Abstract
Eukaryotic cells can initiate several distinct programmes of self-destruction, and the nature of the cell death process (non-inflammatory or proinflammatory) instructs responses of neighbouring cells, which in turn dictates important systemic physiological outcomes. Pyroptosis, or caspase 1-dependent cell death, is inherently inflammatory, is triggered by various pathological stimuli, such as stroke, heart attack or cancer, and is crucial for controlling microbial infections. Pathogens have evolved mechanisms to inhibit pyroptosis, enhancing their ability to persist and cause disease. Ultimately, there is a competition between host and pathogen to regulate pyroptosis, and the outcome dictates life or death of the host.
Cells can die through distinct biochemical pathways that produce different morphological and physiological outcomes. Apoptosis is perhaps the most widely recognized programme of cell death, and is mechanistically defined by the requirement for particular cysteine-dependent aspartate-specific proteases, or caspases, which produce an orchestrated disassembly of the cell1. Apoptotic caspases cleave cellular substrates, resulting in the characteristic features of apoptosis, which include cytoplasmic and nuclear condensation, DNA cleavage and maintenance of an intact plasma membrane. The contents of apoptotic cells are packaged into membrane-enclosed apoptotic bodies, which are targeted for phagocytosis and removal in vivo, resulting in an absence of inflammation2 (BOX 1).
Box 1 | Apoptosis is a programmed process that results in non-inflammatory cell death
Hippocrates was the first to use the term apoptosis in the medical literature (approximately 460–370 BCE)121. After years of exhaustive microscopic evaluation, apoptosis was reintroduced by Kerr et al. in 1972 to describe an active, programmed process that leads to cell death in both healthy and diseased tissues122. Its morphological characteristics included condensation of both the cytoplasm and the nucleus, and the generation of cell fragments called apoptotic bodies, which were phagocytosed by intact cells and subsequently destroyed. Little tissue disruption and a marked lack of inflammation suggested the process was a “general mechanism of controlled cell deletion, which is complementary to mitosis in the regulation of animal cell populations.” (REF. 122) Cell death caused by apoptosis was previously referred to as shrinkage necrosis. By contrast, coagulative necrosis was “invariably caused by noxious stimuli” and resulted from “irreversible disturbance of cellular homeostatic mechanisms.” (REF. 122) These original descriptions are consistent with recent recommendations for using nomenclature that defines cell death, or necrosis, as the end product of processes such as apoptosis3,123. The term apoptosis, which in Greek is used to describe the ‘falling off’ of leaves from a tree, was suggested to indicate the controlled loss of individual cells from the population. Pronunciation provides a clear indication of its Greek roots: “we propose that the stress should be on the penultimate syllable, the second half of the word being pronounced like “ptosis” (with the “p” silent), which comes from the same root “to fall” and is already used to describe drooping of the upper eyelid.” (REF. 122) The ultrastructural features described in this landmark paper are still considered to be hallmarks of apoptosis, and subsequent research has identified the important role of a subset of caspases in mediating the morphological changes observed in this and other early studies1.
Although apoptosis was the first well-recognized programme of eukaryotic cell death, ‘programmed cell death’ is broadly applied to several endogenous genetically defined pathways in which the cell plays an active part in its own destruction3. Other cell death programmes include autophagy, oncosis and caspase 1-dependent programmed cell death (also known as pyroptosis). Pyroptosis is a more recently identified pathway of host cell death that is stimulated by a range of microbial infections (for example, Salmonella, Francisella and Legionella) and non-infectious stimuli, including host factors produced during myocardial infarction4. Caspase 1 was first recognized as a protease that processes the inactive precursors of interleukin 1β (IL-1β) and IL-18 into mature inflammatory cytokines, and was initially called interleukin IL-1β-converting enzyme5. However, caspase 1 activation can result not only in the production of activated inflammatory cytokines, but also rapid cell death characterized by plasma-membrane rupture and release of proinflammatory intracellular contents6,7. Caspase 1-dependent cell death is a programmed process of cellular self-destruction mediated by caspases, and therefore was not initially distinguished from apoptosis8–11. However, the mechanism, characteristics and outcome of caspase 1-dependent cell death are distinct from apoptosis6,7,12. Thus, the term pyroptosis (from the Greek ‘pyro’, relating to fire or fever, and ‘ptosis’, meaning a falling (BOX 1)), is used to described the inherently inflammatory process of caspase 1-dependent programmed cell death13.
Mechanism and features of pyroptosis
Pyroptosis is morphologically and mechanistically distinct from other forms of cell death. Caspase 1 dependence is a defining feature of pyroptosis, and caspase 1 is the enzyme that mediates this process of cell death (FIG. 1). Caspase 1 is not involved in apoptosis, and caspase 1-deficient mice have no defects in apoptosis and develop normally14,15. The apoptotic caspases, including caspase 3, caspase 6 and caspase 8, are not involved in pyroptosis6,10,12,16–20, and substrates of apoptotic caspases, including poly (ADP-ribose) polymerase and inhibitor of caspase-activated DNase (ICAD), do not undergo proteolysis during pyroptosis6,7,9,12. Furthermore, loss of mitochondrial integrity and release of cytochrome c, which can activate apoptotic caspases, do not occur during pyroptosis16,19.
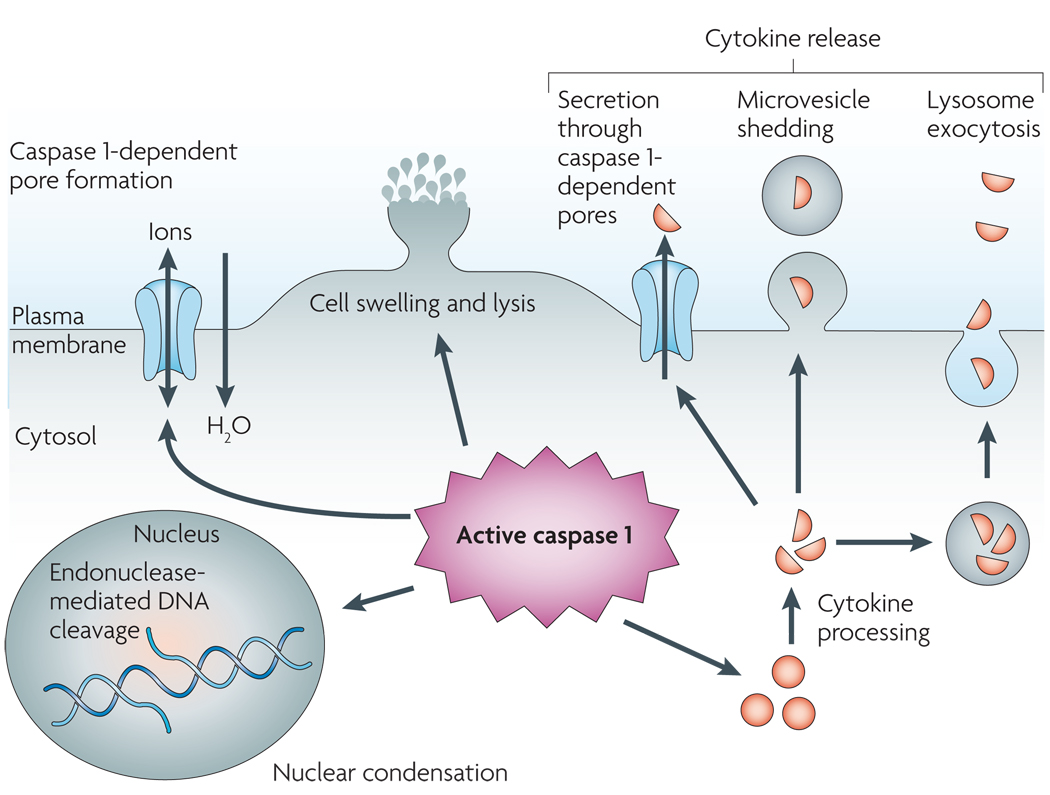
Figure 1. Pyroptosis, an inflammatory host response.
