH of 7.4 at 37 oC. Finally, the rings were exposed to endothelin-1 (0.1μmol/L) or acetylcholine (10 μmol/L) while still in the 0 mmol/L or 2 mmol/L Ca2+ solution. This was followed by the addition of the L-type Ca2+ channel antagonist, nifedipine (10μmol/L). The contractions to ET-1 and ACH are expressed as a percentage of the initial 60K response.
The effect of the α and β estrogen receptor modifiers, ICI 182,780 and tamoxifen, were also evaluated on the 60K-induced contraction. The 60K-induced contraction was evaluated before and after incubation with EtOH, E2, 2-HOE, and 2-MeOH (30 μmol/L). This was done in the presence and absence of a 60 minute incubation period with ICI 182,780 and tamoxifen (10 μmol/L). The isometric tension was amplified by a Transbridge 4M amplifier (World Precision Instruments, Florida, USA) and the data recorded by the Windaq data acquisition system (Dataq Instruments, Ohio, USA).
Chemicals
Acetylcholine HCl, nifedipine, cycloheximide, tamoxifen, 17β-estradiol (E2), 2- hydroxyestradiol (2-HOE), 2-methoxyestradiol (2-MeOH) were purchased from Sigma Aldrich (St. Louis, MO, USA). ICI 182,780 and endothelin-1 were obtained from Tocris Bioscience (Ellisville, MO, USA) and American Peptide Company (Sunnyvale, CA, USA), respectively. The solvent for the estradiols, nifedipine, and ICI 182,780 was ethanol, and stock solutions were made 1000-fold greater than the final bath concentration; thus, the EtOH concentration in the bath was 0.1% (except when generating the concentration-response relationship to the estradiols and EtOH to relax the pre-constricted arterial rings).
Statistical Analysis
Data are expressed as the mean ± S.E.M. for the number (n) of the animals within each group. The EC50 values (−log of the effective concentration to generate a 50% response) were calculated and analyzed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). The relaxation kinetics were determined on each arterial ring by conducting a natural log transformation of the data (r2 > 0.98) using Microsoft Excel (Microsoft Corporation, Redmond, WA); this generated an equation that was used to determine the percent relaxation for each arterial segment. Statistical analyses of the data were performed using a one-way or two-way ANOVA followed by Bonferroni’s post-hoc analysis (SigmaStat 3.5; SyStat Software, Point Richmond, CA), depending on the number of factors of interest. Significance was defined as p < 0.05.
Results
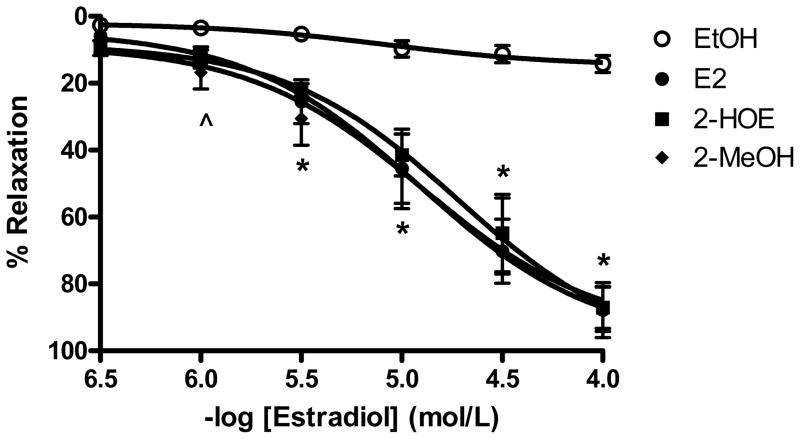
A concentration-response relationship (0.3 μmol/L to 100 μmol/L) to 17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), and 2-methoxyestradiol (2-MeOH) was generated to determine the approximate pD2 value (−log EC50) for each estradiol that would be used in subsequent experiments. As shown in figure 1, all the estradiols similarly relaxed the 60 mmol/L (60K)-induced contraction from 3 μmol/L to 100 μmol/L (n=6). The pD2 values for E2, 2-HOE, and 2-MeOH were 4.91±0.04, 4.72±0.04, and 4.89±0.15, respectively. Likewise, E2, 2-HOE, and 2-MeOH almost achieved complete relaxation to baseline (87.89±8.21%, 87.15±6.25%, and 87.66±6.65%, respectively).
