Abstract
Background
Mechanisms underlying clozapine’s better clinical efficacy in schizophrenia remain poorly understood. The prefrontal cortex (PFC) has been implicated as a primary site for the therapeutic effects of clozapine; however, evidence for how clozapine influences the activity of PFC neurons in behaviorally relevant contexts is lacking.
Methods
Ensemble single unit recording in awake rats was used to measure the activity of PFC neurons in response to clozapine, its main metabolite N-desmethylclozapine (DMClz), and the typical antipsychotic drug haloperidol during baseline conditions and after treatment with the N-methyl-D-aspartate antagonist MK801. Behavioral stereotypy was scored during recording.
Results
Clozapine and DMClz but not haloperidol had an activity-dependent influence on spontaneous firing rate of PFC cells: they increased the activity of neurons with low baseline firing rates and decreased the activity of neurons with higher firing rates. Clozapine and DMClz but not haloperidol also reversed the effect of MK801 on PFC neuronal firing. This reversal was strongly correlated with blockade of MK801-induced behavioral stereotypy.
Conclusions
These findings indicate that clozapine has the capacity to fine-tune spontaneous and disrupted activity of PFC neurons. This effect might contribute, in part, to the therapeutic efficacy of clozapine in schizophrenia.
Keywords: Atypical antipsychotic drugs, cognition, dopamine, ensemble unit electrophysiology, NMDA receptor antagonist, schizophrenia
Despite many decades of research, therapeutic strategies that treat the full spectrum of schizophrenic symptomatology are lacking (Miyamoto et al 2005). This is especially evident for negative symptoms and cognitive deficits, which are growingly acknowledged as the core symptoms of the disease (Carlsson et al 1999; Castner et al 2004; Gold and Weinberger 1995; Meltzer and McGurk 1999; Sharma 1999). Although there is an ongoing discussion about whether atypical antipsychotic drugs offer a better overall efficacy compared with typical drugs (Freedman 2005; Lieberman et al 2005), there is evidence to suggest that clozapine confers advantageous clinical effects, particularly against negative symptoms, some cognitive deficits, and refractory positive symptoms (Davis 2006; Kane et al 1988; Keefe et al 1999; Kumari et al 1999; Lee et al 1999; Wahlbeck et al 2000). Clozapine’s clinical efficacy is also greater in preventing suicidality and improving clinical compliance (Conley and Kelly 2001; McEvoy et al 2006; McGurk 1999; Meltzer et al 2003; Spivak et al 2003). A better understanding of mechanisms responsible for clozapine’s advantageous efficacy might prove helpful in development of novel therapeutic options.
Several studies have suggested that the prefrontal cortex (PFC), a major foci of pathology in schizophrenia (Lewis et al 2005; Winterer and Weinberger 2004), is an important target for the actions of clozapine. For instance, clozapine differentially increases the release of dopamine and glutamate and the expression of c-fos in medial PFC (mPFC) compared with typical antipsychotic drugs such as haloperidol (Deutch and Duman 1996; Karoum and Egan 1992; Kuroki et al 1999; Moghaddam and Bunney 1990; Robertson and Fibiger 1992; Youngren et al 1994, 1999). Clozapine also positively modulates N-methyl-D-aspartate (NMDA) receptor mediated glutamate transmission in PFC (Arvanov and Wang 1999; Arvanov et al 1997; Chen and Yang 2002; Gemperle et al 2003; Jardemark et al 2003). Furthermore, functional imaging studies indicate that symptomatic improvement of cognitive and negative symptoms by clozapine is associated with the structural integrity of PFC and the reversal of abnormal metabolic activity in this region (Arango et al 2003; Konicki et al 2001; Lahti et al 2004; Molina et al 2003).
Several leading theories on the pathophysiology of schizophrenia suggest that an inefficient signal transmission within the PFC might account for some of the symptoms of schizophrenia (Williams and Goldman-Rakic 1995; Winterer and Weinberger 2004). Given the reported efficacy profile of clozapine, we predicted that clozapine modulates the spontaneous and abnormally disrupted activity of PFC neurons in a manner that is different from typical antipsychotic drugs. We tested this prediction with in vivo ensemble unit recording in awake rats under two conditions: 1) at rest when the effect of clozapine on spontaneously active neurons could be measured, and 2) in response to a challenge with the NMDA antagonist MK801. The NMDA antagonist treatment produces some symptoms of schizophrenia in healthy individuals and therefore has face validity as a model of schizophrenia (Javitt and Zukin 1991; Krystal et al 1994). In awake rats, NMDA antagonists disrupt the spontaneous rate and pattern of mPFC neuronal spiking in high correlation with their detrimental effects on behavior ( Jackson et al 2004). Notably, clozapine selectively ameliorates the adverse behavioral effects of NMDA antagonists in humans (Duncan et al 1998; Malhotra et al 1997) and laboratory animals (Bakshi et al 1994; Maurel-Remy et al 1995). In addition to clozapine and haloperidol, we also examined the effect of a main metabolite of clozapine, N-desmethylclozapine (DMClz), because this compound shares clozapine’s ability to potentiate NMDA-receptor activity (Sur et al 2003) and has been proposed as a novel “clozapine-like” antipsychotic agent (Davies et al 2005). Behavioral stereotypy, which is a measure of NMDA antagonist-induced behavioral disruptions, was scored during electrophysiology recording. The results suggest that mPFC neuronal activity is a substrate for different therapeutic effects of clozapine and haloperidol.