Caspase 1 is cleaved and activated in response to multiple stimuli, but once activated, caspase 1 results in a conserved programme of cell death referred to as pyroptosis. Caspase 1 activation also leads to rapid formation of plasma-membrane pores with a diameter of 1.1–2.4 nm. These pores dissipate cellular ionic gradients, allowing water influx, cell swelling and osmotic lysis. The pro-forms of interleukin-1β (IL-1β) and IL-18 are processed by caspase 1 and released during pyroptosis, although the exact mechanism of secretion remains controversial. Secretion does not require lysis and is temporally associated with caspase 1-dependent pore formation, suggesting that these pores facilitate cytokine release. Other suggested secretion mechanisms include caspase 1-independent lysosome exocytosis and microvesicle shedding. Caspase 1 activity results in cleavage of chromosomal DNA by an unidentified endonuclease. Cleavage of DNA does not result in the oligonucleosomal fragments observed during apoptosis. Nuclear condensation is also observed but nuclear integrity is maintained, unlike the nuclear fragmentation observed during apoptosis.
Pyroptosis features rapid plasma-membrane rupture and release of proinflammatory intracellular contents. This is in marked contrast to the packaging of cellular contents and non-inflammatory phagocytic uptake of membrane-bound apoptotic bodies that characterizes apoptosis2. Cell lysis during pyroptosis results from caspase 1-mediated processes8,9,12,17,18,20–24. Salmonella infection or treatment with lethal toxins from Bacillus anthracis produces plasma-membrane pores with a functional diameter of 1.1–2.4 nm7,20, and pore formation is a host cell-mediated process that is dependent on caspase 1 activity7,12,20. Caspase 1-dependent plasma-membrane pores dissipate cellular ionic gradients, producing a net increased osmotic pressure, water influx, cell swelling and, eventually, osmotic lysis and release of inflammatory intracellular contents7. Indeed, cells dying by pyroptosis undergo a measurable size increase7,18 (FIG. 1). In support of this mechanism, the cytoprotective agent glycine non-specifically blocks ion fluxes in damaged eukaryotic cells and thereby prevents swelling and lysis during pyroptosis6,7,21,25,26.
Cleavage of chromosomal DNA is a fatal event that is often assumed to indicate apoptotic cell death3; however, DNA damage also occurs during pyroptosis6,12,24,27,28. During apoptosis, caspase-mediated proteolysis of ICAD releases caspase-activated DNase (CAD). CAD cleaves DNA between nucleosomes, resulting in characteristic oligonucleosomal DNA fragments of approximately 180 bp7. Although purified caspase 1 can cleave ICAD in vitro11, ICAD degradation does not occur during pyroptosis7,12. DNA cleavage during pyroptosis instead results from the activity of an unidentified caspase 1-activated nuclease that does not produce the oligonucleosomal DNA fragmentation pattern that is characteristic of apoptosis7,12,29. DNA cleavage is accompanied by marked nuclear condensation, but unlike apoptosis, nuclear integrity is maintained12,23 (FIG. 1). DNA cleavage and cell lysis are both caspase 1-dependent features of pyroptosis, but cell lysis does not require DNA cleavage7.
Destruction of the actin cytoskeleton has also been observed in cells undergoing pyroptosis, but the mechanism and importance of this destruction remains unclear12,26. Caspase 1-dependent degradation of cellular inhibitor of apoptosis protein (cIAP) also accompanies during pyroptosis, although the exact mechanism that underlies cIAP degradation is also unknown30. Caspase 1 cleaves and inactivates metabolic enzymes during pyroptosis31, and identification of additional proteolytic targets of caspase 1 could yield insight into the mechanism of pyroptosis and novel features of this form of cell death.
TLRs and NLRs
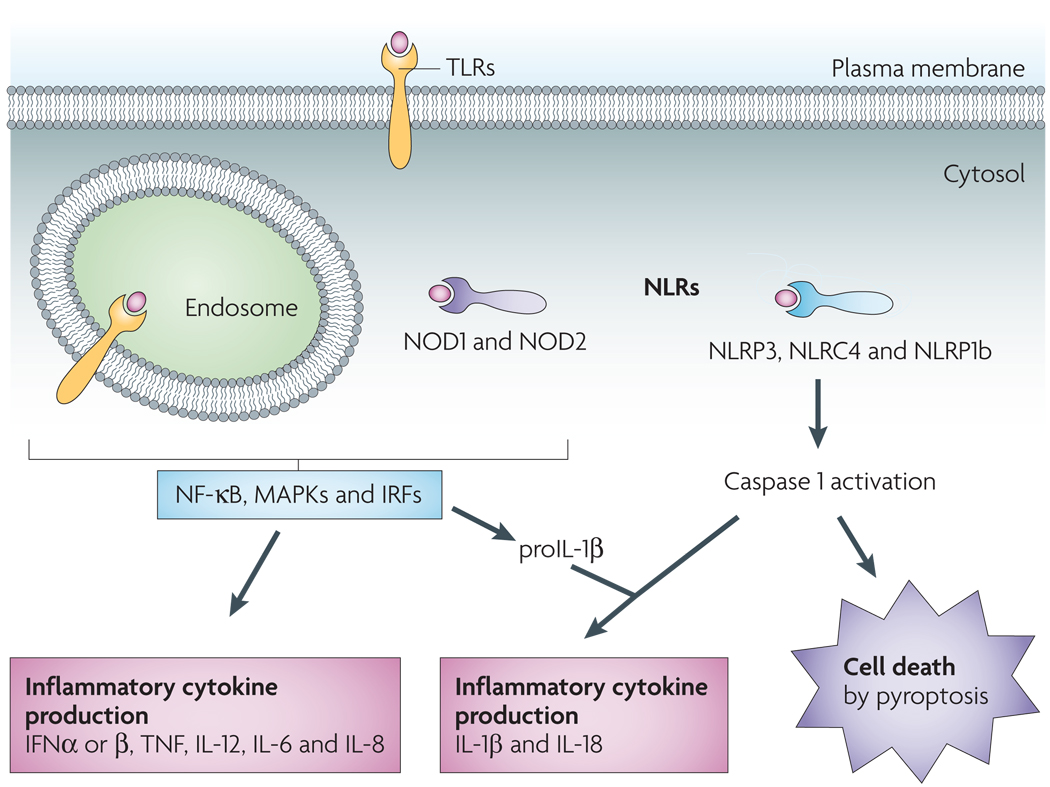
The host can use a range of mechanisms to sense intracellular and extracellular ‘danger’ signals generated by invading pathogenic microorganisms or by the host in response to tissue injury32. Toll-like receptors (TLRs) initiate a signalling cascade that leads to cellular activation and production of inflammatory cytokines, such as tumour necrosis factor (TNF), IL-6, IL-8 and type I interferons (IFNs), in response to extracellular signals33 (FIG. 2). Nod-like receptors (NLRs) function in the recognition of danger signals introduced into the host cell cytosol34. The NLRs nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and NOD2 trigger a signalling cascade following ligand recognition that, similarly to the cascade initiated by TLRs, results in inflammatory cytokine production34 (FIG. 2). Another subset of NLRs trigger activation of the cysteine protease caspase 1 (REF. 35), which leads to caspase 1-dependent pyroptosis and processing and release of the inflammatory cytokines IL-18 and IL-1β3 (FIG. 2). TLRs and caspase 1-activating NLRs often act in concert with TLR stimulation, resulting in enhanced susceptibility to NLR-mediated caspase 1 activation in response to ATP treatment36– 38 and Yersinia infection12. TLRs and NOD1 and NOD2 also stimulate the production and intracellular accumulation of pro-IL-1β33,34. Thus, TLRs and NOD1 and NOD2 prime cells to undergo caspase 1 activation and produce maximal IL-1β in response to subsequent cytosolic recognition of host- or pathogen-derived danger signals.