Figure 1.
17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), and 2-methoxyestradiol (2-MeOH demonstrate a similar ability to induce coronary relaxation. Rings were initially pre-constricted with a 60 mmol/L KCl solution followed by the generation of a cumulative concentration response relationship to each respective estradiol (0.3 μmol/L to 100 μmol/L). Each estradiol-induce relaxation is expressed as percentage (%) of the maximum contractile response to 60 mmol/L KCl (n=6 pigs for each group). ^ indicates 2-MeOH p<0.05 from the EtOH solvent. Also, * suggests p<0.05 for all the groups from the EtOH control using a one-way ANOVA.
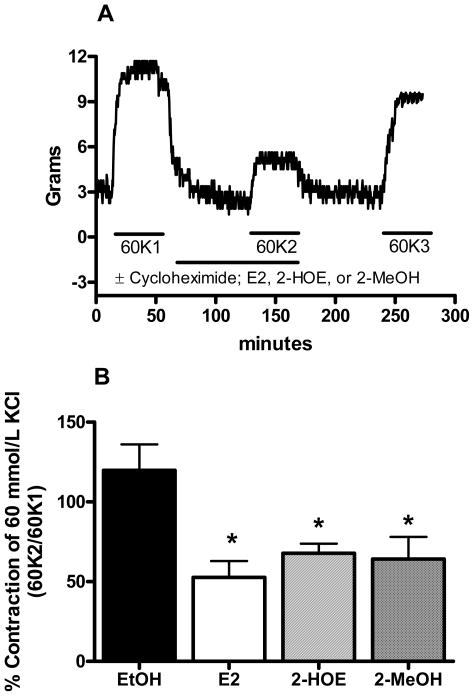
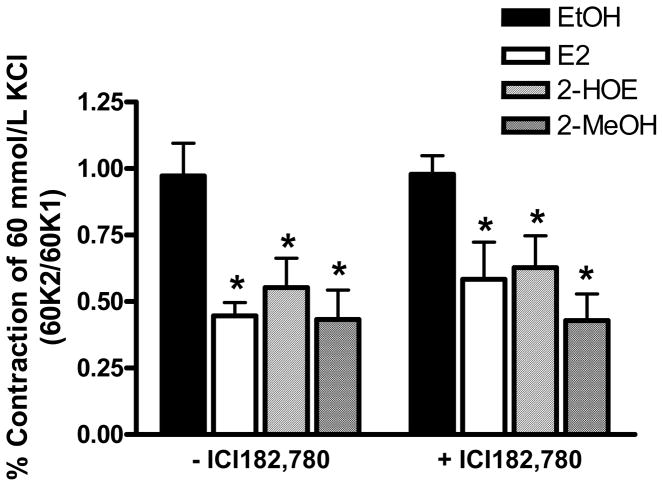
The approximate relaxation pD2 values (10 μmol/L) for E2, 2-HOE, and 2-MeOH also attenuated the contraction to a depolarizing 60K solution (n=5). As shown in figure 2A, the rings were exposed to 60K before and after treatment with E2, 2-HOE, and 2-MeOH. Then after a washout period with a physiological saline solution (PSS), 60K was reapplied to the rings. The percent change from the first (60K1) to the second (60K2) 60K-induced contraction for the ethanol (EtOH) solvent was 119.77±16.28%. In contrast, the 60K2/60K1 ratio for E2, 2-HOE, and 2-MeOH was 52.70±10.20%, 67.76±6.04%, and 64.13±13.92%, respectively (figure 2B). Cycloheximide (10 μmol/L) had no effect on 60K-induced contraction in the presence of EtOH, or the E2, 2-HOE, and 2-MeOH attenuation of the 60K-mediated contraction. After the washout period, the 60K-induced contraction (60K3) was similar to the initial (60K1) 60K contraction. The 60K3/60K1 ratio for EtOH, E2, 2-HOE, and 2-MeOH was 111.06±6.50%, 81.32±5.73%, 97.14±12.08%, and 93.09±11.22%, respectively. The attenuation of the 60K-induced contraction by the estradiols was further investigated using the known estrogen receptor antagonists, ICI 182,780 (n=5) and tamoxifen (n=2). Teoh et al. (1999; 2000b) has previously used these antagonist concentrations to effectively confirm if E2 operates through an estrogen receptor dependent mechanism in porcine coronary arteries. As shown in figure 3, ICI 182,780 (10 μmol/L) had no effect on the estradiols’ antagonism of the contraction by 60K. Tamoxifen (10 μmol/L) also had no effect (data not shown).