Methods and Materials
Subjects
A total of 23 adult male Sprague-Dawley rats weighing 340–420 g were used. Animals were individually housed on a 12-hour light/dark cycle (lights on at 7:00 am), and experiments were performed during the light phase. All experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrophysiology
Methodological details of the recording and isolation of single units have been published previously (Homayoun et al 2004). Briefly, microelectrode arrays (NB Labs, Denison, Texas) consisting of eight 50-µm diameter Teflon-insulated, stainless steel wires were chronically implanted under halothane anesthesia in the medial PFC (mPFC) (target coordinates for the center of the array at anteroposterior + 3.0, mediolateral .7, and dorsoventral −3.5) according to the atlas of Paxinos and Watson (1998). Arrays were arranged in a 2 × 4 pattern measuring approximately .25 × .7 mm. All recordings were performed in a standard transparent rat housing cage with a modified open top. Animals were connected to a field effect transistor (FET) head-stage (NB Labs) with lightweight cabling that passed through a commutator and allowed the animal unrestricted movement during recording. Extracellular unit activity was recorded with multiple channel amplifiers with 500× gain and 220–5900 Hz band pass filters (Plexon, Dallas, Texas). The amplified signal from each electrode was digitized (30 kHz sampling rate) and saved on computer hard disk for off-line spike sorting. Spike sorting was performed with Off-Line Sorter software (Plexon) with a combination of automatic and manual sorting techniques previously described (Homayoun et al 2004). The first three principal components of all waveforms recorded from each electrode were depicted in three-dimensional space. Automatic clustering techniques (K-means clustering and valley seeking methods) were used to produce an initial separation of waveforms into individual units. Each cluster was then checked manually to ensure that the cluster boundaries were well separated and waveform shapes were consistent with action potentials. In addition, four different statistical measures were used to assess the quality of separation between identified clusters and the effects of incremental increases or decreases in the number of units isolated from a channel. These included parametric F statistics of multivariate analysis of variance (MANOVA), the J3 and pseudo F statistics, and the Davies-Bouldin (DB) validity index (Nicolelis et al 2003). The J3 is a measure of the ratio of between-cluster to within-cluster scatter, and DB is a measure of the ratio of the sum of within-cluster scatter to between-cluster separation. Although none of these measures alone provides a clear-cut criterion for separating clusters, using them in conjunction with other criteria for cell isolation (as previously described) improves the quality of unit isolation on a case by case basis (Nicolelis et al 2003). The waveforms in each selected cluster were averaged to produce computer-generated templates that were then used to match spikes recorded during each single session. Obvious artifacts were removed, and the stability of clusters throughout the experiment was confirmed by plotting the first principal component versus the timestamp for each waveform. For each isolated cluster, an interspike interval histogram was constructed and an absolute refractory period of at least 1.1 msec was used to exclude suspected multiple units. We further attempted to separate putative pyramidal neurons from interneurons with the previously reported criteria of firing rate and spike width (McCormick et al 1985; Tierney et al 2004). Most cortical pyramidal units fire at frequencies < 10 Hz and have a wide spike width, whereas the majority of interneurons have higher firing frequencies and a narrow spike width. We used the firing rate as the primary criteria to separate putative subpopulations of neurons into two categories of regular firing (RF; baseline firing rate < 10 Hz, putative pyramidal neurons, 95.6% of total) and fast firing neurons (FF; baseline >10 Hz, putative interneurons, 4.4% of total). The latter group was further examined to find units with a narrow spike width (< 400 msec peak to trough). Only the small number of FF units that met both criteria were designated as putative interneurons. It should be noted that we did not apply the spike width measure to the population of RF units for two reasons: 1) using blunt electrode tips limits the applicability of spike width measure, because units are sampled from a larger area compared with sharp tip electrodes and this increases the variation in spike width measurement on the basis of the distance of the recorded unit from electrode tip; and 2) current extracellular recording methods increase the likelihood of picking up the large-amplitude signals of pyramidal cells versus smaller signals of interneurons, making it likely that the great majority of RF units are pyramidal units. The latter statement is also consistent with the low reported proportion of interneurons in previous recording studies (Baeg et al 2001; Bartho et al 2004; McCormick et al 1985). Because the proportion of recorded FF units was considerably less than RF units, the analysis was focused on the latter group.
Treatment
After 5–7 days of post-surgical recovery, animals were transferred from home cage to the recording chamber and were gently handled and then connected to the head-stage 3 hours daily for 3–4 days to habituate to the recording environment. The animals also received a couple of sham injections during this period to habituate to systemic injection. Thereafter, animals were assigned to 1 of the 12 acute treatment regimens each consisting of two IP injections 20 min apart. Experiment 1 consisted of six groups receiving an injection of either vehicle A, clozapine (1, 5, or 10 mg/kg), DMClz (5 mg/kg), or haloperidol (.1 mg/kg) followed by vehicle B. Experiment 2 consisted of six other groups receiving one of the same six pre-treatments mentioned previously followed by .1 mg/kg of MK801. Vehicle A was acidified water, prepared from .1 mol/L acetic or hydrochloric acid, diluted with water and titrated with .1 mol/L sodium hydroxide to pH 5.5, used as solvent for clozapine, DMClz, and haloperidol. Vehicle B was .9% saline solution used as solvent for MK801. The single dose of haloperidol was chosen on the basis of previous studies to be behaviorally effective against the NMDA antagonist effects, at a level comparable to the higher dose of clozapine used here (Sams-Dodd 1998; Tiedtke et al 1990; Verma and Moghaddam 1996). In all groups, the first injection was preceded by 30 min of baseline recording and was followed by at least 120 min of post-drug recording. Each animal received one to five different acute treatments on the basis of a pseudorandom order with washout periods between 6 and 10 days. Because analysis of results did not show any effect for the order of received treatments, all results obtained from a given treatment regimen were analyzed together. Number of recorded RF units/treatment group was as follows: Vehicle + Vehicle, n = 112; Clozapine 1 mg/kg + Vehicle, n = 143; Clozapine 5 mg/kg + Vehicle, n = 106; Clozapine 10 mg/kg + Vehicle, n = 135; DMClz 5 mg/kg + Vehicle, n = 119; Haloperidol .1 mg/kg + Vehicle, n =118; Vehicle + MK801, n = 111; Clozapine 1 mg/kg + MK801, n = 95; Clozapine 5 mg/kg + MK801, n = 221; Clozapine 10 mg/kg + MK801, n =120; DMClz 5 mg/kg + MK801, n = 140; Haloperidol .1 mg/kg + MK801, n =81.
Data Analysis
Electrophysiological data were analyzed with NeuroExplorer (Plexon) and Matlab. Firing rate statistics were calculated with firing rate histograms with 5-min bins normalized to the mean baseline firing (30 min) of individual units. Normalization was used to allow comparison across the population of neurons with various firing rates. To detect the predominant patterns of responses to treatment, we used a K-means cluster analysis (SPSS, Chicago, Illinois) with the normalized rate histogram bins of each unit as variables. This analysis was performed separately for all units in Experiment 1 (vehicle B as second treatment) and experiment 2 (MK801 as second treatment). The number of clusters used for K-means analysis was initially set at 2 and was incremented to 10 at steps of 1. The clusters isolated at each step were visualized by plotting the average firing rates of all neurons allocated to each cluster and comparing their response patterns. Whenever neurons with similar response patterns had been clustered separately on the basis of a small difference in response magnitude, the clusters were merged. The fewest number of clusters that could explain more than 95% of the variability of response patterns was determined as the number of main clusters of responses. The final grouping was verified by obtaining a significant analysis of variance (ANOVA) with time as repeated measure and cluster membership as factor. The individual rate histograms were visually compared with the average waveform of their assigned cluster to confirm correct classification. Cluster analysis focused on the long duration changes in firing and was used to classify the response types without imposing a priori assumptions about what defines different response types. The distribution of response types between groups in each experiment were compared with the Pearson χ2 test with p < .05 as significance threshold. The duration of periods of significant increase or decrease in firing rate was determined for each spike train by calculating the 99% confidence intervals of the baseline firing rate and detecting the post-injection bins that exceeded this range. A minimum of three consecutive exceeding bins was required for initiation of a significant response, and a minimum of three consecutive non-exceeding bins was required for terminating it. The duration of firing rate increases (or decreases) in all neurons with at least one significant increase (or decrease) response was compared between groups with one-way ANOVA followed by Bonferroni post hoc test. For this and all subsequent post-test analysis, p < .05 was used as criterion for significance.
The relationship between baseline firing rates and post-injection responses to antipsychotic treatment was examined by plotting the average normalized firing rate during the period after first injection against the baseline firing rate for each neuron. Scatter plots were analyzed with first order linear regression. In Experiment 2 two way ANOVAs with time as the repeated measure and treatment as factor was used to compare the temporal profile of firing increases in vehicle and drug pre-treated groups. For the latter analysis, we treated units recorded during various sessions as independent measures because: (1) our methodology could not identify neurons recorded from the same electrode weeks apart as the same neurons and (2) we have observed that old units disappear and new ones become detectable on the same electrode across different recording sessions.