Figure 2. Sensing of host- and microorganism-derived ‘danger’ signals leads to two distinct outcomes: cellular activation and cell death.
Leucine-rich repeat (LRR) domains mediate host recognition of pathogen- and danger-associated molecular patterns. Toll-like receptors (TLRs) are LRR-containing transmembrane proteins that detect danger signals located in the extracellular milieu and within endosomes. TLRs initiate a signalling cascade that leads to cellular activation (through nuclear factor-κB (NF-κB)-, mitogen-activated protein kinase (MAPK)- and interferon (IFN)-regulatory factor (IRF)-dependent pathways) and inflammatory cytokine production (including IFNα, IFNβ, tumour necrosis factor (TNF), interleukin-12 (IL-12), IL-6, IL-8 and pro-IL-1β). Nod-like receptors (NLRs) function in the recognition of danger signals introduced into the host cell cytosol. Like TLRs, NOD1 (nucleotide-binding oligomerization domain-containing protein 1) and NOD2 stimulation results in cytokine production. Another subset of NLRs mediate activation of the cysteine protease caspase 1, which triggers caspase 1-dependent cell death (pyroptosis) and processing and release of the inflammatory cytokines IL-18 and IL-1β. NLRC4, NLR family CARD domain-containing protein 4; NLRP3, NACHT, LRR and PYD domains-containing protein 3. NLRP1b, NAHCT, LRR and PYD domains-containing protein 1b.
Caspase-1-activating NLRs
NLR recognition of bacterial, viral and host molecules, as well as toxic foreign products, can lead to the activation of caspase 1. The NLR protein NLRP3 (NACHT, LRR and PYD domains-containing protein 3; also known as NALP3) responds to multiple stimuli, including pore-forming toxins38–40, extracellular ATP in the presence of various pathogen-associated molecules38,41,42, uric acid crystals43, virus-associated DNA44, RNA45, asbestos46 and ultraviolet B irradiation47. The mechanism by which NLRP3 detects this divergent group of signals is unknown. Cellular potassium efflux is a common response to many of these stimuli, and preventing potassium efflux blocks caspase 1 activation48–50. However, potassium efflux alone does not seem to be sufficient to trigger activation of caspase 1 (REFS 48,51), and preventing potassium efflux also blocks caspase 1 activation that is mediated by another NLR, NLRP1b (also known as NALP1b)20,52,53. This indicates that potassium efflux may not directly signal for NLRP3-dependent caspase 1 activation, but rather creates an environment that is favourable for ligand detection and/or caspase 1 activation49,52,54. It is possible that host cells respond to all of these stimuli by generating one or more secondary factors that bind NLRP3, and further experiments are needed to determine how NLRP3 directly recognizes or participates in the response to such a broad range of molecules.
The NLR protein NLRC4 (NLR family CARD domain-containing protein 4; also known as IPAF) mediates the recognition of diverse bacterial pathogens, which during infection reside extracellularly (for example, Pseudomonas) or intracellularly (for example, Salmonella, Legionella, Listeria and Shigella), and share similar requirements for the activation of caspase 1. These pathogens deliver virulence determinants into host cells through translocation systems that form conduits between the bacteria and host cell cytosol. The same conduits, key to the pathogenesis of infection, also betray the presence of pathogens by introducing flagellin into the host cell, where its recognition is facilitated by NLRC4 (REFS 23,55–59). During infection with cytosolic pathogens, such as Listeria, secreted flagellin has direct access to the cytosol, and a translocation system is not required60. Expression of flagellin in the macrophage cytosol stimulates NLRC4-dependent pyroptosis61, suggesting that NLRC4 directly recognizes flagellin; however, such an interaction has not been demonstrated. Interestingly, NLRC4-dependent caspase 1 activation has been reported during infection with Pseudomonas and Shigella mutants that do not produce flagellin62,63. These studies suggest that NLRC4, like NLRP3, can respond to additional bacterial components that remain unidentified.
The NLR NLRP1b recognizes cytosolic delivery of B. anthracis lethal toxin, a metalloprotease that can cleave host mitogen-activated protein kinases (MAPKs). NLRP1b-mediated caspase 1 activation is not due to structural recognition of the toxin itself, as lethal toxin that contains a point mutation in the catalytic site, but retains its native structure, fails to activate caspase 1 (REFS 20,64). Proteolytic activity of lethal toxin is required for caspase 1 activation, but MAPK cleavage alone is not sufficient, suggesting that as-yet-unidentified lethal toxin substrates are involved20. Proteasome activity is also required for caspase 1 activation in response to lethal toxin treatment20,30,53, suggesting that a lethal toxin-mediated alteration in proteasome function allows caspase 1 activation30.
Several NLR proteins, in addition to those described above, have been implicated in caspase 1 activation35. The NLR neuronal apoptosis inhibitory protein 5 (NAIP5) is required for caspase 1 activation during infection with Legionella, but does not seem to be necessary for all bacteria that activate caspase 1 through NLRC4 (REF. 61), and the exact role of NAIP5 in pyroptosis is unknown. Francisella requires ASC (apoptosis-associated speck-like protein containing a CARD), but not NLRC4 or NLRP3, to stimulate caspase 1 activation24,38, which implicates another NLR in the recognition of this pathogen.
The inflammasome
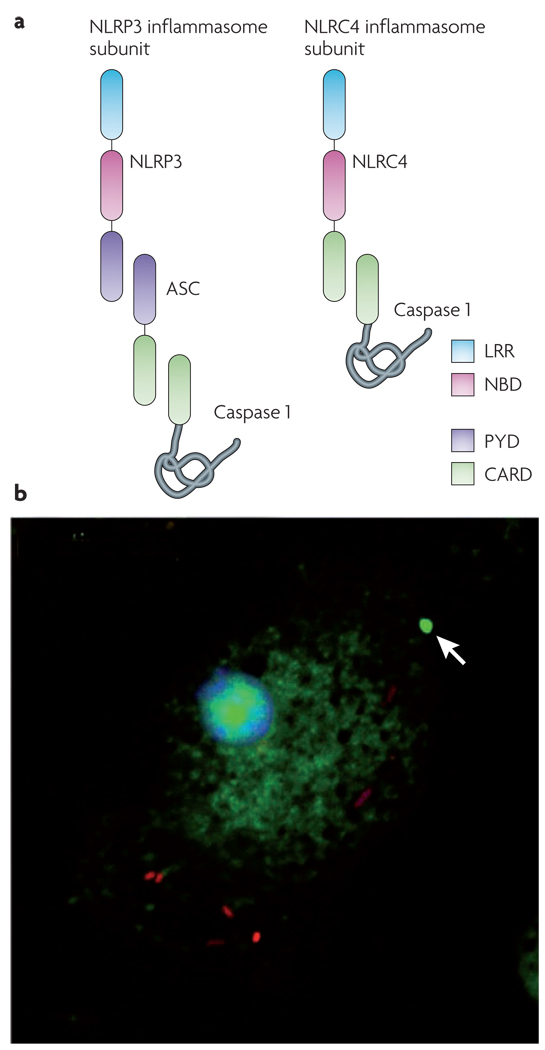
NLRs recognize their cognate host- or microorganism-derived danger signals and trigger formation of a multiprotein complex called the inflammasome, which contains caspase 1 (REFS 35,65). NLRs that have encountered their signal undergo nucleotide-dependent oligomerization using their nucleotide-binding domain66. Some NLRs, including NLRP3, bind to the adapter protein ASC, which contains a caspase activation and recruitment domain (CARD) and interacts with caspase 1 (REF. 35) (FIG. 3a). Other NLRs, such as NLRC4, contain a CARD and can directly interact with caspase 1 when overexpressed67 (FIG. 3a). The association of caspase 1 within this complex allows its processing and activation35.