Figure 2.
17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), and 2-methoxyestradiol (2-MeOH) exhibit a similar nongenomic inhibitory effect on a depolarizing KCl-induced contraction. (A) Typical tracing illustrating the experimental protocol used to detemermine the nongenomic inhibition of the 60 mmol/L KCl (60K)-induced contraction by E2, 2-HOE, and 2-MeOH. Separate arterial rings incubated with either 10 μmol/L of E2, 2-HOE, or 2-MeOH from the same artery (n=5) were incubated in the presence or absence of cycloheximide (10 μmol/L). The horizontal lines indicate the exposure time to each drug. 60K1, 60K2, and 60K3 represent the first, second, and third application of the 60K solution. The composite data are shown in panel (B), which demonstrates that the KCl-induced contraction is attenuated by E2, 2-HOE, and 2-MeOH. The data shown are in the absence of cycloheximide and are expressed as a percentage of the first 60K-mediated contraction (60K2/60K1). * indicates p<0.05 (one-way ANOVA) from the EtOH solvent.
Figure 3.
Inhibition of the KCl-induced contraction by 17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), and 2-methoxyestradiol (2-MeOH) is independent of estrogen receptors. The 60 mmol/L KCl (60K)-induced contraction was evaluated in the absence (60K1) and presence (60K2) of the selective estrogen receptor modifier, ICI 182,780, * indicates p<0.05 from the EtOH solvent (n=5 pigs for each group) using a two-way ANOVA.
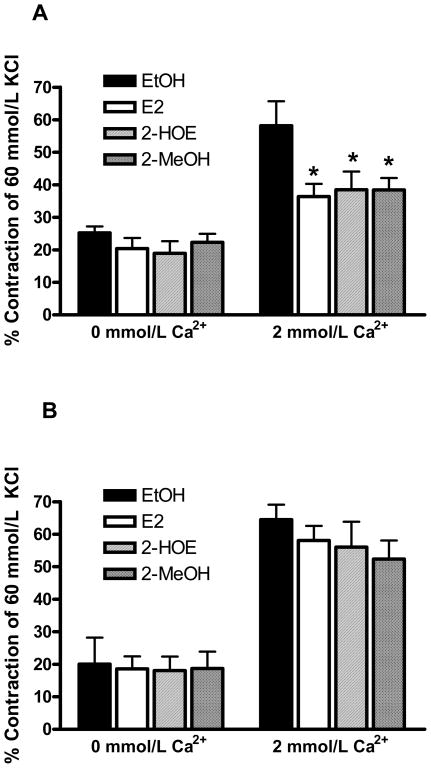
E2, 2-HOE, and 2-MeOH (1 μmol/L and 10 μmol/L) were also evaluated against the potent, vasoconstrictive peptide, endothelin-1 (ET-1, 0.1 μmol/L). As shown in figure 4A, incubation in E2, 2-HOE, and 2-MeOH (10 μmol/L, n=7) similarly attenuated the ET-1-mediated constriction by ~ 36% compared to the EtOH solvent. While the addition of nifedipine (10 μmol/L) relaxed the ET-1-induced contraction to pre- constriction levels in the EtOH group, nifedipine had no further effect on the estradiol-mediated inhibition (data not shown). No attenuation of the ET-1 contraction was present using 1 μmol/L of E2 and its metabolites (data not shown; n=7). As demonstrated in figure 4A, removal of extracellular Ca2+ (0 mmol/L Ca2+) significantly lowered the ET-1-induced contraction in the presence of EtOH and the estradiols (10 μmol/L); however, the estradiols did not effect the contraction to ET-1 in the absence of extracellular Ca2+.
Figure 4.