Behavioral Stereotypy
As an index of the gross behavioral disturbances induced by MK801, stereotypical behavior was scored every 5 min during the recording sessions with a scoring scale described previously (Adams and Moghaddam 1998). In brief, every 5 min animals were observed for 30 continuous sec and received a score of 1 if any of the following behaviors were present during that 30 sec: ambulation, turning, head wagging, grooming, sniffing up or down, digging, rearing, and mouth movement or jaw tremor. Scores for different repetitive behaviors at each 5-min point were summed and temporal profiles of stereotypy behavior across groups were compared with ANOVA with time as the repeated measure, followed by Bonferroni post hoc test. To assess the correlation between behavioral and electrophysiological effects of the drugs, we compared the stereotypy score during each 5-min bin with the average firing rate during the same 5-min bin for each recording session by using Pearson correlation coefficient with the Bartlett χ2 test for significance. For the latter analysis we used only the neurons with an increase firing response (Jackson et al 2004).
Histology
Animals were anesthetized with chloral hydrate and intracardially perfused with saline followed by 10% buffered formalin. Coronal brain sections (200 µm) were prepared and stained with cresyl violet. Tracks of recording electrodes were verified under a light microscope.
Results
Effects of Clozapine, Haloperidol, and DMClz on PFC Spontaneous Neuronal Activity
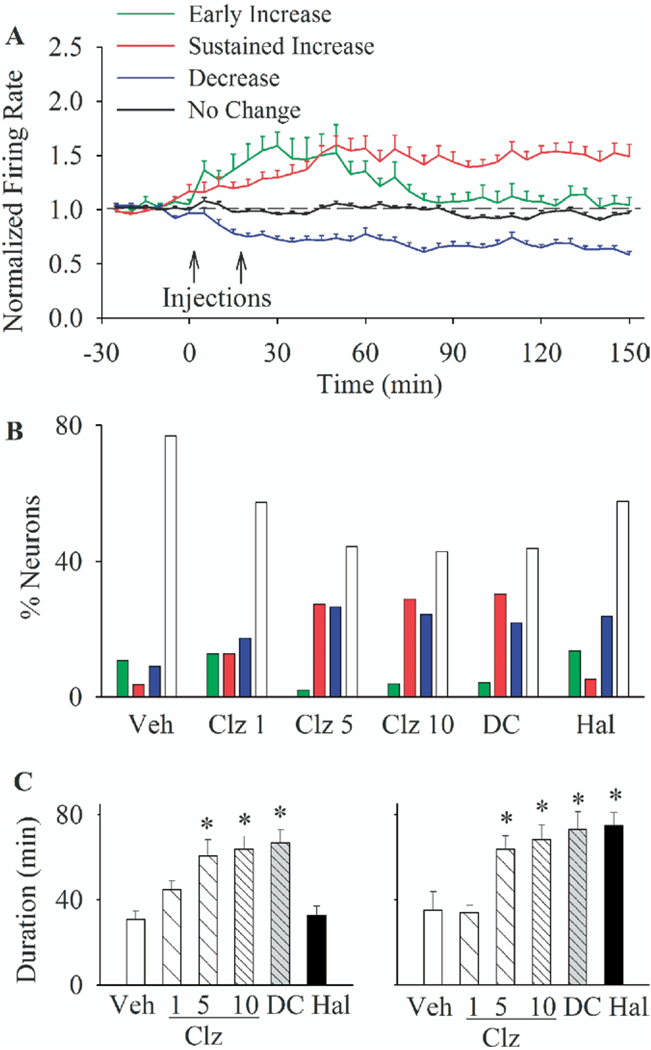
A total of 1501 RF single units with an average baseline firing rate of 3.29 Hz were isolated during 54 recording sessions. To identify the predominant patterns of mPFC neuronal responses to antipsychotic treatment, we used a K-means clustering analysis on the rate histogram of all RF units in Experiment 1 (see Methods). The great majority of units were classified as belonging to one of four clusters showing distinct patterns of firing rate responses to treatment: A) an early transient increase, B) a sustained increase, C) a sustained decrease, or D) no change in firing rate (Figure 1A, average firing rate of each cluster of neurons). The distribution of these four response types were significantly different between treatment groups [Figure 1B, χ2 = 100.13, p < .001]. In vehicle pre-treated group, the majority of neurons showed minimal change in activity (76.8% of all units), whereas most of the remaining units with an increased firing had only a transient response (10.7%). In contrast, both clozapine (in a dose-related manner) and DMClz altered the activity of more than half of PFC neurons, causing sustained firing increases and decreases in two distinct subsets of neurons. Haloperidol also induced sustained decreases in a subset of neurons, but its excitatory effects were mostly transient, similar to the vehicle group. This differential effect of clozapine versus haloperidol was further confirmed by comparing the average duration of all significant increase and decrease responses (Figure 1C). Clozapine (5 and 10 mg/kg) and DMClz significantly enhanced the duration of both increase [ANOVA, F(5,184) = 5.44, p < .001] and decrease [F(5,144) = 6.2, p < .001] responses whereas haloperidol only affected the decrease responses.
Figure 1.
The predominant types of responses of prefrontal neurons to antipsychotic treatments. (A) Firing responses of all units recorded in experiment 1 were clustered into one of four main response types with a K-means clustering analysis on normalized firing rate histograms with 5-min bins. Each color line shows the average firing rate (± SEM) of all neurons in one of the response clusters (early increase, sustained increase, decrease, or no change). Injections are indicated by arrows. (B) The proportion (% of total) of different response types in each treatment group. Treatments included: vehicle (Veh), clozapine (Clz; 1, 5, or 10 mg/kg), N-desmethylclozapine (DC; 5 mg/kg), or haloperidol (Hal; .1 mg/kg) injected at min 0 followed by saline at min 20. Color codes are as in section A. (C) The average duration of periods of significant increase (left) or decrease (right) in firing rates in all responsive neurons after second injection. *p < .05 compared with vehicle + vehicle group.