Figure 3. Components of the inflammasome and visualizing the inflammasome complex.
a | Nod-like receptor (NLR) leucine-rich repeat (LRR) domains are implicated in sensing a range of intracellular ‘danger’ signals. After ligand recognition, the nucleotide-binding and oligomerization domain (NBD) mediates nucleotide-dependent self-association of NLRs. Some NLRs, such as NLRP3 (NACHT, LRR and PYD domains-containing protein 3; also called NALP3), contain a pyrin (PYD) domain that interacts with the adapter protein ASC (apoptosis-associated speck-like protein containing a CARD). ASC contains a caspase activation and recruitment domain (CARD) that binds and facilitates activation of caspase 1. Other NLRs, such as NLRC4 (NLR family CARD domain-containing protein 4; also known as IPAF), contain a CARD and can directly interact with caspase 1. However, ASC is often required for NLRC4-dependent caspase 1 activation, indicating that ASC may participate in NLRC4 inflammasome formation or play an additional part in caspase 1 activation. b | Salmonella (red) infection of macrophages results in activation of caspase 1 (green), which is visualized here using a fluorescently labelled inhibitor of the active enzyme. Active caspase 1 is often concentrated within a single focus (indicated by the arrow) and diffusely distributed throughout the cytoplasm. A similar distribution of active caspase 1 is seen in macrophages treated with Bacillus anthracis lethal toxin20.
It has been proposed that a single NLR mediates caspase 1 activation in response to a given stimulus, and these complexes can be observed in vitro65,66. However, other data suggest that interactions between multiple NLRs might contribute to inflammasome formation. For example, NAIP5 can affect the ability of Legionella to stimulate NLRC4-dependent activation of caspase 1 (REF. 61). NAIP5 can bind NLRC4 and contains a pathogen-sensing leucine-rich-repeat (LRR) domain57, but its exact role in inflammasome formation is unknown. NAIP5 does not seem to play a part in all NLRC4-containing inflammasomes, as NAIP5 is not required for caspase 1 activation by Salmonella61. Similarly, both NLRP3 and NLRC4 have a role in caspase 1 activation in response to Listeria infection60, pore-forming toxins39 and ultraviolet B irradiation47. These data suggest that multiple sensors are present in the same complex and function cooperatively to activate caspase 1. In addition, microbial infection could lead to cell damage and release of host danger signals, such as uric acid and ATP, that stimulate the activation of caspase 1. However, the release of these host ligands by dying cells has not been shown in vivo. Thus, host cells encounter a barrage of caspase 1-activating ligands and are endowed with a diverse sensor array to trigger the common downstream response of pyroptosis efficiently20.
Inflammasomes were observed microscopically during Salmonella infection and treatment with B. anthracis lethal toxin, and active caspase 1 was found to be located within a single inflammasome complex as well as diffusely distributed throughout the cytoplasm20 (FIG. 3b). The adapter protein ASC can self-associate and form similarly sized complexes in the absence of an NLR54, but the extent to which the self-association of ASC contributes to the formation of NLR-containing inflammasomes is unknown (FIG. 3b). However, the fact that Salmonella-mediated activation of caspase 1 is reduced in ASC-deficient macrophages68 suggests that ASC facilitates caspase 1 activation even though it is not absolutely required for the binding of NLRC4 to caspase 1 (FIG. 3a). These data are consistent with the formation of a single, large inflammasome, or aggregation of multiple complexes that contain one or more NLRs, rather than many smaller complexes within a cell. The localization of a large percentage of active caspase 1 within a single complex could limit access to some caspase 1 substrates and allow recruitment of others by a mechanism that is analogous to recruitment of substrates to the proteasome. By this model, regulatory proteins could recruit substrates, control access to the proteolytic regions of the complex and alter the enzymatic function of the complex to regulate substrate cleavage69. Similarly, the catalytic activity of caspase 9 is enhanced when it is bound to the apoptosome, a multiprotein complex that is involved in caspase 9 activation70. Defining the components of native inflammasomes will provide insight into how this complex functions in its regulation of caspase 1 activity.
Inflammasome components can also interact with proteins that activate alternative cellular processes or forms of cell death. Autophagy has been observed during infection of macrophages with Legionella71,72 and Francisella73, which can also induce caspase 1 under other in vitro conditions23,24. Failure to induce robust caspase 1 activation owing to suboptimal ligand production by the pathogen or host mutations does not result in pyroptosis, but instead may allow inflammasome components to interact with other cell death machinery and stimulate alternative cell death pathways23,72. ASC- and caspase 1-deficient macrophages fail to activate caspase 1 in response to multiple stimuli, but are not always protected from cell lysis, suggesting that the absence of caspase 1-dependent pyroptosis allows other cell death processes to predominate, including pyronecrosis and autophagy62,63,74,124. Infection with Shigella or Salmonella triggers caspase 1 activation in wild-type macrophages, but in the absence of caspase 1, infected macrophages display features of autophagy63,75. The induction of autophagy by Shigella requires the NLR protein NLRC4, implicating NLR proteins in stimulation of both pyroptosis and autophagy63.
Caspase 1-dependent processes
Several caspase 1-dependent processes do not directly contribute to cellular demise, but accompany the cell death process and contribute to the inflammatory nature of pyroptosis. In addition, some events that are caspase 1-dependent can occur in the absence of cell death. Caspase 1 activation can result in a combination of the following processes, which are dictated by cell type as well as the nature and magnitude of the stimulus received.
IL-1β and IL-18 processing and secretion
The inflammatory cytokines IL-1β and IL-18 undergo caspase 1-dependent activation and secretion during pyroptosis. IL-1β is a potent endogenous pyrogen that stimulates fever, leukocyte tissue migration and expression of diverse cytokines and chemokines76. IL-18 induces IFNγ production and is important for the activation of T cells, macrophages and other cell types77. Both IL-1β and IL-18 play crucial parts in the pathogenesis of a range of inflammatory and autoimmune diseases76,77. Although neither cytokine is required for the process of cell death37,78, their production contributes to the inflammatory response elicited by cells undergoing pyroptosis. IL-1β and IL-18 lack secretion signals and their mechanism of release has not been definitively determined. Formation of caspase 1-dependent pores in the plasma membrane is temporally correlated with cytokine release in macrophages7, suggesting that cytokine secretion occurs through these pores (FIG. 1). Interestingly, lysis is not required for the release of activated IL-1β and IL-18, because pharmacological inhibition of lysis does not prevent caspase 1-dependent pore formation or cytokine secretion7. Thus, cytokine secretion and cell lysis are both downstream consequences of caspase 1-dependent pore formation (FIG. 1).