17β-estradiol (E2), 2-hydroxyestradiol (2-HOE), and 2-methoxyestradiol (2-MeOH) attenuate the endothelin-1-induced (ET-1) constriction, but not the acetylcholine-mediated constriction. Both agonist-induced contractions were evaluated in the presence (2 mmol/L Ca2+) and absence (0 mmol/L Ca2+) of extracellular Ca2+. (A) E2, 2-HOE, and 2-MeOH (10 μmol/L) demonstrate a similar ability to inhibit the ET-1-induced (0.1μmol/L) contraction in 2 mmol/L Ca2+. * indicates p<0.05 from the EtOH solvent (n=7 pigs for each group). Also, p<0.05 between EtOH groups (2 mmol/L Ca2+ versus 0 mmol/L Ca2+). (B) E2, 2-HOE, and 2-MeOH (10 μmol/L) have no effect on the acetylcholine-induced (10 μmol/L) contraction (n=4 pigs for each group). All data are represented as the % of an initial 60 mmol/L KCl-induced contraction for each arterial ring. Statistical analysis was performed using a two-way ANOVA.
In contrast to ET-1, incubation in E2, 2-HOE, and 2-MeOH (1 μmol/L and 10μmol/L) did not attenuate the contraction induced by the coronary vasoconstrictive agonist, acetylcholine (10 μmol/L, n=4). As similarly demonstrated by ET-1, the absence of extracellular Ca2+ significantly reduced the acetylcholine-induced contraction (figure 4B); this contraction was not effected by the estradiols.
Discussion
Prior studies have suggested that a short-term exposure (< 60 minutes) to E2 elicits a nongenomic relaxation response in the vasculature. These nongenomic responses include the generation of NOS (Mendelsohn, 2002), activation of cAMP (Teoh and Man, 2000), and the inhibition of Ca2+ influx, thus inducing vascular relaxation (Crews and Khalil, 1999; Kitazawa et al., 1997; Salom et al., 2002) and attenuating constrictive agonists (Kitazawa et al., 1997; Teoh et al., 2000a). However, few studies have directly compared the nongenomic response of E2 with its most efficacious metabolites of the vascular system, 2-HOE and 2-MeOH. The purpose of our study was to determine the short-term response of E2 with 2-HOE and 2-MeOH on coronary vasoconstriction. Our results indicated that E2, 2-HOE, and 2-MeOH similarly inhibited a depolarizing KCl-induced contraction, and also the contraction mediated by the vasospastic peptide, endothelin-1. The estradiols failed to inhibit the vasoconstriction induced by another vasospastic agent, acetylcholine. Our results indicate that the estradiols inhibit the voltage-gated entry of extracellular Ca2+, therefore, the difference in the agonist-induced attenuation by the estradiols appears to be due to the Ca2+ influx pathways used to elicit a contraction.
Inhibition of Ca2+ influx by the estradiols appears to be nongenomic, estrogen receptor independent, and occur at supraphysiological concentrations. Because of the high E2, 2-HOE, and 2-MeOH concentrations used to attenuate the contractile activity of the coronary arteries, we predict that the estradiols primarily target the arterial smooth muscle in lieu of the endothelium. Although the aim of this study was not to determine if E2, 2-HOE, and 2-MeOH mediate their contractile inhibition through their action on smooth muscle versus the endothelium, our lab has previously found that the mechanical removal of the endothelium does not change the relaxation kinetics of E2, 2-HOE, and 2-MeOH in this study (unpublished observations). A reoccurring concern with many of the studies that have evaluated the short-term (<60 minutes) effects of E2 on vascular tone is that E2 elicits its effects at superphysiological concentrations. However, our lab has found that a longer exposure time (> 24 hrs) to E2 will increase its efficacy. Using coronary arteries we have found that 1 nmol/L E2 attenuates the arterial constriction in response to a high KCl solution due to the downregulation of voltage-gated Ca2+ channels (Hill and Rusch, 2008). Future studies will be aimed to investigate whether physiological concentrations of the E2 metabolites can elicit a similar genomic effect as E2 based upon a longer exposure time.