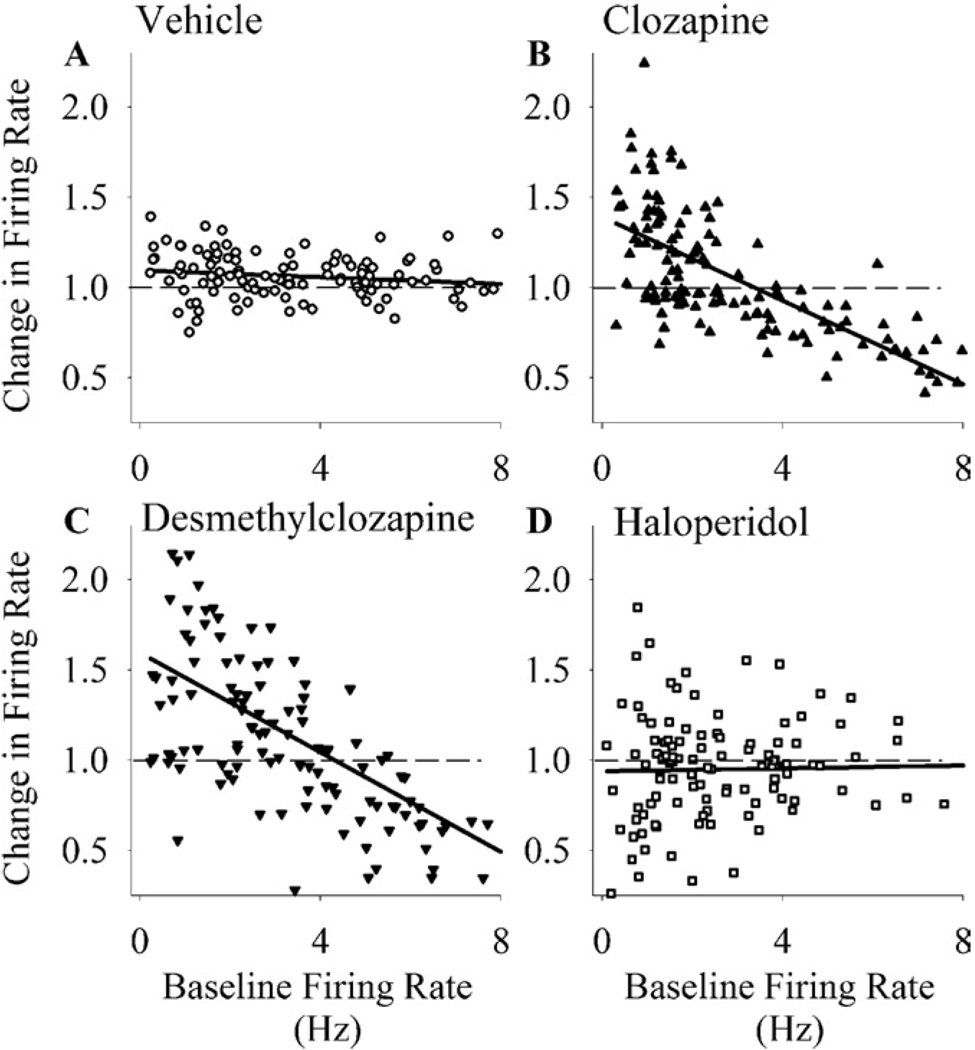
Further examination of data revealed that clozapine exerted a state-dependent effect on firing of individual neurons on the basis of their baseline firing rate: it preferentially inhibited the neurons with a higher baseline rate while exciting the neurons with a lower baseline activity (Figure 2B). Regression analysis showed a significant negative correlation between individual neuronal responses to clozapine and the baseline firing rates [r = .68, p < .001]. A similar state-dependent modulation of firing activity was observed for DMClz [Figure 2C, r = .60, p < .001] but not for haloperidol [Figure 2D, r = .16] or vehicle [Figure 2A, r = .03].
Figure 2.
State-dependent modulation of firing rate by clozapine and N-desmethylclozapine. The normalized firing rates of individual neurons have been plotted against their baseline firing rates. (A) Vehicle + saline. (B) Clozapine (10 mg/kg) + saline. (C) N-desmethylclozapine (5 mg/kg) + saline. (D) Haloperidol (.1 mg/kg) + saline. Baseline firing rates were calculated for the 30-min period before the first injection, and the normalized responses (y axis) were calculated for the period afterwards. The dashed line indicates normalized value of 1 (no change), and the solid line is the linear regression line.
Effects of Clozapine, Haloperidol, and DMClz on NMDA Antagonist Disruption of PFC Activity and Behavior
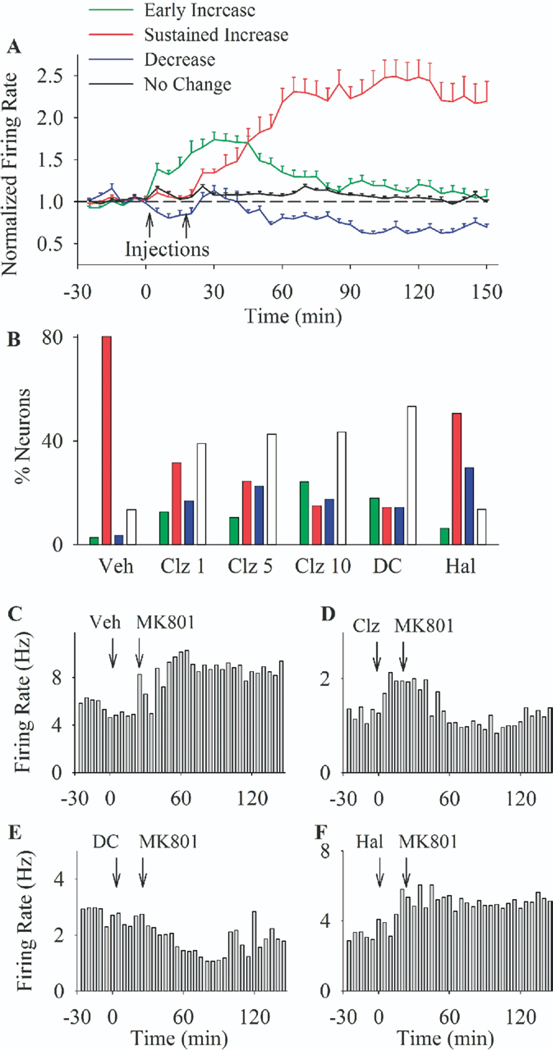
An independent cluster analysis of firing responses performed on all RF units in Experiment 2 (six groups receiving MK801 treatment) identified four major firing response patterns as follows: A) an early transient increase, B) a sustained robust increase, C) a decrease, and D) no change (Figure 3A, average firing of each cluster of neurons; Figure 3C–F, rate histograms of individual single units displaying various response types). Notably, the sustained increase response characteristic of MK801 effect in this group of units was considerably stronger than the moderate sustained increase observed in experiment 1 (Figure 1A). The distribution of the aforementioned response types was significantly different between groups [Figure 3B, χ2 = 189.67, p < .001]. Whereas a sustained increase was the predominant type of response in both vehicle (80% of units) and haloperidol (50%) pre-treated groups, the majority of neurons in clozapine and DMClz pre-treated groups displayed either no change in firing (44.8%), a decrease response (18.6%), or only a transient increase returning to baseline after MK801 (15.4%). In contrast to clozapine, haloperidol only caused a modest reduction in the proportion of sustained increase responses without attenuating the magnitude of MK801 effect on individual neurons. This was confirmed by comparing average firing of all neurons that responded to MK801 by increasing their firing rate (Figure 4A). Repeated measures ANOVA with time and treatment as factors revealed that clozapine (dose-dependently) and DMClz significantly attenuated the magnitude of MK801 effects in responsive neurons, whereas haloperidol was devoid of such effect [two-way ANOVA with time as repeated measures, comparison with vehicle pre-treatment group: Clozapine 1 mg/kg: treatment F(1,132) =5.77, p < .05, time F(35,4620) = 9.02, p < .001, time × treatment interaction F(35,4620) = 5.26, p < .001; Clozapine 5 mg/kg: treatment F(1,167) = 3.94, p < .05, time F(35,5845) = 25.56, p < .001, time × treatment interaction F(35,5845) = 4.04, p < .001; Clozapine 10 mg/kg: treatment F(1,137) = 3.84, p < .05, time F(35,4795) = 27.9, p < .001, time × treatment interaction F(35,4795) = 4.79, p < .001; DMClz: treatment F(1,135) = 5.2, p < .05, time F(35,4725) = 9.24, p < .001, time × treatment interaction F(35,4725) = 6.07, p < .001; Haloperidol: treatment F(1,136) = 1.07, p > .05, time F(35,4760) = 19.17, p < .001, time × treatment interaction F(35,4760) = 1.06, p > .05].