Additional mechanisms of IL-1β and IL-18 release have also been described that occur in the absence of cell lysis. Monocytes package active caspase 1 and cytokine substrates into lysosomes79,80, and secretion of processed cytokines occurs through lysosome fusion with the cell surface80 (FIG. 1). Although this is an elegant mechanism for cytokine secretion in the absence of pyroptosis, recent evidence suggests this may be limited to monocytes81. Release of cytokine-containing vesicles has also been observed in a range of cell types, including dendritic cells, microglial cells and macrophages, during caspase 1 activation in response to treatment with ATP82–85. Two mechanisms have been proposed for vesicle release: fusion of multivesicular bodies with the cell surface82 and direct budding of microvesicles from the plasma membrane83–85 (FIG. 1). Vesicle release has so far only been observed in response to ATP stimulation, and surface microvesicle shedding results in a significant reduction in cell size owing to loss of the plasma membrane83,85. By contrast, in Salmonella- and Burkholderia-infected macrophages, cells increase in size as processed cytokines are released7,18, suggesting that alternative mechanisms also mediate secretion of IL-1β and IL-18.
Additional inflammatory cytokines
Caspase 1 activation is also required for maximal production of inflammatory cytokines other than IL-1β and IL-18. Active caspase 1 has been shown to bind to and facilitate secretion of IL-1α by an unknown mechanism5,86. A modest but significant reduction in TNF and IL-6 secretion by caspase 1-deficient macrophages in response to lipopolysaccharide stimulation has also been reported14,15,87. This is due to caspase 1-mediated cleavage of the TLR adapter protein TIRAP (Toll/interleukin-1 receptor domain-containing adapter protein; also known as MAL). Caspase 1-processed TIRAP signals more efficiently, resulting in enhanced TNF and IL-6 production and macrophage activation in response to TLR2 and TLR4 ligands87. Therefore, in addition to regulating the production of IL-1β and IL-18, caspase 1 activation can also have a role in fine-tuning cytokine responses to microbial stimuli.
Inhibiting growth of intracellular bacteria
Caspase 1 activation helps to restrict the growth of intracellular pathogens. In macrophages that fail to trigger robust caspase 1 activation in response to Legionella infection, the bacteria replicate within an endoplasmic reticulum-derived compartment that resembles an immature autophagosome71. Infection of macrophages that more readily activate caspase 1 results in the rapid caspase 1-dependent delivery of Legionella to lysosomes and degradation of the bacteria23,88. Caspase 1 activity also enhances the killing of mycobacteria by stimulating trafficking of the bacteria to lysosomal compartments89. However, caspase 1 is not required for the degradation of all bacteria88. Legionella, mycobacteria and other pathogens produce virulence factors that modulate the trafficking of intracellular compartments, and further experiments are required to determine how caspase 1 allows macrophages to overcome these bacterial factors and contributes to the control of pathogen replication in vivo.
Cell repair and survival
Caspase 1 activation fails to trigger pyroptosis in all cell types, and somewhat surprisingly, epithelial cells use caspase 1 activation to prevent cell death39. Caspase 1 activation stimulates lipid production and membrane repair in response to the pore-forming toxins aerolysin and α-toxin, and indeed inhibition of caspase 1 activity actually enhances cell lysis39. This suggests that under certain conditions activation of caspase 1 could represent a cellular survival mechanism.
The function of caspase 1 is analogous to the activities of other apoptotic caspases (caspases 3 and 8) in modulating the fate of certain cell types90. Low levels of apoptotic caspase activation prevent autophagic cell death, regulate the proliferation and differentiation of B and T cells, and control the maturation of dendritic cells90. Higher levels of activation of the same apoptotic caspases result in the non-inflammatory elimination of these cells90. Similarly, the magnitude of caspase 1 activation modulates the response to microbial stimuli and host factors that warrant an inflammatory response. Low levels of active caspase 1 stimulate cell survival responses, control intracellular bacterial growth and mediate inflammatory cytokine production. When caspase 1 activation passes a critical threshold level, cells undergo pyroptosis and release inflammatory intracellular contents.
We propose that the level of caspase 1 activation tailors the host response to inflammatory stimuli. In addition, the fate of cells with active caspase 1 could be controlled independently of active enzyme levels by the subcellular localization of caspase 1. Restriction of active caspase 1 to lysosomes by monocytes79,80 could sequester certain substrates to one compartment for cleavage and release, while keeping cellular substrates that mediate cell death in another. In vivo, minimizing pyroptosis and intravascular lysis of circulating monocytes would probably be crucial to avoid an unfocused and potentially lethal systemic inflammatory response. The function of active caspase 1 could also be regulated by its localization within the cytosol. The confinement of active caspase 1 to a single focus within the cell cytosol has been observed20,54 (FIG. 3b), and this restricted localization could limit the access of the active enzyme to certain cellular substrates, as previously discussed. The molecular decision to undergo pyroptosis could be modulated by the presence of death effector proteins within a given cell type. Cells are not uniformly susceptible to this process: several stimuli that trigger pyroptosis in macrophages and dendritic cells fail to do so in epithelial cells39,91.
Caspase 1 in host response and disease pathology
Pyroptosis protects against infection and induces pathological inflammation. Although caspase 1 activity and pyroptosis can have a role as a protective host response to infectious diseases, exuberant or inappropriate caspase 1 activation and pyroptosis can be detrimental (FIG. 4). Mutations in NLR proteins can lead to inappropriate caspase 1 activation, which is associated with hereditary autoinflammatory syndromes92. Furthermore, caspase 1 is involved in the pathogenesis of several diseases, including myocardial infarction4, cerebral ischaemia93, inflammatory bowel disease94, neurodegenerative diseases95 and endotoxic shock14, each of which are characterized by inflammation and cell death. Caspase 1 deficiency, or pharmacological inhibition, provides protection against the inflammation, cell death and organ dysfunction that are associated with these diseases, making caspase 1 an attractive therapeutic target. The protection afforded by caspase 1 deficiency against sepsis and renal failure is not mimicked by neutralization of the cytokine targets, IL-1β and IL-18 (REFS 96–98), suggesting that caspase 1 has an additional role in disease apart from cytokine processing.
Figure 4. Caspase 1 activation in health and disease: fighting infection versus pathological inflammation.
Caspase 1 plays a protective part in the response to microbial infection. a | In response to infection, quiescent cells undergo caspase 1 activation and pyroptosis, allowing cleavage and release of interleukin-18 (IL-18), IL-1β and other inflammatory intracellular contents. Quiescent cells can also undergo ‘activation’ in response to inflammatory mediators, thereby lowering the threshold for caspase 1 activation and pyroptosis and stimulating increased production of IL-1β. b | As infection progresses, the inflammation that occurs as a consequence of pyroptosis leads to an increased population of activated cells that are primed to undergo pyroptosis and have increased inflammatory potential. c | Inflammatory contents produced during pyroptosis recruit and activate immune cells and stimulate the development of adaptive immune responses. This contributes to the control and ultimate resolution of microbial infection, and returns tissues to their resting state. Alternatively, caspase 1 activation can be detrimental, as mutations in Nod-like receptor (NLR) proteins or the persistence of sterile inflammatory stimuli can result in inappropriate and/or excessive caspase 1 activation. The inflammation produced by this process increases the population of activated cells that are primed to undergo pyroptosis and express increased levels of IL-1β, and the amplification cycle persists (b). This potentiates the response and maintains an inflammatory state, which, if uninterrupted, leads to pathology.
Caspase 1 activation helps to clear pathogens, such as Salmonella99,100, Shigella101, Legionella23,57, Francisella24, Anaplasma phagocytophilum102 and Listeria103, during infection in vivo in response to innate immune recognition of microorganism-associated patterns. This phenotype cannot be attributed solely to IL-18 and IL-1β production. Mice that are deficient in caspase 1 are more susceptible to Francisella than mice that lack both IL-1β and IL-18, indicating that cell death itself, or other caspase 1-dependent processes, contributes to the control of infection104.