We confirmed that the short exposure time used in this study represented nongenomic E2, 2-HOE, and 2-MeOH effects by demonstrating that washing out the arterial rings with a physiological saline solution could reverse the estradiols’ inhibition of the KCl-induced contraction. Also, the presence of the protein synthesis inhibitor, cycloheximide, did not effect the inhibition of the KCl contraction. In further support of this nongenomic effect of E2, 2-HOE, and 2-MeOH, we found that the relaxation response and inhibition of the KCl-induced contraction was not effected by the E2 receptor antagonists, ICI 182,780 and tamoxifen. In agreement, Nadal et al. (2000) and Hayes et al. (2002) previously suggested that the acute effects of estadiols occur independent of the classical α and β estrogen receptors.
The estradiols’ inhibition of the depolarizing KCl-induced contraction suggests that that E2, 2-HOE, and 2-MeOH interfere with voltage-gated Ca2+ influx. This corroborates a study by Han et al. (1995) who demonstrated E2 inhibition of Ca2+ influx in female porcine coronary arteries. Likewise, our data indicate the estradiols also inhibited the extracellular Ca2+-dependent vasoconstriction mediated by the coronary vasospastic peptide, endothelin-1 (ET-1). ET-1 is known to mediate force generation by a sustained influx of extracellular Ca2+ into vascular smooth muscle cells primarily by voltage-dependent mechanisms (i.e. L-type Ca2+ channels) (Bowles et al., 1995; Hill et al., 2000). In our study we suspect that the estradiols do primarily inhibit the voltage-dependent mechanisms because (1) the attenuation of the KCl and ET-1-induced contractions (in 2 mmol/L extracellular Ca2+) had a similar magnitude, and (2) in the presence of extracellular Ca2+ the addition of the L-type Ca2+ channel antagonist, nifedipine, had no additional effect on the estradiols’-induced attenuation of the ET-1 contraction.
In contrast to the KCl and ET-1 contractions, E2, 2-HOE, and 2-MeOH had little effect on the acetylcholine-mediated contraction. Because our data suggest that the estradiols inhibit voltage-dependent Ca2+ channels we suspect that acetylcholine must mediate its contractile response via a voltage-independent Ca2+ influx mechanism. Our results are corroborated by a study by Tanz and Nayler (1991) who used pig coronary arteries to demonstrate that the acetycholine-induced contraction is mediated by a Ca2+ influx mechanism that is not sensitive to the L-type Ca2+ channel antagonists, nifedipine and diltiazem. We did not attempt to mechanistically characterized the acetylcholine-induced contraction in these arteries; however, Weirich et al. (2005) has previously identified that acetylcholine may induce Ca2+ influx via Ca2+-permeable nonselective cation channels and store-operated Ca2+ channels.
Overall, our studies suggest that the metabolites of E2 (2-HOE and 2-MeOH) are equally effective as its parent compound, E2, to inhibit voltage-gated Ca2+ influx (i.e. L-type Ca2+ channels) and attenuate agonist-induced extracellular Ca2+-dependent contractions. 2-MeOH has already been evaluated in phase I and II clinical trials with mixed results to treat various myelomas (James et al., 2007; Rajkumar et al., 2007). Rajkumar et al. (2007) has suggested that part of the problem with the oral administration of 2-MeOH (> 1,000 mg/day) is its low bioavailability in the plasma (increase of 1-2 ng/mL). However, Yan et al. (2006) demonstrated that the localized administration of 2-MeOH can reduce cerebral vasospasms and inhibit vascular smooth muscle cell proliferation. The clinical use of the E2 metabolites (particularly 2-MeOH) needs to be further analyzed because 2-MeOH may have a protective role against the development of coronary artery disease.
Acknowledgments
This study was made possible by Grant Number P20 RR-16460 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. We also appreciate Odoms Tennesse Pride Sausage plant (Little Rock, AR) for their donation of the pig hearts.