Figure 3.
The predominant response patterns of prefrontal neurons to MK801 after different pre-treatments. (A) Firing response of all neurons in experiment 2 were clustered into one of four response types. Each color line depicts the average firing rate (± SEM) of all neurons with one of identified response types (early transient increase, late robust increase, decrease, or no change). Injections are indicated by arrows. (B) The proportion (% of total) of response types in each treatment group. Treatments included: vehicle (Veh), clozapine (Clz; 1, 5, or 10 mg/kg), N-desmethylclozapine (DC; 5 mg/kg), or haloperidol (Hal; .1 mg/kg) injected at min 0 followed by MK801 (.1 mg/kg) at min 20. Color codes are as in section A. (C–F) Rate histograms of four individual single units displaying different response types in experiment 2. All neurons were treated with a pre-treatment (indicated above each histogram; (C) vehicle; (D) clozapine 10 mg/kg; (E) N-desmethylclozapine; (F) haloperidol) followed by MK801. Arrows indicate the time of injections, and histograms bins are 5 min.
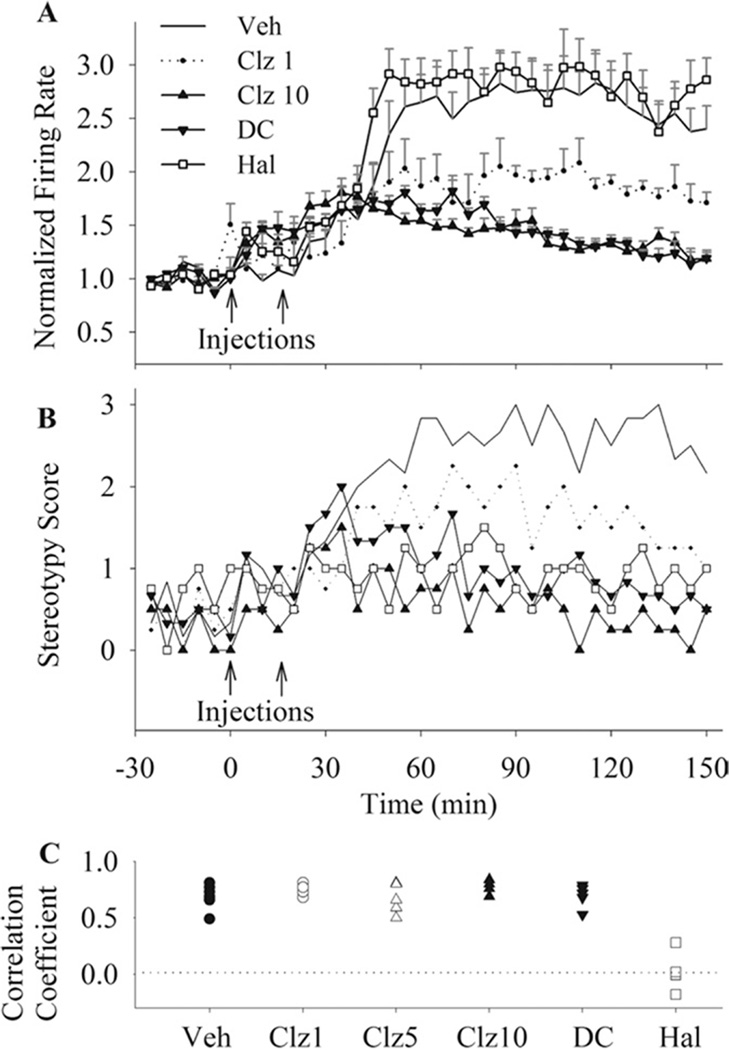
Figure 4.
The effects of antipsychotic drugs on the magnitude of MK801-induced excessive firing and stereotypical behavior. (A) The temporal profile of normalized firing rate (mean ± SEM) of all neurons with an increase response after MK801. All groups received MK801 (.1 mg/kg) as second injection, preceded by vehicle (Veh), clozapine (Clz; 1, 5, or 10 mg/kg), N-desmethylclozapine (DC; 5 mg/kg), or haloperidol (Hal; .1 mg/kg). Arrows indicate injections and bins are 5 min. For brevity, data for clozapine 5 mg/kg group is not shown. (B) Average stereotypy score in the same groups. All conventions are as in section A. For clarity, the error bars are not shown. (C) The coefficients of correlation between stereotypy score and neuronal firing response are shown for individual sessions in each treatment group. There was a high correlation between behavior and electrophysiological activity in all groups except for the haloperidol pre-treated group.
All three drugs (clozapine at the two higher doses) significantly attenuated MK801-induced behavioral stereotypy during recording sessions [Figure 4B, two-way repeated measures ANOVA, clozapine, treatment, F(3,15) = 23.15, p < .001; time × treatment interaction, F(105,525) = 2.98, p < .001; DMClz, treatment, F(1,10) = 29.7, p < .001; time × treatment interaction, F(35,350) = 5.20, p < .001; Haloperidol, treatment, F(1,8) = 19.69, p < .01; time × treatment interaction, F(35,280) = 4.20, p < .001]. To examine whether changes in PFC neuronal activity correlate with the gross behavioral effects of MK801, we examined the correlation between neuronal activity and stereotypy score in individual animals. This analysis revealed a high correlation between behavior and the average firing of mPFC neurons with an increase response to MK801 in vehicle pre-treated as well as in clozapine and DMClz pre-treated animals (Figure 4C). However, changes in PFC firing and stereotypy score had a poor correlation in haloperidol pre-treated animals (average correlation coefficient, .028), indicating a dissociation of two measures in this group.