Caspase 1 activation also influences the development of adaptive immune responses. In conjunction with IL-12, IL-18 plays a major part in stimulating the differentiation of T helper 1 (TH1)-type CD4+ T cells and enhancing their IFNγ production5,77. Caspase 1-deficient mice infected with Candida albicans, Listeria or A. phagocytophilum have an impaired TH1 response compared with wild-type mice102,103,105. CD4+ T cells generated in caspase 1-deficient mice during infection shift towards a TH2 phenotype102,103,105, resulting in impaired resistance to secondary infection by pathogens for which TH1-type responses are required for immunity105. The ability of caspase 1 activation to enhance the development of adaptive immune responses is supported by the finding that the non-microbial activators of caspase 1 can act as adjuvants. Uric acid released from necrotic cells enhances cross-presentation and generation of CD8+ T cells that are specific for exogenous antigens106. Aluminium-containing adjuvants also stimulate caspase 1 activation107 and lead to TH2 CD4+ T cells and robust humoral immune responses108. Mice that cannot activate caspase 1 in response to aluminium-containing adjuvants fail to recruit inflammatory cells109 and cannot stimulate TH2 CD4+ T-cell responses109,110. However, the role of caspase 1 in the regulation of antibody production remains controversial109–112. The contributions of pyroptosis to host resistance are therefore multifaceted. Early in infection, caspase 1-mediated processes, including, but not limited to, IL-1β and IL-18 production, lead to activation and recruitment of immune cells and innate control of infection. During persistent infection, continued caspase 1-dependent inflammation promotes the development of appropriate adaptive immune responses that lead to the resolution of infection (FIG. 4).
Microbial regulation of caspase 1 activation
Active caspase 1 allows the host to control various microbial infections, so it is not surprising that pathogens have evolved mechanisms to limit the activation of caspase 1 in response to infection. Innate recognition is often limited to microbial patterns that are required for pathogen survival, such as peptidoglycan, lipopolysaccharide, and nucleic acids33,34. Flagellin, which is recognized by NLRC4, is not required for the survival or virulence of Salmonella or Legionella in vivo23,113. Legionella and Salmonella use translocation systems to modulate host cell function, but must also avoid introducing flagellin into the cytosol through these translocation systems and stimulating pyroptosis. Both organisms downregulate flagellin production during intracellular growth114,115, which could provide a strategy to avoid pyroptosis, thereby limiting inflammation and allowing continued intracellular replication of the bacteria.
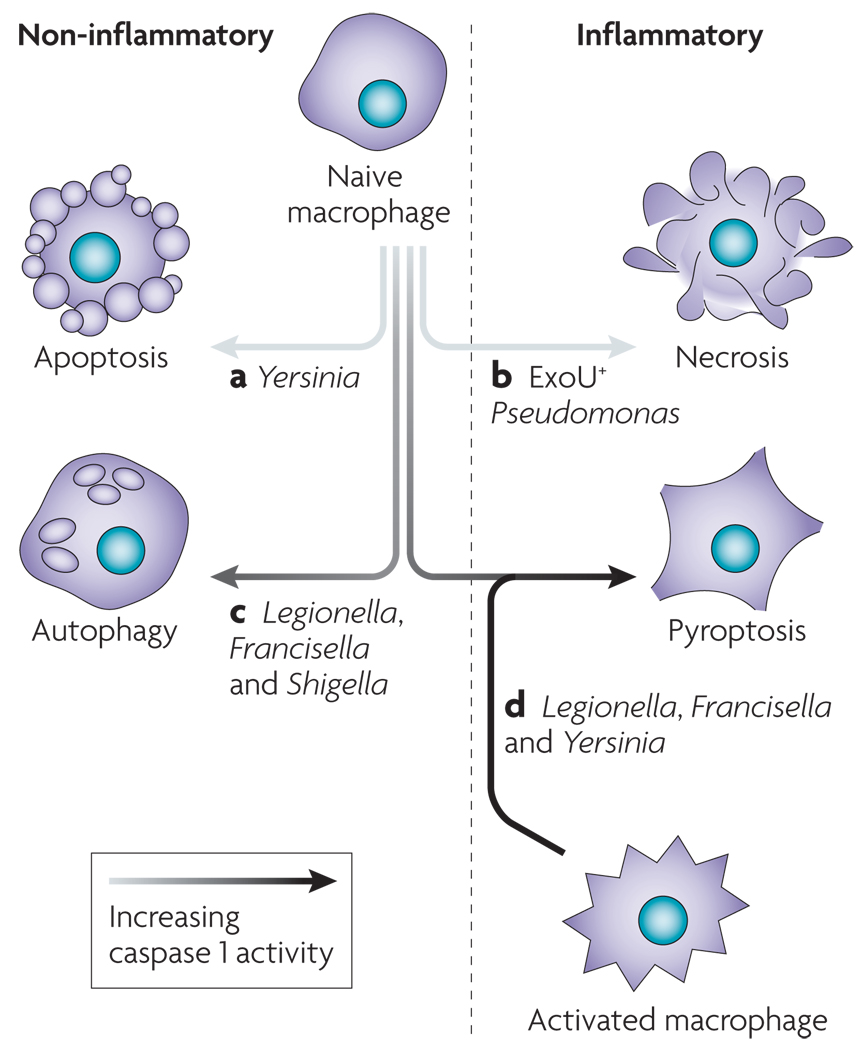
There are multiple examples of pathogens that induce an alternative form of cell death, effectively eliminating cells that would otherwise undergo pyroptosis and stimulate pathogen clearance. Apoptosis kills macrophages by a process that results in the production of anti-inflammatory factors and maintains membrane integrity, thereby preventing release of inflammatory intracellular contents2. The activation of apoptotic caspase 3 also results in cleavage of the caspase 1 substrate IL-18 at an alternate site, rendering it non-inflammatory5. Yersinia can trigger apoptosis in naive macrophages and dendritic cells, which effectively prevents inflammatory pyroptosis12 (FIG. 5). Pseudomonas strains that produce the type III secretion system-secreted protein ExoU induce caspase 1-independent necrosis, resulting in lysis but preventing the cleavage and release of IL-1β and IL-18 (REF. 62). However, 80% of clinical isolates are ExoU negative62, and instead trigger pyroptosis58,59,62 (FIG. 5). Pseudomonas strains that express ExoU are more virulent62, supporting the hypothesis that neutralizing macrophages before they have the opportunity to activate caspase 1 benefits the bacteria during infection.
Figure 5. Susceptibility to pyroptosis is governed by pathogen and host modulation of caspase 1 activation.
Pathogens have mechanisms for modulating cell death by inhibiting caspase 1 activation or inducing an alternative form of cell death that is more conducive to their continued replication. Yersinia (a) and Pseudomonas (b) translocate type III secretion effectors, resulting in apoptosis and necrosis, respectively. Pathogens can fail to induce robust caspase 1 activation owing to suboptimal ‘danger’ signal production by the pathogen (c). In addition, host mutations may not allow sufficient levels of caspase 1 activation to trigger pyroptosis. These infected macrophages often display features that are consistent with autophagy. Robust production of caspase 1-activating ligands by Legionella during infection of a susceptible macrophage triggers pyroptosis (d). Not all cells are uniformly susceptible to pyroptosis, and macrophage activation enhances caspase 1 activation (FIG. 4) in response to Yersinia and Francisella infection, which do not stimulate pyroptosis in naive macrophages.