References
- Bowles DK, Laughlin MH, Sturek M. Exercise training alters the Ca 2+ and contractile responses of coronary arteries to endothelin. J Appl Physiol. 1995;78:1079. doi: 10.1152/jappl.1995.78.3.1079. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19:1034. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. J Hypertens. 2001;37:640. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zheng XL. 2-methoxyestradiol induces cell cycle arrest and mitotic cell apoptosis in human vascular smooth muscle cells. J Hypertens. 2006;47:271. doi: 10.1161/01.HYP.0000199656.99448.dc. [DOI] [PubMed] [Google Scholar]
- Han SZ, Karaki H, Ouchi Y, Akishita M, Orimo H. 17 beta-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Li L, Russell KS, Bender JR. Rapid vascular cell responses to estrogen and membrane receptors. Vascul Pharmacol. 2002;38:99. doi: 10.1016/s0306-3623(02)00133-7. [DOI] [PubMed] [Google Scholar]
- Hill BJF, Katwa LC, Wamhoff BR, Sturek M. Enhanced endothelin A receptor-mediated calcium mobilization and contraction in organ cultured porcine coronary arteries. J Pharmacol Exp Ther. 2000;295:484. [PubMed] [Google Scholar]
- Hill BJF, Rusch NJ. An estrogen-induced decrease in voltage-gated calcium channel expression attenuates coronary artery contractility. FASEB J. 2008;22:1152A. [Google Scholar]
- James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor C, Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- Kawano H, Ogawa H. Endothelial function and coronary spastic angina. Intern Med. 2005;44:91. doi: 10.2169/internalmedicine.44.91. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol. 1997;499(Pt 2):497. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA. 2000;97:11603. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki I, Sasaguri Y, Yagi K. Anti-proliferative effect of 2-methoxyestradiol on cultured smooth muscle cells from rabbit aorta. Atherosclerosis. 1995;113:167. doi: 10.1016/0021-9150(94)05442-l. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Frapier JM, Harricane MC, Albat B, Nargeot J, Richard S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J Clin Invest. 1997;99:185. doi: 10.1172/JCI119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Richardson PG, Lacy MQ, Dispenzieri A, Greipp PR, Witzig TE, et al. Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin Cancer Res. 2007;13:6162. doi: 10.1158/1078-0432.CCR-07-0807. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Caixeta AM, Chierchia S, Arie S, Lopez-Hidalgo M, Pereira WI, et al. Short-term anti-ischemic effect of 17beta-estradiol in postmenopausal women with coronary artery disease. Circulation. 1997;96:2837. doi: 10.1161/01.cir.96.9.2837. [DOI] [PubMed] [Google Scholar]
- Salom JB, Burguete MC, Perez-Asensio FJ, Centeno JM, Torregrosa G, Alborch E. Acute relaxant effects of 17-beta-estradiol through non-genomic mechanisms in rabbit carotid artery. Steroids. 2002;67:339. doi: 10.1016/s0039-128x(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Ko E, Zellner C, Wong HE, Hutchison SJ, Chou TM, Chatterjee K. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation. 1997;96:3626. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- Tanz RD, Nayler WG. Concentration-dependent desensitization of isolated porcine coronary arterial segments to acetylcholine. Arch Int Pharmacodyn Ther. 1991;312:110. [PubMed] [Google Scholar]
- Teoh H, Leung SW, Man RY. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc Res. 1999;42:224. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- Teoh H, Man RY. Enhanced relaxation of porcine coronary arteries after acute exposure to a physiological level of 17beta-estradiol involves non-genomic mechanisms and the cyclic AMP cascade. Br J Pharmacol. 2000;129:1739. doi: 10.1038/sj.bjp.0703252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol. 2000a;129:1301. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H, Quan A, Man RY. Acute impairment of relaxation by low levels of testosterone in porcine coronary arteries. Cardiovasc Res. 2000b;45:1010. doi: 10.1016/s0008-6363(99)00398-3. [DOI] [PubMed] [Google Scholar]
- Weirich J, Dumont L, Fleckenstein-Grun G. Contribution of capacitative and non-capacitative Ca2+-entry to M3-receptor-mediated contraction of porcine coronary smooth muscle. Cell Calcium. 2005;38:457. doi: 10.1016/j.ceca.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Yan J, Chen C, Lei J, Yang L, Wang K, Liu J, Zhou C. 2-methoxyestradiol reduces cerebral vasospasm after 48 hours of experimental subarachnoid hemorrhage in rats. Exp Neurol. 2006;202:348. doi: 10.1016/j.expneurol.2006.06.009. [DOI] [PubMed] [Google Scholar]