Putative Interneurons
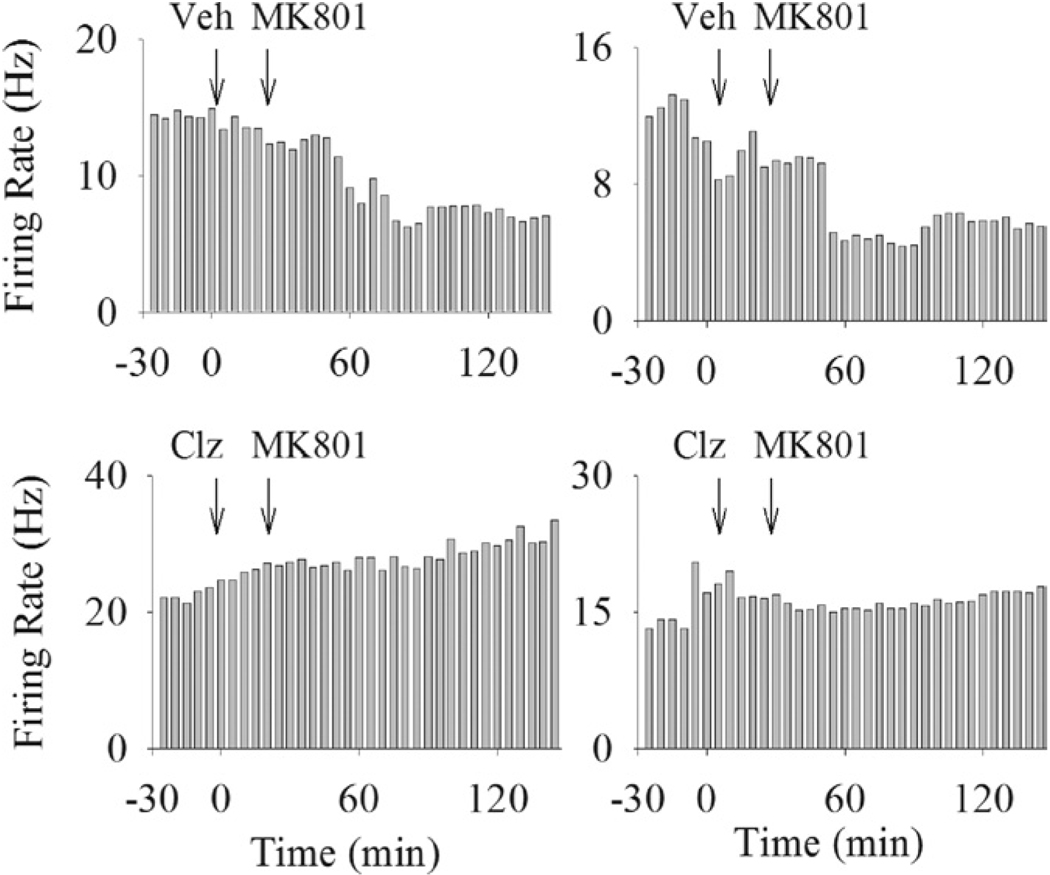
Of the seven putative interneurons recorded in the vehicle + MK801 group, five units (71%) decreased their firing after MK801 injection and two units (29%) did not show a significant response (Figure 5, example units). In contrast, of the seven units pre-treated with clozapine (5 or 10 mg/kg), four units (57%) increased their firing in response to MK801 whereas three units (43%) had no response. The distribution of response types was significantly different between vehicle-pretreated and clozapine-pretreated groups (p < .05). Of the four putative interneurons recorded in haloperidol pre-treated group, three decreased their firing after MK801 and one did not change its activity. The paucity of data did not allow analysis of this type of neuron in other groups.
Figure 5.
Example rate histograms of putative interneurons. Each histogram depicts the response of an individual putative interneuron to treatment with vehicle + MK801 (upper row) or clozapine (10 mg/kg) + MK801 (lower row). Treatments are indicated above each histogram with arrows showing the injection time.
Discussion
Clozapine is widely regarded as having better clinical efficacy for treatment of schizophrenia compared with other antipsychotic drugs (Freedman 2005; Meltzer et al 2003). A more thorough understanding of mechanisms that underlie this effect of clozapine is important for development of more efficacious antipsychotic therapies. The present data show that tonic activity of mPFC neuronal ensembles is differentially modulated by clozapine compared with the classic antipsychotic drug haloperidol. Clozapine produced a state-dependent effect on firing rates, preferentially inhibiting neurons with relatively high baseline activity and exciting neurons with low baseline activity. In comparison, haloperidol produced an indiscriminate inhibitory response in a subpopulation of mPFC neurons. Clozapine also reduced the disruptive effects of NMDA receptor hypofunction on the PFC neurons in a manner that was positively correlated with the expression of behavioral stereotypy.
Both clozapine and haloperidol are thought to treat the positive symptoms of schizophrenia by blocking dopamine D2 receptors (Creese et al 1976; Seeman et al 1976). However, the contribution of D2 receptors—especially in the PFC—to other dimensions of antipsychotic efficacy is less clear. Here, the only shared effect of clozapine and haloperidol was a sustained inhibition of a small subset of PFC neurons, suggesting that this subset of neurons might be especially sensitive to D2 receptor blockade. In contrast, haloperidol did not share clozapine’s state-dependent modulation of firing rates and its sustained excitatory effect on a subset of neurons. These latter effects, therefore, might be mediated through other receptor targets of clozapine, such as D1 dopamine, 5HT2 serotonin, and α-adrenergic and M1 muscarinic receptors. In fact, the unique properties of clozapine have been suggested to arise from its concomitant effects on several receptors rather than modulation of any single receptor per se (Meltzer et al 1989; Roth et al 2004). For example, both the ratio of antagonism at 5HT2 to D2 receptors (Stockmeier et al 1993) and the concomitant activation of α-adrenergic and D2 receptors (Svensson 2003) have been suggested, among others, as possible mechanisms for the unique effects of clozapine. Regardless of the special combination of receptors that mediate clozapine’s effects, it is important to characterize the functional endophenotype that represents these effects at the cellular level. The present data suggest that clozapine modulates PFC neuronal firing on the basis of baseline activity level of different ensembles. This modulatory activity might confer clozapine an ability to fine-tune the PFC function through gating unwanted disturbances in neuronal signal to noise ratio. Interestingly, D1 dopamine receptors have been suggested to exert a similar role in gating the excitability of PFC pyramidal neurons and in establishment of functional neural ensembles, on the basis of in vitro and anesthetized preparation experiments (O’Donnell 2003; Peters et al 2004; Yang and Seamans 1996) and neural modeling (Dreher and Burnod 2002; Durstewitz and Seamans 2002; Durstewitz et al 2000). It is possible that the present in vivo effects might also involve stimulation of D1 receptors, because clozapine is known to selectively enhance the extracellular release of dopamine in the PFC (Moghaddam and Bunney 1990; Yamamoto et al 1994).
Another possible mechanism for clozapine effects is potentiation of NMDA receptor transmission. Recent in vitro studies have shown that clozapine but not haloperidol enhances the excitatory NMDA current in PFC pyramidal neurons (Arvanov and Wang 1999; Arvanov et al 1997; Chen and Yang 2002). The positive modulation of NMDA currents might contribute to excitation of a subset of PFC neurons by clozapine and can prove helpful in conditions associated with NMDA receptor hypofunction. This is consistent with our findings that clozapine selectively reversed the electrophysiological disturbances caused by NMDA receptor antagonism through decreasing both the number and magnitude of response to MK801. It is likely that a potentiation of NMDA currents contributes to this effect of clozapine, because NMDA antagonists may cause a state of hyper-excitability through selective inhibition of γ-aminobutyric acid (GABA)ergic interneurons, leading to excessive non-NMDA glutamatergic activity (Greene 2001; Moghaddam et al 1997). Augmentation of NMDA transmission by clozapine can attenuate the initial disinhibition of GABAergic interneurons, thus blocking the overexcitation cascade. This mechanism is in agreement with our observation of a predominantly inhibitory effect of MK801 on putative interneurons and the blockade of this effect by clozapine. However, this idea remains to be further verified in future studies recording from a larger population of interneurons.