Pathogens also produce factors that can directly inhibit the activation of caspase 1. Poxviruses are DNA viruses that replicate in the cytoplasm and would therefore be readily detected by NLRP3. The poxvirus protein M13L-PYD binds ASC through its pyrin domain (FIG. 3a), thereby disrupting inflammasome formation and preventing binding to and activation of caspase 1. Deletion of this viral protein results in increased caspase 1 activity and impaired replication in host cells in vitro and during infection in vivo116. The influenza virus protein NS1 has also been shown to limit caspase 1 activation and cell death by an unknown mechanism117, which indicates that inhibition of caspase 1 activation could be a common strategy for successful viral pathogens. Yersinia translocates type III secretion proteins that counteract the caspase 1 activating potential of the type III secretion system itself. Yersinia strains that lack all the type III secretion system-translocated proteins have an increased ability to activate caspase 1 (REFS 12,91,118). Analysis of individual effectors suggests that YopE has an important role in the inhibition of caspase 1 activation, probably owing to the ability of YopE to modulate host Rho GTPase function118. Francisella mutants that trigger induction of pyroptosis more quickly than the wild type have been identified, suggesting that Francisella also possesses a mechanism for inhibiting caspase 1 (REF. 119), and Mycobacterium tuberculosis produces a zinc metalloprotease that prevents activation of caspase 1 through an unknown mechanism89. Finally, mutants of Francisella and Mycobacterium that cannot control caspase 1 activation are attenuated in vivo, which is consistent with the idea that increased levels of active caspase 1 and pyroptosis limit bacterial replication89,119 (FIG. 5).
Host cell activation redirects cell death
Caspase 1 activation clearly functions as a host defence mechanism in a wide range of microbial infections. Although localized inflammation during infection could enhance tissue disruption and pathogen dissemination, as infection progresses, caspase 1 activation limits pathogen replication, enhances innate and adaptive immune responses, and improves host survival (FIG. 4). Pathogens require mechanisms for preventing or controlling the potent inflammatory outcome of pyroptosis to persist and cause disease. Likewise, the host has evolved mechanisms to counteract pathogen-mediated regulation of caspase 1 activity and successfully control infection. Activation of macrophages counteracts Yersinia-mediated inhibition of pyroptosis12 and enhances susceptibility to Francisella-induced pyroptosis120. Host recognition of microbial infection may lead to upregulation of NLRs or other unknown accessory proteins that are involved in caspase 1 activation and prime macrophages to undergo pyroptosis. This enhanced sensitivity to pyroptosis allows a shift from the non- or anti-inflammatory modes of cell death triggered by Yersinia and Francisella in naive macrophages (apoptosis and autophagy, respectively) to inflammatory pyroptosis (FIG. 5). The transition from autophagy to pyroptosis is also observed during Legionella infection, perhaps owing to increased production of flagellin by the bacteria72. The ability of macrophage activation to enhance pyroptosis in response to Legionella infection remains unexplored. Activation could sensitize Legionella-infected cells to undergo pyroptosis in response to lower amounts of flagellin. Together, these data clearly indicate that a host-mediated redirection of cell death to pyroptosis can benefit the host by increasing inflammation and facilitating the resolution of infection.
Concluding remarks
A wide range of host and microbial factors stimulate caspase 1 activation, and this leads to an array of caspase 1-dependent processes that include cell death, modulation of inflammatory cytokine production and restriction of pathogen replication. Together, these caspase 1-dependent processes benefit the host in vivo by contributing to the control of microbial infection. Pathogens use virulence factors to limit caspase 1 activation, but the host has mechanisms for priming cells to activate caspase 1 in the presence of this inhibition. Ultimately, there is competition between host and pathogen to regulate caspase 1 activation, and the outcome dictates life or death of the host.
Host and microbial factors that trigger caspase 1 activation, and the host NLR proteins that detect these molecules, have been the focus of recent research. We are only beginning to understand the molecular mechanism of pyroptosis and other processes downstream of caspase 1 activation. Identification of proteins cleaved by caspase 1 in vivo will probably provide a great deal of insight and allow a more thorough mechanistic description of this process. The localization or composition of the inflammasome could have some role in regulating protein processing by caspase 1. It remains to be determined whether the inflammasome complex can determine the fate of cells that have active caspase 1. Importantly, the physiological features downstream of caspase 1 activation, including pyroptosis, are conserved responses to multiple stimuli. Pyroptosis and other caspase 1-dependent processes are therefore relevant to our understanding of important beneficial host responses as well as medical conditions for which inflammation is central to the pathophysiology of disease.
DATABASES
Entrez Genome Project
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj
Anaplasma phagocytophilum | Bacillus anthracis | Candida albicans | Mycobacterium tuberculosis
UniProtKB
ASC | caspase 1 | caspase 3 | caspase 6 | caspase 8 | caspase 9 | IFNγ | IL-1α | IL-1β | IL-6 | IL-8 | IL-18 | NAIP5 | NLRC4 | NLRP1b | NLRP3 | NOD1 | NOD2 | TNF | TIRAP
FURTHER INFORMATION
Brad T. Cookson’s homepage
http://depts.washington.edu/mcb/facultyinfo.php?id=190
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Supplementary Material
Acknowledgements
S.L.F. was supported by National Institutes of Health Grants AI47242 and P50 HG02360 and Poncin and Achievement Rewards for College Scientist Fellowships. T.B. was supported by National Institute of General Medical Sciences Public Health Service National Research Service Award Grant T32 GM07270 and a Helen Riaboff Whitely Fellowship.
Abbreviations
- Caspases
A group of cysteine proteases that, based on their physiological roles, can be divided into two groups: those involved in the initiation and execution of apoptosis (caspase 2, 3, 6, 7, 8, 9 and 10) and those that trigger inflammation (caspase 1 and related caspases).
- Autophagy
A programme of cellular self-digestion in which cytoplasmic components are sequestered and degraded intracellularly in autophagosomes. Autophagic cell corpses are ultimately removed by phagocytosis.
- Oncosis
A caspase-independent pathway of cell death triggered by exposure to toxins or physical damage that features organelle and cell swelling and culminates in cell lysis with release of intracellular contents that stimulate inflammatory responses.
- Toll-like receptor
A transmembrane protein that contains a leucine-rich repeat domain and mediates host recognition of pathogen- and danger-associated molecular patterns located in the extracellular milieu or within endosomes.
- Nod-like receptor
A protein that contains a leucine-rich repeat domain and mediates host recognition of pathogen- and danger-associated molecular patterns in the host cell cytosol.
- Proteasome
A multiprotein complex that recognizes and degrades polyubiquitinated substrates.
- Pyronecrosis
Results from Shigella infection at high MOI (multiplicity of infection), morphologically resembles oncosis and is NLRP3-dependent and caspase 1-independent.
- Microvesicle
A membrane vesicle of less than 0.5 µm in diameter that is shed from the plasma membrane of eukaryotic cells.
- Necrosis
Does not indicate a specific pathway of cell death, but is a post-mortem description of dead cells that have reached equilibrium with their surroundings.
References
- 1.Samali A, Zhivotovsky B, Jones D, Nagata S, Orrenius S. Apoptosis: cell death defined by caspase activation. Cell Death Differ. 1999;6:495–496. doi: 10.1038/sj.cdd.4400520. [DOI] [PubMed] [Google Scholar]
- 2.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nature Rev. Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 3.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frantz S, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J. Mol. Cell. Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 5.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J. Clin. Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 6.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 7. Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. Mechanistic description of the features of pyroptosis, including the identification of caspase 1-dependent membrane pore formation, which leads to cell lysis.
- 8.Hersh D, et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. First description of caspase 1 activity that led to pathogen-induced cell death.
- 10.Hilbi H, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, et al. Nitric oxide induces thymocyte apoptosis via a caspase-1-dependent mechanism. J. Immunol. 2000;165:1252–1258. doi: 10.4049/jimmunol.165.3.1252. [DOI] [PubMed] [Google Scholar]
- 12. Bergsbaken T, Cookson BT. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. This study demonstrated host redirection of cell death from apoptosis to pyroptosis in activated macrophages in response to Yersinia infection.
- 13.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 14.Li P, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 15.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 16.Jesenberger V, Procyk KJ, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 2000;192:1035–1046. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 2003;71:4448–4455. doi: 10.1128/IAI.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun GW, Lu J, Pervaiz S, Cao WP, Gan YH. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell. Microbiol. 2005;7:1447–1458. doi: 10.1111/j.1462-5822.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 19.Cervantes J, Nagata T, Uchijima M, Shibata K, Koide Y. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell. Microbiol. 2008;10:41–52. doi: 10.1111/j.1462-5822.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 20. Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl Acad. Sci. USA. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. This study showed that distinct events which lead to caspase 1 activation, and ultimately cell death, occur through a common pathway of caspase 1-mediated pyroptosis.
- 21.Thumbikat P, Dileepan T, Kannan MS, Maheswaran SK. Mechanisms underlying Mannheimia haemolytica leukotoxin-induced oncosis and apoptosis of bovine alveolar macrophages. Microb. Pathog. 2005;38:161–172. doi: 10.1016/j.micpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J. Immunol. 2003;171:6742–6749. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- 26.Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ. Cytotoxicity and interleukin-1β processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur. J. Immunol. 2002;32:1464–1471. doi: 10.1002/1521-4141(200205)32:5<1464::AID-IMMU1464>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl Acad. Sci. USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect. Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson PR, et al. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect. Immun. 2000;68:3744–3747. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickliffe KE, Leppla SH, Moayeri M. Killing of macrophages by anthrax lethal toxin: involvement of the N-end rule pathway. Cell. Microbiol. 2008;10:1352–1362. doi: 10.1111/j.1462-5822.2008.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Kufer TA, Sansonetti PJ. Sensing of bacteria: NOD a lonely job. Curr. Opin. Microbiol. 2007;10:62–69. doi: 10.1016/j.mib.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 36.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-κB-driven protein synthesis. J. Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 37.Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- 38.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 39.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Koo IC, et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanneganti TD, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Kanneganti TD, et al. Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 43.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 44.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 45.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 46.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 48.Perregaux D, et al. IL-1β maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J. Immunol. 1992;149:1294–1303. [PubMed] [Google Scholar]
- 49.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 50.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 51.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1β release through a dye uptake-independent pathway. J. Biol. Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 52.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 53.Wickliffe KE, Leppla SH, Moayeri M. Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell. Microbiol. 2008;10:332–343. doi: 10.1111/j.1462-5822.2007.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase 1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. This study found that a macromolecular structure which contained ASC and caspase-1 was formed during pyroptosis.
- 55.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 56. Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nature Immunol. 2006;7:576–582. doi: 10.1038/ni1346. Together with References 23 and 55, this study found that bacterial flagellin and the host protein NLRC4 are required for the activation of caspase 1 during infection with Legionella and Salmonella.
- 57.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nature Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 58.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl Acad. Sci. USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 60.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nature Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 65. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. First description of the inflammasome as a caspase 1-activating platform.
- 66.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 67.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 68.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt M, Hanna J, Elsasser S, Finley D. Proteasome-associated proteins: regulation of a proteolytic machine. Biol. Chem. 2005;386:725–737. doi: 10.1515/BC.2005.085. [DOI] [PubMed] [Google Scholar]
- 70.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 71.Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swanson MS, Molofsky AB. Autophagy and inflammatory cell death, partners of innate immunity. Autophagy. 2005;1:174–176. doi: 10.4161/auto.1.3.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl Acad. Sci. USA. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willingham SB, et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 2003;163:1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delaleu N, Bickel M. Interleukin-1β and interleukin-18: regulation and activity in local inflammation. Periodontol. 2000. 2004;35:42–52. doi: 10.1111/j.0906-6713.2004.003569.x. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 78.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell. Microbiol. 2001;3:825–837. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 79.Andrei C, et al. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrei C, et al. Phospholipases C and A2 control lysosome-mediated IL-1β secretion: implications for inflammatory processes. Proc. Natl Acad. Sci. USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1β is cytosolic and precedes cell death. J. Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 82.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 83.Pizzirani C, et al. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 84.Bianco F, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J. Immunol. 2005;174:7268–7677. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 85.MacKenzie A, et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 86.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 87.Miggin SM, et al. NF-κB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc. Natl Acad. Sci. USA. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 89.Master SS, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nature Rev. Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 91.Shin H, Cornelis GR. Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of pro-inflammatory IL-1β. Cell. Microbiol. 2007;9:2893–2902. doi: 10.1111/j.1462-5822.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- 92.Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R86–R98. doi: 10.1152/ajpregu.00504.2006. [DOI] [PubMed] [Google Scholar]
- 93.Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1β converting enzyme-deficient mice. J. Cereb. Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 94.Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl Acad. Sci. USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ona VO, et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 96.Wang W, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am. J. Physiol. Renal Physiol. 2005;288:F997–F1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- 97.Faubel S, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 98.Sarkar A, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am. J. Respir. Crit. Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sansonetti PJ, et al. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 102.Pedra JH, et al. ASC/PYCARD and caspase-1 regulate the IL-18/IFN-γ axis during Anaplasma phagocytophilum infection. J. Immunol. 2007;179:4783–4791. doi: 10.4049/jimmunol.179.7.4783. [DOI] [PubMed] [Google Scholar]
- 103.Tsuji NM, et al. Roles of caspase-1 in Listeria infection in mice. Int. Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 104. Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol. 2007;9:2543–2551. doi: 10.1111/j.1462-5822.2007.01022.x. Identified a role for type I IFN signalling in priming macrophages to undergo pyroptosis in response to Francisella infection.
- 105.Mencacci A, et al. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect. Immun. 2000;68:5126–5131. doi: 10.1128/iai.68.9.5126-5131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 107.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nature Rev. Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 108.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kool M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 110. Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. Showed that the adjuvant activity of alum is due to its ability to stimulate caspase 1 activation, highlighting the role of caspase 1 in the enhancement of adaptive immunity.
- 111.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lockman HA, Curtiss R., 3rd Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hammer BK, Swanson MS. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- 115.Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J. Immunol. 2005;174:7929–7938. doi: 10.4049/jimmunol.174.12.7929. [DOI] [PubMed] [Google Scholar]
- 116. Johnston JB, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. This study identified a microbial protein that is capable of inhibiting caspase 1 activation by disrupting inflammasome formation.
- 117.Stasakova J, et al. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1β and 18. J. Gen. Virol. 2005;86:185–195. doi: 10.1099/vir.0.80422-0. [DOI] [PubMed] [Google Scholar]
- 118.Schotte P, et al. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1β. J. Biol. Chem. 2004;279:25134–25142. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- 119.Weiss DS, et al. In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl Acad. Sci. USA. 2007;104:6037–6042. doi: 10.1073/pnas.0609675104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esposti MD. Apoptosis: who was first? Cell Death Differ. 1998;5:719. doi: 10.1038/sj.cdd.4400430. [DOI] [PubMed] [Google Scholar]
- 122.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandez-Prada CM, Hoover DL, Tall BD, Venkatesan MM. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect. Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.