The characteristic stereotypical behaviors induced by NMDA antagonists in rodents have been compared with psychosis-related behaviors that have perseverative and restricted patterns (Gorelick and Balster 1994). This measure of NMDA antagonist behavioral disruption is dependent on both cortical and subcortical circuitry (Jentsch et al 1998; Takahata and Moghaddam 2003). Clozapine blocked the expression of behavioral stereotypy induced by the NMDA antagonist MK801. This behavioral effect was highly correlated with its effect on PFC neuronal excitability. This correlation suggests that PFC neuronal activity plays a critical role in clozapine action against NMDA antagonists. Indeed, NMDA antagonist stereotypy might arise from an impairment in proper sequencing of behavioral sets caused by persistent and disorganized overactivation of cortical neural ensembles (Jackson et al 2004). The fine tuning of these ensembles by clozapine would restore the PFC capability to choose the appropriate behavioral repertoire. Notably, a dose of haloperidol with comparable effect on stereotypy score had limited effect on PFC neural activity and led to a low correlation between PFC firing and behavior. This discrepancy suggests that the behavioral effect of haloperidol is most probably mediated at the subcortical level (Delfs and Kelley 1990).
N-desmethylclozapine is the main circulating metabolite of clozapine that shares many functional properties with clozapine including the ability to potentiate NMDA receptor- mediated currents (Sur et al 2003) and the induction of c-fos expression in PFC (Young et al 1998). The present data show that DMClz, similar to clozapine, state-dependently modulates basal PFC neuronal firing and normalizes NMDA antagonist-induced disruptions of PFC activity. N-desmethylclozapine also significantly reduces the behavioral effects of MK801, in close correlation with its effect on PFC firing. These findings predict that DMClz might offer beneficial effects comparable to clozapine on PFC-related symptomatology. Notably, a positive correlation between plasma concentration of DMClz and therapeutic response has been reported in schizophrenic patients on clozapine regimen (Piscitelli et al 1994; Weiner et al 2004). At the mechanistic level, DMClz has a high ratio of 5-HT1c to D2 affinities, comparable to clozapine (Kuoppamaki and Hietala 1993). In addition, DMClz is also a potent M1 muscarinic receptor agonist (Davies et al 2005; Sur et al 2003). Considering the reported cognitive enhancing properties of muscarinic potentiators (Friedman 2004), the similarity between DMClz and clozapine effects supports the possibility that positive modulation of M1 receptors might contribute to clozapine’s clinical efficacy.
The computational integrity of PFC, which is thought to be impaired in schizophrenia (Winterer and Weinberger 2004), is critical for executive control over goal-directed behavior (Bechara et al 2000; Funahashi et al 1993; Izaki et al 2001; Manes et al 2002; Sawaguchi and Iba 2001). For optimal function, PFC neural networks integrate external information with internal representations to determine the appropriate sets of actions (Braver and Barch 2002; Fuster 1985; Miller et al 2001). Both hypoactivity and over-engagement of neural ensembles would be expected to attenuate the efficiency of information processing within PFC through disrupting the representative or integrative capacities. Instead, an optimal modulation of PFC function would require fine-tuning of network activity by boosting the least active neural ensembles and dampening the more engaged ones, thus allowing maximal representation without limiting the capacity to shuffle between various perceptual and behavioral sets. Our findings suggest that clozapine might have the capacity to influence both hyperactivity and hypoactivity of cortical neurons and thus might produce its advantageous therapeutic efficacy by improving cortical processing capacity in schizophrenia.
Acknowledgments
This study was supported by the National Institute of Mental Health, Pittsburgh Life Sciences Green House, and Tourette Syndrome Association.
References
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango C, Breier A, McMahon R, Carpenter WJ, Buchanan R. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am J Psychiatry. 2003;160:1421–1427. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- Arvanov V, Liang X, Schwartz J, Grossman S, Wang R. Clozapine and haloperidol modulate N-methyl-D-aspartate- and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharm Exp Thera. 1997;283:226–234. [PubMed] [Google Scholar]
- Arvanov V, Wang R. Clozapine, but not haloperidol, prevents the functional hyperactivity of N-methyl-D-aspartate receptors in rat cortical neurons induced by subchronic administration of phencyclidine. J Pharmacol Exp Ther. 1999;289:1000–1006. [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of rats. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geter MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Braver T, Barch D. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson M. Neurotransmitter interactions in schizophrenia: Therapeutic implications. Biol Psychiatry. 1999;46:1388–1395. doi: 10.1016/s0006-3223(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Castner S, Goldman-Rakic P, Williams G. Animal models of working memory: Insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang C. Interaction of dopamine D1 and NMDA receptors mediates acute clozapine potentiation of glutamate EPSPs in rat prefrontal cortex. J Neurophysiol. 2002;87:2324–2336. doi: 10.1152/jn.2002.87.5.2324. [DOI] [PubMed] [Google Scholar]
- Conley R, Kelly D. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt D, Snyder S. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Davies M, Compton-Toth B, Hufeisen S, Meltzer H, Roth B. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: Is M1 agonism a pre-requisite for mimicking clozapine’s actions? Psychopharmacology (Berl) 2005;178:451–460. doi: 10.1007/s00213-004-2017-1. [DOI] [PubMed] [Google Scholar]
- Davis J. The choice of drugs for schizophrenia. N Engl J Med. 2006;354:518–520. doi: 10.1056/NEJMe058298. [DOI] [PubMed] [Google Scholar]
- Delfs J, Kelley A. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neurosci. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Deutch A, Duman R. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: Cellular localization and pharmacological characterization. Neurosci. 1996;70:377–389. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- Dreher J, Burnod Y. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Networks. 2002;15:583–602. doi: 10.1016/s0893-6080(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Duncan G, Leipzig J, Mailman R, Lieberman J. Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res. 1998;812:65–75. doi: 10.1016/s0006-8993(98)00926-3. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J. The computational role of dopamine D1 receptors in working memory. Neural Networks. 2002;15:651–672. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J, Sejnowski T. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Freedman R. The choice of antipsychotic drugs for schizophrenia. N Engl J Med. 2005;353:1286–1288. doi: 10.1056/NEJMe058200. [DOI] [PubMed] [Google Scholar]
- Friedman J. Cholinergic targets for cognitive enhancement in schizophrenia: Focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 2004;174:45–53. doi: 10.1007/s00213-004-1794-x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce C, Goldman-Rakic P. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas.”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex, mediator of cross-temporal contingencies. Human Neurobiol. 1985;4:169–179. [PubMed] [Google Scholar]
- Gemperle A, Enz A, Pozza M, Luthi A, Olpe H. Effects of clozapine, haloperidol and iloperidone on neurotransmission and synaptic plasticity in prefrontal cortex and their accumulation in brain tissue: An in vitro study. Neurosci. 2003;117:681–695. doi: 10.1016/s0306-4522(02)00769-8. [DOI] [PubMed] [Google Scholar]
- Gold J, Weinberger D. Cognitive deficits and the neurobiology of schizophrenia. Curr Opin Neurobiol. 1995;5:225–230. doi: 10.1016/0959-4388(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Gorelick D, Balster R. Phencyclidine (PCP) In: Bloom F, Kupfer D, editors. Psychopharmacology, The Fourth Generation of Progress. New York: Raven Press; 1994. pp. 1767–1776. [Google Scholar]
- Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11:569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 (mGlu2/3) receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2004;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Maruki K, Hori K, Nomura M. Effects of rat medial prefrontal cortex temporal inactivation on a delayed alternation task. Neurosci Lett. 2001;315:129–132. doi: 10.1016/s0304-3940(01)02366-7. [DOI] [PubMed] [Google Scholar]
- Jackson M, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:6391–6396. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardemark K, Ninan I, Liang X, Wang R. Protein kinase C is involved in clozapine’s facilitation of N-methyl-D-aspartate- and electrically evoked responses in pyramidal cells of the medial prefrontal cortex. Neurosci. 2003;118:501–512. doi: 10.1016/s0306-4522(02)00976-4. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: Behavioral and neurochemical evidence. Psychopharmacol. 1998;138:89–95. doi: 10.1007/s002130050649. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Karoum F, Egan M. Dopamine release and metabolism in the rat frontal cortex, nucleus accumbens, and striatum: A comparison of acute clozapine and haloperidol. Br J Pharmacol. 1992;105:703–707. doi: 10.1111/j.1476-5381.1992.tb09042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: A review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Konicki P, Kwon K, Steele V, White J, Fuller M, Jurjus G, Jaskiw G. Prefrontal cortical sulcal widening associated with poor treatment response to clozapine. Schizo Res. 2001;48:173–176. doi: 10.1016/s0920-9964(00)00130-4. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kuoppamaki MSE, Hietala J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol. 1993;245:179–182. doi: 10.1016/0922-4106(93)90126-t. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer H, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- Lahti A, Holcomb H, Weiler M, Medoff D, Frey K, Hardin M, Tamminga C. Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Eng J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lee M, Jayathilake K, Meltzer H. A comparison of the effect of clozapine with typical neuroleptics on cognitive function in neuroleptic-responsive schizophrenia. Schizophr Res. 1999;37:1–11. doi: 10.1016/s0920-9964(98)00145-5. [DOI] [PubMed] [Google Scholar]
- Lewis D, Hashimoto T, Volk D. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A. Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: A study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Maurel-Remy S, Bervoets K, Millan MJ. Blockade of phencyclidine-induced hyperlocomotion by clozapine and MDL in rats reflects antagonism of 5-HT2A receptors. Eur J Pharmacol. 1995;280:R9–R11. doi: 10.1016/0014-2999(95)00333-g. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, et al. CATIE Investigators. Effectiveness of clozapine versus olan-zapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- McGurk S. The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry. 1999;60 suppl 1:24–29. [PubMed] [Google Scholar]
- Meltzer H, Alphs L, Green A, Altamura A, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- Meltzer H, McGurk S. The effects of clozapine, risperidone, and olan-zapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and seratonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Miller P, Lawrie SM, Hodges A, Clafferty R, Cosway R, Johnstone EC. Genetic liability, illicit drug use, life stress and psychotic symptoms: Preliminary findings from the Edinburgh study of people at high risk for schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2001;36:338–342. doi: 10.1007/s001270170038. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan G, Marx C, Lieberman J. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: An in vivo microdialysis study. J Neurosci. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sarramea F, Sanz J, Francisco Artaloytia J, Luque R, et al. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiat Res. 2003;124:153–161. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peters Y, Barnhardt N, O’Donnell P. Prefrontal cortical up states are synchronized with ventral tegmental area activity. Synapse. 2004;52:143–152. doi: 10.1002/syn.20015. [DOI] [PubMed] [Google Scholar]
- Piscitelli S, Frazier J, McKenna K, Albus K, Grothe D, Gordon C, Rapoport J. Plasma clozapine and haloperidol concentrations in adolescents with childhood-onset schizophrenia: Association with response. J Clin Psychiatry. 1994;55 suppl B:94–97. [PubMed] [Google Scholar]
- Robertson G, Fibiger H. Neuroleptics increase c-fos expression in the forebrain: Contrasting effects of haloperidol and clozapine. Neurosci. 1992;46:315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Roth B, Sheffler D, Kroeze W. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. A test of the predictive validity of animal models of schizophrenia based on phencyclidine and D-amphetamine. Neuropsychopharmacology. 1998;18:293–304. doi: 10.1016/S0893-133X(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Iba M. Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. J Neurophysiol. 2001;86:2041–2053. doi: 10.1152/jn.2001.86.4.2041. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antispsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Sharma T. Cognitive effects of conventional and atypical antipsychotics in schizophrenia. Br J Psychiatry Suppl. 1999;38:44–51. [PubMed] [Google Scholar]
- Spivak B, Shabash E, Sheitman B, Weizman A, Mester R. The effects of clozapine versus haloperidol on measures of impulsive aggression and suicidality in chronic schizophrenia patients: An open, nonrandomized, 6-month study. J Clin Psychiatry. 2003;64:755–760. doi: 10.4088/jcp.v64n0703. [DOI] [PubMed] [Google Scholar]
- Stockmeier C, DiCarlo J, Zhang Y, Thompson P, Meltzer H. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther. 1993;266:1374–1384. [PubMed] [Google Scholar]
- Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci U S A. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson T. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Biol Psychiatry. 2003;27:1145–1158. doi: 10.1016/j.pnpbp.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Tiedtke PI, Bischoff C, Schmidt WJ. MK-801-induced stereotypy and its antagonism by neuroleptic drugs. J Neural Trans. 1990;81:173–182. doi: 10.1007/BF01245040. [DOI] [PubMed] [Google Scholar]
- Tierney P, Degenetais E, Thierry A, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: Modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Essali M. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2000;2 doi: 10.1002/14651858.CD000059. CD000059. [DOI] [PubMed] [Google Scholar]
- Weiner D, Meltzer H, Veinbergs I, Donohue E, Spalding T, Smith T, et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology (Berl) 2004;177:207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:393–401. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger D. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto B, Pehek E, Meltzer H. Brain region effects of clozapine on amino acid and monoamine transmission. J Clin Psychiatry. 1994;55 suppl B:8–14. [PubMed] [Google Scholar]
- Yang C, Seamans J. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: Modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Meltzer H, Deutch A. Effects of desmethylclozapine on Fos protein expression in the forebrain: In vivo biological activity of the clozapine metabolite. Neuropsychopharmacology. 1998;19:99–103. doi: 10.1016/S0893-133X(97)00203-0. [DOI] [PubMed] [Google Scholar]
- Youngren KD, Inglis FM, Pivirotto PJ, Jedema HP, Bradberry CW, Goldman-Rakic PS, et al. Clozapine preferentially increases dopamine release in the rhesus monkey prefrontal cortex compared with the caudate nucleus. Neuropsychopharmacology. 1999;20:403–412. doi: 10.1016/S0893-133X(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Youngren KD, Moghaddam B, Bunney BS, Roth RH. Preferential activation of dopamine overflow in prefrontal cortex produced by chronic clozapine treatment. Neurosci Lett. 1994;165:41–44. doi: 10.1016/0304-3940(94)90704-8. [DOI] [PubMed] [Google Scholar]